Abstract
Over the last few years, several newly developed immune-based cancer therapies have been shown to induce clinical responses in significant numbers of patients. As a result, there is a need to identify immune biomarkers capable of predicting clinical response. If there were laboratory parameters that could define patients with improved disease outcomes after immunomodulation, product development would accelerate, optimization of existing immune-based treatments would be facilitated and patient selection for specific interventions might be optimized. Although there are no validated cancer immunologic biomarkers that are predictive of clinical response currently in widespread use, there is much published literature that has informed investigators as to which markers may be the most promising. Population-based studies of endogenous tumor immune infiltrates and gene expression analyses have identified specific cell populations and phenotypes of immune cells that are most likely to mediate anti-tumor immunity. Further, clinical trials of cancer vaccines and other cancer directed immunotherapy have identified candidate immunologic biomarkers that are statistically associated with beneficial clinical outcomes after immune-based cancer therapies. Biomarkers that measure the magnitude of the Type I immune response generated with immune therapy, epitope spreading, and autoimmunity are readily detected in the peripheral blood and, in clinical trials of cancer immunotherapy, have been associated with response to treatment.
Keywords: Immunity, Biomarkers, T cell, Clinical response, Correlation, Immunotherapy
Introduction
Over the last year, a variety of immune-based approaches for cancer therapy have demonstrated statistically significant therapeutic efficacy in randomized Phase III clinical trials. In 2010, the first therapeutic cancer vaccine was approved for widespread use in the United States. In a double-blind, placebo-controlled Phase III trial, randomizing 512 patients with metastatic prostate cancer to receive a vaccine targeting prostatic acid phosphatase (sipuleucel-T) or a placebo, antigen-specific vaccination resulted in a 22% reduction in the risk of death in treated patients compared to controls (P = 0.03, CI 0.61–0.98). A survival benefit of a median of 4.1 months was significant even after adjusting for those patients who received docetaxel after study completion (P = 0.03) [1]. A Phase III trial of ipilimumab, an anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) monoclonal antibody, evaluated 676 HLA-A2 positive metastatic melanoma patients who were randomized 3:1:1 to receive ipilimumab and a melanoma antigen-specific vaccine, ipilimumab without vaccination and vaccination alone. Both arms containing ipilimumab, whether or not given with a vaccine, demonstrated superior median overall survival (OS) (P < 0.01) when compared with the vaccine alone arm [2]. The addition of a vaccine did not further enhance the therapeutic efficacy of ipilimumab (P = 0.76). Similar to the vaccine trial cited above, the survival benefit was approximately 4 months in these pre-treated patients. Finally, immunotherapy has been found to be beneficial in the treatment of neuroblastoma. Two hundred twenty-six patients with high-risk neuroblastoma, who achieved at least a partial response to initial therapy and stem cell transplant, were randomized to receive isoretinoin alone or isoretinoin administered with immune therapy consisting of a monoclonal antibody targeting GD2, a disialoganglioside which has been shown to be a tumor antigen, GM-CSF, and IL-2. The immunotherapy arm had both a superior disease-free (DFS) (P = 0.01) and overall survival (P = 0.02) when assessed at an interim analysis with a median follow-up of just over 2 years [3].
As a group, these recent studies are notable, not only for the significant clinical benefit associated with cancer immune therapy, but also for the diversity of the potential mechanisms of action of the agents. The first agent, sipuleucel-T, was designed to stimulate antigen-specific effectors, T cells, targeting the tumor. Ipilimumab modulates T cell function by both enhancing T effector cell function and blocking T cell regulatory function via ligation of CTLA-4 expressed on both those cell types [4]. The combination immunotherapy regimen in the neuroblastoma study was aiming to stimulate antibody-dependent cell-mediated cytotoxicity (ADCC) via antibody binding, and triggering of innate immune system cells recruited by GM-CSF and IL-2. IL-2 may serve to generate further proliferation of NK cells enhancing ADCC as well as increasing the activity of anti-tumor T cells primed by tumor-associated antigen-presenting cells (APC) [3]. The diversity in mechanism is matched by the diversity of the responding patient populations. Human malignancies as varied as prostate cancer, melanoma, and neuroblastoma responded significantly to immune-based therapy. Further, the ages of the patients in these studies ranged from children less than 18 months to patients greater than 65 years old. These data would indicate that cancer immune therapy has the potential for significant clinical impact across multiple human malignancies.
The ability to conduct randomized clinical trials of cancer immune-based therapies that result in well-matched patient populations that have either derived a clinical benefit from treatment or not now sets the stage for the potential validation of immunologic biomarkers which may be associated with clinical response. The identification of assays, interrogating specific immune responses, would not only result in a greater understanding of the mechanisms of action of these complex agents, but will also allow rapid optimization of more effective regimens if the endpoint of such optimization studies could be a validated immune biomarker. The challenge is to determine which biomarkers, of the many highly quantitative immune assessments available, have the greatest potential to be explored as correlates to clinical response. The ideal immunologic biomarker would be one that can (1) be measured easily from bodily fluids, such as blood, (2) was quantitative allowing for stratification of patients based on magnitude of response and allowed some qualitative assessment of the response, and (3) reflected the mechanism of action of the agent studied or the direct effect of immunity on cancer, i.e. tissue destruction (Fig. 1). Although assessment of the tumor itself may be the best source of markers which predict disease outcome as discussed below, often tumor sites are inaccessible for sampling for the purpose of clinical trials. Moreover, many immune-based therapies are being tested in the adjuvant setting where malignant tissue may not be available after the patient has been optimally treated. There are indicators in the published literature that blood-based immunologic biomarkers which predict clinical response can be developed. This review focuses on the identification of immunologic markers that may predict response to immune-based therapy or are modulated during the course of treatment. There is evidence that markers present prior to the initiation of immunotherapy could serve to predict clinical response to treatment, e.g. SNP or tumor expression of specific receptors/cell populations.
Fig. 1.
Characteristics of the ideal immunologic biomarker. Points in the arrow demonstrate the progression of analysis from analyte (bodily fluid) to assay (quantitative/qualitative) to outcome measurement which would reflect mechanism
Evaluation of cancer induced immunity provides a foundation for defining the elements of an effective anti-tumor immune response
Over the last several years, there have been a series of population-based studies, evaluating large numbers of cancer tissues, assessing the clinical importance of tissue-based immunologic biomarkers. These studies, for the most part, have examined cancer gene signatures, tumor infiltrating cell populations, and markers directly expressed on or in cancer and stromal cells as immunologic predictors of improved prognosis.
Gene expression profiling of cancer has determined that many human malignancies express an “immunologic signature,” which is associated with improved prognosis. Breast cancer was one of the first malignancies to exploit the use of any gene classifier for clinical decision making [5]. Since then, there have been multiple reports of gene signatures associated with breast cancer prognosis. A recent analysis evaluated the predictive performance of 9 gene expression signatures across 7 large breast cancer data sets, assessing greater than 1,000 patients [6]. The investigators identified a core group of gene modules that were common across all sets; apoptosis, proliferation, focal adhesion, RNA splicing and immunity. A composite signature of the immune and RNA splicing modules was the predictive of improved survival (related to breast cancer) and lack of metastasis (P < 0.001). Moreover, the combined signature was validated as an independent predictor of improved prognosis by multivariate analysis in selected data sets. Even within specific molecular subtypes such as HER-2/neu (HER2) positive breast cancer, expression of immune response-associated genes will identify a further subset of patients with marked survival benefit [7]. Alternatively, downregulation of 7 genes (C1QA, IGLC2, LY9, TNFRSF17, SPP1, XCL2, and HLA-F) associated with immunity was shown to be predictive of an increase in the development of distant metastasis in estrogen receptor negative tumors, P = 0.009, HR 2.02, CI: 1.2–3.4 [8].
Immune signatures of survival can be identified in both “immunogenic” and “non-immunogenic” tumors. Melanoma has long been known to be an immunogenic tumor and is actively immunoedited during the course of the disease [9]. Investigators have demonstrated that a signature highly enriched for immune response genes is associated with an improved prognosis in multivariate analysis (P = 0.02) [10]. Genes identified as prognostic included MHC Class II, T cell activation-, innate immunity-, and cytokine-related genes. A recent analysis of 44 patients with metastatic melanoma also identified a similar gene signature significantly associated with prolonged survival. The signature included genes associated with MHC class II, activated T cells, and other markers of innate and adaptive immunity [11]. Non-small-cell lung cancer has not been thought of as an immunogenic tumor. Investigators defined a 72 gene classifier that predicted relapse-free survival in early stage on small-cell lung cancer patients [12]. The gene signature was strongly enriched for immune response-related genes—interferon, immunoglobulin, and cytokine-associated genes. Multivariate analysis revealed the gene classifier was an independent predictor of both OS (HR 4.8, CI: 2.5–9.4) and relapse-free survival (HR 4.9, CI: 2.4–9.4) in these patients. Gene expression studies were performed in colon cancer—another tumor that was never previously thought to be “immunogenic”. Investigators also identified an immune signature that correlated with prognosis [13]. Although only 75 patients were evaluated, researchers discovered a cluster of genes associated with Type I adaptive immunity and cytotoxic T cells that were differentially regulated between patients with different times to recurrence. The greater the number of upregulated immune genes in the cluster, the less likely patients were to relapse. The cluster included markers, such as, granzyme B, T cell, and interferon-related genes.
The studies cited above are just a representation of the number of published reports of immune response-related gene signatures in a variety of cancers. Common themes identified include expression of interferon-related genes and Type I cytokine expression, upregulation of immune recognition molecules, particularly MHC class II, and activation of T cells. Indeed, the ability of T cells to infiltrate into cancer tissues has, in and of itself, been shown to be a beneficial prognostic marker.
It has long been known that T cells do infiltrate tumors; however, reports were conflicted as to whether this infiltration indicated a good or poor prognosis. It was only after investigators began to examine both the location of the infiltrating T cells as well as their phenotype that important observations concerning the type of T cell that could impact cancer prognosis have emerged. A large study performed, using the tumors of patients with ovarian cancer, demonstrated that T cells must penetrate the tumor stroma (i.e. be intratumoral T cells) to benefit disease outcome [14]. In a study of about 175 tumors derived from patients with Stage III and IV disease, the 5-year survival for those with intratumoral T cells was 38% compared to 4.5% of patients without T cells that penetrated the tumor. Moreover, the presence of intratumoral T cells predicted improved disease-free and overall survival (P < 0.001) [14]. Subsequently, the presence of intratumoral T cells has been shown to be an independent predictor of improved prognosis, as demonstrated in multivariate analyses, for mesothelioma, colorectal cancer, melanoma, endometrial carcinoma, bile duct carcinoma, renal cell cancers, and esophageal carcinoma [15–22]. In many of these reports, CD8+ T cell infiltration appeared to be important for the correlation to clinical benefit. Additional studies in colorectal cancer demonstrated that a high density of CD8+ and infiltrating memory T cells was an independent predictor of both overall and disease-free survival (P < 0.001) when compared with patient tumor samples that had a low density of infiltrating memory T cells, n = 336 samples [23].
Infiltrating T cells that regulate immune responses to self may adversely impact outcome, most likely by limiting the Type I immunity (as indicated by the gene signatures), which drives proliferation and function of infiltrating CD8+ and memory T cells. Indeed, tumor infiltrating T regulatory cells are associated with decreased survival in many diseases. In a study of 237 invasive breast cancer specimens, the presence of a high number of infiltrating T regulatory cells was associated with a higher risk of relapse in estrogen receptor positive breast cancers than in similar patients whose tumors had lower numbers of these infiltrating cells (P = 0.005) [24]. Moreover, the loss of T regulatory cell infiltrate and an increase in intratumoral CD8+ T cells with neoadjuvant chemotherapy were independent predictors of the development of pathologic complete remission at the time of breast resection [25]. In contrast to infiltrating Type I T cells, infiltrating FOXP3+ T regulatory cells have been associated with a poorer prognosis across multiple tumor types including non-small-cell lung cancer, gastric cancer, and renal cell carcinoma to name but a few tumors [26–28]. Of note, there have been studies evaluating the role of infiltrating FOXP3+ T regulatory cells that have demonstrated a correlation with a favorable prognosis, particularly in lymphoma and colorectal cancer [29, 30].
Finally, immune regulatory pathways that enhance or limit the generation of a productive immune response are operative in human malignancies. Potentially, one of the most important is the programmed death-1 (PD-1/B7-H1) pathway, which inhibits T cell activation. Several studies have demonstrated that upregulation of B7-H1 on the surface of tumor cells is correlated with a poor outcome. For example, the presence of tumor-associated B7-H1 in uroepithelial cancers was a more significant prognostic indicator than standard tumor grading systems [31]. In another study, elevated tumor-associated B7-H1 was an independent predictor of increased risk of disease progression and increased risk of death in patients with renal cell carcinoma [32]. Examples of other tumor expressed immune molecules that are independently associated with a poor prognosis are downregulation of MHC class I, and intratumoral TGF-β expression [33–35].
In summary, these multiple studies indicate that the selected patients, representing most human malignancies, develop a tumor-associated immune response. Further evidence suggests that a type I environment, if generated in the tumor, will result in the activation and proliferation of T cells, which are, most likely, the major mediators of anti-tumor immunity. Moreover, if negative regulation of the resultant tumor-associated immune response is not contained, suppressive forces in the tumor microenvironment will overcome the anti-tumor T cell response. The studies described above have focused on delineating important prognostic immune biomarkers in cancer tissue; whether those same biomarkers measured in the blood of cancer patients will be predictive is not known. Much effort has been placed on developing assays that will quantitate Type I T cell immunity in the peripheral blood generated in the context of clinical trials of cancer immunotherapy. Now, that several forms of cancer immunotherapy are achieving measureable clinical benefit, perhaps blood-based immune biomarkers that are predictors of clinical benefit can be defined (Fig. 1).
Immunologic biomarkers associated with clinical response after immune therapy
Studies described above underscore the importance of the development of a Type I adaptive immune response as a positive prognosticator. Type I CD4+ T cells secrete interferon gamma (IFN-γ) and support the development of antigen-specific CD8+ T cells, which are directly linked to tumor cell death [36]. One of the most common methods to assess Type I adaptive immunity is the measurement of tumor-specific immune responses by IFN-γ ELISPOT. Several recent studies, in a number of tumor types, have reported an association with immune responses measured by ELISPOT and improved clinical outcome after immune modulation. A clinical trial of a multi-peptide melanoma antigen targeting vaccine, enrolling 51 patients, demonstrated that individuals who developed a T cell response to immunizing antigen had a greater probability of a longer disease-free survival than patients who did not develop such immunity (P = 0.041) [37]. Another multi-peptide vaccine trial in melanoma, assessing 73 patients (of 120 enrolled) who had both ELISPOT and clinical data available, demonstrated that 34% of patients developed immunity to immunizing antigen. Patients with induced tumor-specific immune responses had a longer OS, 21.3 versus 10.8 months, P = 0.033, than non-immune responders [38]. Immune response was marginally significant (P = 0.073) as a predictor of survival after adjusting for other prognostic factors. A vaccine targeting prostate-specific antigen (PSA) was evaluated in 32 patients with metastatic chemotherapy-naïve prostate cancer and demonstrated that the magnitude of the induced immune response may be a better predictor of clinical response than the generation of detectable immunity. Investigators showed a trend in OS benefit for those patients who had sixfold or greater difference in the magnitude of the PSA-specific ELISPOT response from pre- to post-vaccination compared to those who showed a lesser rise (P = 0.055) [39]. Of note, there was no difference in the induction or magnitude of PSA antibody-specific immunity between short-term and long-term survivors. A similar trend was noted in breast cancer patients who were immunized with a vaccine targeting HER2 [40]. In this study, although the generation of immunity was not associated with OS benefit, the magnitude of immunity (greater vs. less than the median response) was higher in patients with longer survival (P = 0.08). Moreover, a higher magnitude of epitope spreading as assessed by IFN-γ ELISPOT also trended toward improved survival (P = 0.09). Many of the patients in this trial had elevated TGF-β serum levels at study initiation. Significant declines in TGF-β serum levels were associated with significant increases in the magnitude of intramolecular epitope spreading (P = 0.0003, r = 0.614). A clinical trial recently reported immunizing patients with colorectal cancer with an autologous tumor lysate vaccine and demonstrated the development of a tumor-specific IFN-γ ELISPOT response predicted those patients with longer recurrence-free survival (63% at 5 years) when compared to patients who did not develop immunity (18% at 5 years), P = 0.037 [41]. Notably, patients were given a control immunization with KLH, and there was no significant difference in KLH-induced immunity between groups. One of the most direct associations of Type I immunity as measured by ELISPOT and disease response was reported in a clinical trial of a HPV peptide-based vaccine for vulvar intraepithelial neoplasia [42]. The patients who developed a complete response with total resolution of disease during and after active immunization demonstrated a higher number of IFN-γ antigen-specific T cells than those who did not achieve a complete response (P = 0.001). These studies as well as several others demonstrate that the development of high magnitude Type 1 immune responses, both CD4+ and CD8+, may be a predictor of clinical outcome. Of note, although statistically significant, none of the above reports assessed the markers in multivariate analysis documenting the response as an independent predictor of prognosis. Along with the magnitude, the breadth of the immune response elicited may be a predictor of disease outcome as assessed by the development of epitope spreading (discussed below).
Similar to the population-based studies described above, the generation of a T cell memory response after immune-based cancer therapy has also been shown to be predictive of clinical outcome. Delayed type hypersensitivity response (DTH) has long been used as a measure of antigen recall or memory and has been shown to directly correlate with peripheral blood antigen-specific T cell responses [43]. Over a decade ago, DTH responses were shown to correlate with OS in studies of autologous tumor cell lysate vaccines in melanoma. In an evaluation of 81 patients injected with such a vaccine, both induration (P < 0.0001) and erythema (P = 0.0004) correlated strongly with survival benefit when the vaccine without adjuvant was used for skin testing [44]. DTH is commonly used as an immune assessment for cancer vaccines that are composed of whole tumors or tumor lysates. In these preparations, specific antigens are often unknown; thus, DTH via the vaccine preparation will evaluate the entire antigenic repertoire. Similar to the studies cited above for ELISPOT, evaluation of memory as assessed by DTH has shown some utility as a prognostic biomarker for cancer immunotherapy in a wide variety of malignancies. A phase I evaluation of a GM-CSF secreting pancreatic cell-based vaccine revealed that the development of DTH was associated with those patients who demonstrated increased disease-free survival compared to those patients who did not develop such a reaction [45]. More recently, in a study of 50 patients with Stage III and IV melanoma, injected with dendritic cell vaccine pulsed with tumor lysate, DTH-positive patients showed a greater OS (33 months) when compared with DTH-negative patients (11 months), P = 0.0014 [46]. Moreover, DTH-positive patients showed a significant decrease in TGF-β-producing FOXP3+ regulatory T cells when compared to DTH-negative patients (P < 0.0001). This finding, along with the study cited above that related elevated Th1 immunity to decreased serum TGF-β levels, suggests that vaccine induced immune responses are modulating the tumor microenvironment to support further evolution of immunity. Finally, DTH responses after peptide-based immunization have correlated with clinical outcome in melanoma. After immunization with gp100 and tyrosinase peptide-loaded dendritic cells, investigators observed a direct correlation of the ability to detect vaccine induced T cells in the DTH biopsy site and favorable clinical outcome (P = 0.0012) [47]. Assays such as ELISPOT and DTH may potentially be assessing the effect of the vaccine or its potency in inducing immunity. However, these methods may not necessarily be directly related to an anti-tumor response (Fig. 2). There are immune biomarkers that may be more directly related to the mechanism by which treatment results in clinical response.
Fig. 2.
Immunologic biomarkers may measure a variety of biologic responses. Shown are examples of three immune biomarkers reported in clinical trials of cancer immune therapy (boxes at top of panel). A cancer vaccine may induce the biologic response of stimulating T cells (blue circles), a demonstration of the potency of the vaccine which was designed to elicit adaptive immunity. The T cells may then traffic to tumor, secreting Type I cytokines, activating APC and stimulating cross-priming (yellow starbursts), inducing epitope spreading the mechanism by which tissue destruction occurs (red lightning bolts). Tissue destruction results in the generation of autoantibodies, which may directly reflect tumor cell death
What biomarker would be reflective of high levels of Type I T cells homing to the tumor microenvironment and inducing changes which would support evolving immunity? The immunosuppressive environment of the tumor does not support cross-priming, which is necessary for tumor immune recognition [36]. Trafficking T cells secreting IFN-γ could activate local tumor APC, restore key functions and upregulate immune accessory molecules, which would enhance cross-priming. Thus, the measurement of the restoration of effective cross-priming may be an immune biomarker more closely related to the anti-tumor response induced by active immunomodulation. Effective cross-priming could be assessed by the development of epitope spreading. The development of epitope spreading after a cancer vaccine, for example, is measured by the generation of new immunity to antigens not present in the vaccine formulation. Recent studies have suggested that epitope spreading is associated with improved outcomes after the administration of a cancer vaccine. In both melanoma and metastatic renal cell carcinoma, epitope spreading appeared to be a biomarker of clinical response albeit in a limited number of patients [48, 49]. One of the first studies to observe the generation of epitope spreading after vaccination (HER2-specific vaccination in breast cancer patients) recently reported immunologic biomarker data related to survival [50]. Fifty-two Stage III and IV patients had been vaccinated and 21 individuals were determined to be living at a median follow-up time of 112 months. Clinical parameters and several immune biomarkers were evaluated in multivariate analyses for the ability to predict survivors. The number of chemotherapy regimens prior to vaccination (HR 5.7 [CI 95%, 1.5–23] P = 0.001), and the development of epitope spreading after vaccination (HR 0.34 [CI 95%, 0.12–1.0]; P = 0.05) were independent predictors of OS. The median OS for subjects (n = 33) who developed epitope spreading was 84 months versus 25 months for the 16 subjects who did not develop epitope spreading [51]. Epitope spreading is a common phenomenon in autoimmune disease and is the basis of continuing pathologic tissue destruction. Identification of tumor antigens for the screening of epitope spreading can be achieved in many ways such as analyzing for the presence of new responses using “omics” techniques i.e. SEREX, proteomics, or screening of peptide libraries, as well as prospectively identifying antigens commonly expressed in a cancer type and evaluating for the development of new immunity. Theoretically, the development of autoimmunity could be an important immunologic biomarker demonstrating tissue destruction induced by effective cross-priming and epitope spreading (Fig. 2).
Autoimmunity may be the ultimate indicator of an anti-tumor response
There has long been an association of the development of autoimmunity with clinically effective cancer immunotherapy and or tumor regression. A recent prospective observational study enrolled almost 3,000 patients with melanoma to determine clinical parameters that correlated with favorable outcome [52]. Approximately 3% of patients developed vitiligo during the time of observation. In multivariate analysis, the development of vitiligo was an independent predictor of longer time to metastatic disease [Stage III (RR = 4.6)] and longer OS [Stage III (P = 0.03)/IV (P = 0.006)] when compared to those patients who did not develop vitiligo.
In 2001, the NCI Surgical Branch evaluated the laboratory parameters and clinical characteristics of 374 patients who were consecutively treated with IL-2 to determine whether any predictors of treatment response could be identified [53]. The overall response rate was about 15%, and favorable response was related to a greater number of doses of IL-2 received (P < 0.01), skin as the site of metastatic disease (P < 0.01), high lymphocyte counts after therapy (P < 0.01) and the development of autoimmune disease. Indeed, patients who developed thyroid dysfunction (P = 0.01) and/or vitiligo (P < 0.01) after IL-2 treatment were more likely to have an increased survival.
Treatment with anti-CTLA-4 monoclonal antibodies have also been shown to induce autoimmunity, which predicts clinical response. Again, the NCI Surgery Branch reported autoimmune phenomenon in 198 metastatic melanoma or renal cell carcinoma patients treated with ipilimumab [54]. Multiple autoimmune syndromes were noted including enterocolitis. While the response rate in the entire population was 14%, the response rate in the melanoma patients with enterocolitis was 36% compared to those who did not develop colitis at 11% (P = 0.0065). Similarly, the response rate in the renal cell cancer patients who developed colitis was 35% compared to 2% for those who did not develop colitis (P = 0.0016).
The development of autoimmunity has been shown to be an independent predictor of OS in patients with melanoma treated with interferon therapy [55]. In a study of 200 patients with Stage II/III melanoma, 26% developed either clinical or subclinical autoimmunity (evident only by serology). Indeed, the majority of patients developed only chemical evidence of autoimmunity. Only 13% of patients with any evidence of autoimmunity experienced a recurrence of their disease when compared with 73% of patients who had no evidence of autoimmune disease. In multivariate analysis the development of autoimmunity correlated with longer relapse-free survival (P < 0.001) as well as OS (P < 0.001). Although these data could not be validated in other similar studies, the observation that the majority of patients with “autoimmunity” had only serologic evidence of autoimmunity without clinical manifestations calls to question the validity of the evaluation of treatment induced autoimmunity after cancer immunotherapy (Fig. 2) [56]. Perhaps the common assays used to assess autoimmunity underestimate the true rate of incidences.
The studies cited above evaluated only a limited number of classic disease-associated autoantibodies. However, more recent data suggest that a larger repertoire of autoantibodies that could reflect response to treatment may exist. Using high-throughput screening methods for the identification of serum autoantibodies developing after treatment, investigators identified a panel of autoantibodies that were associated with survival in melanoma patients receiving a whole cell autologous melanoma vaccine [57]. Many of these antibodies were directed to proteins involved in tumor cell growth. Similarly, autoantibodies to a panel of 20 proteins were identified and associated with the development of melanoma associated retinopathy [58]. Tissue destruction mediated by CD8+ T cells will result in the generation of a multitude of self-directed antibodies reflecting such destruction. An expanded autoantibody panel may better identify patients experiencing subclinical autoimmunity after cancer immune-based therapy (Fig. 3). Moreover, it has often been observed that the measurement of T cells in the peripheral blood may not directly correlate with the activity of T cells taking place at the site of the tumor [59]. Once antibodies are elicited, they are generated at high levels in the blood and will persist over an extended time facilitating detection.
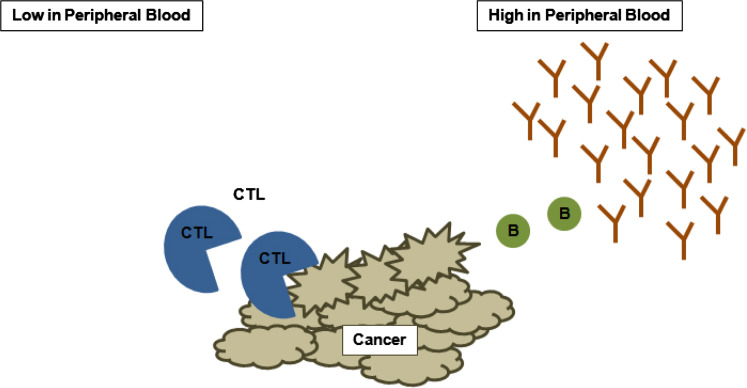
Fig. 3.
Immune biomarkers may be found in differing levels in the peripheral blood. Cancer immunotherapy is often designed to generate cytotoxic T cells (CTL; blue circles) which may have varying levels in the peripheral blood. The majority of induced or infused CTL may be found at the site of the tumor and thus remain undetected. As tumor is destroyed, however, blood-based biomarkers such as antibodies may be present at high levels in the peripheral blood and better reflect tumor destruction (B cells; green circles, antibodies; brown lines)
Conclusions
Studies cited above suggest that there are several immune-based biomarkers that are worthy of exploration as correlates to an anti-tumor response in clinical trials of cancer immunotherapy. Many of these markers have elements of an “ideal immunologic biomarker” (Fig. 1). There are assays that could be defined as those specific to the potency of the immunomodulatory agent, those that may better reflect the mechanism of action of the drug or vaccine, and finally biomarkers that may directly assess immune destruction at the target organ, thus directly reflecting the anti-tumor response (Figs. 2, 3). Currently, most of the immune assays being exploited in clinical trials are a reflection of vaccine potency and not mechanism of action or end result of therapy. There is a role for all these type of assays in the clinical development of immune-based cancer therapies. The ability to identify a marker that accurately predicts responding patients requires the identification of top candidates and application of those candidates to appropriately powered randomized phase III studies.
Acknowledgments
This work was supported by NCI grants R01 CA129517, CA136632 and U01CA154967. I thank Ms. Molly Boettcher for expert assistance in manuscript preparation.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- APC
Antigen-presenting cells
- CI
Confidence interval
- CTLA-4
Cytotoxic T-lymphocyte antigen 4
- DFS
Disease-free survival
- DTH
Delayed type hypersensitivity
- GM-CSF
Granulocyte macrophage colony stimulating factor
- HER2
HER-2/neu
- HPV
Human papilloma virus
- HR
Hazard ratio
- IFN
Interferon
- IL
Interleukin
- KLH
Keyhole limpet hemocyanin
- NCI
National Cancer Institute
- NK
Natural killer
- OS
Overall survival
- PDL
Programmed death ligand
- PSA
Prostate-specific antigen
- RR
Relative risk
- SNP
Single nucleotide polymorphisms
- TGF
Transforming growth factor
Footnotes
This paper is a Focussed Research Review based on a presentation given at the 8th Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 26–28th May, 2010.
References
- 1.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian J, Simon R. What should physicians look for in evaluating prognostic gene-expression signatures? Nat Rev Clin Oncol. 2010;7(6):327–334. doi: 10.1038/nrclinonc.2010.60. [DOI] [PubMed] [Google Scholar]
- 6.Reyal F, van Vliet MH, Armstrong NJ, Horlings HM, de Visser KE, Kok M, Teschendorff AE, Mook S, van ‘t Veer L, Caldas C, et al. A comprehensive analysis of prognostic signatures reveals the high predictive capacity of the proliferation immune response and RNA splicing modules in breast cancer. Br Cancer Res. 2008;10(6):R93. doi: 10.1186/bcr2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staaf J, Ringner M, Vallon-Christersson J, Jonsson G, Bendahl PO, Holm K, Arason A, Gunnarsson H, Hegardt C, Agnarsson BA, et al. Identification of subtypes in human epidermal growth factor receptor 2–positive breast cancer reveals a gene signature prognostic of outcome. J Clin Oncol. 2010;28(11):1813–1820. doi: 10.1200/JCO.2009.22.8775. [DOI] [PubMed] [Google Scholar]
- 8.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8(8):R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamshchikov GV, Mullins DW, Chang CC, Ogino T, Thompson L, Presley J, Galavotti H, Aquila W, Deacon D, Ross W, et al. Sequential immune escape and shifting of T cell responses in a long-term survivor of melanoma. J Immunol. 2005;174(11):6863–6871. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- 10.Mandruzzato S, Callegaro A, Turcatel G, Francescato S, Montesco MC, Chiarion-Sileni V, Mocellin S, Rossi CR, Bicciato S, Wang E, et al. A gene expression signature associated with survival in metastatic melanoma. J Transl Med. 2006;4:50. doi: 10.1186/1479-5876-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogunovic D, O’Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA. 2009;106(48):20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roepman P, Jassem J, Smit EF, Muley T, Niklinski J, van de Velde T, Witteveen AT, Rzyman W, Floore A, Burgers S, et al. An immune response enriched 72-gene prognostic profile for early-stage non-small-cell lung cancer. Clin Cancer Res. 2009;15(1):284–290. doi: 10.1158/1078-0432.CCR-08-1258. [DOI] [PubMed] [Google Scholar]
- 13.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 15.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB, et al. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10(13):4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 16.Ladanyi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, Gaudi I, Timar J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56(9):1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61(13):5132–5136. [PubMed] [Google Scholar]
- 18.Oshikiri T, Miyamoto M, Shichinohe T, Suzuoki M, Hiraoka K, Nakakubo Y, Shinohara T, Itoh T, Kondo S, Katoh H. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol. 2003;84(4):224–228. doi: 10.1002/jso.10321. [DOI] [PubMed] [Google Scholar]
- 19.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–3936. [PubMed] [Google Scholar]
- 21.Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137(4):1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada N, Oizumi S, Kikuchi E, Shinagawa N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T, Torigoe T, et al. CD8 +tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother. 2010;59(10):1543–1549. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 24.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 25.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14(8):2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 26.Liotta F, Gacci M, Frosali F, Querci V, Vittori G, Lapini A, Santarlasci V, Serni S, Cosmi L, Maggi L et al. 2010 Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int. doi:10.1111/j.1464-410X.2010.09555.x [DOI] [PubMed]
- 27.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3 + Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136(10):1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5(5):585–590. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 29.Lee NR, Song EK, Jang KY, Choi HN, Moon WS, Kwon K, Lee JH, Yim CY, Kwak JY. Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma. 2008;49(2):247–256. doi: 10.1080/10428190701824536. [DOI] [PubMed] [Google Scholar]
- 30.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7–H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson RH, Dong H, Kwon ED. Implications of B7–H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13(2 Pt 2):709s–715s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 33.Mizukami Y, Kono K, Maruyama T, Watanabe M, Kawaguchi Y, Kamimura K, Fujii H. Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2008;99(9):1462–1467. doi: 10.1038/sj.bjc.6604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, Ferrone S. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66(18):9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 35.Desruisseau S, Palmari J, Giusti C, Romain S, Martin PM, Berthois Y. Determination of TGFbeta1 protein level in human primary breast cancers and its relationship with survival. Br J Cancer. 2006;94(2):239–246. doi: 10.1038/sj.bjc.6602920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28(29):4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N, Grosh WW, Yamshchikov GV, Neese PY, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13(21):6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 38.Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, Whiteside T, Butterfield LH, Weiner L. Immunogenicity and antitumor effects of vaccination with peptide vaccine ± granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: eastern cooperative oncology group phase II Trial E1696. Clin Cancer Res. 2009;15(4):1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barth RJ, Fischer DA, Wallace PK, Channon JY, Noelle R, Gui J, Ernstoff MS. A randomized trial of ex vivo CD40L activation of a DC vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res. 2010;16(22):5548–5556. doi: 10.1158/1078-0432.CCR-10-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 43.Disis ML, Schiffman K, Gooley TA, McNeel DG, Rinn K, Knutson KL. Delayed-type hypersensitivity response is a predictor of peripheral blood T-cell immunity after HER-2/neu peptide immunization. Clin Cancer Res. 2000;6(4):1347–1350. [PubMed] [Google Scholar]
- 44.Baars A, Claessen AM, van den Eertwegh AJ, Gall HE, Stam AG, Meijer S, Giaccone G, Meijer CJ, Scheper RJ, Wagstaff J, et al. Skin tests predict survival after autologous tumor cell vaccination in metastatic melanoma: experience in 81 patients. Ann Oncol. 2000;11(8):965–970. doi: 10.1023/A:1008363601515. [DOI] [PubMed] [Google Scholar]
- 45.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19(1):145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 46.Lopez MN, Pereda C, Segal G, Munoz L, Aguilera R, Gonzalez FE, Escobar A, Ginesta A, Reyes D, Gonzalez R. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol. 2009;27(6):945–952. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 47.de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, Ruiter DJ, Figdor CG, Punt CJ, Adema GJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23(24):5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 48.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH, Mukherji B, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9(3):998–1008. [PubMed] [Google Scholar]
- 49.Wierecky J, Muller MR, Wirths S, Halder-Oehler E, Dorfel D, Schmidt SM, Hantschel M, Brugger W, Schroder S, Horger MS, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66(11):5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]
- 50.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20(11):2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 51.Salazar LG, Goodell V, O’Meara M, et al. Persistent immunity and survival after immunization with a HER-2/neu vaccine. J Clin Oncol (Meeting Abstracts) 2009;27(15S):2010. [Google Scholar]
- 52.Quaglino P, Marenco F, Osella-Abate S, Cappello N, Ortoncelli M, Salomone B, Fierro MT, Savoia P, Bernengo MG. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21(2):409–414. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 53.Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19(15):3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 54.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354(7):709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 56.Bouwhuis MG, Suciu S, Collette S, Aamdal S, Kruit WH, Bastholt L, Stierner U, Sales F, Patel P, Punt CJ. Autoimmune antibodies and recurrence-free interval in melanoma patients treated with adjuvant interferon. J Natl Cancer Inst. 2009;101(12):869–877. doi: 10.1093/jnci/djp132. [DOI] [PubMed] [Google Scholar]
- 57.Sittler T, Zhou J, Park J, Yuen NK, Sarantopoulos S, Mollick J, Salgia R, Giobbie-Hurder A, Dranoff G, Hodi FS. Concerted potent humoral immune responses to autoantigens are associated with tumor destruction and favorable clinical outcomes without autoimmunity. Clin Cancer Res. 2008;14(12):3896–3905. doi: 10.1158/1078-0432.CCR-07-4782. [DOI] [PubMed] [Google Scholar]
- 58.Hartmann TB, Bazhin AV, Schadendorf D, Eichmuller SB. SEREX identification of new tumor antigens linked to melanoma-associated retinopathy. Int J Cancer. 2005;114(1):88–93. doi: 10.1002/ijc.20762. [DOI] [PubMed] [Google Scholar]
- 59.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26(7):360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]