Abstract
Purpose
In metastatic renal cell carcinoma (mRCC), survival benefit associated with objective response rates of 16–20 % with high-dose interleukin-2 (HDIL-2) is well established and discussed. Based on recently emerged data on efficacy of cancer immunotherapy, we hypothesized that the survival benefit with HDIL-2 extends beyond those achieving objective responses, i.e., to those who achieve stable disease as the best response to treatment.
Materials and methods
All sequential treatment naïve mRCC patients treated with HDIL-2 at the University of Utah (1988–2013) and University of Michigan (1997–2013) were included. Best responses on treatment were associated with survival outcomes using log-rank and COX regression with a landmark analysis at 2 months.
Results
391 patients (75 % male; median age 55 years) were included and belonged to the following prognostic risk categories: 20 % good, 64 % intermediate, and 15 % poor. Best responses on treatment were complete response (9 %), partial response (10 %), stable disease (32 %), progressive disease (42 %), and not evaluable for response (7 %). No significant differences in progression-free survival (HR 0.74, 95 % CI 0.48–1.1, p = 0.14) or overall survival (HR 0.66, 95 % CI 0.39–1.09, p = 0.11) were observed between patients achieving partial response versus stable disease. Significant differences in progression-free survival (HR 0.13, 95 % CI 0.09–0.22, p < 0.0001) and overall survival (HR 0.33, 95 % CI 0.23–0.48, p < 0.0001) were observed between patients achieving stable disease compared to those with progressive disease and who were not evaluable.
Conclusions
Survival benefit with HDIL-2 is achieved in ~50 % patients and extends beyond those achieving objective responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1854-1) contains supplementary material, which is available to authorized users.
Keywords: Metastatic kidney cancer, High-dose interleukin-2, Survival outcomes, Clinical benefit, Immunotherapy
Introduction
High-dose interleukin-2 (HDIL-2), an immunotherapy, is approved for a select group of patients with metastatic renal cell carcinoma (mRCC). Generally, objective response (OR) rates, i.e., complete response (CR) + partial response (PR), of 16–20 % are discussed with patients, and not disease stabilization (SD). Cancer immunotherapy may improve survival without inducing OR. Thus, treatment with HDIL-2 may provide survival benefit to an additional group of patients not experiencing OR, but only SD as the best response.
There is a sense of déjà vu for healthcare providers treating renal cell carcinoma (RCC). A lack of benefit with conventional chemotherapy led to the use of cytokine therapy with interferon alfa or HDIL-2 for RCC [1]. The past decade witnessed the development of vascular endothelial growth factor receptor (VEGFR)–tyrosine kinase inhibitors (TKI) and mammalian target of rapamycin inhibitors (mTORi) highlighting our increased knowledge of the biology of RCC and allowing for oral administration of therapy [2–9]. Interestingly, we are back at the hope of immunotherapy targeting cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death ligand-1 (PD-L1) or its receptor (PD-1) PD-1 for the future of RCC treatment [10]. This begs us to revisit our older immunotherapies as well.
Historically, clinical efficacy and benefit with HDIL-2 have been measured and reported in terms of objective response rates (ORR), a combination of partial response (PR) and complete response (CR), which occurs in approximately 14 % of patients, with durable CRs in 5 % [11, 12]. However, stability of disease (SD) as measure of benefit to HDIL-2 has not been assessed and reported, despite the recent data that cancer immunotherapy may improve survival without inducing OR. Even newer therapies of VEGFR-TKIs and mTORi were approved on the primary end point of progression-free survival (PFS) but with low CR rates of 3 % and almost nonexistent durable CRs [2, 4, 5, 7, 13, 14]. Thus, we hypothesized that treatment with HDIL-2 may provide survival benefit to an additional group of patients not experiencing OR, but only SD as the best response.
Materials and methods
Study cohort
All sequential patients with clear cell mRCC treated with HDIL-2 at the University of Utah Huntsman Cancer Institute from 1988 to 2013 and the University of Michigan from 1997 to 2013 were identified. Patients were excluded if date of last follow-up or death was not available, or date of HDIL-2 administration was not recorded. Patient age, gender, and Karnofsky performance status were collected prior to HDIL-2 therapy. Clear cell histology was confirmed by pathology reports, and number and sites of metastasis prior to HDIL-2 were recorded. Demographics, as well as clinical and laboratory values for prognostic risk stratification as per the International metastatic renal cell carcinoma database consortium (IMDC) criteria were collected. Grade 3 adverse events were collected at University of Utah in patients treated with HDIL-2 from 2000 to 2012, and laboratory values were collected before and after the first week of treatment at the University of Michigan.
HDIL-2 treatment protocol
One course of HDIL-2 consisted of two cycles—cycle one administered over 5–6 days, followed by 1 week off, followed by cycle two over 5–6 days. HDIL-2 dosing comprised the standard regimen of 600,000 IU/kg IV every 8 h for a total of 14 planned doses per cycle. Restaging scans were done approximately 6 weeks after the first course at the University of Michigan and approximately 8 weeks after the first course at the University of Utah. Thereafter, restaging scans were done every 12 weeks.
HDIL-2 response criteria
Best response criteria were based on RECIST 1.0. A PR was defined as a ≥30 % decrease in target lesion size. Progressive disease was a ≥20 % increase in target lesion size or new lesion. CR indicated no presence of disease. Patients not meeting criteria for PR or progressive disease (PD) were considered to have stable disease (SD). Patients without appropriate follow-up between radiographic imaging and treatment, who were lost to follow-up, or died before determining response were classified as not evaluable (NE) and grouped with PD for statistical analysis.
Objectives
The primary objective was progression-free survival (PFS) and overall survival (OS) stratified by response to HDIL-2 response in patients with mRCC, using landmark analysis at 2 months.
Statistical analysis
Descriptive statistics were used to summarize patient and treatment characteristics. Kaplan–Meier method with log-rank tests was used to assess PFS and OS by HDIL-2 response. A landmark analysis at 2 months, the approximate time to first disease assessment, was performed for both PFS and OS. PFS was defined as the time from first HDIL-2 initiation to disease progression, death, or last follow-up. OS was defined as the time from first HDIL-2 administration to death or last follow-up. In the PFS analysis, censoring occurred at the time of treatment discontinuation if treatment was discontinued for any other reason than progression or death. In both the PFS and OS analysis, censoring occurred at the time of last follow-up in those who had not progressed or were still alive at the end of the designated study period. COX proportional hazards models were created with IMDC prognostic risk criteria and best response using a 2-month landmark analysis for PFS and OS. Significance was set at >0.05 for the analysis.
Results
Demographics and disease characteristics
A total of 391 patients were eligible. The median age of the cohort was 55 years [interquartile range (IQR) 49–59 years], and 75 % (n = 294) of the patients were male (Table 1). The IMDC risk categories were favorable in 20 % (n = 80), intermediate in 64 % (n = 251), and poor in 15 % (n = 60) of patients. Demographics and disease characteristics were similar between sites (Table 1).
Table 1.
Demographics and disease characteristics
| Total N = 391 |
Utah n = 176 |
Michigan n = 215 |
|
|---|---|---|---|
| Age | |||
| Years of age, median (IQR) | 55 (49–59) | 55 (49–60) | 55 (49–59) |
| Sex | |||
| Males, n (%) | 294 (75 %) | 140 (80 %) | 154 (72 %) |
| Prior therapy, n (%) | |||
| Nephrectomy | 328 (84 %) | 148 (92 %) | 180 (84 %) |
| Number of metastatic disease sites, n (%) | |||
| 1 | 118 (30 %) | 52 (30 %) | 66 (31 %) |
| 2 | 133 (34 %) | 62 (35 %) | 71 (33 %) |
| 3 | 85 (22 %) | 36 (20 %) | 49 (23 %) |
| ≥4 | 48 (12 %) | 19 (11 %) | 29 (13 %) |
| Unknown | 7 (2 %) | 7 (4 %) | 0 |
| Common sites of metastases, n (%) | |||
| Lung | 289 (74 %) | 118 (69 %) | 171 (80 %) |
| Bone | 116 (30 %) | 68 (40 %) | 48 (22 %) |
| Lymph node | 115 (29 %) | 37 (22 %) | 78 (36 %) |
| Liver | 61 (16 %) | 28 (16 %) | 33 (15 %) |
| Brain | 22 (6 %) | 17 (10 %) | 5 (2 %) |
| IMDC risk categories, n (%) | |||
| Favorable | 80 (20 %) | 36 (20 %) | 44 (20 %) |
| Intermediate | 251 (64 %) | 109 (62 %) | 142 (66 %) |
| Poor | 60 (15 %) | 31 (18 %) | 29 (13 %) |
IMDC International Metastatic Renal Cell Carcinoma Database Consortium
HDIL-2 treatment
The median total dose of IL-2 received was 689 MIU (IQR 470–989 MIU), and the median total dose of IL-2 per kilogram (kg) was 7.9 MIU/kg (IQR 5.8–11.5). One treatment-related death was observed in the entire cohort (<1 %). Grade 3 adverse events are presented in Supplementary Table 1, and the laboratory parameters prior to initiation of HDIL-2 and after the 1 week of treatment at the University of Michigan are presented in Supplementary Table 2. As expected, high percentages of patients experienced grade 3 hypotension (87.1 %), edema or capillary leak (72.9 %), and rigors (56.5 %). Clinically significant changes in platelet levels and serum creatinine were observed pre- and post- first week of HDIL-2 treatment.
Best response
A CR occurred in 9 % (n = 35), a PR in 10 % (n = 39), and SD was reported in 32 % (n = 125) of the cohort (Table 2). Therefore, an objective response was obtained in 19 % (n = 74) and a clinical benefit (CR + PR + SD) was reported in 51 % (n = 199) of patients. Progressive disease was observed in 42 % (n = 164) and 7 % (n = 28) were considered NE, for a total of 49 % (n = 192) of the cohort. Best responses were similar between sites with ORR of 15 and 22 % and a clinical benefit rate of 45 and 50 % for Utah and Michigan cohorts, respectively (Table 2). However, Utah cohort had higher numbers of patients that were non-evaluable due to patients receiving IL-2 in 1980s and 1990s, where imaging and response rates were not uniformly recorded in the medical chart.
Table 2.
Best response achieved to IL-2
| Best response to HDIL-2 | Total N = 391 |
Utah n = 176 |
Michigan n = 215 |
|---|---|---|---|
| Complete response, n (%) | 35 (9) | 16 (9) | 19 (9) |
| Partial response, n (%) | 39 (10) | 11 (6) | 28 (13) |
| Stable disease, n (%) | 125 (32) | 52 (30) | 73 (34) |
| Progressive disease/not evaluable, n (%) | 192 (49) | 97 (55) | 95 (44) |
Survival outcomes
A total of 285 patients were available for the landmark analysis at 2 months. The median PFS and OS was 6.9 and 34.2 months, respectively, at the 2-month landmark (Table 3). The median OS stratified by IMDC criteria was 53.8 months for favorable, 26.4 months for intermediate, and 12.2 months for poor risk groups (Fig. 1a). Significant differences were observed between all IMDC risk groups (favorable vs. intermediate HR 0.57, CI 0.39–0.80, p = 0.0011; favorable vs. poor HR 0.24, 95 % CI 0.15–0.38, p < 0.0001; and intermediate vs. poor HR 0.42, 95 % CI 0.30–0.62, p < 0.0001).
Table 3.
Median PFS and OS with HDIL-2 in mRCC at the 2-month landmark
| Median PFS (months) | Median OS (months) | |
|---|---|---|
| Overall | 6.9 | 34.2 |
| CR versus PR |
113.8 versus 11.0 (HR 0.17, CI 0.08–0.33) |
156.7 versus 37.8 (HR 0.22, CI 0.09–0.48) |
| CR versus SD |
113.8 versus 8.5 (HR 0.13, CI 0.06–0.22) |
156.7 versus 34.4 (HR 0.14, CI 0.06–0.29) |
| SD versus PD |
8.5 versus 2.6 (HR 0.13, CI 0.09–0.18) |
34.4 versus 12.2 (HR 0.33, CI 0.23–0.48) |
| OR versus (SD and PD) |
26.5 versus 4.9 (HR 0.25, CI 0.17–0.35) |
101.2 versus 24.1 (HR 0.26, CI 0.16–0.39) |
| (CR, PR, SD) versus PD |
10.4 versus 2.6 (HR 0.09, CI 0.07–0.13) |
53.9 versus 12.2 (HR 0.24, CI 0.17–0.34) |
| PR versus SD |
11.0 versus 8.5 (HR 0.74, CI 0.48–1.10) |
37.8 versus 34.4 (HR 0.66, CI 0.39–1.09) |
CR complete response, PR partial response, SD stable disease, PD progressive disease, NE not evaluable or unknown, OR objective response, HR hazard ratio, and CI 95 % confidence interval
Fig. 1.
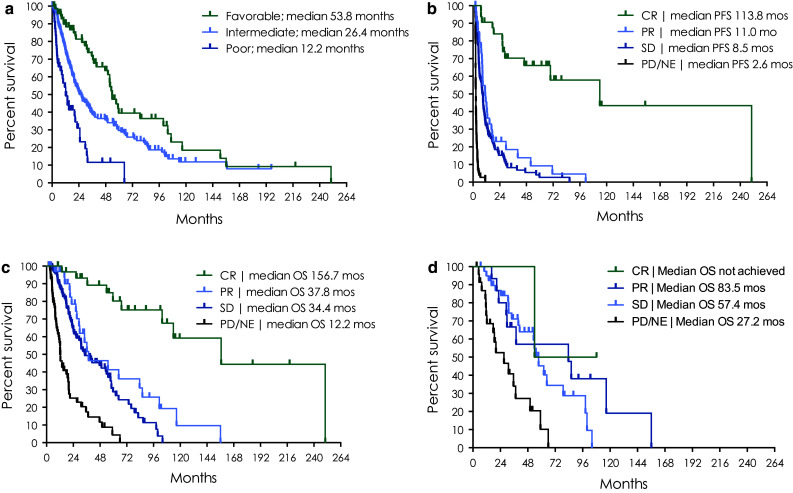
Survival outcomes at 2-month landmark a median overall survival stratified by IMDC risk criteria; b PFS and c OS by best response to HDIL-2; and d OS by best response to HDIL-2 in patients who received a VEGFR-TKI or mTORi post-HDIL-2
The median PFS and OS in patients achieving a CR (n = 35) was 113.8 and 156.7 months, respectively (Table 3; Fig. 1b, c). The median PFS in patients with a PR (n = 39) was 11 months, and for those with SD (n = 52), the median PFS was 8.5 months, which was not significantly different (HR 0.74, 95 % CI 0.48–1.1, p = 0.14). The median OS in patients with a PR was 37.8 months compared to 34.4 months in patients with SD, which also did not differ significantly (HR 0.66, 95 % CI 0.39–1.09, p = 0.11). In patients with PD/NE, the median PFS and OS was 2.6 and 12.2 months, respectively. Significant differences in PFS (HR 0.13, 95 % CI 0.09–0.22, p < 0.0001) and OS (HR 0.33, 95 % CI 0.23–0.48, p < 0.0001) were observed between patients achieving SD compared to PD/NE.
In COX proportional hazards models (OS and PFS) including IMDC criteria and best response, no significant differences in survival were observed in those who obtained a PR versus SD (PFS, HR 0.69, p = 0.0623; OS, HR 0.62, p = 0.0582; Fig. 2). However, those who obtained SD as best response had improved outcomes compared to those with PD reported as the best response (PFS, HR 0.12, p < 0.0001; OS, HR 0.33, p < 0.0001).
Fig. 2.
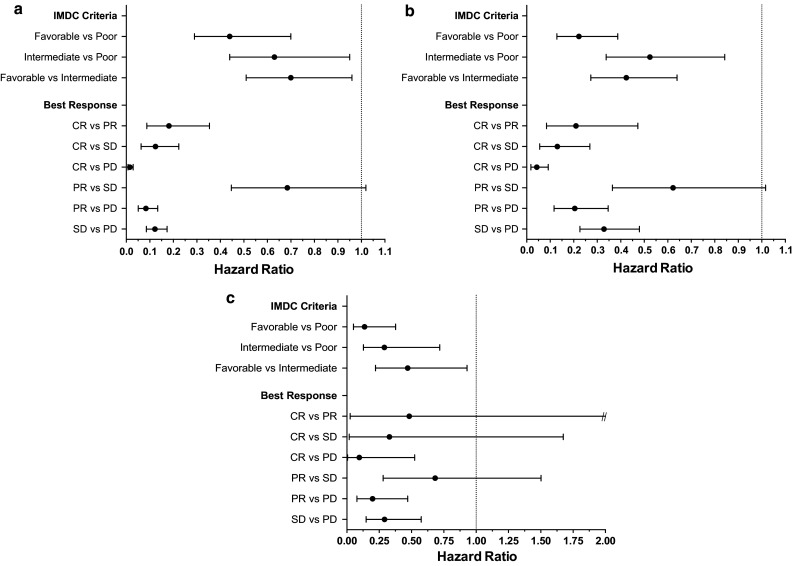
COX proportional hazards model with landmark analysis at 2 months for a PFS and b OS in the entire cohort, and c OS in only those who received VEGFR-TKI or mTORi post-HDIL-2
Effect of post-HDIL-2 treatment
Treatment after HDIL-2 with either a VEGFR-TKI or mTORi occurred in 29 % of all patients. As expected, overall survival was greater in those who received a VEGFR-TKI or mTORi post-IL-2 (53.1 months) compared to those who did not receive these treatments post-HDIL-2 (24.9 months; Table 4). Additionally, in this group of patients receiving post-HDIL-2 treatment, patients with SD had similar OS compared to those with PR as best response to HDIL-2 and significantly improved OS compared to those with PD on HDIL-2 (Figs. 1d, 2c).
Table 4.
Survival outcomes stratified by post-HDIL-2 treatment with VEGFR-TKI or mTORi and response to HDIL-2: 2-month landmark analysis
| Best response and survival outcomes from HDIL-2 | Median OS (months) | HR (95 % CI) | p value* |
|---|---|---|---|
| No VEGFR-TKI or mTORi post-HDIL-2, n = 204 | 24.9 | ||
| Objective response to HDIL-2 | |||
| Yes versus No | 103.7 versus 19.5 | 0.18 (0.10–0.30) | <0.0001 |
| Clinical benefit to HDIL-2 | |||
| Yes versus No | 38.4 versus 12.2 | 0.27 (0.19–0.40) | <0.0001 |
| Best response to HDIL-2 | |||
| CR versus PR | 156.7 versus 34.2 | 0.17 (0.06–0.45) | 0.0004 |
| CR versus SD | 156.7 versus 24.1 | 0.13 (0.05–0.28) | <0.0001 |
| CR versus PD/NE | 156.7 versus 12.2 | 0.06 (0.02–0.13) | <0.0001 |
| PR versus SD | 34.2 versus 24.1 | 0.76 (0.36–1.44) | 0.43 |
| PR versus PD/NE | 34.2 versus 12.2 | 0.35 (0.17–0.66) | 0.0008 |
| SD versus PD/NE | 24.1 versus 12.2 | 0.46 (0.31–0.69) | 0.0001 |
| VEGFR-TKI or mTORi post-HDIL-2, n = 81 | 53.1 | ||
| Objective response to HDIL-2 | |||
| Yes versus No | 83.5 versus 50.0 | 0.44 (0.20–0.90) | 0.0235 |
| Clinical benefit to HDIL-2 | |||
| Yes versus No | 57.4 versus 27.2 | 0.29 (0.15–0.55) | 0.0003 |
| Best response to HDIL-2 | |||
| CR versus PR | NR versus 83.5 | 0.56 (0.03–3.07) | 0.55 |
| CR versus SD | NR versus 57.4 | 0.36 (0.02–1.75 | 0.24 |
| CR versus PD/NE | NR versus 27.2 | 0.12 (0.01–0.62) | 0.0073 |
| PR versus SD | 83.5 versus 57.4 | 0.64 (0.26–1.41) | 0.27 |
| PR versus PD/NE | 83.5 versus 27.2 | 0.21 (0.08–0.51) | 0.0004 |
| SD versus PD/NE | 57.4 versus 27.2 | 0.33 (0.17–0.65) | 0.0016 |
VEGFR-TKI vascular endothelial growth factor receptor–tyrosine kinase inhibitor, mTORi mammalian target of rapamycin inhibitor, and NR not reached
* Log-rank
Discussion
The objective responses observed in 19 % of our patients are in line with previous reports. However, our study demonstrates that patients with SD as the best response (32 % of all patients) derive similar survival benefits as those with PR as the best response to treatment with HDIL-2, indicating that “stable disease,” is a clinically relevant response criterion in this setting. Thus, our study shows that up to 50 % patients derive meaningful survival advantage with HDIL-2. Furthermore, to our knowledge, this is the largest study reporting outcomes in patients with mRCC treated with HDIL-2 to date.
Unlike recently developed immunotherapeutic agents, the mechanism of action of HDIL-2 is not fully understood. Interleukin-2 is a recombinant protein that has a wide range of effects on the immune system, including promoting proliferation and differentiation of CD4(+) T cells into specific effector T cell subsets, of CD8(+) T cells into effector T cells, and into memory cells, but also expansion of immunosuppressive CD4(+)FoxP3 + T regulatory cells in certain situations [15].
According to the current National Comprehensive Cancer Network (NCCN) guidelines, treatment with HDIL-2 is recommended for patients with excellent performance status and normal organ function and is not based on prognostic risk categorization, which applies to treatment decisions for the use of molecularly targeted drugs such as VEGFR-TKIs and mTORis [16]. Since the approval of HDIL-2 in 1992, several studies have shown similar objective responses in the range of 10–20 %, which include complete responses of ~10 %, which are generally durable (Table 5). In the literature and during the treatment decisions for a newly diagnosed patient with mRCC, only ORs and not SD as a possible benefit of HDIL-2 treatment are highlighted and discussed, likely because of the paucity of published data and under recognition of a survival benefit associated with SD, as the best response to HDIL-2. This is despite the recent observations that immunotherapy in general may improve survival outcomes without inducing objective responses. Indeed, one of the prospective databases of mRCC patients treated between 1989 and 2005 demonstrated that of 453 patients treated with either HDIL-2 alone (n = 212), IFN alpha alone (n = 30), or the combination of HDIL-2 and IFN alpha (n = 157), 33 % patients achieved stable disease as the best response, with a resulting median OS of 38.6 months [17]. This was similar to the median OS of 42.8 months in 15 % patients in that study, who achieved PR as the best response to treatment. However, the survival outcomes for these patients with stable disease were not reported specifically for those treated with HDIL-2 alone. More recently, in an observational study, presented as an abstract in the 2014 American Society of Clinical Oncology annual meeting, of 97 mRCC patients treated with HDIL-2 between 2007 and 2012 (i.e., in the era of targeted therapies), the objective response rate with HDIL-2 was 22 % (8 % CR and 14 % PR) [18]. An additional 24 % patients experienced stable disease as the best response. After a median follow-up of 37 months, the overall median OS was 51 months. Clinical benefit of HDIL-2 was seen in patients with CR, PR, as well as in those with SD, none of which reached median OS at the time of the report compared to 37.9 months in patients with PD as the best response to treatment.
Table 5.
Prior studies of mRCC patients receiving HDIL-2 and associated response rates
| Study | Patient inclusion period | No. of patients (n) | CR (%) | PR (%) | SD (%) | PD (%) |
|---|---|---|---|---|---|---|
| Klapper et al. [27] | 1986–2006 | 259 | 23 (9) | 30 (12) | x | x |
| Belldegrun et al. [17] | 1989–2005 | 212 | 16 (8) | 25 (12) | 51 (29) | 110 (52) |
| Yang et al. [28] | 1991–2001 | 155 | 11 (7) | 22 (14) | x | x |
| McDermott et al. [29] | 1997–2000 | 95 | 8 (8.4) | 14 (14.7) | x | x |
| Morse et al. [18] | 2007–2012 | 97 | 7 (7.5) | 13 (14) | 22 (24) | 51 (55) |
| Payne et al. [30] | 1997–2012 | 186 | 12 (7) | 32 (17) | 54 (29) | 88 (47) |
mRCC metastatic renal cell carcinoma, HD high dose, IL-2 interleukin-2, CR complete response, PR partial response, SD stable disease, and PD progressive disease
These results are also closely aligned with melanoma experience with HDIL-2, where CRs are present in approximately 6 % of patients and PRs in 10 % for an ORR of 16 % [19]. This is comparable to our results in mRCC, which demonstrate 9 % CRs and 10 % PRs for an ORR of 19 %. Disease control rates were not reported in this study.
Our study has a patient population not only from pre-molecularly targeted therapy era (71 % patients) but also from the targeted therapy era (29 % patients) and also includes poor risk category patients, likely because most of our patients received HDIL-2 before the first molecularly targeted drug, sunitinib, was approved in 2006, and they had very limited treatment options available. Our data indicate that almost half of all patients (i.e., 51 %) derived survival benefits on treatment with HDIL-2. Even, the overall PFS and OS of our patients (including those 49 % patients with PD as the best response) were 6.9 months and 34.2 months, respectively.
In the molecular targeted therapy era, a PFS of ~9 months was achieved with either pazopanib or sunitinib in the first-line setting of mRCC from a phase III randomized study comparing the efficacy and safety of these agents [20]. The median OS in this trial eligible population was ~29 months. Furthermore, in a recently published, population-based, study of 849 mRCC patients treated with targeted therapies in 13 international cancer centers, the median OS was 18.8 months [21]. Notably, this cohort included both trial eligible and ineligible patients and possibly reflects a more real-world experience with targeted therapies.
Additionally, a new immunotherapy agent for mRCC has recently been FDA-approved based on published data with a programmed death-1 (PD-1) checkpoint inhibitor, nivolumab. In this phase III trial of 821 immunotherapy naïve patients with mRCC, with prior disease progression on anti-angiogenetic therapy, there was a significant improvement in OS on treatment with nivolumab versus everolimus (25 vs. 21.8 months, HR 0.73, p = 0.002) [22]. Median PFS was similar in both groups (4.6 vs. 4.4 months, HR 0.88; p = 0.11). The objective response rates were higher in the nivolumab group (25 vs. 5 %, p < 0.001).
Based on the growing armamentarium of treatment options for mRCC, questions arise on how best to sequence therapies to achieve optimal outcomes. It is our contention that patients eligible for HDIL-2 and can receive treatment at a facility experienced in HDIL-2 administration should receive upfront treatment based on an ORR rate of 19 % and disease control in another 32 % of patients. With frequent disease monitoring (every 2–3 months), patients with progressive disease or patients with lack of clinical benefit (progressive symptoms) should be transitioned to anti-angiogenetic therapies. After failure of anti-angiogenic treatment, checkpoint inhibitors (anti-PD-1) could be considered or other recently approved novel molecularly targeted therapies. Responses to anti-PD-1 after HDIL-2 have been demonstrated in metastatic melanoma, and further investigation in mRCC is warranted [23]. Formal cost-effectiveness/utility studies are needed to assess the net benefit of HDIL-2 compared to anti-PD-1 agents, which will be of great relevance if anti-PD-1 agents are eventually approved in the first-line treatment setting.
Concerns are often raised about HDIL-2 treatment-related mortality. There was only one treatment-related mortality (among 391 patients), which suggests that the incidence of treatment-related mortality may be very low in high-volume HDIL-2 treatment centers. Furthermore, the toxicities associated with HDIL-2 are predominantly acute, occurring during the active treatment, with patients often returning to a baseline symptom level within a few days after completion of a given cycle of HDIL-2. This is to be contrasted with ongoing daily toxicities of molecularly targeted agents and immune-related adverse events associated with novel checkpoint inhibitors, which may be debilitating.
Improved management of these acute and short-term toxicities of HDIL-2 is expected to allow administration of a higher number of doses and eventually improve outcomes by improving tolerability as well as efficacy of HDIL-2. Some of the key players in the mediation of these toxicities include tumor necrosis factor alpha (TNF alpha), and nitric oxide, in addition to several other cytokines, such as type II interferon and transforming growth factor beta (TGF-β), which have been shown to induce autophagy [24]. In a phase I dose escalation clinical trial, HDIL-2 was administered in conjunction with dexamethasone in order to attenuate the HDIL-2-induced release of TNF alpha. In contrast to the high plasma levels of TNF alpha detected in patients receiving IL-2 alone in concurrent studies, TNF alpha levels in most of the dexamethasone-treated patients were undetectable. The concomitant administration of dexamethasone increased the maximum tolerated dose of IL-2 approximately threefold and reduced the severity of hypotension and organ dysfunction commonly seen in this setting. However, given its generalized immunosuppressive effects, dexamethasone has the potential to diminish HDIL-2-mediated tumor-directed immune response, and larger studies are needed to better characterize the efficacy of this combination [25]. Over production of nitric oxide, an endogenous vasodilator, has been shown to result in excessive vascular relaxation and vascular leak syndrome during treatment of HDIL-2. Treatment with a competitive enzyme inhibitor NG-monomethyl-l-arginine (NMA) can diminish nitric oxide production. In a study of 23 patients with mRCC receiving HDIL-2, increasing doses of NMA, ranging from 3 to 36 mg/kg, was concomitantly administered. Anti-hypotensive activity of NMA was observed at all dose levels, and the duration of activity correlated with the dose of NMA. The NMA of 24 mg/kg, with maintenance doses of 8 mg/kg every 4–6 h, was determined to be optimal for the reversal of hypotension and for the reversal of the state of high cardiac output [26]. Inhibiting systemic autophagy during HDIL-2 treatment is another promising avenue. In a murine model, concurrent therapy with an autophagy inhibitor, chloroquine, and the interleukin-2 improved long-term survival and decreased toxicities associated with systemic vascular leak [24]. Each of these combinatorial regimens is promising and has the potential to evolve into effective therapeutic strategies if validated in larger clinical studies.
Limitations of our study include the following: (1) inherent biases that accompany a retrospective analysis, (2) non-evaluability of ~7 % patients in our study (included along with patients with PD for outcomes analysis) because of our inability to view actual radiographic imaging for these patients prior to implementation of electronic medical records, (3) differences in imaging frequency between institutions may have resulted in slight differences in response rates and time to progression between institutions, and (4) lack of documentation of incidence and grading of treatment-related adverse events (other than treatment-related mortality), given the retrospective nature of the data spread over more than 2 decades.
In conclusion, meaningful survival benefit with HDIL-2 occurs in ~50 % patients with mRCC and extends beyond to those with ORs, i.e., to those achieving SD as the best response to treatment. Ongoing trials, such as those looking at a combination of HDIL-2 with immune checkpoint inhibitors, or radiation therapy as an immune modulator, are expected to show further enhancement of the clinical benefit observed with HDIL-2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CR
Complete response
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- HDIL-2
High-dose interleukin-2
- IMDC
International metastatic disease consortium
- IQR
Interquartile range
- mRCC
Metastatic renal cell carcinoma
- mTORi
Mammalian target of rapamycin inhibitors
- NCCN
National Comprehensive Cancer Network
- NE
Not evaluable
- OR
Objective response
- ORR
Objective response rates
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed death-1
- PD-L1
Programmed death ligand-1
- PFS
Progression-free survival
- PR
Partial response
- RCC
Renal cell carcinoma
- SD
Stable disease
- TKI
Tyrosine kinase inhibitors
- VEGFR
Vascular endothelial growth factor receptor
Compliance with ethical standards
Conflict of interest
Authors of this paper have nothing to disclose concerning possible financial or personal relationship with commercial entities that may have a direct or indirect interest in the subject of this paper.
Footnotes
David D. Stenehjem, Michael Toole, and Joseph Merriman have contributed equally to this work.
Contributor Information
Neeraj Agarwal, Phone: 1(801)585-0255, Email: Neeraj.Agarwal@hci.utah.edu.
Ajjai Alva, Email: ajjai@med.umich.edu.
References
- 1.Hudes GR, Carducci MA, Choueiri TK, et al. NCCN Task force report: optimizing treatment of advanced renal cell carcinoma with molecular targeted therapy. J Natl Compr Cancer Netw. 2011;9(Suppl 1):S1–S29. doi: 10.6004/jnccn.2011.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer. 2009;115:2306–2312. doi: 10.1002/cncr.24227. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 10.Dutcher JP. Recent developments in the treatment of renal cell carcinoma. Ther Adv Urol. 2013;5:338–353. doi: 10.1177/1756287213505672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–S57. [PubMed] [Google Scholar]
- 13.Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30:482–487. doi: 10.1200/JCO.2011.37.2516. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2015. J Natl Compr Cancer Netw. 2015;13:151–159. doi: 10.6004/jnccn.2015.0022. [DOI] [PubMed] [Google Scholar]
- 17.Belldegrun AS, Klatte T, Shuch B, et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989–2005): a benchmark for emerging targeted cancer therapies. Cancer. 2008;113:2457–2463. doi: 10.1002/cncr.23851. [DOI] [PubMed] [Google Scholar]
- 18.Morse M, McDermott DF, Daniels GA, et al. High-dose (HD) IL-2 for metastatic renal cell carcinoma (mRCC) in the targeted therapy era: extension of OS benefits beyond complete response (CR) and partial response (PR) J Clin Oncol (ASCO Meet Abstr) 2014;32:4523. [Google Scholar]
- 19.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 21.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong MK, Morse MA, McDermott DF, et al. Overall survival of metastatic melanoma patients treated with HD IL-2 followed by immune checkpoint blockade of the CTLA-4 or the PD-1 pathways: analysis of data on the current use of HD IL-2. J Immunother Cancer. 2015;3:P359. doi: 10.1186/2051-1426-3-S2-P359. [DOI] [Google Scholar]
- 24.Liang X, De Vera ME, Buchser WJ, et al. Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res. 2012;72:2791–2801. doi: 10.1158/0008-5472.CAN-12-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mier JW, Vachino G, Klempner MS, et al. Inhibition of interleukin-2-induced tumor necrosis factor release by dexamethasone: prevention of an acquired neutrophil chemotaxis defect and differential suppression of interleukin-2-associated side effects. Blood. 1990;76:1933–1940. [PubMed] [Google Scholar]
- 26.Kilbourn RG, Fonseca GA, Trissel LA, Griffith OW. Strategies to reduce side effects of interleukin-2: evaluation of the antihypotensive agent NG-monomethyl-l-arginine. Cancer J Sci Am. 2000;6(Suppl 1):S21–S30. [PubMed] [Google Scholar]
- 27.Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 30.Payne R, Glenn L, Hoen H, et al. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a community hospital biotherapy program. J Immunother Cancer. 2014;2:13. doi: 10.1186/2051-1426-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


