Abstract
A central role for T cells in the control of cancer has been supported by both animal models and clinical observations. Accordingly, the development of potent anti-tumor T cell immunity has been a long-standing objective of immunotherapy. Emerging data from clinical trials that test T cell immune-modulatory agents and genetically engineered and re-targeted T cells have begun to realize the profound potential of T cell immunotherapy to target cancer. This review will focus on a description of recent conceptual and technological advances for the genetic engineering of T cells to enhance anti-tumor T cell immunity through the introduction of tumor-specific receptors, both Chimeric Antigen Receptors (CAR) and T cell receptors (TcR), as well as an overview of emerging data from ongoing clinical trials that highlight the potential of these approaches to effect dramatic and potent anti-tumor immunity.
Keywords: Chimeric antigen receptor, T cell receptor, Adoptive T cell transfer, Immunotherapy, Gene transfer, CIMT 2011
T cell immunotherapy: a brief historical overview
The seminal reports by the group of Boon in 1993 [1] which established that T cells that recognized defined antigens expressed by tumors could be identified in the peripheral blood of patients ushered in the modern era of antigen-specific T cell immunotherapy. Since that time, a major focus of immunotherapy-based strategies has been to identify antigens uniquely expressed or over-expressed by tumor cells and to use those antigens as immunogens to trigger antigen-specific T cell responses in patients.
Over the years, a plethora of candidate antigens have been identified using both high-throughput molecular and immunological approaches resulting in an extensive database of candidate antigens and immunologically relevant epitopes, see for example http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm and [2]. Although the search for uniquely tumor-specific antigens with broad expression within and across tumors has not to date met with success, these efforts have resulted in the identification of a number of candidate antigens that are either over-expressed or aberrantly expressed by tumors as well as tissue differentiation antigens. Among these antigens, much effort has been placed on the evaluation of antigens that are either not expressed in normal adult somatic tissues but are expressed developmentally (for example, the cancer-testis (CT) antigens [3], perhaps best exemplified by the MAGE (Melanoma-associated AntiGEn) [4] and NY-ESO-1 families [5]) or are expressed at lower levels in normal tissues and dramatically over-expressed in tumors (for example, Her-2/Neu, EpCAM, cyclinD1). In parallel, a multitude of strategies have been applied to attempt to elicit potent anti-tumor T cell responses to these antigens. Over the past 20 years, essentially every possible modality for vaccination, combined with a wide variety of adjuvants, has been tested in the clinical setting. With few exceptions (see [6] for a recent review), these studies have been disappointing in two fundamental ways: (1) Although objective anti-tumor response rates have occasionally been observed in patients, the overall response rates have been unimpressive and (2) Observed increases in frequencies of antigen-specific T cells post vaccination have often not correlated with anti-tumor activity.
The impact of immune tolerance on immunotherapy
The majority of to-date vaccines studies have attempted to trigger an immune response to self-antigens that are either expressed during normal development or expressed in what is thought to be an immunologically privileged manner. One critical hurdle that such strategies have had to overcome is immunological tolerance, both central and peripheral. Dramatic positive response data in a subset of patients treated with modulators of immune suppression such as anti-CTLA-4, anti-PD-1, anti-CD25, and agonists of CD40 have suggested that the obstacles associated with peripheral tolerance to tumors can be overcome [7–11]; importantly, the nature of the critical targeted antigens recognized by T cells in treatment-responsive patients have not been identified. On the other hand, the principal effect of central tolerance, i.e., the deletion of the high-affinity repertoire to self-antigens during thymic development, remains a fundamental impediment for vaccine-based immunotherapy strategies. Few studies exist that evaluate the effect of central tolerance on the T cell repertoire to self-antigens in humans. However, a few experimental lines of evidence support the notion that central tolerance is a profound obstacle for the establishment of potent immunity to self-antigens. In a general sense, investigators across many studies are intimately familiar with the recurring phenomenon that T cells specific for self-antigens expressed by tumors respond very weakly to target cells that endogenously express the target antigens, as elegantly demonstrated by experiments that evaluated the Her-2/neu specific repertoire in Her-2/neu transgenic animals immunized with Her-2/neu peptides [12]. Precious few studies exist to document the impact of central tolerance on the human T cell repertoire; Friedman et al. showed that the T cell repertoire to the prostate tissue-specific antigen protein was fundamentally different in male versus female healthy donors, with T cells from males recognizing sub-dominant epitopes and with very poor anti-prostate tumor activity, while female-derived T cells showed substantially more potent anti-tumor activity [13].
The striking difference in affinity between T cell receptors specific for self-antigens expressed by tumors and T cell receptors specific for virus antigens has recently been summarized by Cole et al. Such comparative analyses have revealed that TcR from T cells that recognize self-tumor antigens have substantially lower affinities (approximately 0.5 logs) for cognate MHC:peptide complexes compared to their virus-specific counterparts [14]. These observations likely explain at least in part the to-date clinically inadequate results obtained following the activation of self-antigen-specific T cells. Of additional note in this regard is a recent study which demonstrated that strong and clinically relevant T cell immune responses could be generated in patients with vulvar intraepithelial neoplasia following vaccination with long-peptides derived from human papilloma virus serotype 16 (HPV-16) illustrating the potential of peptide-based vaccine strategies to trigger effective anti-tumor immunity [15].
Thus, a consequence of central tolerance is that the T cell repertoire to self-antigens is fundamentally compromised, with T cells that have the potential to respond to the self-target antigens in most cases suboptimal and most likely ineffective in terms of antitumor activity. Indeed, although objective clinical responses have been observed in response to strategies that seek to trigger T cell immune responses to self-antigens (see for example, the successful approval of sipuleucel [16]), there is scant evidence that the relevant immune response is targeted to these antigens.
Approaches to overcome tolerance
A variety of approaches have been developed to circumvent the impact of central tolerance on the ability to stimulate anti-tumor antigen-specific T cell immunity. One general conceptual approach has attempted to reduce the threshold for activation for T cells, under the premise that activated T cells have enhanced sensitivity to subsequent antigen-triggering. Under this premise, investigators have pursued approaches that utilize higher affinity heteroclitic peptides [17], co-stimulatory receptors [18], or adjuvant combinations [19] to improve the avidity of the T cell: APC interaction, to facilitate the productive engagement of antigen-specific T cells and lower the subsequent threshold for T cell re-activation. In a number of cases, such efforts have resulted in the triggering of T cells that respond to the vaccine peptides; however, to date, these efforts have met with minimal clinical success in terms of clinical efficacy.
Adoptive T cell transfer, which involves the ex vivo isolation, expansion, and re-infusion of T cells into patients, is one alternative strategy that circumvents the need to activate and expand a tumor antigen-specific T cell repertoire in patients. Significant effort has been extended over the past few years to evaluate the potential for adoptive T cell transfer to treat cancer, and a variety of approaches using T cells obtained from the periphery [20, 21], from tumor infiltrating lymphocytes (TIL) [22], or enriched for virus-specificities to enhance persistence [23] have been evaluated in clinical trials. Ex vivo activation and expansion of T cells prior to infusion have the potential to circumvent the effects of peripheral tolerance by providing to patients a large, polyclonal, and activated population of T cells. This activated population could contain high-affinity T cells that recognize tumor-unique and to-date undefined antigens and have potent specificity against tumors, as well as T cells specific for “classical” tumor-specific self-antigens that have become sensitized and responsive to antigen-expressing targets.
Turbo-charged TCR and CAR
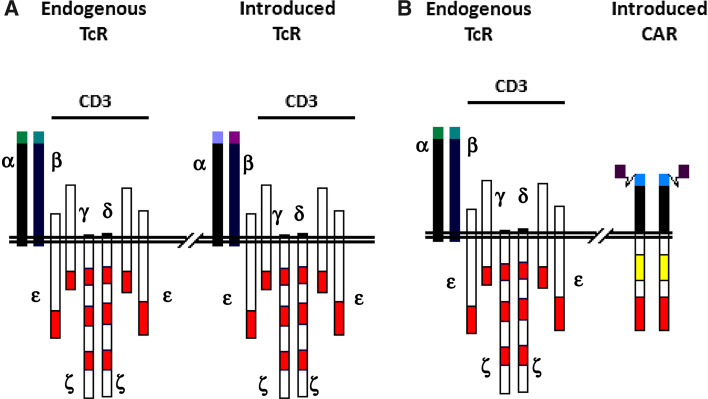
Recent advances in the practical ability to perform high efficiency gene transfer into primary T lymphocytes have allowed for the potential to transfer into T cells receptors with specificity for defined tumor antigens, thus creating large populations of antigen-specific T cells for adoptive transfer. Two basic gene transfer approaches have been pursued to bypass the effects of central and peripheral tolerance on the T cell repertoire; these approaches augment and re-direct anti-tumor T cell activity through the transfer of antigen-specific T cell receptors α and β chains (TcR α/β) or Chimeric Antigen Receptors (CAR) composed of antibody-binding domains fused to T cell signaling domains. In both instances, recipient T cells retain their native specificity while acquiring the second, tumor-specific specificity (Fig. 1).
Fig. 1.
Schematic of T cell surface expressing introduced TcR or CAR polypeptides. a Introduced TcR chains pair with endogenous CD3 γ, δ, ε, and ζ chains re-capitulating a native TcR complex. b CAR are thought to homodimerize and also associate with CD3 γ, δ, ε, and ζ chains. Red bar TcR signaling domains; yellow bar non-TcR complex signaling domains that confer novel functionalities
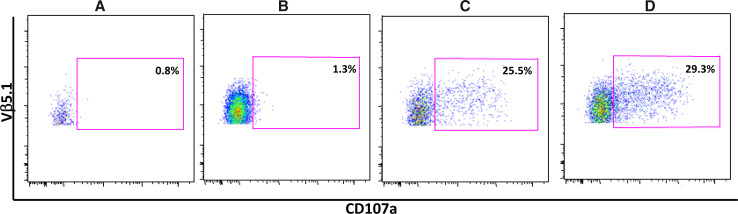
Although transfer of T cell specificity through transfer of the TcR α/β heterodimer was initially demonstrated over 25 years ago [24], demonstration of the feasibility to transfer of T cell specificity into primary T cells through transfer of TcR α/β chains was demonstrated more recently [25, 26]. Tumor antigen-specific T cells, expanded from both cancer patients and healthy volunteers, have been a primary source for isolating tumor-specific TcR α/β heterodimers, and over the years, a large variety of approaches using both peptides and whole antigen have been implemented to expand such T cells. Because of the low frequency of such T cells in peripheral blood, the lack of effective culture and expansion methodologies, and the impact of central tolerance on the repertoire, T cells can only be isolated with considerable difficulty using these approaches; furthermore, such T cells are in general of low affinity and demonstrate weak anti-tumor activity. Approaches to overcome these issues and generate more potent tumor antigen-specific T cells have involved the use of mice transgenic for human HLA-A2 [27], the generation of higher affinity T cells in an allo-reactive context [28], and the generation of T cells to gender-restricted antigens (such as prostate and ovary-restricted antigens) from PBMC of the non-expressing gender [13]. One approach to overcome the issue of the intrinsically low affinity of TcR to self-antigens has been to enhance the affinity of the T cell receptor isolated from such T cells by mutagenesis of the α and β receptor chains. Although such approaches initially involved brute force mutagenesis approaches, recent technological advances have facilitated more elegant molecular and rational high-throughput genetic approaches to affinity enhance TcR [29–32]. Application of these approaches requires significant effort and follow-up to confirm retention of TcR specificity; however, such efforts have resulted in the ability to reproducibly generate TcR with substantially higher affinities for target antigens, often higher by logs [30]. One example of the ability of high-throughput mutagenesis approaches to generate affinity-enhanced TcR that effectively target tumor cells is presented in Fig. 2; whereas T cells non-transduced or transduced with wild-type TcR fail to recognize tumor cells that express physiologic levels of the relevant MHC and antigen (panels a, b), affinity-enhanced receptors generated from these wild-type receptors efficiently redirect T cells to recognize the targets (panel c); importantly, T cells transduced with wild-type TcR efficiently recognized the same target cell line engineered by Lentivirus transduction to over-express the target antigen (panel d), providing evidence for the sub-dominant nature of the target peptide sequence.
Fig. 2.
Affinity enhancement allows for the targeting of subdominant epitopes not recognized by wild-type TcR. Data presented are from a CD107a degranulation assay to measure antigen-specific degranulation of polyclonal T cells populations, either non-modified or modified by Lentivirus transduction to express a tumor-specific TcR α/β heterodimer, detected in b–d by staining through the use of the clonotypic Vβ5.1 antibody reagent. Gating strategy was on live cells >CD3+>CD8+>Vβ5.1+ (allowing gating on the transduced cell population); non-transduced cells had minimal activity in this assay. a Non-transduced bulk expanded T cells, non-modified target cells; b T cells transduced with wild-type tumor-specific receptor, non-modified targets; c T cells transduced with affinity-enhanced T cell receptor, non-modified targets; d T cells transduced with wild-type tumor-specific receptor tested on targets that over-express target antigen
An alternative and potentially complementary strategy to enhance TcR affinity was revealed through a recent report by Kuball et al. these authors demonstrated enhanced functional avidity and improved recognition of tumor cells following introduction of mutations that reduced N-glycosylation on TCR chains [33].
Affinity-enhanced TcR-based transfer approaches afford the opportunity to transfer a signal that can be delivered in a physiological context, which may be relevant for the clinical functionality and persistence of infused T cells. Additionally this approach is not limited to the targeting of cell surface epitopes. On the other hand, this approach suffers from the fact that it remains susceptible to the common tumor escape mechanisms of MHC down-modulation and altered peptide processing. An additional concern with the affinity-enhancement approach is the potential for the degeneration of TcR fine specificity as a consequence of the mutagenesis and enhanced affinity [34]. Finally, the mutagenesis process has the potential to generate neo-epitopes that can be targeted by patient humoral and cellular immune responses.
The concept of CAR was initially proposed by Eshhar et al. [35]. CAR can be described as modular polypeptides composed of 3 distinct modules: an extracellular target-binding module, a transmembrane module anchoring the CAR into the cell membrane, and an intracellular signaling module. The extracellular target-binding module is usually derived from ScFv determinants isolated from antibodies, linked in a single chain through linker polypeptide sequences. Transmembrane modules have typically been derived from molecules involved in T cell function and associated with the TcR supercomplex such as CD8 and CD4, in some cases connected to the binding domain via a “stalk” thought to extend the distance between the binding domain and the T cell surface. The intracellular module almost always consists of the zeta chain of the TcR complex responsible for transmitting TcR engagement-mediated activation signals to cells, although initial studies also explored the signaling modules of the FcεRI-γ chain. As discussed below, next-generation CAR incorporate signaling domains from co-stimulatory and activation molecules such as CD28, CD134, or CD137, alone or in combinations, to attempt to augment zeta signaling in a physiologically relevant manner.
Conceptually, CAR transfer-based strategies afford 2 distinct advantages for the ability to mediate effective anti-tumor activity: on the one hand, CAR provide a mechanism to bypass the fundamental roadblock imposed by central tolerance; On the other hand, CAR bypass common mechanisms that are selected for in tumor cells to blunt T cell immunity. In terms of central tolerance, since the target-binding moiety is derived from antibodies that are generated against target antigens in heterologous species, central tolerance has no impact in shaping the binding affinity to target epitopes. Furthermore, since the number of target epitopes is stoichiometrically equal to the number of target antigen molecules on the cell surface, inefficient processing of target epitopes is not relevant to target cell recognition. The lack of dependence on antigen processing for target recognition by CAR provides a clear advantage in terms of potency since every surface expressed target molecule presents a CAR-triggering epitope. In terms of overcoming immune-evasive mechanisms, since CAR recognition of target cells is neither MHC-restricted nor dependent of proteosomal cleavage and appropriate processing of target epitopes, CAR-based strategies are insensitive to the HLA down-modulation and altered processing escape mechanisms that commonly evolve in tumors.
Additional advantages with CAR-based strategies include the facts that such approaches offer the potential to target glycosylation variants unique to tumor cells, as well as epitopes that are differentially exposed and preferentially exposed to T cells in tumor versus normal tissues.
There are a number of limitations and challenges, both practical and theoretical, associated with CAR-based strategies. In terms of practical limitations, CAR-based approaches are restricted to the targeting of cell surface determinants to which antibodies can be generated in heterologous species. In addition, since CAR are chimeric molecules composed of distinct combinatorial modules that include unique junctional fragments as well as non-human sequences in the scFv domains, there is reasonable potential for CAR-modified T cells to be targeted by patient humoral and cellular immune responses.
In terms of theoretical limitations, because CAR are engineered to deliver TcR and co-stimulation-mediated signals independently from the physiological complex through which natural signaling occurs, it is possible that the signaling cascades initiated through CAR engagement are qualitatively and/or quantitatively distinct from those required to manifest the full range of effector and regulatory consequences of native TcR signaling, a fact that may impact the full range of in vivo functionality for CAR-modified cells. Steric considerations associated with the target epitopes recognized by the antibody determinants on CAR are an additional theoretical limitation. Unlike the TcR α/β heterodimer which has structurally evolved and been biologically selected to recognize peptides located within a sterically defined groove in the major histocompatibility complex (MHC), soluble antibodies target linear or conformational epitopes located throughout the surface of the target antigen. A consequence of this fact is that at least in some cases the grafting of antibody-binding domains onto CAR will create molecules with structural limitations that impact binding to target cells and/or appropriate signaling and effector functionality of the CAR-modified cells.
A summary that describes the advantages and disadvantages of both CAR- and TcR-based approaches is presented in Table 1.
Table 1.
Perceived advantages (*) and disadvantages (Δ) of CAR- and TcR-based gene transfer strategies
| Category | CAR | Affinity-enhanced TcR |
|---|---|---|
| Antigen localization | Surface onlyΔ | Not restricted* |
| Antigen processing | Not required* | DependentΔ |
| MHC expression | Not required* | RequiredΔ |
| Ability to target new antigens | Straightforward-dependent on antibody availability* | Difficult-dependent on identification of relevant T cellsΔ |
| Ligand density | Strictly dependent on target antigen expression* | Dependent on target antigen expression and processing efficiencyΔ |
| Potency of signal | High, can be amplified by including co-stimulatory signaling domains* | Native TcR complex* |
| Surface Expression | Straightforward* | Dependent on efficient α/β paring and availability of other TcR complex chains** |
| Potential immunogenicity | High, antibody domains and fusion junctions** | Low, sequences native* |
| Off-target effects | Low, but enhanced potential to target normal tissues with low levels of expression* | Higher, potential for mispairing, or degeneration of fine specificity as a result of affinity enhancementΔ |
Clinical applications of CAR and TcR
A variety of clinical trials have been initiated to evaluate the potential for the adoptive transfer of CAR- and TcR-modified T cells to mediate anti-tumor activity.
CAR-based approaches have been developed to target a number of well-characterized self-antigens that are expressed on the surface of tumors, and such CAR have been extensively evaluated in animal models and increasingly in clinical trials (see [36] for a recent and comprehensive review). Current and ongoing clinical trials are targeting a variety of antigens including CD19 [37–41], CD20 [42], α-folate receptor [43], GD2 [44], and Her-2/neu [45]. Although each of these trials has been based on the use of a CAR-based targeting moiety, the variety of approaches that have been applied to date in terms of CAR delivery (plasmid electroporation, retrovirus, lentivirus), ex vivo expansion approaches (anti-CD3 and -CD28 beads, OKT3, artificial antigen-presenting cells), the use in some cases of exogenous immunomodulatory cytokines such as IL-2, and the incorporation of various signaling domains (ζ, 28-ζ, CD137-ζ) have precluded head-to-head comparison of the results. Nonetheless at least some of these trials have revealed the dramatic potential of CAR-based approaches. In particular, recent trials that target CD19 have shown potent anti-tumor activity with a number of robust partial responses [38, 39], and in one trial, dramatic and complete responses accompanied by robust expansion, long-term functional persistence, and homing to marrow of CAR-modified T cells together with documented delayed tumor lysis syndrome in one patient [40, 41]. On the other hand, the potency of these approaches has also been revealed by the unfortunate death that resulted from on-target and off-tissue toxicity due to lower levels of expression of target antigens in normal tissues [45, 46]. Additional evidence for the potential for on-target/off-tumor toxicity for CAR has been revealed by the liver dysfunction observed while targeting carbonic anhydrase, an antigen retrospectively identified as being expressed at low levels in liver bile ducts [47], and the delayed tumor lysis syndrome observed in CART19 trials [41].
A number of trials that employ TcR transfer to endow T cells with specificity for antigens expressed by tumors are also underway. These trials have targeted well studied and characterized self-antigens such as NYESO-1 in melanoma and synovial sarcoma [48], CEA in colorectal cancer [49], and MART-1 and gp100 in melanoma [50] and in a recently initiated trials, NYESO-1/LAGE and MAGE-A3/-A6 in multiple myeloma and melanoma. TcR used for these trials have been in some cases affinity enhanced through mutagenesis. Early encouraging and in some cases dramatic results from these trials are also beginning to demonstrate the potential of the affinity-enhanced TcR approach to also mediate effective clinical activity. As with the CAR-based approach, on-target and off-tissue toxicity due to low levels of expression of target antigens in normal tissues is a concern that in fact has been observed in a number of cases [49, 50].
Challenges and future directions
The recent encouraging and occasionally dramatic clinical data obtained using CAR and TcR-based adoptive transfer approaches have promoted a sense of guarded optimism about the potential for this platform to mediate potent anti-tumor immunity. Nonetheless, a number of important challenges remain to be addressed for the broader application of these approaches.
With regard to CAR-based approaches, one challenge relates to the nature of the optimal signaling domain combination needed to mediate the full range of T cell effector activity. To date, most clinical studies have employed the TcR ζ chain either alone or in combination with signaling domains derived from CD28 and more recently CD137 (4-1-BB), both of which have been shown to enhance CAR-mediated potency in animal models [51–53]. Although trials based on such CAR constructs have in some cases demonstrated considerable efficacy, it is possible that inclusion of additional domains may contribute to a more integrated signal that provides biological benefit, such as, for example, skewing CAR-modified cells toward desired phenotypes or secondary functionalities. A recent report from the Baylor group has demonstrated an elegant approach to evaluate different CAR constructs directly in patients by comparing short-term persistence following co-infusion of T cells modified with two CAR constructs with different signaling domain combinations [54]; this approach offers a potentially powerful mechanism to evaluate and compare directly in patients CAR with related but unique structural and/or signaling determinants to identify modules that mediate selective homing or other functionally relevant properties to infused gene-modified T cells.
With regard to TcR-based approaches, one critical challenge relates to the ability to identify T cells with biologically relevant anti-tumor activity and to efficiently isolate TcR receptor pairs from such T cells. Recent technological advances have provided potential breakthroughs in this regard, by enabling the rapid cloning and functional testing of TcR α/β chains from single cells [55, 56]. Application of such technologies on, for example, tumor infiltrating lymphocytes (TIL) specimens has the potential to enable the rapid identification of potent and biologically relevant TcR pairs that recognize antigens (both known and novel) expressed by tumors.
An additional challenge with TcR-based approaches relates to the potential for transferred TcR α and/or β chains to pair with endogenous α/β chains. Such pairings can have two unintended and negative consequences. First, such events reduce the number of relevant TcR pairs on the cell surface, potentially reducing the avidity of the modified lymphocytes for target cells. In addition, this mis-paring has the potential to generate novel TcR complexes with undesired specificities for normal tissues and the potential for autoimmunity. A recent report describes both the impact of such mispairing on T cell effector function as well as an elegant approach based on siRNA to down-modulate endogenous TcR chains and in this manner reduce mispairing and enhance both surface expression and functionality of transferred TcR chains [57]. An alternate strategy to minimize such mispairing involves the introduction of cysteine residues in the transferred TcR chains to facilitate preferential pairing between introduced chains [58].
For both CAR- and TcR-based approaches, the potential for autoimmunity due to on-target off-tissue effects remain as significant challenges to resolve; in the case of affinity-enhanced TcR-based approaches, off-target effects precipitated by the affinity-enhancement process are also a consideration to be kept in mind [47]. This challenge is particularly difficult to address in a comprehensive manner outside of patient trials, since animals models are not suited to address this type of toxicity, which is certainly species specific and at least in some cases likely to be patient unique. One mechanism to overcome this challenge may be to establish reference viable tissue bank repositories to facilitate the molecular and functional analyses of the potential for off-tissue and/or target events.
The potential immunogenicity of CAR- and TcR-modified cells, particularly CAR as discussed above, is another challenge for the field. Although the potential immunogenicity of junctional fragments may be impossible to overcome, approaches to mitigate this potential could involve the humanization of murine ScFv determinants and the removal of cryptic open reading frames shown to be immunogenic [47, 59].
Challenges also remain with regard to the optimal methodology to transfer CAR and TcR into recipient cells. The majority of to-date clinical approaches have employed virus-mediated gene transfer approaches, principally utilizing retroviruses and more recently lentiviruses. Although such approaches result in reasonably efficient transduction of primary T cells, they have considerable limitations in terms of cost to manufacture clinical-grade material, the total size of DNA that can be included in the virus vectors, and the potential, principally for retroviruses, for the integration events to result in insertional oncogenesis. One promising and emerging alternative to virus-mediated gene transfer approaches is RNA transfection [60]. This approach is extremely cost effective and efficient and allows for the potential to co-transfer genes that promote co-stimulation and/or homing to target tissues, provide accessory effector functions, as well as the ability to co-target multiple antigens through the same transfection event. In addition, because the transferred RNA does not stably integrate but rather remains episomal transferred cells are only transiently modified, a fact that may provide considerable advantages should unanticipated toxicities arise. Yet another promising approach for introducing CAR and TcR transgenes into T cell involves the utilization of transposon elements such as sleeping beauty and piggybac [61, 62]. The general issue of eliminating infused and modified T cells is yet another challenge for the field. Initial attempts attempted to introduce ‘suicide genes’ such as herpes simplex virus thymidine kinase (TK) gene; however, these efforts revealed the strong potential for immunologic rejection based on targeting of TK-derived sequences [63]. More recently, an elegant and potentially powerful inducible system based on the use of a modified human caspase-9 fused to a human FK506-binding protein to allow conditional dimerization and delivery of apoptotic signals; in response, a small molecule has been developed [64] and is currently being evaluated in clinical trials.
The final challenge to be discussed in this review relates to the types of cells to be employed in the gene transfer approaches. Principally, on the basis of studying cells that have persisted in patients post-transfer, it has been postulated that cells with longer telomeres fare better upon transfer [65]. More recent data from both primate studies and clinical trials have suggested that central memory cells may be an appropriate cell type to utilize for the gene transfer [66]. Nonetheless, it is perhaps fair to state that the jury is still out on which if any from the plethora of T cell subsets (naïve, central—effector—memory, Th17, NKT, γ/δ, other) is the most appropriate to employ as the vector for delivering CAR and TcR into patients; it is entirely plausible that the choice of cell type to deliver these powerful targeting agents may be linked to the specific homing requirements and immune microenvironment of the disease being targeted.
Acknowledgments
Effort for composing this manuscript was supported in part by funding from the University of Pennsylvania’s Institutional Clinical and Translational Science Award (CTSA), and by the Commonwealth of Pennsylvania/Pennsylvania Department of Health (grant# 4100051725).
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Ninth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, May 25–27, 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother CII. 2005;54(3):187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100(11):2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sang M, Wang L, Ding C, Zhou X, Wang B, Lian Y, Shan B. Melanoma-associated antigen genes—an update. Cancer Lett. 2011;302(2):85–90. doi: 10.1016/j.canlet.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Old LJ. Cancer vaccines: an overview. Cancer Immun. 2008;8(Suppl 1):1. [PubMed] [Google Scholar]
- 6.Bedognetti D, Balwit JM, Wang E, Disis ML, Britten CM, Delogu LG, Tomei S, Fox BA, Gajewski TF, Marincola FM, et al. SITC/iSBTc cancer immunotherapy biomarkers resource document: online resources and useful tools—a compass in the land of biomarker discovery. J Trans Med. 2011;9:155. doi: 10.1186/1479-5876-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70(20):7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 10.Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs. 2010;11(12):1354–1359. [PubMed] [Google Scholar]
- 11.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 12.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34(3):752–761. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 13.Friedman RS, Spies AG, Kalos M. Identification of naturally processed CD8 T cell epitopes from prostein, a prostate tissue-specific vaccine candidate. Eur J Immunol. 2004;34(4):1091–1101. doi: 10.1002/eji.200324768. [DOI] [PubMed] [Google Scholar]
- 14.Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, Price DA, Gao GF, Sewell AK, Jakobsen BK. Human TCR-binding affinity is governed by MHC class restriction. J Immunol. 2007;178(9):5727–5734. doi: 10.4049/jimmunol.178.9.5727. [DOI] [PubMed] [Google Scholar]
- 15.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW et al. (2009) Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. New Eng J Med 361(19):1838–1847 [DOI] [PubMed]
- 16.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 17.Shang X, Wang L, Niu W, Meng G, Fu X, Ni B, Lin Z, Yang Z, Chen X, Wu Y. Rational optimization of tumor epitopes using in silico analysis-assisted substitution of TCR contact residues. Eur J Immunol. 2009;39(8):2248–2258. doi: 10.1002/eji.200939338. [DOI] [PubMed] [Google Scholar]
- 18.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–298. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 19.Chiang CL, Kandalaft LE, Coukos G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. Int Rev Immunol. 2011;30(2–3):150–182. doi: 10.3109/08830185.2011.572210. [DOI] [PubMed] [Google Scholar]
- 20.Levine BL. T lymphocyte engineering ex vivo for cancer and infectious disease. Expert Opin Biol Ther. 2008;8(4):475–489. doi: 10.1517/14712598.8.4.475. [DOI] [PubMed] [Google Scholar]
- 21.Morgan RA, Dudley ME, Rosenberg SA. Adoptive cell therapy: genetic modification to redirect effector cell specificity. Cancer J. 2010;16(4):336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31(8):742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yague J, White J, Coleclough C, Kappler J, Palmer E, Marrack P. The T cell receptor: the alpha and beta chains define idiotype, and antigen and MHC specificity. Cell. 1985;42(1):81–87. doi: 10.1016/S0092-8674(85)80103-3. [DOI] [PubMed] [Google Scholar]
- 25.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163(1):507–513. [PubMed] [Google Scholar]
- 26.Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol. 2000;74(17):8207–8212. doi: 10.1128/JVI.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustgarten J, Theobald M, Labadie C, LaFace D, Peterson P, Disis ML, Cheever MA, Sherman LA. Identification of Her-2/Neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD.8. Hum Immunol. 1997;52(2):109–118. doi: 10.1016/S0198-8859(96)00292-3. [DOI] [PubMed] [Google Scholar]
- 28.Amir AL, Steen DM, Loenen MM, Hagedoorn RS, de Boer R, Kester MD, Ru AH, Lugthart GJ, Kooten C, Hiemstra PS, et al. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res. 2011;17(17):5615–5625. doi: 10.1158/1078-0432.CCR-11-1066. [DOI] [PubMed] [Google Scholar]
- 29.Chervin AS, Aggen DH, Raseman JM, Kranz DM. Engineering higher affinity T cell receptors using a T cell display system. J Immunol Methods. 2008;339(2):175–184. doi: 10.1016/j.jim.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23(3):349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 31.Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180(9):6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udyavar A, Alli R, Nguyen P, Baker L, Geiger TL. Subtle affinity-enhancing mutations in a myelin oligodendrocyte glycoprotein-specific TCR alter specificity and generate new self-reactivity. J Immunol. 2009;182(7):4439–4447. doi: 10.4049/jimmunol.0804377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuball J, Hauptrock B, Malina V, Antunes E, Voss RH, Wolfl M, Strong R, Theobald M, Greenberg PD. Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. J Exp Med. 2009;206(2):463–475. doi: 10.1084/jem.20082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, Li Y, Molloy PE, Dunn SM, Jakobsen BK, et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179(9):5845–5854. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transpl. 2010;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Trans Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified t cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buning H, Uckert W, Cichutek K, Hawkins RE, Abken H. Do CARs need a driver’s license? Adoptive cell therapy with chimeric antigen receptor-redirected T cells has caused serious adverse events. Hum Gene Ther. 2010;21(9):1039–1042. doi: 10.1089/hum.2010.131. [DOI] [PubMed] [Google Scholar]
- 47.Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, Kershaw MH. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118(3):499–509. doi: 10.1182/blood-2011-01-325266. [DOI] [PubMed] [Google Scholar]
- 48.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, Quintas-Cardama A, Larson SM, Sadelain M. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(18 Pt 1):5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 54.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Investig. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birkholz K, Hofmann C, Hoyer S, Schulz B, Harrer T, Kampgen E, Schuler G, Dorrie J, Schaft N. A fast and robust method to clone and functionally validate T-cell receptors. J Immunol Methods. 2009;346(1–2):45–54. doi: 10.1016/j.jim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Seitz S, Schneider CK, Malotka J, Nong X, Engel AG, Wekerle H, Hohlfeld R, Dornmair K. Reconstitution of paired T cell receptor alpha- and beta-chains from microdissected single cells of human inflammatory tissues. Proc Natl Acad Sci USA. 2006;103(32):12057–12062. doi: 10.1073/pnas.0604247103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okamoto S, Mineno J, Ikeda H, Fujiwara H, Yasukawa M, Shiku H, Kato I. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 2009;69(23):9003–9011. doi: 10.1158/0008-5472.CAN-09-1450. [DOI] [PubMed] [Google Scholar]
- 58.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109(6):2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, Oosterwijk E, Sleijfer S, Debets R, Gratama JW. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117(1):72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 60.Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M, Carroll RG, June CH, Grupp SA (2011) Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther [DOI] [PMC free article] [PubMed]
- 61.Wilson MH, Coates CJ, George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15(1):139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 62.Hackett PB, Largaespada DA, Cooper LJ. A transposon and transposase system for human application. Mol Ther. 2010;18(4):674–683. doi: 10.1038/mt.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marktel S, Magnani Z, Ciceri F, Cazzaniga S, Riddell SR, Traversari C, Bordignon C, Bonini C. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood. 2003;101(4):1290–1298. doi: 10.1182/blood-2002-08-2351. [DOI] [PubMed] [Google Scholar]
- 64.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ, Jr, Rosenberg SA, Hodes RJ. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007;30(1):123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]