Abstract
A subset of patients with a variety of cancers shows evidence of a natural adaptive immune response against their tumor, as evidenced by spontaneous T-cell infiltration, circulating anti-tumor T cells, or antibody responses. Evidence has indicated that such natural immune responses have positive prognostic import in early stage disease and may be predictive of clinical response to immunotherapeutics in advanced disease. However, these observations raise a new critical fundamental question—what innate immune signals might be generated in the context of non-pathogen-induced cancers that drive productive antigen presentation toward induction of an adaptive immune response? Gene expression profiling in melanoma revealed that tumors having high expression of T-cell markers also show evidence of a type I IFN transcriptional signature. Mechanistic experiments in mice have revealed that a spontaneous CD8+ T-cell response against transplantable tumors depends on host type I IFN signaling, through a mechanism dependent upon CD8α+ dendritic cells (DCs). The requirement for type I IFN production by host DCs has suggested a subset of innate immune sensing receptors and signaling pathways that might be involved with initiating this process. Elucidating further these innate immune mechanisms should provide new insights into cancer immunotherapy.
Keywords: Tumor immunity, Type I IFNs, Dendritic cells, T cells, PIVAC 11
Evidence for spontaneous T-cell responses against cancer
Contrary to the preconceptions of many in the field, a spontaneous immune response against human cancers is a relatively frequent occurrence. Infiltration with activated T cells into the tumor microenvironment has been observed in a subset of patients with a variety of cancer histologies. In early stage colorectal cancer, the presence of effector/memory CD8+ T cells in the tumor has powerful prognostic importance [1], having recently been reported to be more predictive of outcome than TNM stage [2]. Similar positive prognostic import has been observed in breast cancer and in ovarian cancer [[3], [4]]. Primary melanoma lesions have long been categorized based on the presence or absence of tumor-infiltrating lymphocytes [5]. In metastatic melanoma, the presence of an inflamed tumor microenvironment that includes a T-cell infiltrate appears to predictive for clinical response to several immunotherapies. This includes clinical benefit to several cancer vaccines, the anti-CTLA-4 mAb ipilimumab, and IL-2 [6–11]. Thus, the presence of an ongoing dialog between a tumor and the host immune response may predict the ability of an immunotherapeutic intervention to tip the balance in favor of immune-mediated tumor control.
While the presence of a T-cell infiltrate in the tumor microenvironment may be an interesting biomarker, it is important to know whether among those T cells might be a subset having specificity for tumor antigens. In melanoma, the use of peptide/class I MHC multimers has confirmed the presence of CD8+ T cells that recognize shared tumor antigens within the tumor microenvironment [12–14]. Spontaneous generation of CD8+ T cells detected among circulating PBMCs also has been observed, against NY-Eso1, Melan-A, and MAGE-10 [15–17]. Thus, even though additional T cells may be recognizing unique or mutated antigens, these results suggest that an immune response directed against the tumor is being mounted. This data suggesting spontaneous immunity against tumor antigens should not be completely surprising, as they are in keeping with established knowledge of immune-mediated paraneoplastic syndromes that are caused by cross-reactive antibodies generated against tumor-associated molecules [18]. Spontaneous antibodies against a broad range of proteins has been observed to occur early in prostate cancer development [19], arguing that immune responses against tumor-associated molecules extends beyond melanoma and may be a common occurrence.
If natural immune responses are indeed generated against cancers, at least in a subset of patients, the question arises as to why those tumors are not spontaneously rejected. In most cases, the presence of an apparent host immune response has positive prognostic and/or predictive value, arguing that this process is favoring a better clinical outcome even if it is not complete. Analysis of melanomas having an “inflamed” phenotype that includes a T-cell infiltrate has demonstrated high level of expression of putative negative regulatory mechanisms that likely suppress T-cell function. This includes the tryptophan-catabolizing enzyme indoleamine-2,3-dioxygenase (IDO); PD-L1, the main ligand for the inhibitory receptor PD-1; CD4+CD25+FoxP3+ regulatory T cells (Tregs); and also T-cell anergy, a T-cell-intrinsic hyporesponsive state [20]. Strategies to block or reverse these immune-inhibitory pathways are attractive approaches for cancer immunotherapy. Each strategy has been confirmed to be active in preclinical models [21–24], and several approaches are currently being explored clinically. Thus, for the subset of patients with a T-cell-inflamed tumor microenvironment in which negative regulation appears dominant, there are great expectations that effective immunotherapies are becoming established. However, for the major subset of patients with tumors that lack this natural immune response and have non-inflamed tumor phenotypes, these therapies may have limited value. Thus, attention is shifting toward understanding the mechanisms that underlie the presence or absence of a spontaneous anti-tumor immune response, with the hopes of ultimately transforming those tumors into the inflamed subtype and thus expand the subset of patients capable of responding to immune-based therapies.
A role for host type I IFN signaling in innate tumor recognition
In order to begin to investigate potential mechanisms of innate immune recognition of melanoma that might bridge to an adaptive immune response, gene-expression profiles of human melanoma metastases [7] were interrogated for transcriptional patterns associated with T-cell markers. Indeed, expression of a set of genes known to be driven by type I IFNs was observed to correlate with TCR transcripts. This raised the possibility that type I IFN signaling might be involved in natural immune responses against tumors. In order to test this hypothesis experimentally, a set of mouse models was utilized in which tumors were engineered to express a model antigen against which T-cell response could be measured. Indeed, implantation of these transplantable tumors into syngeneic mice in vivo led to a CD8+ T-cell response that was detectable in the draining lymph node around 6 days later. This model system allowed for the utilization of knockout mice lacking specific host immune components to examine for their necessity upstream from this T-cell priming. Mice deficient in the type I IFN receptor or the downstream signaling molecule Stat1 showed markedly diminished spontaneous T-cell responses, arguing for the necessity of this pathway in vivo. A corresponding increase in IFN-β transcripts was detected by qRT-PCR in the tumor-draining lymph node that preceded the T-cell response, most of which was produced by CD11c+ dendritic cells (DCs). Mechanistically, the requirement for type I IFNs mapped to the antigen-presenting cell/DC compartment. The vast majority of DC properties and functions were found to be intact in type I IFN−/− receptor or Stat1−/− animals. However, it was found that the CD8α+ DC subset failed to accumulate in the tumors in the absence of host type I IFN signaling. Batf3−/− mice, which lack the CD8α+ DC subset, also showed defective T-cell priming against tumor-associated antigens. Mixed bone marrow chimera studies mapped the major type I IFN signaling activity to the CD8α+ DC lineage. Together, these data argue that host DCs “sense” something derived from the implanted tumor in vivo, which drives IFN-β production and cross-priming via CD8α+ DCs [25]. These data are consistent with the work of Reis e Sousa and colleagues who have demonstrated a role for Clec9a, a receptor highly expressed on CD8α+ DCs, in the cross-presentation of antigen from dying cells [26].
A similar set of experiments has been performed by the laboratory of Dr. Robert Schreiber. Using a panel of immunogenic tumors that are spontaneously rejected by immunocompetent mice, they also found a role for host type I IFN signaling for this potent spontaneous immune response. In vitro, they provided evidence that one role for type I IFN is to improve DC-mediated cross-presentation [27]. In vivo, they also have demonstrated a role for the CD8α+ DC lineage for spontaneous rejection of these tumors [28]. These data are, therefore, quite in agreement and support a critical role for host type I IFNs in the generation of natural anti-tumor T-cell responses.
What are the host innate sensing pathways that drive type I IFN production?
The identification of type I IFN production by host APCs as an important step during the innate immune recognition of tumors in vivo during the bridge to adaptive immunity has pushed the problem further upstream toward the identification of the receptor system and tumor-derived ligand that mediate this effect. The field of innate immune sensing is growing rapidly, with several distinct families of proteins having been identified and additional family members still uncharacterized. The toll-like receptor system was the first to be identified, members of which signal via the adaptors MyD88 or TRIF to lead to production of inflammatory cytokines and upregulated expression of costimulatory ligands [29]. Other classes include the NOD-like receptors (NLRs, many of which activate the inflammasome) [30], C-type lectin receptors [31], and cytosolic nucleotide sensors that recognize either RNA or DNA and have been defined in the context of viral infection [32–34]. In addition, the extracellular ATP sensor P2X7R has been suggested to detect the presence of ATP that may be released from dead or dying cells [35, 36]. It is conceivable that tumor-derived products could engage one or more of these receptor systems and initiate innate immune activation. Experiments performed killing cancer cells with specific chemotherapeutic agents or radiation has supported the potential role for TLR signaling via HMGB1 released from dying tumor cells, and also for the inflammasome [37, 38]. However, preliminary data from our laboratory have excluded a mandatory role for either MyD88, Trif, or P2X7R in the spontaneous immune response against tumor-associated antigens in vivo (unpublished data). Additional work will be needed to define the relevant pathways during a natural immune response against tumors in which the mechanisms of death of a subset of cells within a growing tumor mass may be distinct.
Clinical implications
There are several implications of this fundamental work toward clinical application. First, the opportunity has arisen to begin to investigate the molecular mechanisms that explain why a subset of patients develops a spontaneous immune response against their tumor while a major subset does not. One potential explanation may reside at the level of polymorphisms in immune regulatory genes that impact on thresholds of innate immune sensing. Within the type I IFN pathway itself, numerous gene polymorphisms have been identified that have strong association with clinical lupus [39, 40]. Thus, pursuit of germline gene polymorphisms that may be associated with the presence or absence of a T-cell infiltrate is an attractive consideration. Somatic mutation differences between tumors of individual patients also may exist that could activate specific accessory oncogene pathways that either activate or repress the expression of immunoregulatory genes by the tumor cells. A systematic interrogation of patient tumor samples for genetic alterations associated with an immune infiltrate also should be pursued. Once key mechanisms are established, then it will be of interest to know whether immune selection pressure against a tumor phenotype that stimulates innate immunity might arise through immune editing, much as adaptive T-cell responses can sculpt tumor phenotype.
A second major clinical implication of these findings is that they may open the possibility of new immunotherapeutic interventions based on augmenting innate immune activation in the tumor context. Introducing local expression of innate immune cytokines or activators of innate immune sensing pathways could have therapeutic utility. In fact, converting a “non-inflamed” tumor microenvironment into the “inflamed” phenotype that can support the effector phase of the anti-tumor T-cell response may represent one of the biggest current challenges to the field. Examples to be considered include introduction of type I IFNs, TLR agonists, or ligands for the NLR or nucleic acid sensing pathways. The most effective local immune modulators at the level of the tumor microenvironment would subsequently need to be formulated in such a way as to allow systemic administration but preferential accumulation in metastatic tumor sites. Such therapeutic approaches could ultimately combined with vaccination of adoptive T-cell therapy as methods to increase specific T-cell frequencies, or with blockade of negative regulatory pathways such as PD-L1/PD-1 interactions (Fig. 1).
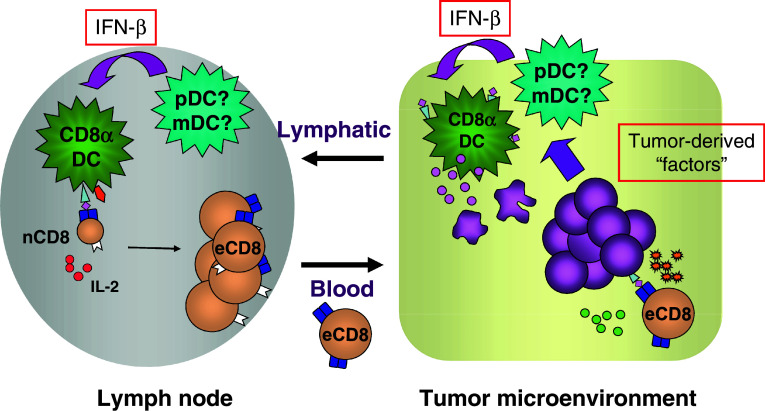
Fig. 1.
Model for the role of host type I IFNs in the innate immune sensing of cancer. Still unidentified tumor-derived substances appear to induce the production of IFN-β from host CD11c+ DCs. This, in turn, acts to promote cross-presentation of tumor-derived antigens to host CD8+ T cells, via the CD8α+ subset of DCs. This innate immune activation and inflammation in the tumor microenvironment also may facilitate the effector phase of the anti-tumor T-cell response and support the final steps of tumor rejection
Acknowledgment
This work was supported by P01 CA97296 from the National Cancer Institute, USA.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Eleventh International Conference on Progress in Vaccination against Cancer (PIVAC 11), held in Copenhagen, Denmark, 10th–13th October 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 2.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Elder DE, Van Belle P, Elenitsas R, et al. Neoplastic progression and prognosis in melanoma. Semin Cutan Med Surg. 1996;15:336–348. doi: 10.1016/S1085-5629(96)80047-2. [DOI] [PubMed] [Google Scholar]
- 6.Gajewski TF, Zha Y, Thurner B, Schuler G. Association of gene expression profile in melanoma and survival to a dendritic cell-based vaccine. J Clin Oncol. 2009;27:9002. [Google Scholar]
- 7.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan RJ, Hoshida Y, Brunet J, et al. A single center experience with high-dose IL-2 treatment for patients with advanced melanoma and pilot investigation of a novel gene expression signature as a predictor of response. J Clin Oncol. 2009;27:15S. [Google Scholar]
- 10.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji RR, Chasalow SD, Wang L et al (2011) An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed]
- 12.Harlin H, Kuna TV, Peterson AC, et al. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appay V, Jandus C, Voelter V, et al. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177:1670–1678. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 14.Mortarini R, Piris A, Maurichi A, et al. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535–2545. [PubMed] [Google Scholar]
- 15.Valmori D, Dutoit V, Rubio-Godoy V, et al. Frequent cytolytic T-cell responses to peptide MAGE-A10(254–262) in melanoma. Cancer Res. 2001;61:509–512. [PubMed] [Google Scholar]
- 16.Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- 17.Jager E, Chen YT, Drijfhout JW, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darnell RB, Posner JB. Paraneoplastic syndromes affecting the nervous system. Semin Oncol. 2006;33:270–298. doi: 10.1053/j.seminoncol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Yu J, Sreekumar A, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 20.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 21.Blank C, Brown I, Peterson AC, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.CAN-03-3259. [DOI] [PubMed] [Google Scholar]
- 22.Brown IE, Blank C, Kline J, et al. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 23.Kline J, Brown IE, Zha YY, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 24.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 25.Fuertes MB, Kacha AK, Kline J et al (2011) Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med [DOI] [PMC free article] [PubMed]
- 26.Sancho D, Joffre OP, Keller AM, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildner K, Edelson BT, Purtha WE, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol. 2002;270:81–92. doi: 10.1007/978-3-642-59430-4_5. [DOI] [PubMed] [Google Scholar]
- 30.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 31.Sancho D, Mourao-Sa D, Joffre OP, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCartney S, Vermi W, Gilfillan S, et al. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–2976. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lister MF, Sharkey J, Sawatzky DA, et al. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller T, Vieira RP, Grimm M, et al. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol. 2011;44:456–464. doi: 10.1165/rcmb.2010-0129OC. [DOI] [PubMed] [Google Scholar]
- 37.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 38.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 39.Niewold TB, Hua J, Lehman TJ, et al. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salloum R, Franek BS, Kariuki SN, et al. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients. Arthr Rheum. 2010;62:553–561. doi: 10.1002/art.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]