Abstract
Context
Gastric type I carcinoid is a rare neoplasm, deriving from enterochromaffin-like cells (ECL), mainly affecting women with autoimmune gastritis. The approach to treatment, either endoscopic, medical or surgical, is not well defined, particularly in multifocal tumours or carcinoids with rapid growth/frequent recurrence.
Objective
To determine whether an anti-G17 vaccination might interfere on the natural history of gastric type I carcinoid.
Setting
Padua teaching Hospital, outpatient clinic.
Design and patients
Three patients with type I gastric carcinoid in autoimmune gastritis were administered, after informed consent and ethic committee approval, with a vaccine against gastrin 17 (G17), a synthetic peptide that stimulates specific and high-affinity anti-G17 antibodies, and followed up endoscopically and clinically for a mean of 36 months.
Main outcome measures
Gastric histology and specifically carcinoid growth/recurrence and trend in time in gastrin, G17, pepsinogens, chromogranin A and clinical parameters.
Results
Following vaccination, carcinoid regression was observed in 2/3 patients and, in one of the patients, even the disappearance of ECL hyperplasia, with a reduced ECL cells stimulation, confirmed by a significant reduction in chromogranin A levels. Regression was observed in the two patients that showed a more clear local response to the vaccine. Increased autoantibody titre was observed, but no appearance of new autoimmune diseases.
Conclusions
Anti-G17 vaccination induced regression of type I gastric carcinoid and could be considered for the treatment of this tumour, when endoscopic removal is not indicated.
Keywords: Gastric carcinoid, Cancer vaccine, Gastrin, G17, Autoimmune gastritis, ECL hyperplasia
Gastric type I carcinoid is a rare neuroendocrine tumour (1–2/100.000 cases per person/year, about 4% of digestive neuroendocrine tumours in Italy) [1], mainly affecting women with autoimmune atrophic gastritis (AAG) [2]. The tumour derives from enterochromaffin-like cells (ECL cells), the most represented endocrine cells in the stomach [2], characterized by chromogranin A (CgA), neuron-specific enolase and synaptophisin immunostaining [3, 4]. These cells play a relevant physiologic role, releasing histamine that contributes to HCl secretion by parietal cells [5]. In the context of autoimmune gastritis, these cells are hyper-stimulated by the increased levels of gastrin, whose subunit G17 acts as a growth factor for ECL cells, inducing a spectrum of hyperplastic and dysplastic changes [5–7]. Linear, micronodular, nodular and adenomatous hyperplasia may indeed develop, and eventually carcinoids that are often multiple and sometimes invasive [6–9].
Type I carcinoids, however, develop in a small minority of AAG patients [10], and factors other than gastrin hypersecretion must be involved. To support this, it is useful to observe how in type II carcinoids that are induced by hypergastrinaemic conditions such as sporadic Zollinger–Ellison syndrome (ZES) and ZES associated with MEN-I syndrome, the incidence of gastric carcinoids is lower in the first condition and higher in the latter [11, 12], thus suggesting a relevance of genetic factors.
Gastric carcinoids may be single, but multifocality is more frequent, and tend to be small, rarely >2 cm. Endoscopically, they appear as small polipoid lesions, but sometimes, they are an incidental finding in biopsies obtained from flat mucosa [13].
Type I gastric carcinoids are classified as well-differentiated endocrine tumours even though their biologic behaviour is somehow unpredictable. The risk of aggressive evolution is small, but metastases, even though extremely rare, may be observed in carcinoids exceeding 2 cm with a high (>2%) mitotic index [13].
Therapeutic algorithms for carcinoids have been recently published [13], and endoscopic treatment is advocated in most cases [14, 15]. Patients with type I gastric carcinoid, diagnosed during endoscopic follow-up of AAG, enter a more intensive protocol of endoscopic treatment and follow-up, in a sort of never-ending story that the patients feel as a nightmare. The alternative is surgery, whether a total gastrectomy, with the obvious implications for the patient in terms of side effects and complications, or antrectomy associated with local removal of the tumour, a less invasive procedure aimed at abrogating the gastrin stimulus [16].
In patients not fit for surgery or that refuse the procedure, treatment with octreotide has been proposed to inhibit gastrin release as well as ECL cell proliferation [17, 18]. This is a lifelong treatment, in patients that are often young, with treatment-associated complications and enormous costs.
Recently, a vaccine against G17 has been synthesized: a peptide obtained coupling an epitope derived from the amino-terminal aminoacid residues 1–9 of G17 with diphtheria toxoid (human G17DT immunogen, INSEGIA*, Aphton Corporation, USA1) that stimulates specific and high-affinity anti-G17 and anti-Gly-G17 antibodies. The vaccine was intended for the treatment of advanced gastric and colorectal cancer, based on the evidence that gastrin (G) acts as a growth factor for both these tumours [19, 20].
Given the obvious role of gastrin in the development of gastric type I carcinoid and the need for an alternative treatment in patients with multifocal recurring disease, we wondered whether INSEGIA* could be effective in the treatment of the disease and whether medical abrogation of gastrin stimulus may induce tumour regression.
Aphton kindly provided the vaccine, and the study was formally approved by the ethical committee of Padua University Hospital.
Three patients were selected for the pilot study (A, B and C) and gave their informed consent; they all were affected by autoimmune atrophic gastritis, aged 52–60, had either multiple carcinoids (2 pts) or had already been operated on, with an ‘a la demande’ gastric resection, for gastric carcinoid (1 pts).
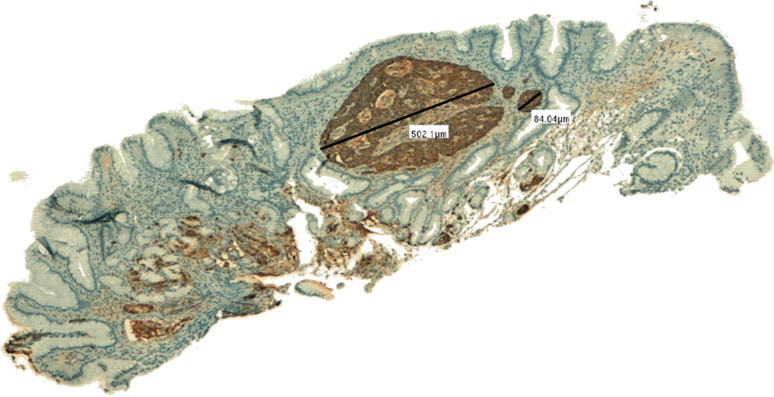
The time 0 features of the three patients (A,B,C), all harbouring low-risk gastric carcinoids, are summarized in Table 1, and an example of the tumour is shown in Fig. 1.
Table 1.
Patients age and features with trend in time of gastric histology and biochemical parameters
| Patients and parameters | t0 | t1 (6 mths) | t2 (12 mths) | t3 (18 mths) | t4 (24 mths) | t5 (36 mths) | |
|---|---|---|---|---|---|---|---|
| A (52 y.o). | Endoscopy | 4 polyps < 1 cm at the corpus | 1 polyp < 1 cm at the body | Flat oedematous mucosa | Flat oedematous mucosa | 1 polyp < 1 cm at the body | Flat mucosa |
| Histology | Carcinoid | M-ECL-h | L/M-ECL-h | M-ECL-h | N-ECL-h (focal) | M-ECL-h | |
| G | 1,200 | 2,900 | 1,000 | 1,140 | 1,251 | 1,731 | |
| G17 | 480 | 1742 | 479 | 254 | 233 | 140 | |
| CgA | 282 | 179 | 267 | 231 | 151 | 180 | |
| PGA/PGC | 0.1 | 0.3 | 0.6 | 0.1 | 0.4 | 0.6 | |
| Vit B12 | 368 | 497 | 346 | – | 331 | – | |
| B (53 y.o). | Endoscopy | 1 polyp of 0.5 mm at the body | Flat mucosa | Flat mucosa | Flat mucosa | Flat mucosa | Flat mucosa |
| Histology | Carcinoid | M-ECL-h | N-ECL-h | L/M-ECL-h | Negative for ECL-h | L-ECL-h | |
| G | 700 | 17,400 | 4,902 | 2256 | 5,834 | 3,388 | |
| G17 | 200 | 15,700 | 2,550 | 1,720 | 1,673 | 1,418 | |
| CgA | 260 | 95 | 138 | 131 | 167 | 144 | |
| PGA/PGC | 0.5 | 0.2 | 0.5 | 0.1 | 0.4 | 0.2 | |
| Vit B12a | 2,000 | 2,000 | 2,000 | 2,000 | 2,000 | – | |
| C (60 y.o). | Endoscopy | 4 polyps <1 cm at the body | 1 polyp <1 cm at the body | 1 polyp <1 cm at the body | Flat mucosa | 3 polyps 0.5 mm at the body | Flat mucosa |
| Histology | Carcinoid | Carcinoid | Carcinoid | N-ECL-h | Carcinoid | Carcinoid | |
| G | 2,200 | 38,400 | 9,695 | 4,506 | 3,217 | 3,265 | |
| G17 | 302 | 24,000 | 7,800 | 2,660 | 1,367 | 1,661 | |
| CgA | 265 | 109 | 106 | 96 | 113 | 147 | |
| PGA/PGC | 0.7 | 0.4 | 0.6 | 0.1 | 0.8 | 0.5 | |
| Vit B12* | 818 | – | 729 | – | 990 | – | |
The age is referred at the moment of the beginning of the INSEGIA administration
G Gastrin (n.v. 37–70 ng/l), G17 Gastrin-17 n.v. 1–15 pmol/l, CgA n.v. 0–98 ug/l, L-ECL-h Linear ECL Hyperplasia, M-ECL-h Micronodular ECL Hyperplasia, N-ECL-h Nodular ECL Hyperplasia, Vit B12 vitamin B12 (n.v.180–900 ng/l)
aPatients i.m. supplemented with B12
Fig. 1.
A 500-μ carcinoid tumour confined to the oxyntic, atrophic mucosa and adjacent to foci of ECL hyperplasia (linear and micronodular) in patient A (chromogranin immunostaining-5x)
All patients underwent staging with abdominal US, total body CT and Octreoscan, with no signs of extra-gastric disease being detected. The patients were negative for the menin gene mutations. They were then treated with 3 doses of INSEGIA* (0.250 μg of INSEGIA* in 0.2 ml of emulsion) (2 pts) or 2 doses (1 pt) i.m. at 0, 2 and 6 weeks, as suggested by Aphton. In one patient, the 3rd dose was not administered due to appearance of side effects (see beyond).
The patients underwent 6-month endoscopic examination, with multiple random biopsies of the body and fundic mucosa of the stomach (at least 12 in the absence of focal changes in order to reduce any sampling error) plus biopsies of any macroscopic lesion. Mean follow-up is presently 36 months, and 2/3 patients already underwent five post-treatment endoscopies. At time 0 and every 6 months, we determinated blood parameters and autoantibodies levels (i.e. intrinsic factor and parietal cell auto-antibodies, ANA, AMA, SMA, anti-LKM, anti-tyreoglobulin and anti-thyroid peroxidase).
G (RIA Kit, MP Biomedicals) and G17 levels (Elisa Kit, Biohit) sharply rose after vaccination (at time 1), with a subsequent decrease at next follow-up, that was continuous for G17. Pepsinogen A (PGA), pepsinogen C (PGC) and PGA/PGC levels (Elisa Kit, Biohit) did not change substantially (Table 1). With the obvious limitations of the sample size, the changes in G (RIA), G17 (Elisa) and chromogranin A (CgA) from peak values to t4 were statistically significant (P < 0.05 and <0.01, respectively). The above data suggest that after the administration of the G17DT immunogen, the levels of G and G17 rose in an attempt to stimulate acid output, most likely due to the inefficacy of the circulating G17, complexed with the antibody. The subsequent drop was probably due to an exhaustion of the capacity of antral G-cells to produce the hormone. The reduction in CgA levels confirmed a reduced ECL cell stimulation, while PGA/PGC ratio fluctuated but were substantially unchanged over time, suggesting a stabilization of body gland mass.
ANA and SMA autoantibodies titre rose, but no clinical signs of autoimmune disease appearance or worsening were observed, nor any change in liver function tests.
Two patients developed a previously reported and expected side effect: a ‘cold’ abscess in the deltoid region, were the vaccine was injected. As already reported, this precluded the administration of the third dose in one patient. These abscesses are sterile and are due to an overwhelming local immunoresponse. One was treated with steroids, but required a surgical drainage. All patients are free of symptoms at the end of follow-up.
Patients A and B, who had the more evident clinical response to the vaccine, developing ‘cold’ abscesses, had:
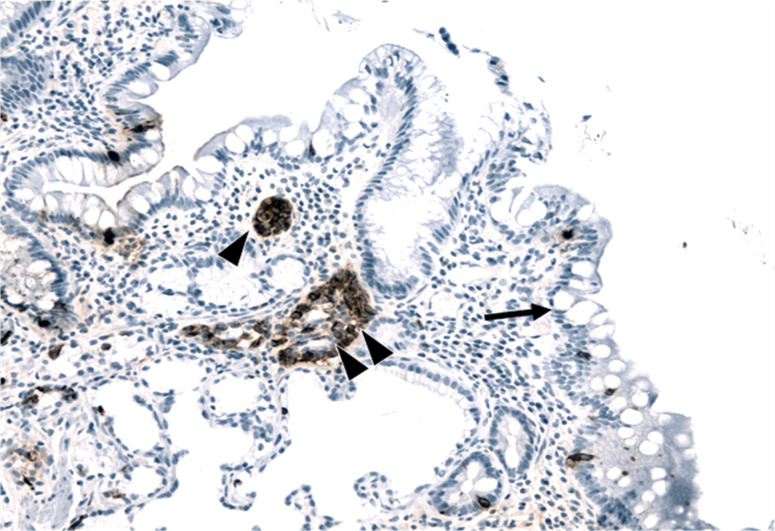
Patient A: nodular hyperplasia at time 4 and micronodular at time 5 (Fig. 2).
Patient B: absence of hyperplasia at time 4 (Table 1).
Fig. 2.
Atrophic mucosa of the gastric body with intestinal metaplasia (goblet cells, see arrow) associated with linear (arrowhead) and micronodular (double arrowhead) enterochromaffin-like (ECL) cell hyperplasia in the same patient at time 5 (chromogranin immunostaining, 20×)
In patient C, a carcinoid was documented at time 1 and 2; at time 3, only nodular hyperplasia was observed, but then, the tumour was again documented.
After discussion of the case with our surgeons, endoscopic follow-up is scheduled for the patient C with persisting carcinoid.
The results of the study on the administration of vaccination with human G17DT immunogen indicate that the administration of the vaccine causes:
an initial increase in G/G17 production, probably depending on an unmet attempt to stimulate HCl production, due to the presence of an ineffective, antibody-complexed hormone, followed by a progressive reduction;
a reduction in ECL cell stimulation, with a drop in chromogranin A levels;
an active and generalized immunoresponse, but with no appearance or exacerbation of autoimmune diseases;
the appearance of side effects that may require minimally invasive procedures but that apparently heralds an effective response to the vaccine;
finally and most importantly, carcinoid regression in 2/3 patients, at short and medium terms, and even, in one patient, disappearance of ECL hyperplasia.
The above findings are to be confirmed by a longer-term follow-up and by larger size studies but indicate the possibility of a new therapeutic approach to gastric type I carcinoids, when endoscopic resection is not feasible.
Acknowledgments
No author declares potential conflicts of interest. I (FF) had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Aphton is no longer responsible for the development of the vaccine.
References
- 1.Corleto VD, Panzuto F, Falconi M, Cannizzaro R, Angeletti S, Moretti A, Delle Fave G, Farinati F. Oncology study section of the Italian society of gastroenterology. Digestive neuroendocrine tumours: diagnosis and treatment in Italy. A survey by the oncology study section of the Italian society of gastroenterology (SIGE) Dig Liv Dis. 2001;33(3):217–221. doi: 10.1016/S1590-8658(01)80710-6. [DOI] [PubMed] [Google Scholar]
- 2.Bordi C. Gastric carcinoids. Ital J Gastroenterol Hepatol. 1999;31(Suppl 2):S94–S97. [PubMed] [Google Scholar]
- 3.Modlin IM, Kidd M, Pfragner R, Eick GN, Champaneria MC. The functional characterization of normal and neoplastic human enterochromaffin cells. J Clin Endocrinol Metab. 2006;91(6):2340–2348. doi: 10.1210/jc.2006-0110. [DOI] [PubMed] [Google Scholar]
- 4.Baudin E, Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin JC, Bidart JM, Cailleux AF, Bonacci R, Ruffié P, Schlumberger M. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. 1998;78(8):1102–1107. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldum HL, Kleveland PM, Brenna E, Bakke I, Qvigstad G, Martinsen TC, Fossmark R, Gustafsson BI, Sandvik AK. Interactions between gastric acid secretagogues and the localization of the gastrin receptor. Scand J Gastroenterol. 2009;44(4):390–393. doi: 10.1080/00365520802624219. [DOI] [PubMed] [Google Scholar]
- 6.Solcia E, Bordi C, Creutzfeld W, et al. Histopathological classification of gastric non antral growth in man. Digestion. 1988;41:185–200. doi: 10.1159/000199786. [DOI] [PubMed] [Google Scholar]
- 7.Bordi C, D’Adda T, Azzoni C, Pilato FP, Caruana P. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol. 1995;19(Suppl 1):S8–S19. [PubMed] [Google Scholar]
- 8.Solcia E, Fiocca R, Villani L, et al. Hyperplastic, dysplastic, and neoplastic enterochromaffin-like-cell proliferation on the gastric mucosa. Am J Surg Pathol. 1995;19(Suppl 1):S1–S7. [PubMed] [Google Scholar]
- 9.Rindi G, Solcia E. Endocrine hyperplasia and dysplasia in the pathogenesis of gastrointestinal and pancreatic endocrine tumors. Gastroenterol Clin North Am. 2007;36(4):851–865. doi: 10.1016/j.gtc.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Varro A, Ardill JE. Gastrin: an analytical review. Ann Clin Biochem. 2003;40(Pt 5):472–480. doi: 10.1258/000456303322326380. [DOI] [PubMed] [Google Scholar]
- 11.Von Rosenvinge EC, Wank SA, Lim RM. Gastric masses in multiple endocrine neoplasia type I-associated Zollinger-Ellison syndrome. Gastroenterology. 2009;137(4):1222. doi: 10.1053/j.gastro.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Burkitt MD, Pritchard DM. Pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther. 2006;24(9):1305–1320. doi: 10.1111/j.1365-2036.2006.03130.x. [DOI] [PubMed] [Google Scholar]
- 13.Delle Fave G, Capurso G, Milione M, Panzuto F. Endocrine tumours of the stomach. Best Pract Res Clin Gastroenterol. 2005;19(5):659–673. doi: 10.1016/j.bpg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Kokkola A, Sjoblom SM, Haapiainen R, et al. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scand J Gastroenterol. 1998;33:88–92. doi: 10.1080/00365529850166266. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy. 2003;35:203–206. doi: 10.1055/s-2003-37256. [DOI] [PubMed] [Google Scholar]
- 16.Modlin IM, Lye KD, Kidd M. Carcinoid tumors of the stomach. Surg Oncol. 2003;12:153–172. doi: 10.1016/S0960-7404(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 17.Fykse V, Sandvik AK, Qvigstad G, Falkmer SE, Syversen U, Waldum HL. Treatment of ECL cell carcinoids with octreotide LAR. Scand J Gastroenterol. 2004;39:621–628. doi: 10.1080/00365520410005225. [DOI] [PubMed] [Google Scholar]
- 18.Fykse V, Sandvik AK, Waldum HL. One-year follow-up study of patients with enterochromaffin-like cell carcinoids after treatment with octreotide long-acting release. Scand J Gastroenterol. 2005;40:1269–1274. doi: 10.1080/00365520510023684. [DOI] [PubMed] [Google Scholar]
- 19.Gilliam AD, Watson SA. G17DT: an antigastrin immunogen for the treatment of gastrointestinal malignancy. Expert Opin Biol Ther. 2007;7(3):397–404. doi: 10.1517/14712598.7.3.397. [DOI] [PubMed] [Google Scholar]
- 20.He AR, Marshall JL. Clinical experiences with G17DT in gastrointestinal malignancies. Expert Rev Anticancer Ther. 2006;6(4):487–492. doi: 10.1586/14737140.6.4.487. [DOI] [PubMed] [Google Scholar]