Abstract
The global prevalence of Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) stands at approximately 43 % among individuals who have previously had acute COVID-19. In contrast, in the United States, the National Center for Health Statistics (NCHS) estimates that around 11 % of individuals who have been infected with SARS-CoV-2 go on to experience long COVID. The underlying causes of PASC remain under investigation, and there are no currently established FDA-approved therapies. One of the leading hypotheses for the cause of PASC is the persistent activation of innate immune cells with increased systemic inflammation. Naltrexone is a medication with anti-inflammatory and immunomodulatory properties that has been used in other conditions that overlap with PASC. We performed a retrospective review of a clinical cohort of 59 patients at a single academic center who received low-dose naltrexone (LDN) off-label as a potential therapeutic intervention for PASC. The use of LDN was associated with a fewer number of symptoms, improved clinical symptoms (fatigue, post-exertional malaise, unrefreshing sleep, and abnormal sleep pattern), and a better functional status. This observation warrants testing in rigorous, randomized, placebo-controlled clinical trials.
Keywords: Naltrexone, LDN, long COVID, treatment, Post-Acute Sequelae of COVID-19, long Haulers, PASC
Introduction
The NIH has provided guidelines on treating acute COVID-19 for hospitalized and non-hospitalized adults and children. However, recommendations are lacking for patients with Long COVID, also known as Post-Acute Sequelae of SARS-CoV-2 (PASC) [1]. In September 2021, the NIH launched the Researching COVID to Enhance Recovery (RECOVER). Initiative to understand the clinical manifestations and pathobiology of post-COVID conditions and test interventions for patients with PASC [2]. One of the leading hypotheses of PASC pathophysiology is immune and inflammatory dysregulation. Therefore, the therapeutic use of immune modulators and anti-inflammatory medications warrants investigation[3,4]. Naltrexone hydrochloride is an oral μ-opioid receptor antagonist that has immune-modulating properties. Off-label low-dose naltrexone (LDN) has been used for fibromyalgia, Crohn’s disease, multiple sclerosis, complex regional pain syndrome, Hailey-Haile disease, cancer, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), and PASC [5–8]. There is a significant overlap between ME/CFS and PASC symptoms [9]. In a cohort of 140 patients with PASC, 46 % also met the diagnostic criteria for ME/CFS [10]. Here, we report patients’ experience at a single academic PASC clinic who received LDN as a potential therapeutic intervention for PASC.
Material and Methods
We employed a detailed retrospective review of Electronic Health Records (EHR) from 468 adult patients in the Stanford PASC clinic with a history of SARS-CoV-2 infection and symptoms attributable to PASC [9]. Our study population were predominant White and lower proportions of Hispanic, Asian, and Black. Majority did not require hospitalization for their acute infection. We reviewed the charts for patients who received LDN for the treatment of PASC between May 18, 2021, to March 17, 2023. LDN was typically prescribed as individualized dose-titration ranging from 0.5 mg daily to 6.0 mg daily. All patients had a documented previous positive test for SARS-CoV-2 (PCR, antigen detection, or a positive serology before SARS-CoV-2 vaccination), and persistent symptoms for ≥28 days following infection. Any SARS-CoV-2 infection before December 1, 2021, was considered an infection caused by a non-Omicron variant. We also recorded the SARS-CoV-2 vaccination status. Symptoms were ascertained using a detailed questionnaire that was completed by patients within seven days before each clinic visit (Questionnaire is included in the supplementary file). The questionnaire included (i) 29 symptoms capturing commonly reported in patients with acute COVID-19, aiming to cover different conditions such as ME/CFS, autonomic, respiratory, cardiac, neurological, psychiatric, olfactory, and gastrointestinal disorders; (ii) the severity of each symptom based on the Likert scale (none = 0, mild = 1, moderate = 2, severe = 3 very severe = 4 and incapacitating = 5) (iii) the Post COVID-19 Functional Status Scale (FSS) modified from Klok et al. [11], which classifies patients as either asymptomatic (level I), symptomatic without limitations (level II), symptomatic with reduced daily activity (level III), symptomatic with a struggle to perform daily activities (level IV), or incapacitated and bedridden (level V). Of the 29 symptoms captured, the seven most frequently identified symptoms from our overall clinic PASC cohort were selected for our primary comparative pre- and post-treatment analyses based on graded Likert scale (1 to 5): headache, fatigue, brain fog, unrefreshing sleep, sleep difficulties, post-exertional malaise, and lightheadedness. A sum composite score was created by adding the severity score of the selected seven symptoms (fatigue, brain fog, post exertional malaise, unrefreshing sleep, abnormal sleep pattern, lightheadedness, and headache) before and after LDN, and the change was grouped into three categories: Same = no score change, Better = negative score (Better), and Worse = higher score.
We used Research Electronic Data Capture (REDCap) and Excel platforms for data collection. The current study falls under the Stanford Post-Acute COVID-19 Syndromes: Patient Database, which received approval from the Stanford Institutional Review Board; protocol #62996. Of the 468 patients seen in the clinic during the specified period, LDN was prescribed by a clinic provider for 207 patients (44.2 %). LDN was prescribed by a provider outside of our clinic for an additional 15 patients (2.8 %). For 59 (27.0 %) of the 207 patients who were prescribed LDN, symptom-based questionaries were completed at each clinic visit (Excel supplementary data includes days on LDN, doses of LDN, FSS, number of symptoms, severity of the selected symptoms sum composite scores).
Statistical Analysis
Patient characteristics and changes in symptom severity were summarized using median and interquartile ranges and compared using the Mann-Whiney rank test according to the time since initial COVID-19 infections (<365 days vs ≥365 days). Changes in symptoms severity and functional status scale between patients who did and did not receive LDN were also compared using Mann-Whiney rank test. The number of symptoms and symptom severity before and after LDN were summarized and compared using the Wilcoxon rank sum test. The correlation coefficient was calculated between the LDN dose and clinical symptom scores. The statistical significance level was set at the two-sided 0.05.
Results
Among the 59 PASC patients from our clinic who received LDN and completed all questionnaires, the median age was 45 years (IQR 34–59), and the majority were women (n = 40; 67.8 %). The median duration of symptoms from initial SARS-CoV-2 infection to LDN use was 361 days (range 61–708 days). The median number of symptoms at baseline was 14 (IQR 9–16). At baseline, the functional status scale (FSS) was II in 1 patient (2 %), III in 23 patients (36 %), IV in 29 patients (51 %), and V in 6 patients (12 %). Most of the SARS-CoV-2 infections (83.3 %) occurred in the pre-Omicron era. Fifty-two (88 %) of patients had received at least one dose of the COVID vaccine (7 (12 %) 2 doses, 42 (71 %) >3doses). At the last office evaluation, the LDN dose ranged from 0.5 to 6 mg; the median dose was 2.0 mg [IQR 1–5]. The median number of days on LDN was 143 days [IQR 77–255]. We found a significant improvement in the total number of symptoms, severity in subset of symptoms (fatigue, post-exertional malaise, unrefreshing sleep, and abnormal sleep pattern) and FSS among patients taking LDN. (Table 1, Fig. 1-Box plot and Sankey diagram). There were no correlations between LDN dose and scored responses to the selected symptoms (correlation coefficient: headache 0.18, fatigue 0.10, brain fog 0.07, unrefreshing sleep 0.13, abnormal sleep pattern 0.13, post-exertional malaise 0.13 and lightheadedness 0). Symptom responses were grouped by the duration of symptoms pretreatment by comparing patients with symptoms ≥365 days (one year) against patients with symptoms <365 days. Twenty-eight patients had symptoms for <365 days (47.5 %), and 31 had symptoms for ≥365 days (52.5 %). There was no statistical difference between these two groups regarding age, days on LDN, number of symptoms, changes in the number of symptoms, or improvement in the selected symptoms (Table 2). A sum composite score showed LDN improvement in 32 (54.2 %), worsening 19 (32.2 %) of the selected symptoms (Excel supplementary data).
Table 1.
Symptoms and Functional Status Scale before and after initiation of Low Dose Naltrexone.
| Before LDN | After LDN | P-value | |
|---|---|---|---|
| Number of symptoms | 14 [9, 16], 13.07* | 12 [6.5, 14], 10.93* | 0.000 |
| 11.9% V | 3.4% V | ||
| Fatigue | 4 [3, 5], 3.97 | 4 [3, 4], 3.41 | 0.013 |
| Brain fog | 4 [2, 4], 3.14 | 3 [2, 4], 2.78 | 0.097 |
| Post exertional malaise | 4 [4, 5], 3.76 | 3 [2, 5], 3.14 | 0.010 |
| Unrefreshing sleep | 4 [2.5, 5], 3.48 | 3 [1.5, 5], 2.85 | 0.010 |
| Abnormal sleep pattern | 3 [0, 5], 2.76 | 2 [0, 4], 2.05 | 0.016 |
| Lightheadedness | 2 [0, 3], 1.90 | 2 [0, 3], 1.68 | 0.304 |
| Headache | 3 [2, 4], 2.61 | 3 [1, 4], 2.42 | 0.428 |
Median [inter-quartiles], mean
Figure 1.
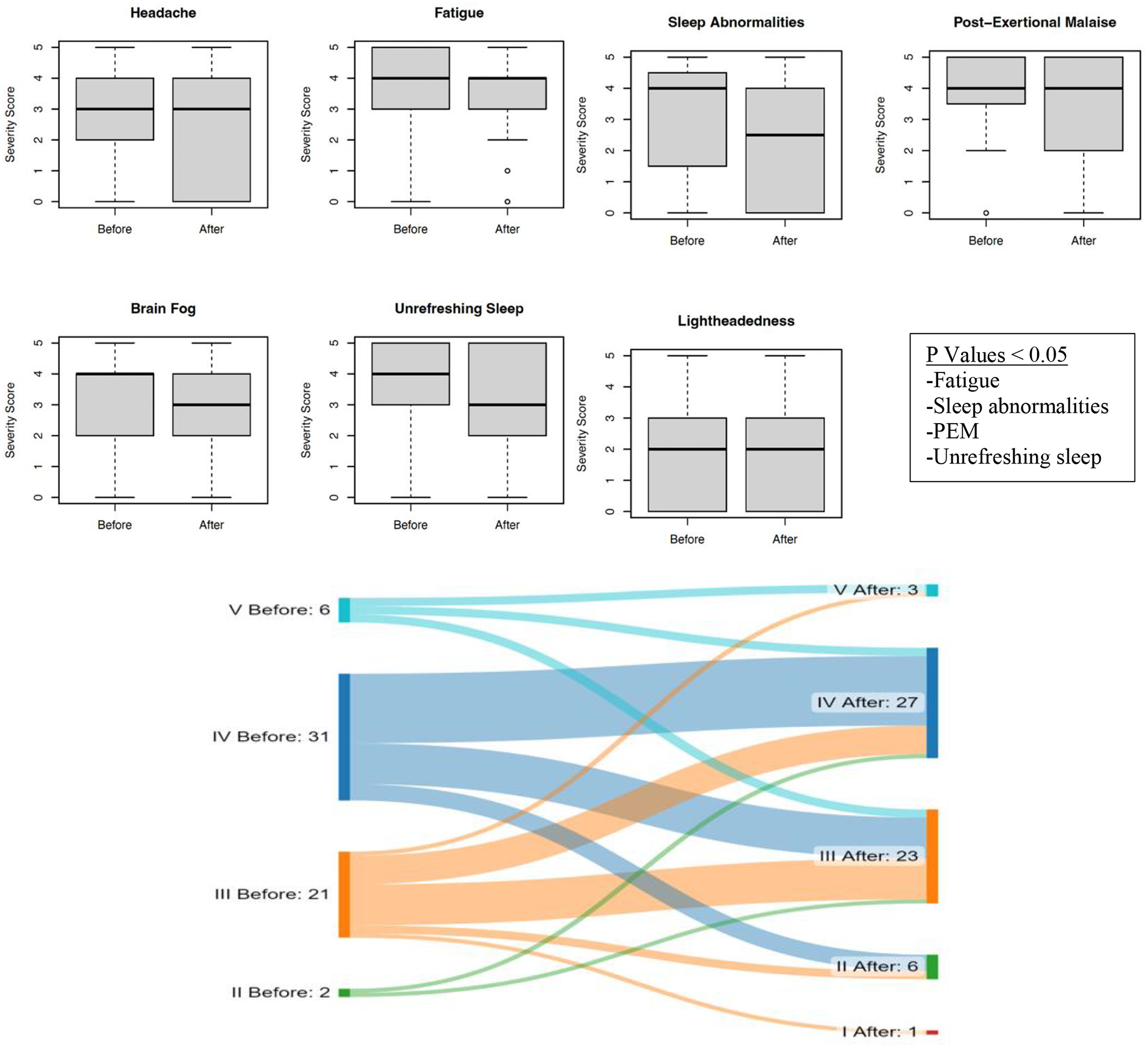
Plot box interquartile of the Likert score (0–5) based on the severity of each seven.
selected symptoms (Headache, fatigue, brain fog, unrefreshing sleep, sleep abnormalities, post-exertional malaise, and lightheadedness) before (left) and after (right) a Low Dose of Naltrexone.
Supplementary Material. In the bottom the Sankey diagram compares the FSS (I-V) before (left) after (right) LDN and the thickness of the arrows represents the magnitude of the change and the direction of FSS changes.
Table 2.
Characteristics and symptom changes in patients with PASC receiving Low Dose Naltrexone (0.5–6 mg) by the duration of infection.
| >=365 days | <365 days | P-value | |
|---|---|---|---|
| Cases | 28 (47.5%) | 31 (52.5%) | |
| Number of symptoms at baseline | 14.5 [9, 16.25], 13.107* | 14 [8.5, 15.5], 13.032 | 0.790 |
| Days since infection | 514 [469, 615], 533 | 273 [146, 329], 244 | |
| Age | 44 [33, 54.25], 44.89 | 50 [35, 60.5], 48.58 | 0.425 |
| Female | 22 (78.6%) | 18 (58.1%) | 0.105 |
| Days on LDN** | 133 [70, 162], 137 | 168 [86, 343], 217 | 0.083 |
| Change in number of symptoms | −1 [−4, 0], −1.46 | −2 [−4.5, 0], −2.74 | 0.368 |
| Change in Fatigue | −1 [−1, 0], −0.61 | 0 [−1.5, 1], −0.52 | 0.495 |
| Change in Brain fog | 0 [−1,0.25], −0.29 | 0 [−1, 0], −0.42 | 0.729 |
| Change in Post exertional malaise *** | 0 [−1, 0], −0.43 | 0 [−1.5, 0], −0.81 | 0.789 |
| Change in Unrefreshing sleep | 0 [−2, 0], −0.82 | 0 [−1.5, 0.5], −0.45 | 0.351 |
| Change Abnormal sleep pattern | −1 [−2.25, 0], −0.86 | 0 [−2, 0], −0.58 | 0.695 |
| Change in Headache | −0.5 [−1, 0], −0.39 | 0 [−1, 1], 0.000 | 0.333 |
| Change in Lightheadedness | 0 [0, 0.25], 0.00 | 0 [−1, 0.5], −0.42 | 0.393 |
Median [inter-quartiles] mean;
Low Dose Naltrexone;
Post Exertional Mala
Discussion
The overall global prevalence of PASC is estimated at 43 % of acute cases (54 % among hospitalized and 36 % among non-hospitalized) [9]. The CDC estimates that 11 % of adults (over 24 million people) have long COVID symptoms, and this proportion is three times higher among 50–59-year-olds than individuals over 80 [12,13]. The most prevalent symptoms include fatigue/weakness, myalgia/arthralgia, depression, anxiety, memory loss, concentration difficulties, dyspnea, and insomnia [9]. Although the underlying biological mechanisms remain elusive, chronic inflammation driving end-organ disease is one of the main proposed hypotheses [3,4,14]. There are currently no FDA-approved therapies for treating PASC symptoms, and there is an urgent need to find effective interventions.
In vitro studies had demonstrated the immunoinhibitory impact of opioids on the immune system via μ-opioid receptor downregulating the immune system (decrease function on macrophages, neutrophils, NK cells, microglia, and astrocyte) [15,16]. Naltrexone, an μ-opioid receptor blocker, has regulatory tissue growth, anti-inflammatory and immunomodulatory effects. The regulatory tissue growth is mediated by antagonist opioid growth factor receptor [17,18] and the anti-inflammatory and immunomodulator mechanisms through attenuation of proinflammatory cytokines (IL-1, IL-6, TNF-α, and INF-β), nitric oxide, suppressive effects on microglia cells, Toll-like receptor-4 (TLR4) antagonist, and regulatory effects on NK cell function [19–24].
Although the possible benefit of LDN in PASC is bases on clinical observational reports such ours, there is no data thus far from randomized controlled studies [25]. A recently published open-label study evaluated the use of LDN in a cohort of 38 patients with Long COVID without a control group. Six evaluated parameters improved over time in the 36 patients who completed two months of treatment, with the largest effects on a significant reduction in pain and low mood, personality change, joint pain, chest tightness and cough. In this study two patients (5 %) discontinued the drug due to diarrhea and fatigue [25]. In our study cohort of 59 patients who received LDN for PASC, LDN use was associated with lower number of symptoms, improvement in the severity of primary symptoms assessed in our study (fatigue, PEM, unrefreshing sleep, and abnormal sleep pattern), and improvement in the FSS, and LDN was well tolerated. While the majority of individuals with acute SARS-CoV-2 infection do recover, a subset may experience lingering symptoms that persist for months or even years. It’s important to note that the extent of recovery can vary significantly and often time depend. To mitigate the time factor on recovery in our study population, we compared the effect of LDN in individuals with ≥365 days of symptoms to those with <365 days of symptoms. In this PASC cohort, the duration of symptoms did not affect the clinical response to LDN for the selected symptoms. Therefore, this retrospective EHR review of patients seen in our PASC clinic who received LDN suggests a potential benefit of LDN in some individuals with PASC that warrants further testing in rigorous, randomized, placebo-controlled clinical trials. Given the heterogeneity of PASC, it will be important to select the suitable patient subpopulation that may best respond to LDN such as ME/CFS. This illness is characterized by debilitating fatigue, post-exertional malaise, unrefreshing sleep, cognitive impartment and orthostatic intolerance [26] clinical features that overlap with PASC, and accumulating studies from clinical cohorts indicate that approximately half of the patients with PASC have the ME/CFS phenotype [10,27,28] and may represent the best candidates for a trial of LDN for persistent symptoms. In another cohort of patients with non-COVID related ME/CFS, the use of LDN showed clinical benefit in 73.9 % of patients [6]. In that study, majority of patients experienced improved vigilance/alertness and physical and cognitive performance. Some patients even reported less pain and fever, without any severe adverse events or long term symptom effects [6].
Finally, we did not find a correlation between LDN dose and changes in the seven responsive clinical symptom scores. This finding could be explained by multiple mechanistic pathways proposed in patients with long COVID (micro-clots, persistent virus, and haywire immune system) [3,4]. However, we cannot exclude the possibility of a placebo effect that could result in either positive or negative response [29]. A larger study of LDN in 160 participants in a randomized parallel group double-blinded placebo-controlled trial is underway [30].Our study is, to date, the largest observational study of LDN in PASC.
However, our study has several limitations. First, this is a retrospective, observational, real-world setting without a placebo control or randomization. In addition, this study represents the experience of a single center in Northern California located in a generally affluent area with a bias toward specific populations. The selection of this cohort may skew our population in the following ways, 1) this is a referred population with multiple and more severe symptoms, and 2) our clinics have a lower proportion of underrepresented minority populations such as blacks and Hispanics, which lessen the generalizability of our findings. Symptom survey data was also not complete on follow-ups in a large portion of patients who were originally prescribed LDN leading to a significant percentage of patients not included in the analyses, and therefore, may have led to bias in the findings. Finally, we did not assess the impact of other medications and supplements that patients were taking while on LDN.
In summary, we describe a cohort of 59 patients with PASC who received LDN off-label as a potential therapeutic intervention for PASC. Overall, the use of LDN was associated with improved self-reported clinical symptoms of fatigue, PEM, unrefreshing sleep, and sleep pattern, and a reduced total number of symptoms with better functional status and no serious AEs. These results suggest that LDN warrants further investigation of LDN in a randomized clinical trial. In addition, exploring the mechanism of action for naltrexone in neuroinflammatory conditions may also provide new insight into the pathogenesis of PASC.
Supplementary Material
Highlights.
-The off-label use of low-dose naltrexone (LDN) is a potential drug intervention for the management of post-COVID conditions.
-LDN has anti-inflammatory and immunomodulatory properties which may benefit those with PASC where persistent inflammation is the causative pathway.
- LDN warrants testing in rigorous randomized, placebo-controlled clinical trials.
Acknowledgements
We would like to thank Stanford Health Care and the Stanford Department of Medicine for the supporting the clinic, and we thank all the patients of the Stanford Long COVID clinic. We also acknowledge the RECOVER project for bringing several of these authors together.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Vincent C. Marconi received support from the Emory Center for AIDS Research (P30AI050409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health or the Emory Center for AIDS Research.
Competing interests
V.C.M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences, and ViiV.
GM has received research funding from Genentech, Roche, Merck, Pfizer, Redhill, Cognivue, and consultations fees (all unrelated to this work) from Merck, Janssen, Gilead, Theratechnologies, and ViiV.
LG has received research funding from Pfizer and advisory fees from United Healthcare.
HB has received research funding from Pfizer and advisory fees from United Healthcare.
LE reports serving on the advisory boards for AstraZeneca and Regeneron.
All other authors report no potential conflicts.
Footnotes
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References:
- 1.NIH. COVID-19 Treatment Guidelines. April, 20 2023; https://www.covid19treatmentguidelines.nih.gov/
- 2.NIH. RECOVER: Researching COVID to Enhance Recovery. April, 23 2023; https://recovercovid.org
- 3.Couzin-Frankel J Clues to long COVID. Science. Jun 17 2022;376(6599):1261–1265. doi: 10.1126/science.add4297 [DOI] [PubMed] [Google Scholar]
- 4.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. May 2022;28(5):911–923. doi: 10.1038/s41591-022-01810-6 [DOI] [PubMed] [Google Scholar]
- 5.Bruun KD, Amris K, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: protocol for a double-blind, randomized, placebo-controlled trial. Trials. Nov 15 2021;22(1):804. doi: 10.1186/s13063-021-05776-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polo OPP, Tuominena E. Low-dose naltrexone in the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Fatigue: Biomedicine, Health & Behavoir. December 23th 2019;doi: 10.1080/21641846.2019.1692770 [DOI] [Google Scholar]
- 7.Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. Feb 2013;65(2):529–38. doi: 10.1002/art.37734 [DOI] [PubMed] [Google Scholar]
- 8.Toljan K, Vrooman B. Low-Dose Naltrexone (LDN)-Review of Therapeutic Utilization. Med Sci (Basel). Sep 21 2018;6(4)doi: 10.3390/medsci6040082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global Prevalence of Post COVID-19 Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis. Apr 16 2022;doi: 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilla HQT, Tiwari A, Bonilla AE, Miglis M, Yang P, Eggert L, Sharifi H, Horomanski A, Subramanian A, Smirnoff L, Simpson N, Halawi H, Sum-Ping O, Kalinowski A, Patel Z, Shafer R, Geng L. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front Neurol. 2023; 14:1090747. doi: 10.3389/fneur.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok FA, Boon G, Barco S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. Jul 2020;56(1)doi: 10.1183/13993003.01494-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. National Center for Health Statistics. Nearly One in Five American Adults Who Have Had COVID-19 Still Have “Long COVID”. 2022; [Google Scholar]
- 13.Ford ND, Slaughter D, Edwards D, et al. Long COVID and Significant Activity Limitation Among Adults, by Age - United States, June 1–13, 2022, to June 7–19, 2023. MMWR Morb Mortal Wkly Rep. Aug 11 2023;72(32):866–870. doi: 10.15585/mmwr.mm7232a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peluso MJ, Deeks SG. Early clues regarding the pathogenesis of long-COVID. Trends Immunol. Apr 2022;43(4):268–270. doi: 10.1016/j.it.2022.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenstein TK. The Role of Opioid Receptors in Immune System Function. Front Immunol. 2019;10:2904. doi: 10.3389/fimmu.2019.02904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaveriaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc Natl Acad Sci U S A. May 26 1998;95(11):6326–30. doi: 10.1073/pnas.95.11.6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Tian Z, Wei H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front Immunol. 2017;8:930. doi: 10.3389/fimmu.2017.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, McLaughlin PJ, Zagon IS. Ontogeny of the opioid growth factor, [Met5]-enkephalin, preproenkephalin gene expression, and the zeta opioid receptor in the developing and adult aorta of rat. Dev Dyn. Apr 1998;211(4):327–37. doi: [DOI] [PubMed] [Google Scholar]
- 19.Franchi S, Moretti S, Castelli M, et al. Mu opioid receptor activation modulates Toll like receptor 4 in murine macrophages. Brain Behav Immun. Mar 2012;26(3):480–8. doi: 10.1016/j.bbi.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 20.Kucic N, Racki V, Sverko R, Vidovic T, Grahovac I, Mrsic-Pelcic J. Immunometabolic Modulatory Role of Naltrexone in BV-2 Microglia Cells. Int J Mol Sci. Aug 5 2021;22(16)doi: 10.3390/ijms22168429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selfridge BR, Wang X, Zhang Y, et al. Structure-Activity Relationships of (+)-Naltrexone-Inspired Toll-like Receptor 4 (TLR4) Antagonists. J Med Chem. Jun 25 2015;58(12):5038–52. doi: 10.1021/acs.jmedchem.5b00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cant R, Dalgleish AG, Allen RL. Naltrexone Inhibits IL-6 and TNFalpha Production in Human Immune Cell Subsets following Stimulation with Ligands for Intracellular Toll-Like Receptors. Front Immunol. 2017;8:809. doi: 10.3389/fimmu.2017.00809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabanas H, Muraki K, Staines D, Marshall-Gradisnik S. Naltrexone Restores Impaired Transient Receptor Potential Melastatin 3 Ion Channel Function in Natural Killer Cells From Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Front Immunol. 2019;10:2545. doi: 10.3389/fimmu.2019.02545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skolnick P, Davis H, Arnelle D, Deaver D. Translational potential of naloxone and naltrexone as TLR4 antagonists. Trends Pharmacol Sci. Sep 2014;35(9):431–2. doi: 10.1016/j.tips.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 25.O’Kelly B, Vidal L, McHugh T, Woo J, Avramovic G, Lambert JS. Safety and efficacy of low dose naltrexone in a long covid cohort; an interventional pre-post study. Brain Behav Immun Health. Oct 2022;24:100485. doi: 10.1016/j.bbih.2022.100485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medicine Io. Recommendations. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. 2015. The National Academies Collection: Reports funded by National Institutes of Health.
- 27.Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of Cardiopulmonary Stress Testing for Patients With Unexplained Dyspnea Post-Coronavirus Disease. JACC Heart Fail. Dec 2021;9(12):927–937. doi: 10.1016/j.jchf.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. Aug 30 2022;13(1):5104. doi: 10.1038/s41467-022-32507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pozgain I, Pozgain Z, Degmecic D. Placebo and nocebo effect: a mini-review. Psychiatr Danub. Jun 2014;26(2):100–7. [PubMed] [Google Scholar]
- 30.Nacul L Low-dose Naltrexone for Post-COVID Fatigue Syndrome. University of British Columbia. https://clinicaltrials.gov/ct2/show/NCT05430152. 2022; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


