Abstract
Because cancer is associated with aging, immunological features in the aged should be considered in anticancer immunotherapy. In this study, we investigated antitumor immunity in aged mice using a CT26 colon carcinoma model. The tumor growth of CT26 was accelerated in aged mice compared with that in young mice, but this difference was not observed in nude mice. The serum levels of IL-6 and TNF-α were higher in aged mice than those in young mice, irrespective of the CT26-bearing state. The in vitro induction of CT26-specific CTLs from aged mice that were vaccinated with doxorubicin (DTX)-treated CT26 cells was impaired. In vivo neutralization of IL-6, but not TNF-α, showed a tendency to restore the in vitro induction of CT26-specific CTLs from vaccinated aged mice. Analyses on tumor-infiltrating immune cells as early as day 5 after CT26 inoculation revealed that monocytic and granulocytic MDSCs preferentially infiltrated into tumor sites in aged mice compared with young mice. Alternatively, oral administration of Lentinula edodes mycelia (L.E.M.) extract, which has the potential to suppress inflammation in tumor-bearing hosts, decreased the serum levels of IL-6 in aged mice. When administration of L.E.M. extract was started 1 week earlier, CT26 growth was retarded in aged mice and the in vivo priming of tumor-specific CTLs was improved in CT26-vaccinated aged mice. These results indicate early infiltration of MDSCs is related to impaired immunity of aged hosts and that oral administration of L.E.M. extract can mitigate the impairment.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1857-y) contains supplementary material, which is available to authorized users.
Keywords: Aging, Inflammation, IL-6, MDSC, T cell immunity
Introduction
Cancer frequently occurs in the elderly, and their immunological competence is reduced [1]. Therefore, age-associated impairment of immunity, particularly T cell immunity, should be considered at the setting of immunotherapy. However, much attention has not been paid on this issue. Thus far, several reasons have been proposed to explain immune dysfunction in the aged [1]: the decreased number of T cells due to thymic atrophy with aging [2], decreased proliferation and cytokine production by T cells [3, 4], reduced cytotoxic activity of CTLs [5], decreased naïve T cells capable of reacting to new antigens [6], and deficiencies in APCs, such as DCs [7]. Additionally, several studies have indicated that aged hosts have higher numbers of regulatory T cells (Tregs) and MDSCs [8–10]. Moreover, proinflammatory cytokines, including IL-6 and TNF-α, are increased in the aged [11, 12]. The accumulation of these alterations may lead to immune dysfunction in aged hosts, resulting in the promotion of tumor growth [1]. However, several studies have reported that tumor growth is retarded in aged mice compared with young mice [13–16]. Overall, the underlying mechanisms of impaired antitumor immunity in aged hosts are complicated, and the precise mechanisms have not been fully elucidated.
The cancer-bearing state is accompanied by inflammation [17]. Particularly, IL-6 and TNF-α are the main mediators of cancer-associated inflammation and are known to induce cachexia at high levels [18]. Alternatively, these cytokines are also increased in the aged [11, 12]. IL-6 inhibits the type 1 helper (Th1) response and induction of CTLs [11]. Additionally, inflammation and IL-6 increase MDSCs [19, 20], and MDSCs suppress the Th1 response via IL-6 production [21].
We recently reported that oral ingestion of Lentinus edodes mycelia (L.E.M.) extract mitigates Treg-mediated immunosuppression and restores melanoma-reactive CD8+ T cells in melanoma-bearing mice [22]. Additionally, this reagent can prolong vaccine-induced tumor-specific CTLs in vivo [23]. Furthermore, oral ingestion of L.E.M. extract can suppress an increase in the serum level of IL-6 in mice that were inoculated with colon carcinoma into the cecum [24], suggesting that this reagent has the potential to attenuate inflammation with either cancer and/or aging.
In the present study, we tried to elucidate the mechanisms of impaired antitumor immunity in aged hosts by comparing CT26 colon carcinoma-bearing young and aged mice. Our results indicate that IL-6 and TNF-α were increased in aged mice and that monocytic and granulocytic MDSCs were preferentially infiltrated into tumor sites. Additionally, our findings suggested that oral administration of L.E.M. extract can mitigate impaired antitumor immunity to the level of young hosts and restore the efficacy of anticancer vaccine to some degree.
Materials and methods
Mice and tumor cell lines
BALB/c and BALB/c nu/nu female mice [H-2d: 6–7 weeks (W) old] were purchased from CLEA Japan, Inc. (Tokyo, Japan), and Japan SLC, Inc. (Hamamatsu, Japan), respectively. These mice were used as young mice. Retired BALB/c mice (45 W) were purchased from CLEA Japan. Experiments were performed according to the ethical guidelines for animal experiments of the Shimane University Faculty of Medicine (IZ26-5) and for animal experiments of the Kobayashi Pharmaceutical Co., Ltd. CT26 is a colon carcinoma cell line derived from BALB/c mice and was maintained in RPMI 1640 supplemented with 10 % FBS.
L.E.M. reagent
Dried powdered extract of L.E.M. was prepared with hot water before germination and after culturing mycelia in a medium composed of bagasse and rice bran, as previously described [25]. L.E.M. extract was diluted in PBS at a dose of 0.25 g/mL and was orally administered using gavage at a volume of 200 and 300 μl for young and aged mice, respectively. As a control, the same volume of PBS was administered.
Vaccine with cancer cells and in vitro induction of tumor-specific CTLs
To inactivate cancer cells, CT26 cells were treated with doxorubicin (DTX) (Kyowa Hakko Co. Ltd., Tokyo, Japan) at a dose of 50 μg/mL for 12 h. After washing, 1 × 106 CT26 cells were injected to the left flank of mice. After 2 weeks, spleen cells were harvested and cultured with an H-2Ld-binding peptide (SPSYVYHQF), a tumor antigen peptide of CT26 derived from the envelope protein (gp70) of an endogenous murine leukemia virus [26], in the presence of IL-2 (20 U/mL) for 4 days. This peptide was designated AH1 in the current study. AH1 peptide was >80 % pure and was purchased from Invitrogen (Carlsbad, CA, USA). Thereafter, its cytotoxicity was measured using a 5-h 51Cr-release assay.
Flow cytometry
To assess the subsets of the spleen, the cell suspension was treated with RBC-lysing buffer, stained with the indicated mAbs and analyzed using flow cytometry with a FACSCalibur (Becton–Dickinson, Fullerton, CA, USA). The following mAbs were used for staining: APC-conjugated anti-CD45 (BioLegend, San Diego, CA, USA), PE-conjugated anti-CD4 (AbD Serotec, Oxford, UK), PE-conjugated anti-CD8 (Southern Biotech, Birmingham, AL, USA), PE-conjugated anti-CD11b (BioLegend), FITC-conjugated anti-Gr-1 (R&D systems, Minneapolis, MN, USA), and PE/cy7-conjugated anti-Ly6C (BioLegend). To examine Tregs, the cell suspension was stained with APC-conjugated anti-CD45 (BioLegend) and PE-conjugated anti-CD4 (AbD Serotec). After fixing with IntraPrep Permeabilization Reagent (Beckman Coulter, Brea, CA, USA), these cells were stained with anti-Foxp3 mAb (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
In some experiments, 1 × 106 CT26 cells were inoculated s.c. to four different sites in one mouse. On day 5, the tumor tissues were harvested from the four sites, pooled and examined by flow cytometry. The pooled cell suspension was individually pooled from each mouse.
ELISA
Serum levels of IL-6 and TNF-α were determined using the ELISA Development Kit (PeproTech, Rocky Hill, NJ, USA). To examine the ability of spleen cells to produce cytokines, spleen cells were cultured with or without LPS (List Biological Laboratories, Inc, Campbell, CA, USA) (5 μg/mL) or anti-CD3 mAb (clone 145-2C11; BioLegend) (2 μg/mL) for 48 h.
In vivo neutralization of IL-6 and/or TNF-α
To neutralize either or both IL-6 and TNF-α in vivo, anti-mouse IL-6 antibody (rat IgG1: R&D Systems, Inc., Minneapolis, MN, USA) and 100 μg anti-mouse TNF-α antibody (rat IgG1: Southern Biotech, Birmingham, AL, USA), at a dose of 100 μg, were injected i.p. twice, one day before and simultaneously with vaccination of cancer cells. As a control, rat IgG (Sigma-Aldrich, Saint Louis, MO, USA) was injected.
Protective immunity assay
To compare protective antitumor immunity, we used mice that were vaccinated s.c. in the left flank with 1 × 106 DTX-treated CT26 cells. Two weeks later, these mice were inoculated s.c. in the right flank with 5 × 105 viable CT26 cells. Several mice groups received PBS or L.E.M. extract administered orally. The L.E.M. extract was administered 1 week prior to and 1 week after vaccination for a total of 2 weeks. The tumor size was measured thereafter.
Statistical analysis
Data were evaluated using unpaired two-tailed Student’s t test and analysis of variance (ANOVA) with Bartlett’s test. A P value of <0.05 was considered to indicate statistical significance.
Results
Accelerated growth of CT26 in aged compared with young mice
First, we compared the growth of CT26 in young (7 W) and aged (45 W) BALB/c mice. Three different doses of CT26 were inoculated s.c. to the right flank (Fig. 1a). The tumor size was larger in aged mice compared with that in young mice when 1 × 105 CT26 cells were inoculated (Fig. 1b). A similar result was observed when 1 × 105 CT26 cells were inoculated into young (7 W) and aged (60–65 W) mice (Fig. 1c, left), and the tumor weight on day 28 in aged mice was significantly higher than that in young mice (Fig. 1c, right).
Fig. 1.
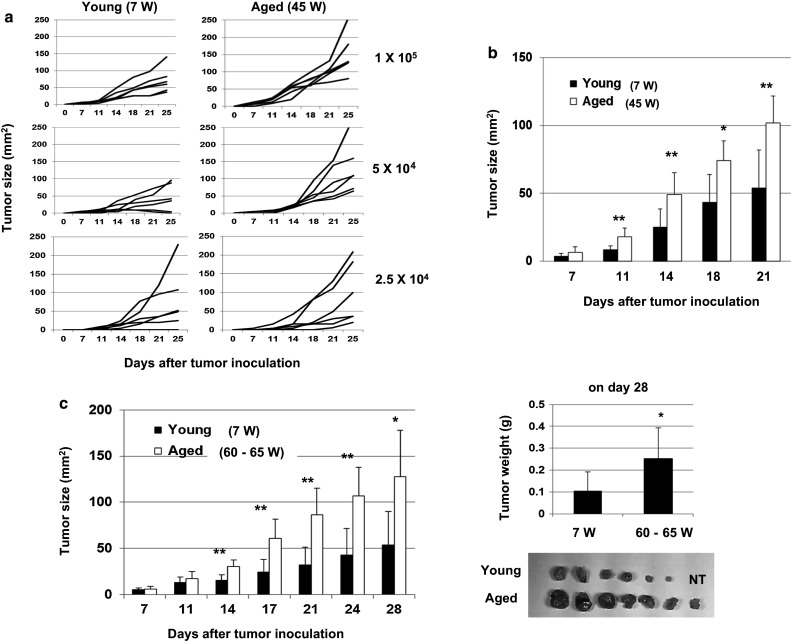
Promotion of tumor growth in aged mice. a Young (7 W) and aged (45 W) BALB/c mice (n = 6) were inoculated s.c. with the indicated doses of CT26 cells. Each line represents the tumor growth of an individual mouse. b A representative result of tumor growth in BALB/c mice that were injected s.c. in the right flank with 1 × 105 CT26 cells is shown. *P < 0.05, **P < 0.01. Similar results were obtained in two independent experiments. c (Left) Young (7 W) and aged (60–65 W) BALB/c mice (n = 7) were inoculated s.c. with 1 × 105 CT26 cells. *P < 0.05, **P < 0.01. Similar results were obtained in three independent experiments. (Right) On day 28, tumor weight was measured. *P < 0.05. NT no tumor
Cell number and subsets of spleen cells from young and aged mice
Next, we compared the cell number and subsets of spleen cells from young (7 W) and aged (60 W) BALB/c mice. The spleen cell number and positive percentages of B220+ B cells were higher in aged mice, whereas the positive percentage of CD8+ T cells was lower in spleen cells from aged mice (Suppl. Figure 1a). No difference was observed in the percentages of CD4+ T cells and Tregs. Additionally, we examined the percentages of myeloid cells. MDSCs can be divided into monocytic CD11b+ Gr-1low Ly6C+ cells and granulocytic CD11b+ Gr-1+ Ly6C− cells [27, 28]. The staining and gating strategies to examine myeloid cells are shown in Suppl. Figure 1b. Splenic monocytes were defined as CD11b+ F4/80+ cells. All myeloid cells showed a tendency to increase in aged mice, and increases in the percentages of total and monocytic MDSCs were statistically significant.
Elevation of IL-6 and TNF-α in aged naïve mice
We next measured the serum levels of IL-6 and TNF-α in young (7 W) and aged (60 W) mice that were naïve or inoculated s.c. with 5 × 105 CT26 cells 10 days prior. The results showed that the serum levels of IL-6 and TNF-α were significantly elevated in aged mice compared with those of young mice and that the tumor-bearing state showed the tendency to increase IL-6, but the difference was not significant (Fig. 2a). IL-6 and TNF-α were detected in the culture supernatants of CT26 cells (1 × 105 cells/mL for 48 h) at levels of 120 pg/mL and 63 pg/mL, respectively. We also measured the potential of spleen cells to produce IL-6 and TNF-α and found that the spleen cells from aged mice produced higher levels of IL-6 upon LPS stimulation than young mice (Fig. 2b). Additionally, spleen cells from aged mice produced higher levels of IFN-γ in response to anti-CD3 mAb compared with young mice. We further determined whether these findings could be observed in nude mice. No difference in tumor growth was observed between young (7 W) and aged (45 W) nude mice (Fig. 2c). We also measured the serum levels of IL-6 and TNF-α in young (7 W) and aged (45 W) nude mice that were naïve or inoculated s.c. with 1 × 105 CT26 cells 18 days prior. As a result, the level of IL-6 was elevated in the serum of aged nude mice, whereas that of TNF-α was not increased in aged nude mice (Fig. 2d). These results indicate that aging is accompanied by increased levels of proinflammatory cytokines, including IL-6 and TNF-α, and the increase in IL-6 in aged mice is T cell-independent.
Fig. 2.
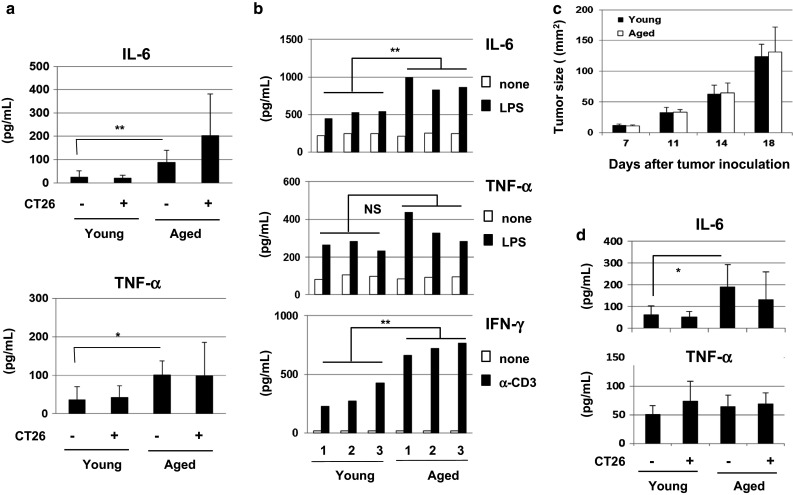
Increased levels of serum IL-6 and TNF-α in naïve aged mice. a Serum levels of IL-6 and TNF-α in naïve and CT26-bearing (on day 10) young (7 W) and aged (60 W) BALB/c mice were measured by ELISA. Mice were injected s.c. with 1 × 105 CT26 cells. Each group consisted of five or six mice. *P < 0.05, **P < 0.01. Similar results were obtained in two independent experiments. b The spleen cells from three young (7 W) and three aged (60 W) BALB/c mice were examined for their production of IL-6 and TNF-α during two days in culture in the presence or absence of LPS (5 μg/mL) or anti-CD3 mAb (2 μg/mL). **P < 0.01. NS not significant. Similar results were obtained in two independent experiments. c Nude young (7 W) and aged (45 W) mice were injected s.c. with 1 × 105 CT26 cells, and tumor growth was measured. Each group consisted of five mice. d Serum levels of IL-6 and TNF-α in naïve and CT26-bearing (on day 18) young (7 W) and aged (45 W) nude mice were measured by ELISA. Each group consisted of five mice. *P < 0.05
Effects of IL-6 and TNF-α on in vivo priming and in vitro induction of tumor-specific CTLs
We compared the vaccine efficacy of inactivated cancer cells in the in vivo priming of tumor-specific CTLs between young and aged mice. Two weeks after s.c. inoculation of DTX-treated CT26 cells, spleen cells were in vitro stimulated with a tumor antigenic AH1 peptide and examined for cytotoxicity against CT26 cells (Fig. 3a). Although tumor-specific CTLs were induced successfully from the spleen cells of vaccinated young mice, the vaccine efficacy was weak in aged mice. Given that the serum levels of IL-6 and TNF-α were elevated in aged mice (Fig. 2a), we examined the effects of these cytokines on the in vitro induction of antitumor CTLs. The spleen cells from vaccinated young and aged mice were stimulated in vitro with a tumor antigenic AH1 peptide and IL-2 for 4 days in the presence of IL-6 (200 pg/mL) and/or TNF-α (100 (pg/mL). The results showed that anti-CT26 CTL activity was enhanced only when TNF-α was added to the culture of the spleen cells from young mice (Suppl. Figure 2). Alternatively, we examined the effects of IL-6 and TNF-α on the in vivo priming of tumor-specific CTLs in aged mice (Fig. 3b). The aged (45 W) mice were i.p. injected with either or both anti-IL-6 and/or anti-TNF-α neutralizing antibodies twice, one day before and simultaneously with s.c. injection of DTX-treated CT26 cells. As a result, neutralization of TNF-α, but not IL-6, at the timing of cancer vaccine suppressed the induction of CT26-specific CTLs from aged mice. We additionally performed the anti-IL-6 neutralizing experiments using aged (60 W) mice. As a result, neutralization of IL-6 augmented the induction of CT26-specific CTLs from spleen cells of vaccinated aged (60 W) mice, but this increase was not significant (Fig. 3c). Overall, these results suggest that TNF-α exerts a stimulatory effect on the in vivo priming of tumor-specific CTLs, but that IL-6 has the tendency to inhibit it.
Fig. 3.
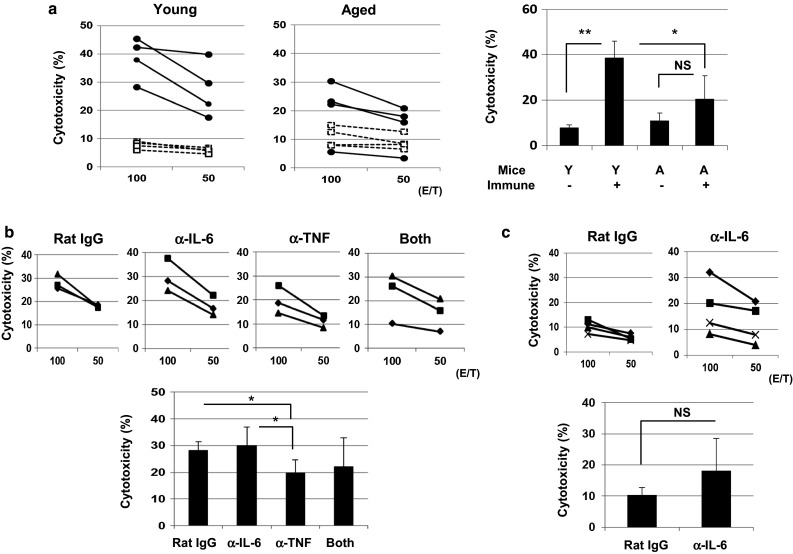
Effects of IL-6 and TNF-α on the in vitro induction and in vivo priming of tumor-specific CTLs. a (Left) Naïve young (7 W) and aged (60 W) BALB/c mice were injected s.c. with DTX-treated 1 × 106 CT26 cells. Two weeks later, spleen cells were harvested and cultured with the AH1 peptide and IL-2 (20 U/mL) for 4 days. These cultured cells were examined for their cytotoxicity against CT26 cells using the 51Cr-release assay. Each group consisted of four mice. (Right) The results at an E/T ratio of 100 are shown *P < 0.05, **P < 0.01. NS not significant. Y young, A aged. Similar results were obtained in two independent experiments. b (Upper) Naïve aged (45–50 W) BALB/c mice were injected s.c. with DTX-treated 1 × 106 CT26 cells. Anti-IL-6 or anti-TNF-α neutralizing mAb (100 μg) was i.p. injected twice (1 day before and on the same day of immunization with DTX-treated CT26). Two weeks later, spleen cells were harvested and cultured with the AH1 peptide and IL-2 (20 U/mL) for 4 days. These cultured cells were examined for their cytotoxicity against CT26 cells using the 51Cr-release assay. Each group consisted of three mice. (Lower) The results at an E/T ratio of 100 are shown *P < 0.05. c The similar experiment described in (b) was performed using aged (60 W) mice. Each group consisted of four mice. NS not significant
Early infiltration of monocytic and granulocytic MDSCs into the tumor sites in aged mice
Inflammation generally increases the trafficking ability of immune cells [29]. Additionally, MDSCs can be induced by inflammation [20, 30, 31]. Therefore, we examined the tumor-infiltrating immune cells, focusing especially on MDSCs, in young and aged mice as early as 5 days after CT26 inoculation. The staining and gating strategies to detect two different sets of MDSCs in tumors are shown in Fig. 4a. To obtain a sufficient amount of infiltrating cells to perform flow cytometric analysis, each mouse was inoculated s.c. with 1 × 106 CT26 cells to four different sites, and all tumor tissues in each mouse were pooled. The tumor sizes of young and aged mice on day 5 were 17.3 ± 2.0 and 24.0 ± 1.8 mm2, respectively (P < 0.01). As a result, the percentages of CD45+ immune cells were higher in aged mice than in young mice (Fig. 4b). However, there was no difference between young and aged mice in the percentage of CD11b+ cells among CD45+ cells in tumor sites. Additionally, there was no difference in the percentages of CD11b+ Gr-1low Ly6C+ monocytic and CD11b+ Gr-1+ Ly6C – granulocytic MDSCs. Similarly, no difference was observed in the percentages of CD11b+ Gr-1− Ly6C− cells, which were probably tumor-associated macrophages. These results indicate that CD45+ immune cells preferentially infiltrate into tumor sites in aged mice compared with young mice at the early tumor-bearing stage.
Fig. 4.
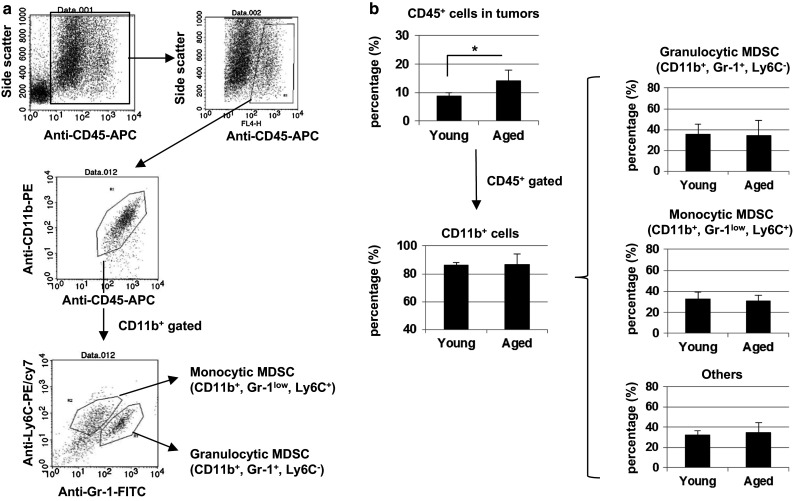
Infiltration of CD45+ immune cells into the tumor sites. Young (7 W) and aged (60 W) BALB/c mice were injected s.c. into four sites with 1 × 106 CT26 cells. On day 5, the tumors were harvested from each mouse separately and analyzed by flow cytometry. a The staining and gating strategies are shown. b The percentages of tumor-infiltrating CD45+ immune cells, CD11b+ cells among CD45+ cells, and myeloid-derived cells among CD11b+ cells are shown. The mean ± SD of four mice is shown *P < 0.05
Effect of oral administration of L.E.M. extract on CT26 growth in aged mice
As described in Introduction, oral ingestion of L.E.M. extract mitigates an increase in serum IL-6 in mice that were inoculated with CT26 into the cecum [24]. We next determined whether oral administration of L.E.M. extract could ameliorate the impaired antitumor immunity in aged mice. Since the body weights of aged mice were almost 1.5 times higher than those of young mice (Fig. 5a), we suspended L.E.M. extract in PBS at a dose of 0.25 g/mL and orally administered it at volumes of 200 and 300 μl to young and aged mice, respectively. In the first experiment, we started to administer L.E.M. extract on the next day following s.c. inoculation of CT26, but no antitumor effect was observed in young or aged mice (Fig. 5b). Therefore, given the elevated levels of IL-6 and TNF-α in naïve aged mice, we next examined the antitumor effect when oral administration of L.E.M. extract was started 1 week before CT26 inoculation. As a result, CT26 growth in aged mice was significantly suppressed by oral administration of L.E.M. extract to the same level of young mice on days 10 and 14 after CT26 inoculation (Fig. 5c). The serum levels of IL-6 of untreated aged and L.E.M.-administered aged mice on day 12 after CT26 inoculation were 390.8 ± 98.2 and 256.9 ± 44.3 (pg/mL), respectively (P < 0.01). In contrast, no difference was observed in the levels of TNF-α. We also examined the effect of oral administration of L.E.M. extract for 1 week on the serum levels of IL-6 and TNF-α in naïve aged (45 and 60 W) mice. Although the difference was not statistically significant in aged (45 W) mice, oral administration of L.E.M. extract significantly decreased serum levels of IL-6, but not TNF-α in 60-W aged mice (Fig. 5d).
Fig. 5.
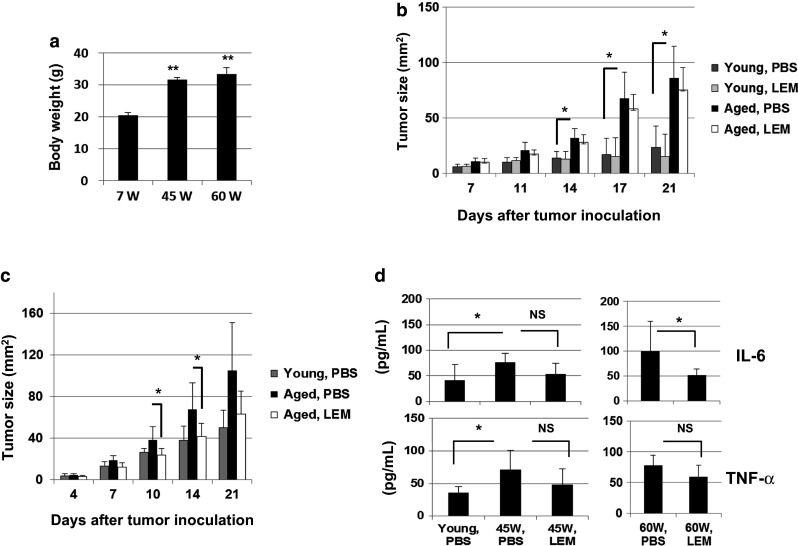
Effect of oral administration of L.E.M. extract on CT26 growth in aged mice. a Body weights of naïve young (7 W) and aged (45 or 60 W) BALB/c mice were measured. Each group consisted of five mice. **P < 0.01. b Young (7 W) and aged (60 W) BALB/c mice were inoculated s.c. with 1 × 105 CT26 cells. From the following day, these mice were administered orally with PBS or L.E.M. extract 6 days per week throughout the experiment. Tumor growth was measured. Each group consisted of seven mice. *P < 0.05. Similar results were obtained in two independent experiments. c Similarly, young (7 W) and aged (45–50 W) BALB/c mice were inoculated s.c. with 1 × 105 CT26 cells. PBS or L.E.M extract administration was initiated 1 week before CT26 inoculation and continued throughout the experiment. *P < 0.05. Each group consisted of seven mice. Similar results were obtained in two independent experiments. d PBS or L.E.M. extract was administered orally for 1 week to naïve young (7 W) and aged (45 or 60 W) BALB/c mice that were orally administered with PBS or L.E.M. extract for 1 week, and the sera were collected. The IL-6 and TNF-α levels were measured by ELISA. Each group consisted of six or seven mice. *P < 0.05. NS, not significant
Amelioration of impaired anticancer vaccine efficacy in aged mice by oral administration of L.E.M. extract
We next examined the effect of oral administration of L.E.M. extract on the in vivo priming with inactivated CT26 cells. Aged (45–50 W) mice were administered L.E.M. extract orally 1 week before and were injected s.c. with DTX-treated CT26 cells. Oral administration of L.E.M. extract was continued until the spleen cells were harvested. Two weeks after vaccination, spleen cells were harvested, and their anti-CT26 CTL activity was examined after in vitro culture with the AH1 peptide. As a result, oral administration of L.E.M. extract increased the CT26-specific cytotoxicity of DTX-vaccinated aged mice (Fig. 6a). Subsequently, a similar experiment was performed using young (7 W) mice (Fig. 6b). Vaccine alone induced anti-CT26 CTLs effectively, but oral administration of L.E.M. extract had no augmenting effect on this CTL induction. Lastly, we examined the effect of oral administration of L.E.M. extract on protective immunity (Fig. 6c). DTX-treated CT26 cells were vaccinated s.c. into young (7 W) and aged (60 W) mice; after 2 weeks, 5 × 105 CT26 cells were challenged. Some mice were received L.E.M. extract orally, beginning 1 week before and lasting until 1 week after vaccination. Vaccine alone induced more prominent protective immunity in young mice than in aged mice. Six of seven young mice rejected the CT26 re-challenge compared with one of the six aged mice. The tumor size decreased to 20 % in young mice, but to only 50 % in aged mice. Oral administration of L.E.M. extract led to a slight decrease in tumor growth of CT26 in aged mice, but this decrease was not significant. These results indicate that oral administration of L.E.M. extract can partially restore the impaired anticancer vaccine efficacy in aged mice.
Fig. 6.
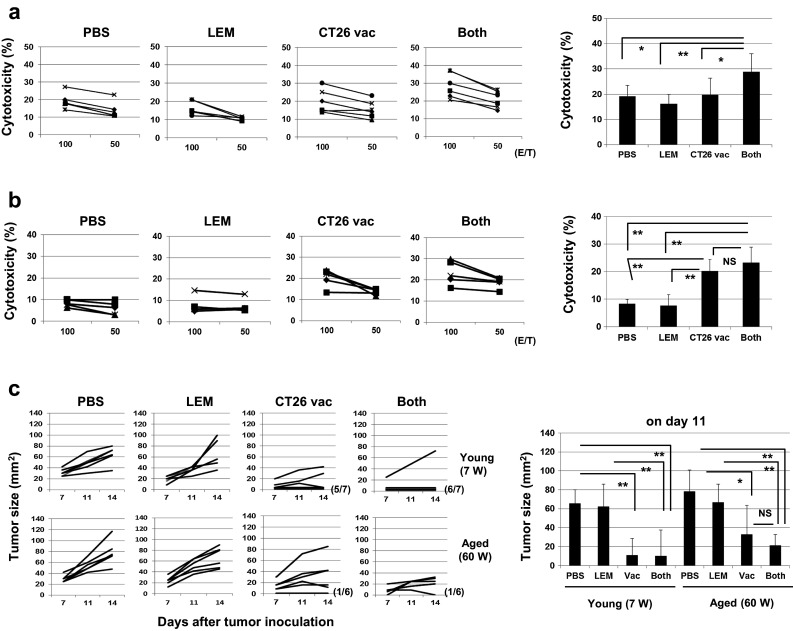
Effect of oral administration of L.E.M. extract on the induction of anti-CT26 CTLs and protective immunity. a (Left) Similarly to Fig. 3a, naïve aged (45 W) BALB/c mice were vaccinated with 1 × 106 DTX-treated CT26 cells. In some groups, oral administration of PBS or L.E.M extract was initiated 1 week before CT26 vaccination and continued until spleen cell harvesting. Two weeks after CT26 vaccination, the spleen cells were harvested and cultured with the AH1 peptide and IL-2 (20 U/mL) for 4 days and examined for their cytotoxicity against CT26 cells using the 51Cr-release assay. Each group consisted of six mice. *P < 0.05. **P < 0.01. (Right) The results at an E/T ratio of 100 are shown. b Similar experiments were performed using young (7 W) mice. Each group consisted of five mice. The results at an E/T ratio of 100 are shown. **P < 0.01. NS not significant. c (Left) Naïve young (7 W) and aged (60 W) BALB/c mice were vaccinated s.c. with DTX-treated 1 × 106 CT26 cells. In some groups, oral administration of PBS or L.E.M extract was initiated 1 week before CT26 vaccination and continued until 1 week after vaccination. Two weeks after CT26 vaccination, 5 × 105 viable CT26 cells were inoculated s.c. and tumor growth was measured. Each group consisted of six or seven mice, and each line represents the tumor growth of an individual mouse. (Right) The tumor sizes on day 11 after CT26 challenge are shown. *P < 0.05. **P < 0.01. NS not significant
Discussion
Age-associated attenuation of immunological competence urges us to take precautions in anticancer immunotherapy for aged hosts. Until now, several studies have revealed the mechanisms underlying why antitumor immunity in aged hosts is impaired, as described in Introduction. Nevertheless, the underlying mechanisms and therapies to relieve impaired antitumor immunity in aged hosts have not been investigated fully. Therefore, we undertook the present study and subsequently tested the possibility that an orally available immunomodulator, L.E.M. extract, could alleviate age-associated impairment of antitumor immunity.
After observation that the growth of CT26 carcinoma was accelerated in aged mice (Fig. 1), we compared tumor growth in young and aged nude mice; however, no difference in tumor growth was observed (Fig. 2c). These results suggest that the difference in tumor growth between young and aged mice was ascribed mainly to the impairment of antitumor T cell immunity in this tumor model. Additionally, the levels of serum IL-6 and TNF-α were increased in aged mice compared with young mice (Fig. 2a), consistent with previous reports [11, 12, 19]. What types of cells produce IL-6 and TNF-α in aged mice? The spleen cells from aged mice produce higher levels of IL-6 upon LPS stimulation than those from young mice (Fig. 2b). Regarding IL-6, LPS might trigger IL-6 production by macrophages and B cells in the spleen via TLR4-mediated signaling. We suppose that these cells in aged mice increase the potential to produce IL-6 as a result of an age-associated inflammatory environment. However, some reports have suggested that macrophages from aged mice impair the production of proinflammatory cytokines, such as IL-6 and TNF-α, upon LPS stimulation compared with young mice [31, 32]. In contrast, other studies have reported that aged mice are more sensitive to LPS stimulation than young mice [33, 34]. Of note is that serum IL-6, but not TNF-α, was elevated in aged nude mice (Fig. 2d). This finding indicates that IL-6 was increased in a T cell-independent manner in aged mice. Interestingly, a recent report suggests that the interaction between adiposity and macrophages is a critical factor for cytokine storm after stimulatory immunotherapy with anti-CD40 antibody and IL-2 in young obese mice [35]. In addition, caloric restriction prevents an elevation of IL-6 and TNF-α in aged mice [12, 35]. Adiposity seems to be involved in the age-associated increase in proinflammatory cytokines. Alternatively, spleen cells from aged mice produced a higher level of IFN-γ in the presence of anti-CD3 antibody (Fig. 2b). This observation is inconsistent with other studies reporting that Th1 responses are impaired in aged hosts [11, 36]. Whereas other studies examined IFN-γ production by antigen-specific T cells, we examined bulk IFN-γ production by polyclonal T cells using anti-CD3 mAb. Although a component of unprimed naïve T cells could be diminished in aged hosts, effector or memory-like T cells capable of producing Th1 type cytokines might be accumulated during aging. Further studies are needed to elucidate the underlying mechanism.
The efficacy of the anticancer vaccine to induce tumor-specific CTLs was impaired in aged mice. To use this vaccine, we applied DTX to inactivate cancer cells because DTX is a useful drug to induce immunogenic cancer cell death [37], and we previously reported that DTX-treated immunization can induce protective immunity [38]. Thereafter, we determined whether IL-6 and/or TNF-α have any effect on the in vivo priming and in vitro induction of tumor-specific CTLs. In vivo neutralization of TNF-α at the in vivo priming with DTX-treated cancer cells inhibited CTL induction (Fig. 3b), whereas that of IL-6 showed the tendency to restore this induction (Fig. 3c). Given that tumor-specific CTLs may not be induced/primed for only 4 days in in vitro culture, TNF-α could exert a positive role in in vivo priming of tumor-specific CTLs in vaccinated aged mice. Presumably, TNF-α enhanced the antigen-presenting capacity of DCs to prime tumor-specific CTLs, because DCs play a central role in the in vivo priming of cancer-specific CTLs after cell-based vaccines [39] and because TNF-α can augment the antigen-presenting capacity of DCs [40]. Alternatively, in vitro addition of TNF-α slightly increased tumor-specific CTLs in the spleen cells of vaccinated young mice (Suppl. Figure 2). In this in vitro system, TNF-α might affect the killing activity of tumor-specific CTLs. Indeed, it has been reported that TNF-α enhances the cytotoxicity of tumor-specific CTLs by preventing an inhibitory effect of TGF-β [41] and that CTLs induce cytolysis of cancer cells via secreted or membrane-associated forms of TNF-α [42]. In contrast, the age-associated increase in IL-6 seems to counteract the effect of TNF-α, and in vivo blockade of IL-6 activity tended to enhance the induction of tumor-specific CTLs in vaccinated aged mice (Fig. 3c). However, IL-6 showed no effect on the in vitro induction of tumor-specific CTL activity in spleen cells from vaccinated mice (Suppl. Figure 2). This result suggests that IL-6 indirectly exerted an inhibitory effect on the in vivo priming of tumor-specific CTLs in vaccinated mice. In fact, it has been reported that IL-6 inhibits the Th1 response and the induction of CTLs [11] and increases MDSCs [19, 20].
Inflammation and IL-6 are known to induce MDSCs [19, 20]. Therefore, given an increase in serum levels of IL-6 and TNF-α in naïve aged mice, we tested the possibility that MDSCs were preferentially infiltrated into the tumor sites of aged mice. Particularly, we focused on MDSCs in tumor sites at the early tumor-bearing state. As a result, monocytic and granulocytic MDSCs infiltrated into tumor sites more preferentially in aged mice than in young mice on day 5 after tumor inoculation (Fig. 4b). These early infiltrating MDSCs were at least partially responsible for accelerated tumor growth in aged mice.
We previously reported that oral ingestion of L.E.M. extract can suppress CT26 growth in the cecum and increase serum IL-6 in young mice [24]. Therefore, we tested whether oral administration of L.E.M. extract could suppress the s.c. inoculated CT26 growth and attenuate increases in serum IL-6 and TNF-α in aged mice. Oral administration of L.E.M. extract significantly decreased serum IL-6 in aged mice (Fig. 5d). Treatment with L.E.M. extract led to the tendency toward decreased TNF-α in aged mice, but the difference was not significant. Importantly, oral administration of L.E.M. extract beforehand retarded CT26 growth in aged mice to the same level in young mice (Fig. 5c). Additionally, oral administration of L.E.M. extract beforehand restored anticancer vaccine efficacy by inducing tumor-specific CTLs in aged mice (Fig. 6a). These results suggest that oral administration of L.E.M. extract can restore anticancer vaccine efficacy to some degree even in aged tumor-bearing hosts.
In conclusion, we demonstrated that the age-associated increase in IL-6/TNF-α and preferential and early infiltration of MDSCs into tumor sites, at least partially, contribute to impaired T cell immunity in aged mice. In addition, oral administration of L.E.M. extract beforehand retarded tumor growth, probably via attenuating aging-associated inflammation. L.E.M. extract can be administered on an outpatient basis [43]. Given that an orally available immunomodulator is not a burden for aged patients, this type of drug will be useful in aged cancer patients treated with anticancer immunotherapies, including anticancer vaccines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are grateful to Ms. Tamami Moritani for her technical assistance. This study was supported in part by JSPS KAKENHI Grant (No. 25430150 to N. Harashima, and No. 24501331 to M. Harada) and from the Shimane University “SUIGANN” Project.
Abbreviations
- DTX
Doxorubicin
- L.E.M.
Lentinus edodes mycelia
- Th1
Type I helper
- Treg
Regulatory T cell
Compliance with ethical standards
Conflict of interest
Mamoru Harada received donations for research from the Kobayashi Pharmaceutical Co., Ltd. All other authors declare that they have no conflict of interest.
References
- 1.Lustgarten J. Cancer, aging and immunotherapy: lessons learned from animal models. Cancer Immunol Immunother. 2009;58:1979–1989. doi: 10.1007/s00262-009-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 3.Song I, Kim Y, Chopra R, et al. Age-related effects in T cell activation and proliferation. Exp Gerontol. 1993;28:313. doi: 10.1016/0531-5565(93)90058-L. [DOI] [PubMed] [Google Scholar]
- 4.Engwerda C, Fox B, Handwerger B. Cytokine production by T lymphocytes from young and aged mice. J Immunol. 1996;156:3621. [PubMed] [Google Scholar]
- 5.Bloom E, Umehara H, Bleackley R, et al. Age-related decrement in cytotoxic T lymphocyte (CTL) activity is associated with decreased levels of mRNA encoded by two CTL-associated serine esterase genes and the perforin gene in mice. Eur J Immunol. 1990;20:2309. doi: 10.1002/eji.1830201021. [DOI] [PubMed] [Google Scholar]
- 6.Thoman M. Effects of the aged microenvironment on CD4+ T cell maturation. Mech Ageing Dev. 1997;96:75–88. doi: 10.1016/S0047-6374(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 7.Saurwein-Teissl M, Romani N, Grubeck-Loebenstein B. Dendritic cells in old age- neglected by gerontology? Mech Ageing Dev. 2000;121:123. doi: 10.1016/S0047-6374(00)00203-7. [DOI] [PubMed] [Google Scholar]
- 8.Nishioka T, Shimizu J, Iida R, et al. CD4+ CD25+ Foxp3+ T cells and CD4+ CD25+ Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Dominguez A, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 10.Grizzle W, Xu X, Zhang S, et al. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred B × D12 mice. Mech Ageing Dev. 2007;128:672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto H, Senju S, Matsumura K, et al. IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age. Nat Commun. 2015;6:6702–6716. doi: 10.1038/ncomms7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-α and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/S0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 13.Tomihara K, Shin T, Hurez VJ, et al. Aging-associated B7-DC+ B Cells enhance anti-tumor immunity via Th1 and Th17 induction. Aging Cell. 2012;11:128–138. doi: 10.1111/j.1474-9726.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donin N, Sinai J, Staroselsky A, et al. Comparison of growth rate of two B16 melanomas differing in metastatic potential in young versus middle-aged mice. Cancer Invest. 1997;15:416–421. doi: 10.3109/07357909709047580. [DOI] [PubMed] [Google Scholar]
- 15.Kanno J, Wakikawa A, Utsuyama M, et al. Effect of restraint stress on immune system and experimental B16 melanoma metastasis in aged mice. Mech Ageing Dev. 1997;93:107–117. doi: 10.1016/S0047-6374(96)01827-1. [DOI] [PubMed] [Google Scholar]
- 16.Itzhaki O, Kaptzan T, Skutelsky E, et al. Age-adjusted antitumoral therapy based on the demonstration of increased apoptosis as a mechanism underlying the reduced malignancy of tumors in the aged. Biochim Biophys Acta. 2004;1688:145–159. doi: 10.1016/j.bbadis.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Cahlin C, Körner A, Axeisson H, et al. Experimental cancer cachexia: the role of host-derived cytokines interleukin (IL)-2, IL-12, interferon-gamma, and tumor necrosis factor alpha evaluated in gene knockout, tumor-bearing mice on C57Bl background and eicosanoid-dependent cachexia. Cancer Res. 2000;60:5488–5493. [PubMed] [Google Scholar]
- 19.Liu Q, Tan Q, Zheng Y, et al. Blockade of fas signaling in breast cancer cells suppresses tumor growth and metastasis via disruption of fas signaling-initiated cancer-related inflammation. J Biol Chem. 2014;289:11522–11535. doi: 10.1074/jbc.M113.525014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Bunt SK, Yang L, Sinha P, et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukamoto H, Nishikata R, Senju S, et al. Myeloid-derived suppressor cells attenuate TH1 development through IL-6 production to promote tumor progression. Cancer Immunol Res. 2013;1:64–76. doi: 10.1158/2326-6066.CIR-13-0030. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Ishikawa S, Matsui Y, et al. Oral ingestion of Lentinula edodes mycelia extract inhibits B16 melanoma growth via mitigation of regulatory T cell-mediated immunosuppression. Cancer Sci. 2011;102:516–521. doi: 10.1111/j.1349-7006.2010.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K, Ishikawa S, Matsui Y, et al. Combining a peptide vaccine with oral ingestion of Lentinula edodes mycelia extract enhances anti-tumor activity in B16 melanoma-bearing mice. Cancer Immunol Immunother. 2012;61:2143–2152. doi: 10.1007/s00262-012-1275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K, Matsui Y, Ishikawa S, et al. Oral ingestion of Lentinula edodes mycelia extract can restore the antitumor T cell response of mice inoculated with colon-26 cells into the subserosal space of the cecum. Oncol Rep. 2012;27:325–332. doi: 10.3892/or.2011.1549. [DOI] [PubMed] [Google Scholar]
- 25.Kojima H, Akaki J, Nakajima S, et al. Structural analysis of glycogen-like polysaccharides having macrophage-activating activity in extracts of Lentinula edodes mycelia. J Nat Med. 2010;64:16–23. doi: 10.1007/s11418-009-0357-1. [DOI] [PubMed] [Google Scholar]
- 26.Huang AY, Gulden PH, Woods AS, et al. The immunedominant major histocompatibility complex class I-restricted antigens of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youn JI, Nagaraj S, Colloazo M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha P, Parker KH, Horn L, et al. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-γ and IL-4Rα. Eur J Immunol. 2012;42:2052–2059. doi: 10.1002/eji.201142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beury DW, Parker KH, Nyandio M, et al. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc Biol. 2014;96:1109–1118. doi: 10.1189/jlb.3A0414-210R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehmer ED, Goral J, Faunce DE, et al. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 32.Chelvarajan RL, Collons SM, Van Willigen JM, et al. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77:503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 33.Bouchlaka MN, Sckisel GD, Chen M, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210:2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohman RA, Crowell B, Kusnecov AW. Differential sensitivity to endotoxin exposure in young and middle-age mice. Brain Behav Immun. 2010;24:486–492. doi: 10.1016/j.bbi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirsoian A, Bouchalaka MN, Sckisel GD, et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med. 2014;211:2373–2383. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young MR, Kolesiak K, Achille NJ, et al. Impact of aging on immune modulation by tumor. Cancer Immunol Immunother. 2001;50:315–320. doi: 10.1007/s002620100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesniere A, Apetoh L, Ghiringhelli F, et al. Immunogenic cancer cell death: a key–lock paradigm. Curr Opin Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Tongu M, Harashima N, Yamada T, et al. Immunogenic chemotherapy with cyclophophamide and doxorubicin against established murine carcinoma. Cancer Immunol Immunother. 2010;59:769–777. doi: 10.1007/s00262-009-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Bosch ML, Salgaller ML. Current methods for loading dendritic cells with tumor antigen for the induction of antitumor immunity. J Immunother. 2002;25:289–303. doi: 10.1097/00002371-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Mcllroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother. 2003;52:583–591. doi: 10.1007/s00262-003-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorelik L, Bar-Dagan Y, Mokyr MB. Insight into the mechanism(s) through which TNF promotes the generation of T cell-mediated antitumor cytotoxicity by tumor bearer splenic cells. J Immunol. 1996;156:4298–4308. [PubMed] [Google Scholar]
- 42.Ratner A, Clark WR. Role of TNF-α in CD8+ cytotoxic T lymphocyte- mediated lysis. J Immunol. 1993;150:4303–4314. [PubMed] [Google Scholar]
- 43.Yoshioka Y, Tamesada M, Nagayama A. The Safety of Excessive Intake of the Food Containing Extract of Cultured Lentinula edodes Mycelia (L.E.M.) in Healthy Adult Volunteers. JCAM. 2009;6:9–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


