Abstract
No second-line treatment significantly prolongs the survival of malignant mesothelioma patients who have a high unmet medical need. Here, we comment on the therapeutic potential of cytotoxic T-lymphocyte-associated protein (CTLA)4-blockade by the anti-CTLA4 monoclonal antibody (mAb) tremelimumab of refractory malignant mesothelioma patients. We also focus on the critical role of an accurate tumor assessment in the course of treatment with immunomodulating mAb. Finally, treatment with potentially effective, second-generation checkpoint(s) inhibiting mAb, as well future combination strategies in this deadly disease, will be discussed.
Keywords: Mesothelioma, Anti-CTLA4 mAb, Tremelimumab, Immunotherapy, NIBIT-2013
Achievements, failures, and future role of cytotoxic and targeted therapy
First-line and maintenance therapy
Malignant mesothelioma (MM) is a very poor prognosis human neoplasm, whose incidence is increasing worldwide due to exposure to asbestos [1]. Caused by its insidious pattern of local spreading, only few patients are candidate to surgery, generally within a multimodality treatment including chemotherapy and radiotherapy [2]. The role of surgery, however, is highly debated, and most patients receive chemotherapy alone as first-line therapy [3]. Based on the results of a Phase III trial comparing the combination of cisplatin and pemetrexed with cisplatin alone, this doublet regimen has been set as the standard first-line treatment for pleural MM, with a response rate (RR) of 41.3 %, a median time to progression (TTP) of 5.7 months, and a median overall survival (OS) of 12.1 months [4]. Aiming to reduce cisplatin toxicity, schedules with carboplatin have been implemented, with comparable results in terms of disease control rate (DCR) and survival outcomes, but with a more favorable toxicity profile [5]. Studies evaluating 3-drug regimens with the addition of a targeted agent (particularly bevacizumab) have shown no improvement in RR and OS [6]. There is no consensus on the optimal duration of first-line chemotherapy in MM; therefore, 4–6 cycles are usually administered [2]. Maintenance strategies are also being explored in order to prolong disease control in responding patients. A randomized Phase II study of continuation maintenance with pemetrexed versus observation alone is ongoing; recently, the negative results of a switch maintenance trial with the anti-angiogenic compound thalidomide have been reported [7].
Second-line therapy
Second-line therapies are being increasingly used in MM clinical practice, but their role is still unproven, and the optimal regimen remains to be defined [8]. Published studies have frequently severe limitations, due to the small number and to the heterogeneity of patients included, and often to a retrospective design. In the few patients not previously treated with pemetrexed, data from an expanded access program and from a randomized Phase III study support the use of single-agent pemetrexed as a standard second-line treatment [9]. However, most patients are pretreated with first-line pemetrexed-based chemotherapy; thus, no standard of care exists in this increasing group of patients that remains an ideal field in which to test new therapeutic agents [8]. The better understanding of the biology of MM has led to the assessment of a number of targeted agents in clinical trials. However, most studies with targeted therapies have shown disappointing results. In a Phase III study enrolling 660 patients, platinum-/pemetrexed-pretreated patients were randomly assigned to receive vorinostat, a histone deacetylase inhibitor, or placebo; no difference in OS was observed in the intent-to-treat population [10]. Based on a strong preclinical rationale, great hope was placed in the use of angiogenesis inhibitors in the second-line setting of MM; however, their clinical use has been more challenging than anticipated. RR has been generally less than 10 %, with 40–80 % of patients achieving short-lived disease control; median TTP has been invariably around 3 months [11]. Second-line chemotherapy, mainly vinorelbine or gemcitabine, has limited efficacy in unselected patients [8]. In MM cases with a prolonged response to first-line chemotherapy, re-treatment with a pemetrexed-based regimen represents the best available therapy [12]. New treatment options are therefore eagerly awaited in the second-line setting of MM, and several ongoing studies are exploring new strategies such as validation of biomarkers in patient selection for chemotherapy, mesothelin-targeted therapies, and immunotherapy [13–15].
The evolving role of CTLA4 blockade
Targeting immune checkpoint(s) by immunomodulatory mAb is a novel and rapidly evolving strategy that will likely change the therapeutic landscape of human malignancies of different histotype, allowing achieving a long-term disease control and a significantly prolonged survival. Along this line, the therapeutic success achieved first in metastatic melanoma patients by the anti-CTLA4 mAb ipilimumab, has broadened its clinical investigation in multiple tumor types, and has prompted the clinical development of additional checkpoint blocking mAb [16–18].
Based on the upcoming evidences above, the MESOT-TREM-2008 study (Clinicaltrial ID: NCT01649024) investigated the therapeutic potential of the anti-CTLA4 mAb tremelimumab (15 mg/kg once every 3 months) in unresectable MM patients progressing to a first-line platinum-based regimen. Two of the 29 treated patients achieved a long-lasting partial response, 7 had a prolonged stabilization of disease (median duration 12.4 months), and the disease control rate was 31 % [15]. Selected patients experienced disease progression followed by a long-lasting partial response, as already demonstrated in melanoma patients treated with ipilimumab [19, 20]. This evidence broadens the notion that, at variance to cytotoxic/target therapies, treatment with anti-CTLA4 mAb can induce atypical patterns of clinical response and that a careful clinical and instrumental tumor assessment is mandatory before discontinuation of anti-CTLA4 therapy, as clinical benefits may be delayed in selected patients [20]. This latter aspect is even more relevant in MM patients in which tumor assessment requires specific radiologic expertise and ad hoc criteria, as further discussed in a separate section of this manuscript [21]. A long-lasting stable disease was achieved by 24 % of patients treated within the MESOT-TREM-2008 study; moreover, landmark analysis at 1 and 2 years identified survival rates of 48.3 and 36.7 % that compare favorably with available data in MM patients [15, 22]. Therefore, the MESOT-TREM-2008 study identified a proportion of MM patients obtaining long-term clinical benefit from the treatment with tremelimumab, which is consistent with clinical data with ipilimumab in metastatic melanoma patients [23].
A critical and unsettled issue in the course of CTLA4 blockade derives from the lack of reliable predictive biomarkers of response to treatment. Treatment of MM patients with tremelimumab also demonstrated a significant association between an early increase in circulating CD4+ICOS+ T lymphocytes and improved survival [15]. Once more, this finding corroborated previous data obtained with ipilimumab and suggested that the evaluation of CD4+ICOS+ T lymphocytes might represent a pharmacodynamic tool to guide the use of anti-CTLA4 mAb, as discussed elsewhere [23, 24].
To corroborate the initial findings providing proof of concept that CTLA4 blockade bears an encouraging clinical activity in MM patients, and in light of pharmacokinetic studies with tremelimumab further discussed in this manuscript, we activated the MESOT-TREM-2012 study (Clinicaltrial ID: NCT01655888). This second study explored a more intensive schedule of administration of tremelimumab at 10 mg/kg i.v. on day 1, q4 weeks (wks) for 6 doses (induction phase), followed by q12-week dosing (maintenance phase), until progressing disease (PD) or severe toxicity in second-line MM patients (Table 1). The study has enrolled the planned 29 patients, and preliminary data have been presented at the ASCO meeting 2014 [25].
Table 1.
Clinical studies with tremelimumab in malignant mesothelioma patients
| Study | Agent | Phase | Accrual | No. patients enrolled |
|---|---|---|---|---|
| MESOT-TREM-2008 (NCT01649024) |
Tremelimumab second line |
II | Completed | 29 |
| MESOT-TREM-2012 (NCT01655888) |
Tremelimumab second line |
II | Completed | 29 |
| D4880C00003 (NCT01843374) |
Tremelimumab versus PBO second/third line |
II | Recruiting | 564 |
A model indication to re-explore the role of tremelimumab
Development and progression of MM may have an immune-mediated component; thus, an agent such as tremelimumab that enhances T cell immune function might have relevant antitumor activity in MM patients [26, 27].
In a Phase III melanoma trial in the first-line metastatic setting, tremelimumab (15 mg/kg Q90 days for 4 doses in total) demonstrated a response rate of 11 %, and a median OS of 13 months compared to 11 months in the chemotherapy arm (DTIC/temozolomide) failing to demonstrate a statistically significant survival advantage of treatment with tremelimumab over standard-of-care chemotherapy [28]. One potential explanation for the failure of this study could be related to the underexposure that most patients experienced with the dose and schedule of tremelimumab utilized in that trial (15 mg/kg Q90 days). A retrospective analysis of pharmacokinetic data from Phase II and Phase III studies of tremelimumab (15 mg/kg Q90 days) in patients with melanoma clearly showed an improvement in the OS for those patients who achieved a higher drug exposure, as measured by the area under the concentration–time curve from time 0 to 90 days (AUC90). The survival analysis for patients in the tremelimumab arm of the Phase III study analyzed by AUC90 above or below the median AUC for the tremelimumab-treated arm showed a more favorable and prolonged median OS for subjects who had an AUC above the median, compared to those with an AUC below the median (16 vs. 10 months). This effect was consistent even in the multivariate analyses controlling for confounding factors (e.g., lactate dehydrogenase, C-reactive protein level, and M1c stage status). The PK modeling developed at MedImmune also suggests that a higher tremelimumab exposure can be reached with a more frequent dosing schedule. These latter results were also confirmed in MM patients treated with tremelimumab (data not shown).
Based on data demonstrating that tremelimumab can augment activation of the human immune system in MM, the biologic background of mesothelioma, the immunosuppression induced by asbestos, and the activity seen to date in the Italian investigator-sponsored MESOT-TREM-2008 study, MedImmune has initiated a randomized, double-blind, placebo-controlled study in adults with unresectable pleural or peritoneal MM, who progressed on 1 or 2 prior systemic treatment regimens including pemetrexed (or other anti-folate) in combination with a platinum agent in first line (Clinicaltrial ID: NCT01843374) [29]. Subjects will be randomized in a 2:1 ratio to receive either tremelimumab or placebo. Randomization will be stratified by the European Organization for Research and Treatment of Cancer (EORTC) status (low-risk vs. high-risk), line of therapy (second vs. third), and anatomical site (pleural vs. peritoneal). Approximately 564 subjects will be enrolled at approximately 180 study centers in multiple countries (Table 1).
Immunotherapy of MM: a radiologic challenge
Current imaging
Imaging plays an essential role in the evaluation of pleural MM. Chest radiography typically shows a unilateral pleural effusion in 30–80 % of patients that may obscure underlying pleural thickening or tumor masses, until thoracentesis is performed. Computed tomography (CT) is superior to radiography for the identification of early abnormalities in patients with MM to evaluate extension and morphology of the disease and represents the primary imaging modality used for diagnosis, staging, and response evaluation of MM [30, 31]. CT imaging suggesting radiologic diagnosis of MM includes unilateral pleural effusion in 70 % of patients and nodular pleural thickening in 90 %; nodular thickening can be discrete or diffuse with involvement of fissures. Calcified pleural plaques are found on CT in approximately 20 % of patients [32]. Although CT is the most commonly used modality for the evaluation of lymph node, its accuracy remains suboptimal because enlarged nodes alone do not prove nodal involvement [33]. Magnetic resonance imaging (MRI) can provide additional staging information; the excellent contrast resolution of MRI can allow improved detection of tumor extension, especially to the chest wall and to the diaphragm. A recent study showed that MRI is superior to CT in revealing invasion of the diaphragm and of the endothoracic fascia, or a single chest wall focus [34].
Novel tumor evaluation criteria
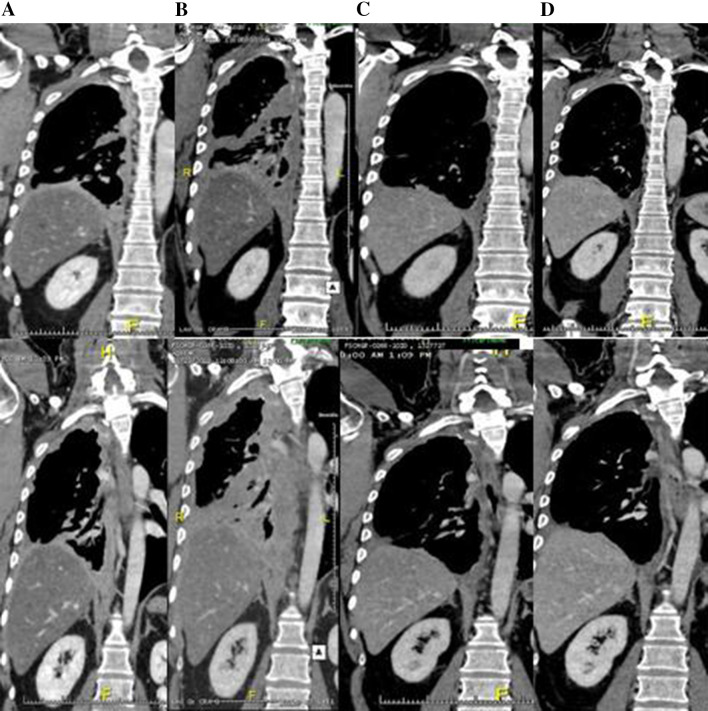
Assessment of response with conventional criteria based on CT measurement is challenging in MM, because the growth pattern of the disease makes the use of response evaluation criteria in solid tumors (RECIST) difficult. Modified RECIST (mRECIST) were therefore developed based upon speculation that RECIST did not address the unique shape of mesothelioma tumors with the pleural rind and thus led to inaccurate measurement [35]. The mRECIST guideline is currently the standard for the assessment of treatment response in MM, while bidimensionally measurable lesions, such as mediastinal lymph nodes and abdominal lesions, continue to be recorded unidimensionally as for RECIST.1.1 [21]. Although mRECIST are being used in most current clinical trials, they have been criticized based on the high degree of inter-observer variability documented in the assessment of tumor response classification in MM [36]. Furthermore, increasing clinical experience indicates that traditional response criteria may not be sufficient to fully characterize the clinical activity of immunotherapy, due to the different profile of response in which an initial increase in tumor burden can be followed by an objective tumor response once an effective anti-tumor immune response develops (Fig. 1). In these specific cases, conventional RECIST may not adequately assess the activity of immunotherapeutic agents, because initial PD does not necessarily reflect therapeutic failure. Immuno-related response criteria were therefore proposed to avoid this issue and should be adopted in these setting [20]. In spite of these considerations, radiology expertise is crucial to improve the accuracy of response evaluation along with the development of alternative measurement modalities using direct assessment of tumor volume and metabolic imaging.
Fig. 1.
CT scans of a patient with pleural MM treated with tremelimumab. Tumor assessment was done at baseline (a), W12 (b), W24 (c), and W36 (d). The patient achieved a partial response (c, d) after initial PD at w12 (b)
Immunomodulating antibodies in MM: the future ahead
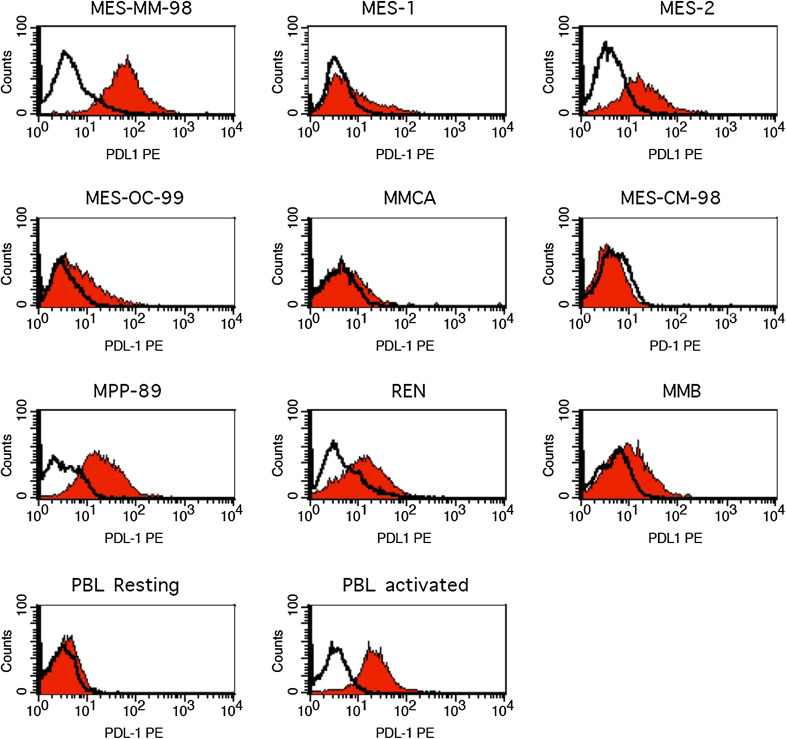
New treatment horizons are opening for MM patients, in which CTLA4 blockade represents a novel and highly promising clinical strategy that may eventually lead to a therapeutic paradigm shift. A further step to improve the clinical outcome of MM patients will likely involve targeting or co-targeting of additional immune checkpoint(s) by immunomodulating mAb that are currently under active clinical development in other tumor types. Among these, targeting the co-inhibitory receptor programmed cell death 1 (PD-1) and its main counter-receptor PD ligand 1 (PD-L1) is showing encouraging clinical activity in patients with metastatic melanoma, renal and lung cancer; along this line, the combination of PD-1 and CTLA4 blockade seems to have superior efficacy though with an increased toxicity [37, 38]. Opposite to CTLA4, PD-1 is primarily involved in modulating T cell activity in peripheral tissues and in the tumor microenvironment by interacting with its ligands, PD-L1 and PD-L2. In addition to its prognostic potential, it has been recently suggested that PD-L1 expression on tumor tissues may represent a predictive marker of response to treatment with anti-PD-1/PD-L1 blocking mAb [39–41]. In spite of these initial findings, emerging evidences demonstrate that PD-L1-negative tumors can also respond to treatment with PD-1/PD-L1 blocking mAb, though to a lower extent [42]; therefore, the search for predictive biomarker to treatment with anti-CTLA4 blocking mAb and with novel immunomodulating mAb remains an area of active investigation. To provide initial insights on the foreseeable role of PD-L1 in MM, we demonstrated that 60 % of investigated MM cell lines expressed PD-L1 (Table 2; Fig. 2). Though preliminary, these results corroborate the rationale to design a clinical study with PD-1/PD-L1 pathway blockade in MM patients.
Table 2.
Expression of PD-L1 in human mesothelioma cell lines
| Cells | Histology | PD-L1 | |
|---|---|---|---|
| % | MFI | ||
| PBMC resting (CTR−) | 1 | 4 | |
| PBMC activated (CTR+) | 89 | 39 | |
| MES-MM-98 | Sarcomatoid | 98 | 75 |
| MES-1 | Epithelioid | 26 | 11 |
| MES-2 | Epithelioid | 81 | 21 |
| MES-OC-99 | Sarcomatoid | 23 | 7 |
| MMCA | Epithelioid | 34 | 8 |
| MES-CM-98 | Epithelioid | 13 | 6 |
| MPP-89 | Epithelioid | 84 | 38 |
| REN | Epithelioid | 53 | 17 |
| MMB | Epithelioid | 49 | 12 |
PD-L1 expression in human MM cell lines was analyzed by flow cytometry using anti-PD-L1 mAb as well as isotype control mAb IgG. Peripheral blood mononuclear cells (PBMC) resting and activated were used as negative and positive controls (CTR), respectively
MFI mean fluorescence intensity
Fig. 2.
Immunofluorescence analysis of PD-L1 expression in human MM cell lines. PD-L1 expression in human MM cell lines was analyzed by flow cytometry using a mouse anti-human PD-L1 mAb (red histograms) (clone MIH1, Becton–Dickinson) as well as an isotype-matched control mAb IgG (empty histograms). Resting and activated PBMC were used as negative and positive controls, respectively
Additional ways to improve the efficacy of checkpoint(s) blocking mAb will likely derive from combinations with other immunomodulatory compounds, including DNA hypomethylating agents (DHA) [43]. Along this line, we recently hypothesized that immunomodulatory agents acting at tumor site can represent useful therapeutic “partners” of CTLA4 blocking mAb, in order to target host’s immune system on one hand, and to improve the immunogenicity and immune recognition of neoplastic cells on the other [43]. Ongoing experiments will hopefully provide experimental support to this hypothesis, eventually leading to test also this combination in the clinical setting of MM.
Much remains to be gained in the therapeutic landscape of MM; however, immune checkpoint(s) blockade is definitively revitalizing the clinical role of immunotherapy in this still highly deadly disease.
Acknowledgments
This work was supported by unrestricted grants from the Associazione Italiana per la Ricerca sul Cancro, Istituto Toscano Tumori, and MedImmune.
Conflict of interest
Luana Calabrò served on Advisory Boards for Bristol-Myers Squibb. Giovanni Luca Ceresoli declares no conflict of interest. Alessandra di Pietro is a full time employee of MedImmune. Ornella Cutaia declares no conflict of interest. Aldo Morra declares no conflict of interest. Ramy Ibrahim is a full time employee of Astrazeneca. Michele Maio served on Advisory Boards for Bristol-Myers Squibb, Roche-Genentech, and MedImmune.
Abbreviations
- AUC
Area under the curve
- CT
Computed tomography
- CTLA4
Cytotoxic T-lymphocyte antigen4
- DCR
Disease control rate
- DHA
DNA hypomethylating agent
- ICOS
Inducible costimulator
- Ig
Immunoglobulin
- Iv
Intravenous
- mAb
Monoclonal antibody
- MM
Malignant mesothelioma
- MRI
Magnetic resonance imaging
- OS
Overall survival
- PD
Progressing disease
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- PD-L2
Programmed cell death ligand 2
- RECIST
Response evaluation criteria in solid tumors
- RR
Response rate
- TTP
Time to progression
- W
Week
- Wks
Weeks
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Eleventh Meeting of the Network Italiano per la Bioterapia dei Tumori (NIBIT) on Cancer Bio-Immunotherapy, held in Siena, Italy, 17th–19th October 2013. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89:716–724. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceresoli GL, Gridelli C, Santoro A. Multidisciplinary treatment of malignant pleural mesothelioma. Oncologist. 2007;12:850–863. doi: 10.1634/theoncologist.12-7-850. [DOI] [PubMed] [Google Scholar]
- 3.Datta A, Smith R, Fiorentino F, Treasure T. Surgery in the treatment of malignant pleural mesothelioma: recruitment into trials should be the default position. Thorax. 2014;69:194–197. doi: 10.1136/thoraxjnl-2013-203846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 5.Santoro A, O’Brien ME, Stahel RA, Nackaerts K, Baas P, Karthaus M, Eberhardt W, Paz-Ares L, Sundstrom S, Liu Y, Ripoche V, Blatter J, Visseren-Grul CM, Manegold C. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol. 2008;3:756–763. doi: 10.1097/JTO.0b013e31817c73d6. [DOI] [PubMed] [Google Scholar]
- 6.Kindler HL, Karrison TG, Gandara DR, Lu C, Krug LM, Stevenson JP, Jänne PA, Quinn DI, Koczywas MN, Brahmer JR, Albain KS, Taber DA, Armato SG, 3rd, Vogelzang NJ, Chen HX, Stadler WM, Vokes EE. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol. 2012;30:2509–2515. doi: 10.1200/JCO.2011.41.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buikhuisen WA, Burgers JA, Vincent AD, Korse CM, van Klaveren RJ, Schramel FM, Pavlakis N, Nowak AK, Custers FL, Schouwink JH, Gans SJ, Groen HJ, Strankinga WF, Baas P. Thalidomide versus active supportive care for maintenance in patients with malignant mesothelioma after first-line chemotherapy (NVALT 5): an open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2013;14:543–551. doi: 10.1016/S1470-2045(13)70125-6. [DOI] [PubMed] [Google Scholar]
- 8.Ceresoli GL, Zucali PA, Gianoncelli L, Lorenzi E, Santoro A. Second-line treatment for malignant pleural mesothelioma. Cancer Treat Rev. 2010;36:24–32. doi: 10.1016/j.ctrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Jassem J, Ramlau R, Santoro A, Schuette W, Chemaissani A, Hong S, Blatter J, Adachi S, Hanauske A, Manegold C. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol. 2008;26:1698–1704. doi: 10.1200/JCO.2006.09.9887. [DOI] [PubMed] [Google Scholar]
- 10.Krug LM, Kindler H, Calvert H, VANTAGE 014 et al. Vorinostat in patients with advanced malignant pleural mesothelioma (MPM) who have failed prior pemetrexed and either cisplatin or carboplatin therapy: a phase III, randomized, double-blind, placebo-controlled trial. Eur J Cancer. 2011;47(Suppl 2):2–3. doi: 10.1016/S0959-8049(11)70098-3. [DOI] [Google Scholar]
- 11.Ceresoli GL, Zucali PA. Anti-angiogenic therapies for malignant pleural mesothelioma. Expert Opin Investig Drugs. 2012;21:833–844. doi: 10.1517/13543784.2012.681641. [DOI] [PubMed] [Google Scholar]
- 12.Ceresoli GL, Zucali PA, De Vincenzo F, Gianoncelli L, Simonelli M, Lorenzi E, Ripa C, Giordano L, Santoro A. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer. 2011;72:73–77. doi: 10.1016/j.lungcan.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Busacca S, Sheaff M, Arthur K, Gray SG, O’Byrne KJ, Richard DJ, Soltermann A, Opitz I, Pass H, Harkin DP, Quinn JE, Fennell DA. BRCA1 is an essential mediator of vinorelbine-induced apoptosis in mesothelioma. J Pathol. 2012;227:200–208. doi: 10.1002/path.3979. [DOI] [PubMed] [Google Scholar]
- 14.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, Kreitman RJ, Miettinen MM, Steinberg SM, Fowler DH, Pastan I. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5(208):208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L, Maio M. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 16.Eggermont A, Kroemer G, Zitvogel L. Immunotherapy and the concept of a clinical cure. Eur J Cancer. 2013;49:2965–2967. doi: 10.1016/j.ejca.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Calabrò L, Danielli R, Sigalotti L, Maio M. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol. 2010;37(5):460–467. doi: 10.1053/j.seminoncol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 19.Calabrò L, Maio M. Immune checkpoint blockade in malignant mesothelioma: a novel therapeutic strategy against a deadly disease? OncoImmunology. 2014;3:e27482. doi: 10.4161/onci.27482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immunotherapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 21.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 22.Zucali PA, Simonelli M, Michetti G, Tiseo M, Ceresoli GL, Collovà E, Follador A, Lo Dico M, Moretti A, De Vincenzo F, Lorenzi E, Perrino M, Giordano L, Farina G, Santoro A, Garassino M. Second-line chemotherapy in malignant pleural mesothelioma: results of a retrospective multicenter survey. Lung Cancer. 2012;75(3):360–367. doi: 10.1016/j.lungcan.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Di Giacomo AM, Calabrò L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C, Biagioli M, Altomonte M, Maio M. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62:1021–1028. doi: 10.1007/s00262-013-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrò L, Maio M. Biomarkers for immune checkpoint inhibitors. Lancet Oncol. 2014;15(1):e1–e2. doi: 10.1016/S1470-2045(13)70573-4. [DOI] [PubMed] [Google Scholar]
- 25.Calabro’ L, Morra A, Fonsatti F, Cutaia O, Fazio C, Danielli D, Giannarelli D, Altomonte M, Di Giacomo AM, Maio M (2014) A phase 2 single-arm study with tremelimumab at an optimized dosing schedule in second-line mesothelioma patients. J Clin Oncol 32:5s (suppl: abstr 7531)
- 26.Maeda M, Nishimura Y, Kumagai N, Hayashi H, Hatayama T, Katoh M, Miyahara N, Yamamoto S, Hirastuka J, Otsuki T. Dysregulation of the immune system caused by silica and asbestos. J Immunotoxicol. 2010;7(4):268–278. doi: 10.3109/1547691X.2010.512579. [DOI] [PubMed] [Google Scholar]
- 27.Bograd AJ, Suzuki K, Vertes E, Colovos C, Morales EA, Sadelain M, Adusumilli PS. Immune responses and immunotherapeutic interventions in malignant pleural mesothelioma. Cancer Immunol Immunother. 2011;60(11):1509–1527. doi: 10.1007/s00262-011-1103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, Lorigan P, Kendra KL, Maio M, Trefzer U, Smylie M, McArthur GA, Dreno B, Nathan PD, Mackiewicz J, Kirkwood JM, Gomez-Navarro J, Huang B, Pavlov D, Hauschild A. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumagai-Takei N, Maeda M, Chen Y, Matsuzaki H, Lee S, Nishimura Y, Hiratsuka J, Otsuki T. Asbestos induces reduction of tumor immunity. Clin Dev Immunol. 2011;2011:481439. doi: 10.1155/2011/481439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller BH, Rosado-de-Christenson ML, Mason AC, Fleming MV, White CC, Krasna MJ. Malignant pleural mesothelioma: radiologic–pathologic correlation. Radiographics. 1996;16:613–644. doi: 10.1148/radiographics.16.3.8897628. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZJ, Reddy GP, Gotway MB, Higgins CB, Jablons DM, Ramaswamy M, Hawkins RA, Webb WR. Malignant pleural mesothelioma: evaluation with CT, MR imaging and PET. Radiographics. 2004;24:105–119. doi: 10.1148/rg.241035058. [DOI] [PubMed] [Google Scholar]
- 32.Leung AN, Miller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR. 1990;154:487–492. doi: 10.2214/ajr.154.3.2106209. [DOI] [PubMed] [Google Scholar]
- 33.Boiselle PM, Patz EF, Jr, Vining DJ, Weissleder R, Shepard JA, McLoud TC. Imaging of mediastinal limph nodes: CT, MR and FDG-PET. Radiographics. 1998;18:1061–1069. doi: 10.1148/radiographics.18.5.9747607. [DOI] [PubMed] [Google Scholar]
- 34.Heelan RT, Rusch VW, Begg CB, Panicek DM, Caravelli JF, Eisen C. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR. 1999;172:1039–1047. doi: 10.2214/ajr.172.4.10587144. [DOI] [PubMed] [Google Scholar]
- 35.van Klaveren RJ, Aerts JG, de Bruin H, Giaccone G, Manegold C, van Meerbeeck JP. Inadequacy of the RECIST criteria for the evaluation of response in patient with malignant pleural mesothelioma. Lung Cancer. 2004;43(1):63–69. doi: 10.1016/S0169-5002(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 36.Armato SG, 3rd, Ogarek JL, Starkey A, Vogelzang NJ, Kindler HL, Kocherginsky M, MacMahon H. Variability in mesothelioma tumor response classification. AJR. 2006;186:1000–1006. doi: 10.2214/AJR.05.0076. [DOI] [PubMed] [Google Scholar]
- 37.Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov. 2013;12:489–492. doi: 10.1038/nrd4066. [DOI] [PubMed] [Google Scholar]
- 38.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98(6):751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 40.Pardoll D. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 42.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigalotti L, Fratta E, Coral S, Maio M. Epigenetic drugs as immunomodulators for combination therapies in solid tumors. Pharmacol Ther. 2014;142(3):339–350. doi: 10.1016/j.pharmthera.2013.12.015. [DOI] [PubMed] [Google Scholar]