Abstract
Cytokines play a key role in the regulation of cells of the immune system and also have been implicated in the pathogenesis of malignant diseases. The aim of this study was to evaluate cytokine profiles in patients with differentiated thyroid cancer (DTC) before and 7 days after radioactive iodine (131-I) therapy. Cytokine levels were determined in supernatants obtained from phytohemagglutinin-stimulated whole blood cultures of 13 patients with DTC and 13 control subjects. The concentrations of selected cytokines: Th1—interferon gamma (IFN-γ), interleukin 2 (IL-2) and tumor necrosis factor alpha (TNF-α); Th2—interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 13 (IL-13) and interleukin 10 (IL-10); Th9—interleukin-9 (IL-9); and Th17—interleukin 17 (IL-17A) were measured using multiplex cytokine detection systems for Human Th1/Th2/Th9/Th17/Th22. We have shown that peripheral blood cells of DTC patients produce significantly higher concentrations of Th2/Th9 cytokines (IL-5, IL-13 and IL-9) than control subjects. The 131-I therapy led to reduced secretion of Th2 cytokines (IL-4, IL-5 and IL-13). Despite this, the calculated cytokine ratios (Th1/Th2) in DTC patients before and 7 days after 131-I therapy were not different from those in healthy subjects. DTC patients have significantly higher concentrations of Th2/Th9 cytokines (IL-5, IL-13 and IL-9) than control subjects. There is no influence of hypothyroidism or stage of disease on cytokine production in DTC patients before 131-I therapy. The radioactive 131-I therapy leads to reduced secretion of Th2 cytokines (IL-4, IL-5 and IL-13). Additional studies are needed to determine the significance of these findings.
Keywords: Cytokines, Differentiated thyroid cancer, Radioactive iodine therapy, Th1/Th2 ratio
Introduction
Thyroid cancer is the most common malignancy of the endocrine system, with an increasing incidence during the past 20 years [1]. Among thyroid malignancies, differentiated thyroid cancers (DTCs) are the most frequent (more than 90 %) and include papillary and follicular histological types [2]. Similarly to the thyroid cells from which they derive, DTC cells have the ability to concentrate iodine. This property enables administration of radioactive iodine (131-I) for ablation of remnant thyroid tissue and for treatment of iodine-avid metastases [3]. According to the European Association of Nuclear Medicine (EANM) guidelines, after surgery, some patients with DTC need to receive radioactive iodine 131 (131-I) therapy [4].
Cytokines play a key role in the regulation of cells of the immune system and also have been implicated in the pathogenesis of malignant diseases [5, 6]. Although most studies argue that secretion of T-helper-1 (Th1) cytokines is protective, while T-helper-2 (Th2) cytokines contribute to progression of malignancy [7, 8], there are other findings indicating a protective role for Th2 cytokines in anti-tumor immunity [9]. The concentrations of selected cytokines in patients with different types of cancers have been measured [10–13], but seldom in DTC patients [14, 15]. In one study, a Th2 or mixed type of response in tumor infiltrating and peripheral blood lymphocytes of patients with papillary thyroid carcinoma was shown [15]. Data regarding cytokine secretion in DTC patients treated with radioactive iodine are still rare. In one study, a significant increase of IL-6 two months after radioactive iodine therapy was found, while TNF-α level did not change after therapy [14]. The effect of radioactive iodine therapy on cytokine production in patients with hepatocellular carcinoma and Graves’ disease has been analyzed. In patients with hepatocellular carcinoma, iodine-125 implantation stimulated the anti-tumor immune response by promoting Th2/Th1 deviation [16]. Also, in patients with Graves’ disease treated with radioactive 131-I, a transient increase of Th2 cytokines (IL-4, IL-6 and IL-10) was obtained [17]. The aim of this study was to evaluate the cytokine profiles in DTC patients before and 7 days after 131-I therapy. Cytokine concentrations in patient’s sera do not only reflect the ability of the immune cells to produce cytokines, as the levels may be influenced by the presence of various inhibitors, such as soluble receptors, anti-cytokine antibodies and receptor antagonists [18], as well as products from cells outside the immune system [19], including thyroid cancer cells [20]. To assess the cytokine-producing ability of peripheral blood cells in DTC patients, as well as the possible influence of radioactive iodine therapy, cytokine concentrations were measured in phytohemagglutinin (PHA)-stimulated whole blood cultures in vitro. We analyzed the secretion of selected Th1 cytokines: interferon gamma (IFN-γ), interleukin 2 (IL-2) and tumor necrosis factor alpha (TNF-α); and Th2 cytokines: interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 13 (IL-13) and interleukin 10 (IL-10); the Th9 cytokine: interleukin 9 (IL-9) and the Th17 cytokine interleukin 17 (IL-17A). We showed that peripheral blood cells of DTC patients produce significantly higher amounts of Th2/Th9 cytokines (IL-5, IL-13 and IL-9) than control subjects. There was no influence of hypothyroidism or stage of disease on cytokine production in DTC patients before 131-I therapy. The 131-I therapy led to reduced secretion of Th2 cytokines (IL-4, IL-5 and IL-13). Despite this, the calculated cytokine ratios (Th1/Th2) in DTC patients before and 7 days after 131-I therapy were not different from those in healthy subjects.
Patients and methods
The study population included thirteen well-DTC patients (11 females and 2 males) of mean age 51.23 ± 14.9 years. Among them, ten (76.9 %) had papillary carcinomas, while three (23.1 %) had follicular carcinomas. All DTC patients included in this study underwent total thyroidectomy. Four to six weeks after surgery, and 10 days after a low-iodine diet, the patients were treated at the Nuclear Medicine Department of the Clinical Center, Kragujevac, according to the EANM guidelines [4], with fixed nominal activities of 3.7 GBq (100 mCi) of sodium (131-I)iodide administered orally. At the time of 131-I administration, all patients were hypothyroid after thyroid hormone withdrawal [concentration of thyroid-stimulating hormone (TSH) > 30 mIU/l]. None of the patients had acute infections, chronic inflammatory or autoimmune diseases or other conditions that could affect the tested parameters. The control group was composed of 13 healthy subjects (11 females and 2 males) of mean age 45.75 ± 12.89 years. The control subjects had no acute infectious, chronic inflammatory or autoimmune diseases. All control subjects were evaluated for thyroid function and thyroid antibodies. The mean concentration of TSH was 1.46 ± 0.72 mIU/l (range 0.4–3.5 mIU/l), and thyroid antibodies were negative. After venipuncture, blood samples (5 ml) from patients and control subjects were collected in tubes (vacutainer). Blood was taken from control subjects only once, while samples from DTC patients were obtained both before and 7 days after 131-I treatment.
The study was planned according to ethical guidelines following the Declaration of Helsinki. The institutional review committee approved our study protocol (number 01-5868) according to local biomedical research regulations. All patients and control subjects gave informed consent prior to enrollment in the study.
Whole blood culture
Whole blood (0.5 ml per subject) was added to 5 ml of RPMI-based complete medium containing fetal bovine serum, l-glutamine and PHA (GIBCO™ PB-MAX™ karyotyping medium, Invitrogen, CA, USA) and incubated at 37 °C for 72 h. The supernatant was harvested by centrifugation (1,400g for 12 min) and then stored at −20 °C until required.
Cytokine measurements
Cytokines were determined in supernatants obtained from whole blood cultures of DTC patients and controls. The supernatant samples were thawed and analyzed with the multiplex cytokine detection systems for Human Th1/Th2/Th9/Th17/Th22 13plex, FlowCytomix Multiplex (ebioscience Cat. no. BMS817FF) according to the manufacturer’s instructions. All samples were acquired and analyzed on a FC500 Beckman Coulter flow cytometer. Collected data were analyzed using FlowCytomix™ Pro Software.
Statistical analysis
The data were expressed as range (minimum–maximum), mean ± SD, and median values. The commercial SPSS version 10.0 for Windows was used for statistical analysis. Student’s t test was employed for comparison of paired samples. For nonparametric variables, differences between two independent groups were determined by the Mann–Whitney U test, while the Wilcoxon test was used for dependent groups. The observed variables were compared by the bivariate correlation test and determination of Pearson/Spearman coefficient. p values <0.05 were considered to be statistically significant.
Results
We analyzed the cytokine-producing ability of peripheral blood cells from thirteen control subjects and thirteen DTC patients before and 7 days after 131-I therapy. Cytokine concentrations were measured in the supernatants of 72-h PHA-stimulated whole blood cultures in vitro.
The mean leukocyte count in healthy controls was 6.44 ± 1.85 × 109/l (range 3.19–8.34 × 109/l); in DTC patients before 131-I therapy, it was 6.77 ± 1.02 × 109/l (range 5.6–8.2 × 109/l), and in DTC patients after 131-I therapy 5.72 ± 1.02 × 109/l (range 4.6–7.0 × 109/l). There was no significant difference in mean leukocyte counts between healthy controls and DTC patients before therapy, while the difference between DTC patients before and 7 days after therapy was significant (p < 0.001). Since the measured values of cytokines were corrected to 1,000 stimulated cells, whereby dilution was taken into account, the cytokine concentrations are expressed as pg/ml per 1,000 stimulated leukocytes. In this way, the influence of differences in the number of PHA-stimulated cells on cytokine concentrations was excluded.
Cytokine production in PHA-stimulated whole blood cultures of control subjects and DTC patients before and after 131-I therapy
The cytokine concentrations in supernatants from PHA-stimulated whole blood cultures of control subjects and DTC patients before and after 131-I therapy are shown in Table 1.
Table 1.
Cytokine concentrations in PHA-stimulated whole blood cultures of control subjects, DTC patients before and 7 days after 131-I therapy (p values indicate the significance of differences)
| Cytokine | Control subjects | DTC patients before 131-I therapy | DTC patients 7 days after 131-I therapy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range (minimum–maximum) (pg/ml) | X ± SD (pg/ml) | Median (pg/ml) | Range (minimum–maximum) (pg/ml) | X ± SD (pg/ml) | Median (pg/ml) | Range (minimum–maximum) (pg/ml) | X ± SD (pg/ml) | Median (pg/ml) | |
| IFN-γ | 430–58,040 | 22,940 ± 19,182 | 21,774 | 4,468–48,490 | 27,343 ± 15,691 | 28,821 | 4,386–54,190 | 30,150 ± 16,660 | 28,146 |
| IL-2 | 0.0–8,124 | 2,141 ± 3,014 | 694 | 447–10,893 | 4,322 ± 3,768 | 2,998 | 472–2,423 | 1,419 ± 656 | 1,308 |
| TNF-α | 0.0–849 | 336 ± 291 | 291 | 0.0–8,071 | 1,330 ± 2,732 | 448 | 194–734 | 360 ± 169 | 306 |
| IL-4 | 0.0–823 | 286 ± 293 | 288 | 181–1,134 | 494 ± 312d | 396 | 0.0–393 | 193 ± 170 | 240 |
| IL-5 | 0.0–2,412 | 869 ± 842a | 740 | 518–4,763 | 2,232 ± 1608e | 1,396 | 0.0–2,769 | 826 ± 1,135 | 350 |
| IL-13 | 0.0–1,300 | 460 ± 475b | 419 | 317–2,730 | 1,602 ± 954f | 1,783 | 450–1,666 | 709 ± 417 | 500 |
| IL-10 | 0.0–1,415 | 702 ± 513 | 738 | 144–5,390 | 1,319 ± 1,744 | 643 | 304–2,580 | 770 ± 778 | 492 |
| IL-9 | 0.0–142 | 42.4 ± 60.7c | 0 | 0.0–293 | 125.8 ± 87.3 | 122 | 0.0–226 | 89.1 ± 87.9 | 65 |
| IL-17 | 1,101–14,970 | 4,891 ± 4,365 | 4,075 | 976–14,983 | 7,743 ± 4,561 | 8,252 | 1,041–25,465 | 7,262 ± 8,259 | 3,596 |
aStatistical significant difference between DTC patients before therapy and control subjects, p = 0.046
bStatistical significant difference between DTC patients before therapy and control subjects, p = 0.012
cStatistical significant difference between DTC patients before therapy and control subjects, p = 0.038
dStatistical significant difference between DTC patients before and 7 days after therapy, p = 0.031
eStatistical significant difference between DTC patients before and 7 days after therapy, p = 0.038
fStatistical significant difference between DTC patients before and 7 days after therapy, p = 0.037
There were no statistically significant differences in the production of the Th1 cytokines, IFNγ, IL-2 and TNFα between 72-h PHA-stimulated whole blood cultures from DTC patients and controls. On the other hand, PHA-stimulated whole blood cultures from DTC patients had significantly higher concentrations of the Th2 cytokines: IL-5 and IL-13, than control subjects. Also, PHA-stimulated whole blood cultures of DTC patients produced significantly more IL-9. There were no significant differences in production of IL-4, IL-10 and IL-17A between cell cultures of DTC patients and controls.
The mean concentration of TSH in our DTC patients before 131-I therapy was 108.99 ± 81.13 mIU/l. There were no significant correlations between the serum concentrations of TSH and the following cytokines in whole blood culture: IL-13 (Pearson r = 0.209, p = 0.620), IL-5 (Pearson r = 0.242, p = 0.563) and IL-9 (Pearson r = 0.517, p = 0.190). Also, there were no statistically significant differences in concentrations of IL-13 (p = 0.737), IL-5 (p = 0.349) and IL-9 (p = 0.381) between the subgroup of patients with TSH values below 100 mIU/l (56.38 ± 23.80 mIU/l) and that with TSH values above 100 mIU/l (196.67 ± 58.80 mIU/l).
To examine the impact of stage of disease on the production of cytokines, DTC patients were divided into two subgroups: patients with nodal metastases (TxNxM0) and patients without any proven metastases (TxN0M0). The results showed that no significant differences in concentrations of IFN-γ (p = 0.924), IL-2 (p = 0.126), TNF-α (p = 0.528), IL-4 (p = 0.108), IL-5 (p = 0.120), IL-13 (p = 0.412), IL-10 (p = 0.307), IL-9 (p = 0.130) and IL-17 (p = 0.317) between the patients without and with metastases before 131-I therapy.
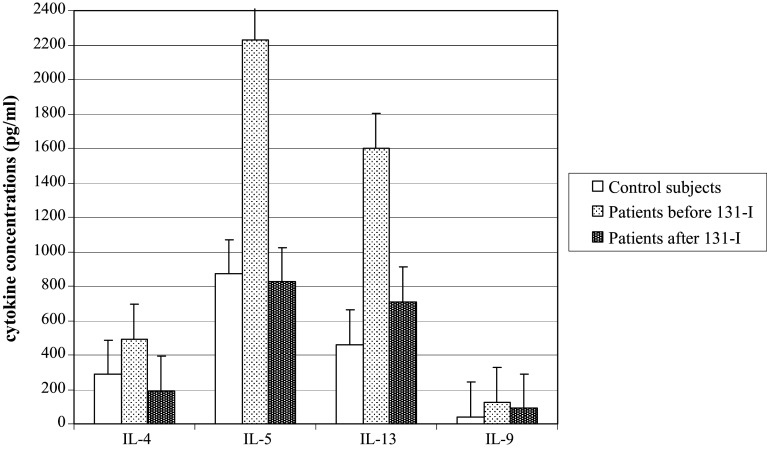
Cytokine concentrations in PHA-stimulated whole blood cultures of DTC patients 7 days after 131-I therapy were significantly less than those before therapy for IL-13, IL-4 and IL-5 (Table 1).
The concentrations of four cytokines for which statistically significant differences were found are shown in Fig. 1.
Fig. 1.
The concentrations of IL-4, IL-5, IL-13 and IL-9 in control subjects and DTC patients before and 7 days after 131-I therapy
Th1/Th2 cytokine ratios in PHA-stimulated whole blood cultures of control subjects and DTC patients before and 7 days after 131-I therapy
Table 2 summarizes the mean values of Th1/Th2 cytokine ratios (IFN-γ or TNF-α vs. IL-4, IL-5 or IL-10) calculated for each DTC patient (before and after 131-I therapy) and control subjects individually.
Table 2.
Mean values (X ± SD) of Th1/Th2 cytokine ratios for control subjects, DTC patient before and 7 days after 131-I therapy
| Th1/Th2 | Control subjects | DTC patients before 131-I therapy | DTC patients 7 days after 131-I therapy |
|---|---|---|---|
| X ± SD | X ± SD | X ± SD | |
| IFN-γ/IL-4 | 46.2 ± 46.4 | 66.6 ± 60.4 | 47.9 ± 53.6 |
| IFN-γ/IL-5 | 24.0 ± 21.9 | 17.9 ± 14.0 | 18.2 ± 21.3 |
| IFN-γ/IL-10 | 29.4 ± 22.8 | 43.4 ± 31.3 | 59.5 ± 39.2 |
| TNF-α/IL-4 | 0.67 ± 0.63 | 3.98 ± 8.66 | 0.84 ± 0.79 |
| TNF-α/IL-5 | 0.34 ± 0.27 | 0.45 ± 0.52 | 0.56 ± 1.01 |
| TNF-α/IL-10 | 0.48 ± 0.50 | 0.77 ± 0.54 | 0.64 ± 0.29 |
There were no significant differences between DTC patients before therapy and healthy controls for Th1/Th2 cytokine ratios (IFN-γ/IL-4, IFN-γ/IL-5, IFN-γ/IL-10, TNF-α/IL-4, TNF-α/IL-5 and TNF-α/IL-10). Also, there were no statistically significant changes in Th1/Th2 cytokine ratios (IFN-γ/IL-4, IFN-γ/IL-5, IFN-γ/IL-10, TNF-α/IL-4, TNF-α/IL-5 and TNF-α/IL-10) after treatment in any patient.
Discussion
The aim of this study was to evaluate the cytokine-producing ability of peripheral blood cells in DTC patients before and 7 days after 131-I therapy. Cytokine concentrations were measured in the supernatants of PHA-stimulated whole blood cultures in vitro. We showed that DTC patients produce significantly higher amounts of Th2/Th9 cytokines (IL-5, IL-13 and IL-9) than control subjects. Radioactive 131-I therapy led to reduced secretion of Th2 cytokines (IL-4, IL-5 and IL-13). Despite this, the Th1/Th2 ratios in DTC patients before and 7 days after 131-I therapy were not significantly different from those in healthy subjects.
It is generally accepted that Th1-type cytokines are critical for the induction and maintenance of anti-tumor cytotoxic T lymphocyte responses in vivo, while Th2-type cytokines may downregulate cell-mediated immunity, providing a microenvironment conducive to malignant disease progression [7, 8]. Cytokine concentrations, including the balance of Th1 and Th2 types, have been frequently studied in cancer patients [10–13, 21]. Cytokines were measured in patients’ sera [13], samples of tumor tissue [10, 11, 22], cells isolated from peritoneal washings [12] and blood cells stimulated in vitro [23].
Imbalances between Th1 and Th2 immune responses have been attributed to immune dysregulation in patients with malignancies [24, 25]. A Th2 bias was described in both metastatic melanoma and renal cell carcinoma [13, 26]. Patients with renal cell carcinoma with significantly increased Th2 type cytokine production do not develop an effective anti-tumor immune response, despite significant infiltration by lymphocytes [27]. These data suggest that the malignancy may play an active role in ‘‘reprogramming’’ systemic immunity toward Th2 dominance that may be permissive for tumor progression/metastases. In this study, we detected an increase of Th2 cytokines (IL-5 and IL-13) in PHA-stimulated whole blood cultures from DTC patients compared to those from healthy controls. In addition, there was also an increase in IL-9 concentration. This indicates that, in response to a nonspecific stimulus, peripheral blood cells of DTC patients are directed to produce more Th2/Th9 cytokines than controls. On the basis of our results, we cannot consider the cause or the potential significance of these findings. However, autocrine production of IL-4 and IL-10 by thyroid cancer cells has been shown earlier [20]. Both cytokines are known as inducers of a Th2 type response.
Another possibility is that the Th2 type of immune response in DTC patients before 131-I was induced as a part of the anti-tumor defense. While it is generally accepted that Th2 cytokines contribute to disease progression [7, 8], there are studies indicating a protective role for them in anti-tumor immunity [9]. Thus, particular components of the Th2-mediated immune response, such as IL-4 and eosinophils, can decrease tumor growth and initiate anti-tumor activity [28]. The basic biological activity of IL-5 is stimulation of eosinophil production in the bone marrow, as well as regulation of survival and activation of eosinophils [29]. Eosinophils have been found in peritumoral infiltrate from a number of tumors (colon carcinoma, planocellular carcinoma, lung adenocarcinoma and urinary bladder carcinoma) and are usually associated with a favorable prognosis (reviewed in references [28, 30]). In experimental animal models, it was shown that eosinophils activated by IL-5 suppress the growth of hepatocellular carcinoma in vivo and in vitro [31], and they can directly kill cells of genetically modified murine fibrosarcoma in vitro [32]. Furthermore, it was shown that tumor-specific CD4+ T lymphocytes with a Th2 cytokine profile can remove an established metastasis of melanoma in lungs, whereby eosinophil degranulation was induced in the tumor tissue [33].
Since two Th2 type cytokines, IL-13 and IL-4, have common and different receptors, their respective roles in tumor immunity are incompletely understood [9]. Recent findings suggest a negative influence of IL-4 on tumor growth [34–36], while data on the role of IL-13 are contradictory [37–39]. According to findings indicating an anti-tumor effect of IL-13, the effect was probably mediated by accumulation of neutrophils and macrophages [37, 39], although action of IL-13 through eosinophils could not be excluded [9].
IL-9 was first described as a Th2 cytokine, and many of its effects are similar to those of IL-4 [40]. In the presence of IL-4 and TGFβ, both Th0 and Th2 cells can differentiate into TH9 lymphocytes, which secrete IL-9 and IL-10. Besides TH9 lymphocytes, other subpopulations of T lymphocytes may secrete IL-9 under certain conditions. IL-9 exerts pleiotropic effects on CD4+ T cell subsets and influences the development and maintenance of effectors’ cells (reviewed in reference [40]). Thus, IL-9 regulates the development of inflammation and hyper-responsiveness in experimental autoimmune encephalomyelitis and rheumatoid arthritis [40]. Moreover, IL-9 may have a protective role in tumor immunity. In an experimental study, Purwar et al. [41] showed that abundant production of IL-9 suppressed the growth of melanoma cells in mice, while IL-9-blocking antibodies enhanced tumor growth.
Although numerous studies with animals [42, 43] and human subjects [44] have shown an influence of hypothyroidism on immune function, our results do not confirm that. Thus, statistical analysis excluded the association of any cytokine concentration with TSH values. This could be explained by differences in study design. Namely, we analyzed the selected cytokines in culture medium after stimulation with PHA in vitro. It is possible that strong mitogen stimulation in vitro prevents or significantly reduces the effect of hypothyroidism on cytokine production in blood cells. Given that our severely hypothyroid DTC patients produce more Th2/Th9 cytokines than controls, while production of cytokines Th1/Th17 is similar in both groups, we believe that the differences in cytokine profile were not caused by hypothyroidism.
Data regarding the cytokine secretion in DTC patients treated with radioactive iodine are very scarce. In a slightly different study design, Barsegian et al. [45] analyzed the production of pro-inflammatory cytokine IFN-γ and anti-inflammatory cytokine IL-10 in DTC patients treated with radioactive 131-I. Upon stimulation with microbial antigen at the single cell level (using ELISPOT assay), there was an increase of IFN-γ production in DTC patients 1 day after 131-I therapy with return to pretreatment values 7 days after therapy. Our results are in agreement with this. Namely, in both studies, the production of IFN-γ and IL-10 at day 7 after radioactive 131-I therapy was similar to the values before therapy.
In this study, we obtained a statistically significant decrease of Th2 cytokine production (IL-4, IL-5 and IL-13) in DTC patients 7 days after 131-I therapy. In patients with hepatocellular carcinoma, it was earlier shown that iodine-125 implantation stimulates the anti-tumor immune response by promoting Th2/Th1 deviation [16]. Also, in patients with Graves’ disease treated with radioactive 131-I, a transient increase of Th2 cytokines (IL-4, IL-6 and IL-10) with longer term increases in IFN-γ production was obtained [17]. In that study, the cytokine-producing cells were evaluated by ELISPOT assay. We believe that the results obtained in patients with Graves’ disease might be different from ours with DTC patients, due to differences in the inflammatory cells included in thyroid cancer and autoimmune thyroid diseases. The reduction of Th2 cytokine in DTC patients after 131-I therapy could be beneficial if it allows the development of a stronger Th1 type of response to the tumor.
Analysis of the Th1/Th2 cytokine ratios showed no statistically significant difference between DTC patients before 131-I therapy and controls. Moreover, in our study, no statistically significant difference of the Th1/Th2 cytokine ratios in DTC patients before and after 131-I therapy was detected. These results indicate that, in spite of induction of the Th2 type of response and cytokine secretion in DTC patients before 131-I therapy, there was no marked shift from the Th1 toward the Th2 type immune response. Moreover, radioactive iodine therapy did not change the Th1/Th2 cytokine ratio in DTC patients.
The advantages of this study are: a simple assay for detection of the cytokine-producing potential of blood cells, no cytokines secreted from other cells (e.g., keratinocytes, osteoblasts, endothelial cells, adipose tissue cells, smooth muscle cells and skeletal muscle cells) and no interference by inhibitory molecules present in the patient’s sera (soluble receptors, anti-cytokine antibodies, receptor antagonists). Despite the limitations of our investigation (small sample size, large individual variations of secreted cytokines, mitogen stimulation of blood cells in vitro), increased production of Th2/Th9 cytokines in patients with DTC before radioactive iodine therapy and reduced capacity of secretion of Th2 cytokines 7 days after 131-I therapy was demonstrated for the first time. As the role of the Th2/Th9 immune response in patients with tumors is still insufficiently clarified, new studies are necessary for evaluation of the role of IL-4, IL-5, IL-13 and IL-9 in tumor immune surveillance and tumor immunity during progression of malignancy.
In conclusion, DTC patients produce significantly higher concentrations of Th2/Th9 cytokines than control subjects, while Th1/Th17 cytokine levels did not differ from those in control subjects. There is no influence of hypothyroidism or stage of disease on cytokine production in DTC patients before 131-I therapy. Radioactive 131-I therapy leads to reduced secretion of Th2 cytokines. Additional studies are needed to determine the significance of these findings.
Acknowledgments
The study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant Nos. III41010 and ON175069).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- 131-I
Iodine 131
- DTC
Differentiated thyroid cancer
- EANM
European Association of Nuclear Medicine
- IFN-γ
Interferon gamma
- IL-2
Interleukin 2
- IL-4
Interleukin 4
- IL-5
Interleukin 5
- IL-9
Interleukin 9
- IL-10
Interleukin 10
- IL-13
Interleukin 13
- IL-17A
Interleukin 17A
- PHA
Phytohemagglutinin
- SD
Standard deviation
- Th1
T-helper-1
- Th2
T-helper-2
- Th9
T-helper-9
- Th17
T-helper-17
- TNF-α
Tumor necrosis factor alpha
- TSH
Thyroid-stimulating hormone
- TxN0M0
Patients without any proven metastases
- TxNxM0
Patients with nodal metastases
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger M, Sherman SI. Endocrine tumors: approach to the patient with advanced differentiated thyroid cancer. Eur J Endocrinol. 2012;166:5–11. doi: 10.1530/EJE-11-0631. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Luster M, Clarke SE, Dietlein M, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–1959. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
- 5.Punnonen R, Teisala K, Kuoppala T, et al. Cytokine production profiles in the peritoneal fluids of patients with malignant or benign gynecologic tumors. Cancer. 1998;83:788–796. doi: 10.1002/(SICI)1097-0142(19980815)83:4<788::AID-CNCR24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Clerici M, Shearer GM, Clerici E. Cytokine dysregulation in invasive cervical carcinoma and other human neoplasias: time to consider the TH1/TH2 paradigm. J Natl Cancer Inst. 1998;90:261–263. doi: 10.1093/jnci/90.4.261. [DOI] [PubMed] [Google Scholar]
- 7.Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197–1213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- 8.Bodelon C, Polley MY, Kemp TJ, et al. Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann Oncol. 2013;24:2073–2079. doi: 10.1093/annonc/mdt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70(1):1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 10.Baier PK, Wolff-Vorbeck G, Eggstein S, et al. Cytokine expression in colon carcinoma. Anticancer Res. 2005;25:2135–2139. [PubMed] [Google Scholar]
- 11.Mocellin S, Provenzano M, Rossi CR, et al. Use of quantitative real-time PCR to determine immune cell density and cytokine gene profile in the tumor microenvironment. J Immunol Methods. 2003;280:1–11. doi: 10.1016/S0022-1759(03)00274-6. [DOI] [PubMed] [Google Scholar]
- 12.Ikeguchi M, Matsumoto S, Murakami D, et al. Gene expression levels of cytokines in peritoneal washings from patients with gastric cancer. Tumour Biol. 2004;25:117–121. doi: 10.1159/000079143. [DOI] [PubMed] [Google Scholar]
- 13.Montero AJ, Diaz-Montero CM, Millikan RE, et al. Cytokines and angiogenic factors in patients with metastatic renal cell carcinoma treated with interferon: association of pretreatment serum levels with survival. Ann Oncol. 2009;20:1682–1687. doi: 10.1093/annonc/mdp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Özata M, Ergun H, Öziflik G, et al. Effect of radioiodine therapy on several hematological and immune parameters in patients with differentiated thyroid carcinoma. Turk J Endocrinol Metab. 2000;2:45–50. [Google Scholar]
- 15.Mardente S, Lenti L, Lococo E, et al. Phenotypic and functional characterization of lymphocytes in autoimmune thyroiditis and in papillary carcinoma. Anticancer Res. 2005;25:2483–2488. [PubMed] [Google Scholar]
- 16.Xiang GA, Chen KY, Wang NH, et al. Immunological influence of iodine-125 implantation in patients with hepatocellular carcinoma resection. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:292–294. [PubMed] [Google Scholar]
- 17.Jones BM, Kwok CCH, Kung AWC. Effect of radioactive iodine therapy on cytokine production in Graves’ disease: transient increases in interleukin-4 (IL-4), IL-6, IL-10, and tumor necrosis factor-α, with longer term increases in interferon-γ production. J Clin Endocrinol Metab. 1999;84:4106–4110. doi: 10.1210/jcem.84.11.6128. [DOI] [PubMed] [Google Scholar]
- 18.Heney D, Whicher JT. Factors affecting the measurement of cytokines in biological fluids: implications for their clinical measurement. Ann Clin Biochem. 1995;32:358–368. doi: 10.1177/000456329503200402. [DOI] [PubMed] [Google Scholar]
- 19.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/S0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 20.Stassi G, Todaro M, Zerilli M, et al. Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res. 2003;63:6784–6790. [PubMed] [Google Scholar]
- 21.Bais AG, Beckmann I, Lindemans J, et al. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. 2005;58:1096–1100. doi: 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulbe H, Chakravarty P, Leinster DA, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duramad P, McMahon CW, Hubbard A, et al. Flow cytometric detection of intracellular Th1/Th2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol Biomark Prev. 2004;13:1452–1458. [PubMed] [Google Scholar]
- 24.Ito N, Nakamura H, Metsugi H, et al. Dissociation between T helper type 1 and type 2 differentiation and cytokine production in tumor-infiltrating lymphocytes in patients with lung cancer. Surg Today. 2001;31:390–394. doi: 10.1007/s005950170127. [DOI] [PubMed] [Google Scholar]
- 25.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nevala WK, Vachon CM, Leontovich AA, et al. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15:1931–1939. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyth GP, Stapleton PP, Barden CB, et al. Renal cell carcinoma induces prostaglandin E2 and T-helper type 2 cytokine production in peripheral blood mononuclear cells. Ann Surg Oncol. 2003;10:455–462. doi: 10.1245/ASO.2003.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 29.Fujisawa T, Terada A, Atsuta J, et al. IL-5 as a strong secretagogue for human eosinophils. Int Arch Allergy Immunol. 1997;114:81–83. doi: 10.1159/000237726. [DOI] [PubMed] [Google Scholar]
- 30.Gatault S, Legrand F, Delbeke M, et al. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61:1527–1534. doi: 10.1007/s00262-012-1288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kataoka S, Konishi Y, Nishio Y, et al. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004;23:549–560. doi: 10.1089/dna.2004.23.549. [DOI] [PubMed] [Google Scholar]
- 32.Simson L, Ellyard JI, Dent LA, et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 33.Mattes J, Hulett M, Xie W, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modesti A, Masuelli L, Modica A, et al. Ultrastructural evidence of the mechanisms responsible for interleukin-4-activated rejection of a spontaneous murine adenocarcinoma. Int J Cancer. 1993;53:988–993. doi: 10.1002/ijc.2910530622. [DOI] [PubMed] [Google Scholar]
- 35.Musiani P, Allione A, Modica A, et al. Role of neutrophils and lymphocytes in inhibition of a mouse mammary adenocarcinoma engineered to release IL-2, IL-4, IL-7, IL-10, IFN-alpha, IFN-gamma, and TNF-alpha. Lab Invest. 1996;74:146–157. [PubMed] [Google Scholar]
- 36.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 37.Lebel-Binay S, Laguerre B, Quintin-Colonna F, et al. Experimental gene therapy of cancer using tumor cells engineered to secrete interleukin-13. Eur J Immunol. 1995;25:2340–2348. doi: 10.1002/eji.1830250833. [DOI] [PubMed] [Google Scholar]
- 38.Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 39.Ma HL, Whitters MJ, Jacobson BA, et al. Tumor cells secreting IL-13 but not IL-13Ralpha2 fusion protein have reduced тumorigenicity in vivo. Int Immunol. 2004;16:1009–1017. doi: 10.1093/intimm/dxh105. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Rostami A. IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neuroimmune Pharmacol. 2010;5:198–209. doi: 10.1007/s11481-009-9186-y. [DOI] [PubMed] [Google Scholar]
- 41.Purwar R, Schlapbach C, Xiao S, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–1253. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valli E, Sterle HA, Méndez-Huergo S, et al. Regulatory T cells mediate immunosupression induced by experimental hypothyroidism (P1215) J Immunol. 2013;190:188.10. [Google Scholar]
- 43.Nieto P, Peñaloza H, Salazar F, et al. Gestational hypothyroidism in mice makes female offspring more resistant to pneumococcal pneumonia (MUC5P.869) J Immunol. 2014;192:134.12. [Google Scholar]
- 44.Botella-Carretero JI, Prados A, Manzano L, et al. The effects of thyroid hormones on circulating markers of cell-mediated immune response, as studied in patients with differentiated thyroid carcinoma before and during thyroxine withdrawal. Eur J Endocrinol. 2005;153:223–230. doi: 10.1530/eje.1.01951. [DOI] [PubMed] [Google Scholar]
- 45.Barsegian V, Müller SP, Horn PA, et al. Lymphocyte function following radioiodine therapy in patients with thyroid carcinoma. Nuklearmedizin. 2011;50:195–203. doi: 10.3413/nukmed-04241108. [DOI] [PubMed] [Google Scholar]