Abstract
In pre-clinical models, the only two chemotherapy drugs which have been demonstrated to directly reduce the number of myeloid-derived suppressor cells (MDSCs) are gemcitabine and 5-fluorouracil. Here we analyze the dynamics of MDSCs, phenotyped as Lin-DR-CD11b+, in patients with advanced pancreatic cancer receiving the combination of gemcitabine and capecitabine, a 5-FU pro-drug. We found no evidence that gemcitabine and capecitabine directly reduce MDSC% in patients. Gemcitabine and capecitabine reduced MDSCs in 42 % of patients (n = 19) and MDSC% fell in only 3/9 patients with above-median baseline MDSCs. In 5/8 patients with minimal tumour volume change on treatment, the MDSC% went up: increases in MDSC% in these patients appeared to correlate with sustained cancer-related inflammatory cytokine upregulation. In a separate cohort of 21 patients treated with gemcitabine and capecitabine together with concurrently administered GV1001 vaccine with adjuvant GM-CSF, the MDSC% fell in 18/21 patients and there was a significant difference in the trajectory of MDSCs between those receiving GV1001 and GM-CSF in combination with chemotherapy and those receiving chemotherapy alone. Thus, there was no evidence that the addition of low-dose adjuvant GM-CSF increased Lin-DR-CD11b+ MDSC in patients receiving combination chemoimmunotherapy. 9/21 patients developed an immune response to GV1001 and the MDSCs fell in 8 of these 9 patients, 6 of whom had above-median pre-vaccination MDSC levels. A high pre-vaccination MDSC% does not preclude the development of immunity to a tumour-associated antigen.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1502-y) contains supplementary material, which is available to authorized users.
Keywords: Myeloid-derived suppressor cell, Chemotherapy, Gemcitabine, Capecitabine, Pancreatic cancer
Introduction
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous family of immature myeloid cells arrested in their differentiation program by a variety of tumour-secreted factors. MDSCs inhibit the activity of cytotoxic T lymphocytes (CTLs) in a variety of ways: high levels of intracellular arginase in MDSCs deplete the cellular micro-environment of arginine, an essential amino acid for T-cell activation [1]; uptake and depletion of cystine by MDSCs deplete T cells of a further amino acid required for T-cell activation [2]; MDSC-mediated downregulation of L-selectin, a molecule that targets T-cells to lymph nodes [3]; the production by MDSCs of the free radical peroxynitrite, which causes the nitration/nitrosylation of the T-cell receptors and CD8 molecules of CTLs, thus preventing their recognition of the peptide–MHC complex on tumour cells [4]. MDSC production of peroxynitrite also causes tyrosine nitrosylation of the MHC class I molecules on tumour cells thus preventing the binding of peptide epitopes [5]. We have recently demonstrated significant elevations of MDSCs in patients with pancreatic cancer, and in multivariate analysis, MDSC levels were shown to be an independent prognostic factor for survival [6]. We have extended our studies in pancreatic cancer patients to examine the effects of gemcitabine and capecitabine chemotherapy on MDSC levels and the impact of GM-CSF given as an adjuvant with a cancer vaccine.
The TeloVac study was a large randomized phase III chemoimmunotherapy trial in patients with advanced pancreatic cancer, the final results of which have been presented elsewhere [7]. Participants were randomized to one of three arms. In arm 1, patients received gemcitabine and capecitabine chemotherapy (GemCap). Arm 2 patients received an initial two full courses of GemCap followed by vaccination with the promiscuous class II telomerase peptide vaccine GV1001 and on subsequent progression re-commenced GemCap if they had not initially progressed on their first two cycles of GemCap. In arm 3, patients received concomitant chemo-immunotherapy with GV1001 vaccination with low-dose GM-CSF as adjuvant and GemCap given concurrently from day 1. Peripheral blood mononuclear cells (PBMCs) were collected from arm 2 and arm 3 patients at various time points for subsequent immunological analyses.
The design of TeloVac allowed us to further explore two important issues in MDSC biology in cancer patients. Firstly, the only two chemotherapy drugs which impact qualitatively and quantitatively on MDSCs in pre-clinical models are gemcitabine and fluorouracil (capecitabine is a fluorouracil pro-drug) [8, 9]. Gemcitabine significantly reduced the number of splenic MDSCs in tumour-bearing mice at 48 h: the numbers of CD4+, CD8+ and B cells were not affected [8]. When splenocytes from animals bearing large tumours were added to a mixture of tumour cells and CTLs, the growth inhibitory effects of the CTLs was lost. The addition of an equal number of splenocytes from tumour-bearing animals treated 48 h earlier with gemcitabine had no suppressive effect. Vincent and colleagues showed that the administration of gemcitabine caused a significant reduction in the percentage of CD11b+ MDSCs in the tumour beds of mice [9]. 5-FU also significantly reduced the percentage of MDSCs and to a greater degree than gemcitabine. Cyclophosphamide, doxorubicin, oxaliplatin, and paclitaxel had no such effect. We thus investigated the effect of gemcitabine and capecitabine given together on MDSCs in humans by analyzing the longitudinal changes in MDSC% in patients treated on arm 2 of the Telovac study during their initial two cycles of chemotherapy prior to the commencement of GV1001 vaccination.
Secondly, there is pre-clinical data that GM-CSF increases MDSCs in the tumour micro-environment [10] and clinical data that low-dose GM-CSF given as a vaccine adjuvant increases the number of MDSCs in the blood [11]. We thus investigated the effect of GV1001 given with adjuvant GM-CSF together with gemcitabine and capecitabine on MDSCs by analyzing the longitudinal changes in MDSC% in patients treated on arm 3 of the Telovac study. We examined whether the concurrent administration of GM-CSF as vaccine adjuvant resulted in appreciable differences in the trajectory of MDSC% compared with that seen with GemCap alone and whether GemCap appeared to offset any adverse impact of GM-CSF administration on MDSC%.
We demonstrate that there does not appear to be a consistent effect of gemcitabine and capecitabine on MDSC levels independent of the effect of treatment on tumour bulk. We found no evidence that the use of low-dose GM-CSF as an adjuvant in a combination chemo-immunotherapy approach leads to consistent increases in MDSC levels. Furthermore, a high pre-vaccine MDSC level did not preclude an immune response to GV1001. However, in patients with high baseline MDSC levels who mounted an immune response to GV1001 delivered concomitantly with chemotherapy, there was generally a fall in MDSC levels.
Methods
Patients
20–30 ml of venous blood was collected from pancreatic cancer patients (n = 40) participating in the TeloVac study (ISRCTN 43482138) and age/sex-matched normal healthy controls (n = 24). Age and sex-matched healthy controls were recruited from surgical minor operation clinics at the Royal Surrey County Hospital, UK. The patients described herein represent a sample of patients randomized to arm 2 and arm 3 of TeloVac and for whom suitable samples were available for sequential MDSC analysis. No subject had a history of autoimmune disease or of recent steroid therapy and no control donor had a prior history of cancer. All subjects provided written informed consent approved by the local human investigators committee. All patients were treated with combination gemcitabine and capecitabine chemotherapy (GemCap): gemcitabine was given intravenously at 1,000 mg/m2 weekly × 3 every 4 weeks together with capecitabine administered orally at 1,660 mg/m2/day (830 mg/m2 twice daily) for 3 weeks followed by 1 week’s rest. We analyzed the trajectory of MDSCs in 19 patients treated on arm 2 of TeloVac during treatment with their initial two cycles of GemCap and prior to the delivery of any vaccine. We also examined the trajectory of MDSCs in 21 patients who received GV1001 concurrently with GemCap on arm 3 of the study. The primary vaccination schedule consisted of 0.56 mg GV1001 vaccine i.d. on days 1, 3, 5 during week 1 followed at weekly intervals weeks 2, 3 and 4 then at week 6 and then at week 10. GM-CSF was given as an adjuvant at a dose of 75 mg given intra-dermally 10–15 min prior to each GV1001 administration at the same site.
Peripheral blood samples were taken prior to and following chemotherapy. For patients receiving gemcitabine and capecitabine alone (arm 2), blood was drawn after 7 weeks of treatment, 1 week following the fifth gemcitabine infusion, immediately prior to the sixth gemcitabine infusion and whilst the patient was taking capecitabine. In 16 patients receiving gemcitabine and capecitabine concomitantly with GV1001 (arm 3), blood was drawn after 10 weeks of treatment to coincide with an immunomonitoring time point, 1 week after the seventh gemcitabine infusion immediately prior to the eighth gemcitabine infusion and whilst the patient was taking capecitabine. 3 patients in arm 3 had blood drawn at week 14 and 2 at week 18 which coincided with the delivery of a booster vaccination of GV1001. These latter 5 patients were still receiving concurrent GemCap.
Blood was drawn into li-heparin tubes (BD Biosciences, Europe) or CPT tubes for shipment to the biomarker repository at the Liverpool Cancer Trials Unit, UK. PBMC were isolated using Ficoll-Hypaque gradients. PBMC were counted, frozen at −80 °C and stored in liquid N2 for subsequent batch analysis.
Immunophenotypic analysis of cells
Peripheral blood mononuclear cells were recovered and washed in 0.15 M phosphate-buffered saline, Dulbecco’s A (Oxoid, UK). Cells were aliquoted for MDSC analysis. The LIVE/DEAD Cell Stain Kit (Invitrogen, UK) was used to differentiate viable and dead cells. After washing in binding buffer (BD Biosciences, Europe), the following anti-human monoclonal antibodies were used for flow cytometry: anti-HLA-DR-APC-Cy7, anti-Lin1(CD3,14,16,19,20,56)-FITC and anti-CD11b-PECy7 (BD Biosciences, Europe). Following immunostaining, cells were washed in binding buffer and analyzed using a MACSQuant flow cytometer with MACSQuantify software (Miltenyi Biotec).
Delayed-type hypersensitivity (DTH) skin tests
GV1001 (100 μg) was administered intra-dermally in the lower abdomen, contra-laterally to the vaccination site. Patients were asked to record the size of the DTH reaction 48 h after administration and report to the clinician. A positive DTH reaction was defined as erythema and induration with an average diameter of 5 mm.
In vitro proliferation assays
Thawed PBMCs were seeded in 48-well plates (ThermoFisher Scientific, USA) at 2 × 106 cells/well in X-VIVO 15 (Lonza, UK) with 10 % pooled human serum (Innovative Research, USA) and 20 ug/ml GV1001 peptide. Following 3 days of culture, 10 units/ml IL-2 (Peprotech, UK) was added to the media. On day 11, the GV1001-enriched cells were harvested and plated at 1 × 105 cells/well (50 μl) in a round bottom 96-well plate. To the pre-stimulated cells, irradiated (45 Gy) autologous PBMCs (1 × 105 cells/well, 50 μl) were added to act as antigen-presenting cells (APCs). GV1001-specific proliferation was tested for by the addition of 100 μl of either control media, GV1001 (20 μg/ml) or positive control PHA (5 μg/ml). After incubation for a further 2 days, 1 μCi/well of 3H-thymidine was added for 16 h before counting. A positive proliferative response to GV1001 was defined as a stimulation index (SI) above 1.8 with a significant difference in counts per minute from four replicates.
Cytokine analysis
Cytokine levels were assayed on patient serum collected at the same time as PBMCs using the BioRad BioPlex 27 Pro Cytokine, Chemokine and Growth Factor Assay (BioRad Laboratories, USA) using the BioRad BioPlex Instrument following manufacturer’s instructions.
Tumour burden assessment
Independent assessment of tumour burden pre- and post-chemotherapy was performed by a radiologist blinded to MDSC results, using RECIST v1.1 criteria for measurement of evaluable lesions on CT imaging [12]. The sum of the long axis measurement of tumour lesions and short axis measurement of pathological lymph nodes was used to measure tumour burden in millimetres.
Statistical analysis
Median levels of Lin-DR-CD11b+ cells were compared in pancreatic cancer patients versus controls using an unpaired t test with Welch’s correction. The correlation between baseline MDSC and cytokine was analyzed with Spearman’s rank test, and the nonparametric Mann–Whitney test was used to identify significant differences in cytokines when dichotomized at median MDSC levels. Paired Wilcoxon test was used to compare pre- and post-chemotherapy cytokine levels.
Pre-treatment values of MDSC were subtracted from post-treatment values to give absolute change in MDSC and percentage change was calculated by dividing the absolute change by the pre-treatment value. These data have highly skewed distributions so they are graphically represented on the log scale but all analyses carried out are on the original scale using a nonparametric approach. Changes within each treatment group are tested using a Wilcoxon signed ranks test. Arms 2 and 3 were compared in terms of the pre-treatment value, post-treatment values, absolute change and percentage change using Wilcoxon two-sample tests. Patients with disease control (PR, SD) and progressive disease (PD) were compared in terms of absolute and percentage change using Wilcoxon two-sample tests. Sensitivity analysis for consistency of trends included (1) a re-analysis that included only the patients with SD to remove the effect of change in tumour size and (2) a re-analysis that included only the patients in arm 3 with post-treatment timing of 10 weeks.
Results
Levels of pro-inflammatory cytokines do not correlate with baseline levels of Lin-DR-CD11b+ cells in pancreatic cancer patients
Cryopreserved PBMCs from 40 patients with advanced pancreatic cancer treated with gemcitabine and capecitabine chemotherapy were analyzed. 21 of the patients received concomitant therapy with the telomerase vaccine GV1001. Their baseline pre-treatment values are included in the calculation of the pre-treatment medians and the correlations of baseline levels of pro-inflammatory cytokines with MDSC levels. In the analysis of the effect of gemcitabine and capecitabine on Lin-DR-CD11b+ cells, only sequential samples in the 19 patients who received gemcitabine and capecitabine alone were analyzed. This phenotype was used to mark MDSCs based on the work of Kotsakis and colleagues [13].
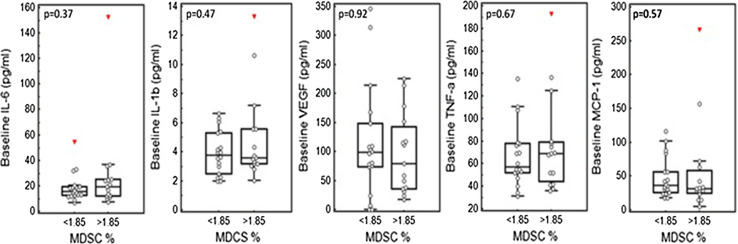
There was a significant increase in Lin-DR-CD11b+ cells in pancreatic cancer patients (n = 40) compared with controls (p < 0.0001). The median baseline of Lin-DR-CD11b+ cells in patients (expressed as a % of live PBMCs) was 1.85 (range 0.62–8.45): corresponding values in 24 controls were median 0.82 (range 0.16–2.2). There was no correlation between baseline levels of pro-inflammatory cytokines and baseline MDSCs in the 33 pancreatic cancer patients for whom we had full cytokine data (Spearman’s coefficient: IL-6 = 0.153, IL-1β = 0.22, VEGF = −0.0389, TNFα = 0.0587, MCP-1 = −0.226) (data not shown). When MDSC levels were dichotomized at the median, there were no significant differences in the baseline level of these cytokines in patients with high MDSCs compared with those with low MDSCs (Fig. 1).
Fig. 1.
No significant differences in the level of pro-inflammatory cytokines in patients with high MDSCs compared with those with low MDSCs. Peripheral blood mononuclear cells from 33 pancreatic cancer patients obtained pre-treatment (at baseline) were immunostained for HLA-DR-APC-Cy7, Lin1(CD3,14,16,19,20,56)-FITC and CD11b-PECy7 and with ViViD to discriminate live and dead cells. Following immunostaining, cells were analyzed using a MACSQuant flow cytometer with MACSQuantify software. In addition, serum cytokines TNFα, MCP-1, IL-1b, IL-6 and VEGF were determined using the Bio Rad Bio Plex 27 Pro Cytokine, Chemokine and Growth Factor assay using the Bio Rad Bio Plex Instrument. Baseline MDSCs dichotomized at median (1.85) plotted with baseline IL-6, IL-1b, VEGF, TNFα and MCP-1 (pg/ml). P value generated for Mann–Whitney test shows no significant differences
Gemcitabine and capecitabine therapy does not consistently reduce Lin-DR-CD11b+ cell numbers—the contribution of disease control and the degree of cancer-related inflammation
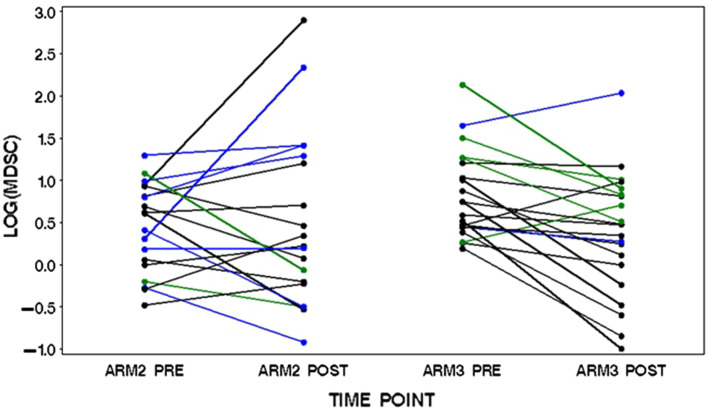
In the patients receiving chemotherapy alone (arm 2 patients, n = 19), gemcitabine and capecitabine therapy resulted in a fall in Lin-DR-CD11b+ cells in 8/19 patients (42 %) (Fig. 2; Table 1). In the 7 patients with progressive disease (PD), the Lin-DR-CD11b+ cell level went up in 5 and down in 2 (range −60 to +662 %). In the 10 patients with stable disease (SD), Lin-DR-CD11b+ cell levels increased in 6 and fell in 4 (range −68 to +604 %). Both patients achieving a partial response had falls in the Lin-DR-CD11b+ %. We obtained accurate tumour measurements, and in 8/10 of the patients with stable disease, there was no significant increase or decrease in the sum of the longest diameters. In these patients where the relative contribution of a change in Lin-DR-CD11b+ % as a direct result of any significant change in tumour bulk would be minimal, Lin-DR-CD11b+ % went up in 5 patients and down in 3. These data suggest that there is no consistent reduction in Lin-DR-CD11b+ % secondary to gemcitabine and capecitabine chemotherapy per se. Changes in Lin-DR-CD11b+ % had a tendency to track with tumour response. This is well demonstrated in the patients with baseline Lin-DR-CD11b+ greater than the median, the group where a fall in MDSCs would be most likely to be of the greatest immunological benefit (Table 2). In these patients, the Lin-DR-CD11b+ % increased in 6: 3 of these patients had progressive disease as best response to therapy and 3 had 0 % tumour volume change. In the 3 patients in whom Lin-DR-CD11b+ % fell, 1 obtained a partial response and another an 11 % reduction in tumour volume. In only 3 of these patients was the Lin-DR-CD11b+ % less than the baseline median following gemcitabine and capecitabine therapy. When arms 2 and 3 were combined, the absolute changes in MDSC were related to response (p = 0.02) with levels in the 9 patients with PD in the study increasing on average (median = 0.47) and in the 31 with disease control decreasing (median = −0.49).
Fig. 2.
The trajectory of MDSC levels in patients receiving GemCap chemotherapy alone (arm 2) and GemCap chemotherapy concomitantly with GV1001 vaccination and low-dose adjuvant GM-CSF (arm 3). PBMCs were obtained pre- and post-treatment and immunostained for HLA-DR-APC-Cy7, Lin1(CD3,14,16,19,20,56)-FITC and CD11b-PECy7 as well as ViViD to discriminate live and dead cells. Following immunostaining cells were analyzed using a MACSQuant flow cytometer with MACSQuantify software. The graph depicts the log percentage change in Lin-DR-CD11b+ MDSCs from baseline in patients during treatment with gemcitabine and capecitabine. Blue denotes patients with progressive disease on treatment, black stable disease and green partial response by RECISTv1.1
Table 1.
Summary statistics for MDSC values in patients receiving GemCap chemotherapy alone (arm 2) and GemCap chemotherapy concomitantly with GV1001 vaccination and low-dose adjuvant GM-CSF (arm 3)
| N | Median | Inter-quartile range | Range | |
|---|---|---|---|---|
| Arm 2 | ||||
| Pre | 19 | 1.84 | 1.00, 2.54 | 0.62, 3.65 |
| Post | 19 | 1.25 | 0.80, 3.64 | 0.40, 18.16 |
| Absolute change | 19 | 0.16 | −0.90, 0.95 | −2.02, 15.58 |
| Percentage change | 19 | 9 % | −46, 48 % | −68, 662 % |
| Arm 3 | ||||
| Pre | 21 | 2.11 | 1.56, 3.34 | 1.21, 8.45 |
| Post | 21 | 1.61 | 1.00, 2.30 | 0.37, 7.67 |
| Absolute change | 21 | −0.54 | −1.31, −0.19 | −5.98, 2.46 |
| Percentage change | 21 | −23 % | −63, −11 % | −78, 69 % |
Cryopreserved PBMCs obtained from patients with advanced pancreatic cancer before (baseline) and after treatment with gemcitabine and capecitabine chemotherapy were immunostained for HLA-DR-APC-Cy7, Lin1(CD3,14,16,19,20,56)-FITC and CD11b-PECy7 and ViViD to discriminate live and dead cells. Following immunostaining, cells were analyzed using a MACSQuant flow cytometer with MACSQuantify software
Table 2.
Changes in Lin-DR-CD11b+ cells during chemotherapy in patients with baseline values > median
| Number | Stage | Baseline Lin-DR-CD11b+ | Post therapy Lin-DR-CD11b+ | Change (%) | Radiological response |
|---|---|---|---|---|---|
| 1 | III | 2.58 | 18.16 | +604 | SD (0 %) |
| 2 | IV | 3.65 | 4.12 | +13 | PD |
| 3 | III | 2.25 | 3.33 | +48 | SD (0 %) |
| 4 | IV | 2.54 | 1.59 | −37 | SD (−11 %) |
| 5 | IV | 2.0 | 1.08 | −46 | SD (0 %) |
| 6 | IV | 2.69 | 3.64 | +35 | PD |
| 7 | IV | 2.23 | 4.12 | +85 | PD |
| 8 | IV | 2.96 | 0.94 | −68 | PR |
| 9 | III | 1.86 | 2.02 | +9 | SD (0 %) |
Cryopreserved PBMCs obtained from patients with advanced pancreatic cancer before (baseline) and after treatment with gemcitabine and capecitabine chemotherapy were immunostained for HLA-DR-APC-Cy7, Lin1 (CD3,14,16,19,20,56)-FITC and CD11b-PECy7 and ViViD to discriminate live and dead cells. Following immunostaining, cells were analyzed using a MACSQuant flow cytometer with MACSQuantify software. Numbers represent % change in the Lin-DR-CD11b+ cells only in those patients with baseline Lin-DR-CD11b+ greater than the median. There was no consistent reduction in Lin-DR-CD11b+ % secondary to gemcitabine and capecitabine chemotherapy. SD = stable disease and PR = partial response and PD = progressive disease
Given the lack of a strict association of MDSC level changes with objective response to therapy, we next analyzed whether the changes in MDSC level tracked with changes in the degree of cancer-related inflammation using as a surrogate changes in IL-6 and other inflammatory cytokines during treatment. We hypothesized that in patients with stability of tumour volume but in whom the MDSC% went up, there was continued tumour-related inflammation, which continued to drive MDSC production. IL-6 levels went up in 7/19 arm 2 patients during gemcitabine and capecitabine treatment: 4 of these patients had progressive disease. The other 3 had stable disease and in these 3 patients MDSC% increased. Table 3 shows the changes in MDSC% against the changes in inflammatory cytokines during chemotherapy in the 10/19 patients with stable disease. In the four patients in whom there was a fall in MDSC%, IL-6 fell in all 4 and in one of these patients (patient 8) a fall in IL-6 level from 152.72 to 8.66 pg/ml over 7 weeks of chemotherapy was associated with a fall in MDSC% from 2.54 to 1.59. In the six patients with stable disease in whom the MDSC% increased, in 3 the baseline MDSC level was below the median and remained so following chemotherapy. In two patients where an above-median baseline MDSC level continued to rise on treatment, there was a significant increase in IL-6 (patients 1 and 3). VEGF levels also increased from 37.59 to 70.69 pg/ml in patient 3, the only patient without progressive disease to show such an increase.
Table 3.
Changes in MDSC% during gemcitabine and capecitabine chemotherapy correlated with changes in serum TNFα, MCP-1, IL-1b, IL-6 and VEGF in patients with stable disease on therapy
| Patients | MDSC (%) | TNFα (pg/ml) | MCP-1 (pg/ml) | IL-1b (pg/ml) | IL-6 (pg/ml) | VEGF (pg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 2.58 | 18.16 | 36.24 | 30.02 | 5.19 | 10.04 | 2.82 | 2.37 | 11.13 | 266.67 | 79.08 | 45.66 |
| 2 | 0.75 | 1.41 | 34.27 | 13.87 | 17.45 | 6.22 | 2.27 | 1.13 | 9.11 | 4.40 | 148.52 | 38.89 |
| 3 | 2.25 | 3.33 | 69.34 | 69.93 | 23.21 | 17.00 | 3.06 | 2.51 | 24.29 | 87.04 | 37.53 | 70.69 |
| 4 | 0.62 | 0.8 | 135.53 | 74.27 | 33.57 | 30.69 | 6.31 | 4.69 | 20.65 | 19.39 | 97.05 | 60.86 |
| 5 | 1.00 | 1.25 | 47.07 | 67.00 | 42.59 | 47.1 | 2.99 | 3.51 | 11.67 | 18.19 | 100.04 | 376.92 |
| 6 | 1.86 | 2.02 | 71.8 | 60.48 | 69.62 | 58.53 | 4.22 | 4.54 | 22.47 | 18.73 | 82.76 | 29.18 |
| 7 | 1.06 | 0.82 | 54.65 | 53.69 | 26.08 | 30.45 | 3.62 | 3.71 | 12.98 | 11.74 | 6.87 | 3.05 |
| 8 | 2.54 | 1.59 | 192.74 | 25.14 | 266.73 | 17.35 | 10.62 | 1.68 | 152.72 | 8.66 | 213.81 | 10.26 |
| 9 | 2.00 | 1.08 | 68.17 | 40.02 | 71.78 | 68.12 | 3.16 | 1.73 | 12.83 | 9.86 | 225.10 | 51.15 |
| 10 | 1.84 | 0.59 | 78.58 | 72.61 | 30.67 | 44.86 | 6.08 | 7.77 | 54.33 | 36.62 | 108.04 | 29.81 |
PBMC from ten patients with stable disease during gemcitabine and capecitabine treatment were analyzed both pre- and post-treatment by flow cytometry for changes in Lin-DR-CD11b+ cells and changes in serum cytokines TNFα, MCP-1, IL-1b, IL-6 and VEGF by the Bio Rad Bio Plex 27 Pro Cytokine, Chemokine and Growth Factor assay
Low-dose GM-CSF delivered as an adjuvant to GV1001 vaccination does not increase Lin-DR-CD11b+ MDSCs in patients receiving concomitant GemCap
We analyzed the trajectory of the changes in MDSCs in patients receiving GemCap alone (arm 2) and GemCap together with GV1001 vaccination with low-dose GM-CSF as adjuvant (arm 3) and compared the two. Summary statistics for the MDSC values pre- and post-treatment on arms 2 and 3 are shown in Table 1 and the values are plotted graphically on the log scale in Fig. 1. The MDSC values pre-treatment were higher for arm 3 than arm 2 (p = 0.08). There was a significant decrease in MDSC from this higher level in arm 3 (p = 0.007 and p = 0.006 for absolute and percentage change, respectively), whereas there was no significant change in arm 2 (p = 0.60 and p = 0.62 for absolute and percentage change, respectively). This gave values on arms 2 and 3 that were not significantly different post-treatment (p > 0.99). This differential behaviour between arms 2 and 3 in terms of change in MDSC was borderline statistically significant (p = 0.04 and p = 0.06 for absolute and percentage change, respectively). Sensitivity analysis showed the differential trends observed between arms 2 and 3 remained consistent for the SD subgroup and the post-treatment assessment at 10-week subgroup.
We analyzed the development of an immune response to GV1001 in the 21 arm 3 patients receiving concomitant gemcitabine and capecitabine and GV1001 (positive proliferation assay, and/or the development of a positive DTH, to GV1001). Nine patients in this cohort developed an immune response. In 8/9 of these patients, the MDSC% fell during chemo-immunotherapy. 6/9 had baseline Lin-DR-CD11b+ % greater than the patient median and in all of these the MDSC level fell (Supplementary Table 1). All immune responders had radiological disease control (either PR or SD) at the time that blood was drawn for the analysis of proliferation response, which coincided with the timing of the sequential MDSC assay in these patients.
Discussion
This study demonstrates that there appears to be no consistent reduction in MDSC level in pancreatic cancer patients treated with a combination of gemcitabine and capecitabine. We analyzed a homogeneously treated group of patients obtaining samples at the same time point in all, immediately prior to a gemcitabine infusion, 1 week following the last gemcitabine infusion and whilst the patient was taking daily capecitabine. This time point correlated with the first radiological assessment of tumour response. MDSC changes tended to track with tumour volume: most patients with progressive disease demonstrated an increase in MDSC level during chemotherapy whilst those who obtained a partial response a decreased level. In patients with no significant change in tumour volume during therapy, gemcitabine and capecitabine did not consistently reduce MDSC levels as would be expected if GemCap reduced MDSC levels independently of response, through a direct effect on MDSCs. In patients with stable disease as best response to GemCap, the MDSC level was just as likely to go up as to go down. In spite of the pre-clinical data, gemcitabine and fluoropyrimidine treatment in cancer patients does not appear to reproducibly result in an independent reduction in MDSC level. Any decrease is likely to reflect predominantly the effect of the resultant disease control, although even in patients with no change at all in disease volume the MDSC level may actually go up. We did not assess the individual impact of gemcitabine and capecitabine on MDSC level as all patients in the TeloVac study received the combination GemCap. However, both of these drugs reduce MDSC level pre-clinically, and it seems unlikely that clinically an effect of one of the drugs in reducing MDSCs would be negated by the other increasing MDSCs. These data support two previously published studies examining the effects of chemotherapy on MDSC levels but are important because they are from patients homogeneously treated with a combination of the only two chemotherapy drugs which pre-clinically have been shown to reduce MDSC levels directly. In the first of these studies where the chemotherapy was not specified, MDSC numbers tracked with tumour volume in 6 patients, with numbers falling with response and increasing with progression [14]. Similarly, in 25 patients with advanced colorectal cancer, MDSCs were significantly higher (and increased from baseline) in patients with progressive disease compared with those with a response to chemotherapy. Again, the regimen was not specified [15].
We hypothesized that longitudinal changes in MDSC levels during gemcitabine and capecitabine chemotherapy might in part be related to temporal changes in the degree of tumour-related inflammation. The degree of inflammation in any tumour is likely to be somewhat independent of tumour response and this might explain the differential effects on MDSC levels in patients with stable disease. We used sequential changes in IL-6 and other inflammatory cytokines as a surrogate for the status of cancer-related inflammation and correlated changes in MDSC levels with changes in the levels of IL-6 during treatment with gemcitabine and capecitabine. We demonstrated that in patients with stable disease, the direction of MDSC change tracked well with the direction of IL-6 change. These data support a model whereby significant increases in tumour burden during chemotherapy are likely to be associated with MDSC increases and significant decreases in tumour burden with decreased MDSC level, whilst in those with little change in tumour bulk, the direction of MDSC change will be determined by the longitudinal change in the degree of cancer-related inflammation. Continued significant tumoural inflammation, in spite of stabilization of tumour volume, is likely to support the continued production of MDSCs.
Given the above we were initially surprised to see no association between baseline levels of pro-inflammatory cytokines and baseline MDSCs. Using a 4T1 mammary carcinoma model, Bunt and colleagues demonstrated significant elevations of the pro-inflammatory cytokines IL-6, MCP-1, TGFβ and IL1β in tumour tissue showing that tumour growth is associated with an inflammatory milieu [16]. Intratumoral inflammatory cytokine levels were significantly reduced, and accumulation of CD11b+ Gr1+ MDSCs in the blood was delayed in IL-1R−/− mice. Overexpression of IL-6 in 4T1 cells compensated for the loss of IL-1R with no delay in MDSC accumulation. However, it is important to note that the delay in accumulation of MDSCs in the IL-1R−/− mice was short-lived, and by day 26 from inoculation, MDSC levels were at the same high level as in BALBc mice. This could explain a lack of correlation between baseline IL-6 (and other inflammatory cytokines including IL1β) and MDSC levels at a time when the cancer is already well established.
GM-CSF appears to have more profound effects on MDSC biology than Il-1β and IL-6 in the 4T1 model [17], and this is supported by data demonstrating the critical role of GM-CSF in the induction of human CD33+ MDSCs from normal PBMCs [18]. GM-CSF plays a key role in the generation of MDSCs in KPC mice where oncogenic KRAS and mutant p53 are targeted to the pancreas [19]. Levels of IL-6 were generally low in the KPC cell lines and tended to be higher in the normal ductal cells. GM-CSF drove GR-1+ CD11b+ cell generation, whereas IL-6 was minimally effective: neutralization of GM-CSF completely abrogated MDSC generation from c-kit + precursors by conditioned media whereas anti-IL-6 had no effect. 19/20 human pancreatic ductal adenocarcinoma samples expressed GM-CSF by immunocytochemistry. These data fit with the clinical observation of an increase in the frequency of suppressive monocytic MDSCs (CD14+ CD11b+ DR−) in patients with melanoma who received GM-CSF as an adjuvant with a heat shock protein-based vaccine [11]. The same dose of GM-CSF was delivered as in the TeloVac study but was given for 3 days rather than one. We thus analyzed the effects of vaccination with GV1001 with GM-CSF as adjuvant in pancreatic cancer patients receiving concomitant GemCap on the frequency of Lin-DR-CD11b+ MDSCs. We found no evidence that low-dose GM-CSF as adjuvant increased the % of Lin-DR-CD11b+ MDSCs. Indeed, there was a significant decrease in patients receiving GM-CSF as adjuvant with GV1001 vaccination and concomitant GemCap. No significant decrease was seen in patients receiving GemCap alone and this differential behaviour between arms 2 and 3 in terms of change in MDSC was borderline statistically significant. The MDSC% fell in 18/21 patients receiving vaccine with GM-CSF plus GemCap compared with only 8/19 of patients receiving GemCap alone. These data do not necessarily contradict those of Fillipazzi and colleagues who also found no evidence that low-dose GM-CSF increased the Lin-DR-CD11b+ cell population [11].
One of the limitations of this study is that it utilized cryopreserved MDSCs. This reflects the difficulties inherent in performing immunological analyses in large multi-centre studies. Kotsakis and colleagues demonstrated that freezing had a profound effect on the function of recovered MDSCs [13]. Thus, we did not perform any specific functional assays. However, we sought to understand the functional effect of any gemcitabine and capecitabine-mediated quantitative effects on MDSCs by correlating the effects of chemotherapy on MDSC levels with the development of an immune response to the GV1001 vaccine. The MDSC level fell in 8 of the 9 patients who developed an immune response to GV1001 administered concomitantly with gemcitabine and capecitabine. In 6 of these patients, the initial pre-vaccination MDSC level was above the median for the cancer patient cohort. Thus, a high pre-vaccination MDSC level is clearly not an absolute bar to the development of immunity to a tumour-associated antigen. However, it is reasonable to hypothesize that this is most likely to occur with any concomitantly administered treatment which checks the progress of increasing immunosuppression which will occur with increasing volumes of disease and/or increasing levels of cancer-related inflammation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was funded by BRIGHT: Better Research Into Gastrointestinal cancer Health and Treatment, registered charity number 1064857. The TeloVac Trial was funded by Cancer Research UK and KAEL-Gemvax.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Nicola E. Annels and Victoria E. Shaw have contributed jointly to this work.
References
- 1.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4- induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid- derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton GW, Valle JW, Wadsley J, Propper D, Coxon FY, Ross PJ et al (2013) A phase III randomized trial of chemoimmunotherapy comprising gemcitabine and capecitabine with or without telomerase vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer. J Clin Oncol 31(suppl; abstr LBA4004) [DOI] [PubMed]
- 8.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 9.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5- Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 10.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero MC, Mariani L, Parmiani G, Rivoltini L, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381(1–2):14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, Garrett-Mayer E, Montero AJ, Bronte V, Mandruzzato S. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118(8):2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67(20):10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 18.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21(6):822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.