Abstract
Intra-tumor injection of immunotherapeutic agents is often the most effective, likely because of concomitant modification of tumor microenvironment. We tested an immunotherapeutic regimen consisting of CpG oligonucleotides and of adenovirus-mediated gene delivery of CCL16 chemokine directly into orthotopically implanted prostate tumors by ultrasound-guided injection, followed by systemic administration of an anti-IL-10R antibody. This combination treatment induced rapid stromal rearrangement, characterized by massive leukocyte infiltration and large areas of necrosis, a scenario that eventually led to complete tumor rejection and systemic immunity in 75 % of the treated mice. In vivo T lymphocyte depletion experiments demonstrated that the efficacy of CCL16/CpG/anti-IL-10R combination treatment relies upon CD8 T lymphocytes whereas CD4 T cells are dispensable. The results underlie the feasibility of echo-guided local immunotherapy of tumors located in visceral organs that are not easily accessible.
Keywords: Local cancer immunotherapy, Ultrasound-guided immunotherapy, Combination treatment, CpG oligonucleotides, CCL-16, Prostate tumor
Introduction
The failure of most immunotherapeutic approaches against established tumors is mainly due to unfavorable local environment dominated by immunosuppression [1].
The local delivery of cytokines can change the number and the phenotype of infiltrating immune cells, but when given as single agents, they generally fail to change tumor outcome [2]. However, the combination of vaccines, cytokines or chemokines with CpG oligonucleotides or other Toll-like receptor agonists is quite effective in several experimental models and could find clinical application [3]. Chemokines delivered as coding cDNAs ensure substantial local production, and adenoviral vectors are the most used because of transduction efficacy and transient, but elevated, transgene expression [4–6]. This approach that requires tumor accessibility for local injection has not been effectively tested in case of deep/visceral tumors being most preclinical works done by using subcutaneously implanted tumors [7].
To mimic visceral growing tumors, we established a new tumor cell line from an ex vivo transgenic adenocarcinoma of the mouse prostate (TRAMP) that is able to grow both subcutaneously and orthotopically in the prostate of syngeneic mice [8]. The tumor mass growing in the prostate can be evaluated by ultrasound in vivo imaging since early time points after tumor injection, when tumor diameter is still very small. These growing tumors can be treated locally by ultrasound-guided injection of adenoviral vectors in combination or not with CpG oligonucleotides. We show here that adenoviral-vector-mediated local delivery of the CCL16 chemokine, together with CpG oligonucleotides and systemic injection of anti-IL-10 receptor mAb, cures established orthotopically implanted prostate tumors and induces specific immunity to SV40 large T oncogene in line with the idea that local immunotherapy could be amenable to treat prostate tumors [9].
Materials and methods
Tumor cells and adenovirus production
The murine prostate adenocarcinoma cell line T1525p was derived from a TRAMP mouse and has been previously described [8]. Cells were cultured in DMEM with 10 % FBS, supplemented with penicillin, streptomycin and l-glutamine (Biowhittaker). HEK293 are human embryo kidney cells transformed by shared adenovirus type 5 DNA, used to complement the growth of E1-defective adenoviral vectors [10]. HEK293 cells were cultured in EMEM (Biowhittaker) supplemented as described above. T23 cell line, used in in vitro cytotoxic assay, was similarly derived and maintained [8].
Adenoviral vector coding for CCL16 was produced and purified as previously described [7].
Mice and in vivo procedures
Eight- to ten-week-old male C57BL/6J mice were the transgenic negative siblings of TRAMP mice (originally purchased from The Jackson Laboratory) from our colony. Animals were maintained in filtered top cages at the Fondazione IRCCS Istituto Nazionale Tumori, Milano. All procedures involving animals were approved by the Institute Ethical Committee and performed in accordance with institutional guidelines and national law (DL116/92).
Orthotopic tumor implants were obtained injecting 2 × 106 T1525p cells into the anterior lobe of surgically exposed prostate. Beginning 10 days after tumor implant, mice were evaluated for tumor growth by ultrasound in vivo imaging (Vevo™ 770, VisualSonics); when tumors reached the volume of 30–50 mm3, echography was used to guide the injection of local treatments. Treatment consisted of 1 × 109 pfu adenoviral particles carrying the CCL16 cDNA (AdCCL16) or the empty vector as control (Addl70-3) with or without 5 μg of CpG 1668 (MWG), also given as single agent for control. Some mice also received or not 200 μg anti-IL10R antibody (1B1.3a; IgG1) intraperitoneally [11]. Treatment was repeated once 10 days apart.
For in vivo depletion of CD4+ and CD8+ cells, 5-week-old mice were injected i.p. with 500 μg/mouse of anti-CD4 (GK1.5, Lyt2; ATCC) or anti-CD8 (clone 2.43; ATCC) mAb 3 days before the first and the second treatment. FACS analysis of peripheral blood confirmed that depletion was never inferior to 95 %.
Tumor growth was monitored weekly and expressed as percentage of tumor-free mice over total injected mice; tumor size was measured by in vivo imaging with Vevo instrument using VisualSonics software and expressed in mm3.
Histology, immunohistochemistry and immunofluorescence
For histological evaluation, tissue samples were fixed in 10 % neutral buffered formalin, embedded in paraffin, sectioned at 4 μm and stained with hematoxylin and eosin. Toluidine blue staining was performed on treated and untreated tumors to evaluate the presence of mast cells.
For immunofluorescence analysis, tissue sections were incubated, by sequential immunostaining, with primary anti-mouse CD68 (Abcam) and anti-mouse IL10 (eBioscience) antibodies. After Fc blocking, binding of the primary antibodies was revealed by Alexa 568- and Alexa 488 Fluor-conjugated secondary antibodies (Invitrogen). Nuclear staining was performed by incubation with DAPI (4′,6-diamidino-2-phenylindole, Invitrogen). Slides were evaluated using a Leica DM2000 microscope equipped with a Leica DFC320 digital camera.
FACS analysis of leukocyte infiltration
Tumor masses were collected at the specific time points and digested with collagenase/hyaluronidase solution (Stem Cell Technologies). Cell suspension after extensive washing and filtering with 70-μm cell strainer (BD) was stained with the following antibodies for multicolor cytofluorimetric analysis: CD45.2-APC-Alexa780, CD11b-PE, CD4-PeCy7 and CD8-PerCP5.5. Samples were acquired using a BD Fortessa FACS instrument and analyzed with FlowJo software (TreeStar). All samples were analyzed in single; in each experiment, at least four samples were analyzed for each group.
Evaluation of CD8+ T lymphocyte response
Splenocytes (5 × 105 cells/ml) from treated and vaccinated mice were restimulated in vitro with irradiated T1525P cells or H2-Kb peptide Tag IV (1 μg/ml; Primm) [12] and tested, 5 days later, for cytotoxic activity in a standard 4-h 51Cr release assay as previously reported [13]. Concanavalin A-stimulated syngeneic splenocytes pulsed or not with Tag IV peptide were used as targets.
To evaluate degranulation by activated CD8+ T cells, the expression of CD107a on the cell surface was evaluated upon a 5-h in vitro boost with H2-Kb peptide Tag IV in the presence of Alexa Fluor 647-conjugated anti-CD107a mAb (clone: 1D4B, eBioscience), IL-2 (20 IU/ml, Peprotech) and the secretion inhibitor monensin (BD) as described [14]. Then, cells were stained with mAb to CD8 (FITC-conjugated: clone 53–6.7, eBioscience) and for intracellular IFNγ (PE-conjugated, clone: XMG1.2, eBioscience) and analyzed in flow cytometry (Fortessa, BD) gating on CD8+ cells to show the double-positive CD107a+ IFNγ + cytotoxic activated lymphocytes. For these experiments, lymphocytes were obtained from spleens and tumors of treated and untreated mice, using Ficoll-Hypaque (Sigma Aldrich) density centrifugation, 5 days after the second treatment.
Statistical analysis
Data were analyzed using Student’s t or Mann–Whitney test using Prism software (GraphPad Software). Differences were considered significant at p < 0.05.
Results
Combinatory treatment of CCL16, CpG oligonucleotides and anti-IL-10R antibody cures orthotopic prostate tumors
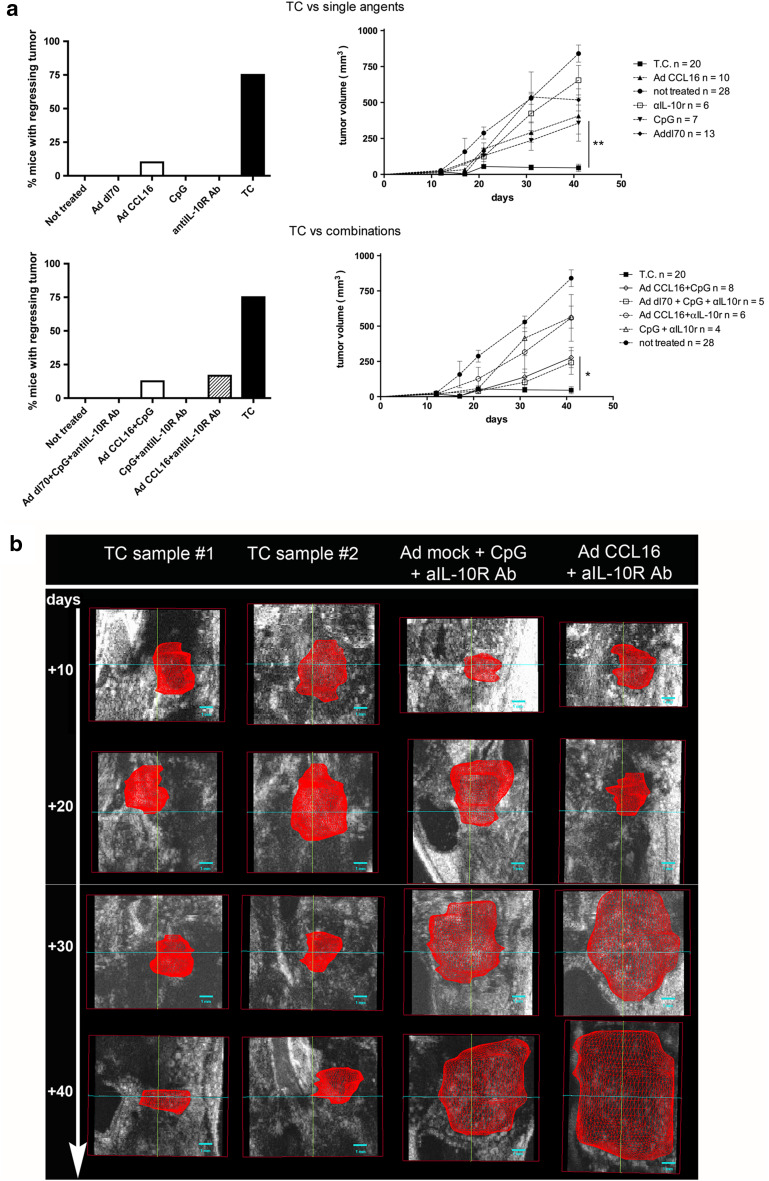
We aimed to test whether a treatment regimen that successfully cured established mammary carcinomas subcutaneously implanted [7] was also efficient in case of tumors located in visceral organs, such as prostate cancer. Therapeutic treatment consists of local injection of adenoviral vector carrying the CCL16 cDNA and of CpG oligonucleotides, followed by systemic IL-10 neutralization by means of anti-IL-10R mAb [11]. A new TRAMP cell line (T1525p) has been established that grows within the prostate of C57BL/6 mice retaining the expression of SV40 large T antigen and adenocarcinoma characters [8]. The orthotopic surgical implant of T1525p cells into the prostate anterior lobe was followed for tumor growth by ultrasound inspection. When tumor reached the size of 30–50 mm3, ultrasound-guided injection of adenovirus and CpG or controls was administered and repeated once 10 days apart. The triple combination of CCL16, CpG oligonucleotides and anti-IL-10R mAb regimen was superior to any single or dual treatment in inducing tumor rejection. Figure 1 shows the cumulative data of 15 out of 20 mice with regressing tumors after treatment with the triple combination (TC) versus single agents (Fig. 1a upper panels) or versus double combinations (Fig. 1a lower panels). Mice with regressing tumor became completely tumor free in 15–20 days and remained as such for the whole observation period, at least 80–100 days. All double combinations between CCL16 and CpG or anti-IL-10R mAb resulted in delayed size enlargement of tumors that eventually steadily progressed (Fig. 1b right panel). Representative ultrasound images show tumor regression beginning after the second injection of the triple combination while steadily progressing in mice treated with control adenoviral vector + CpG + anti-IL-10R mAb or with dual AdCCL16 + anti-IL-10R mAb treatment (Fig. 1b).
Fig. 1.
Combinatory CCL16/CpG +anti-IL-10R mAb treatment cures mice from orthotopic prostate tumors. a Left panels percentage of tumor-free mice after intra-prostatic T1525p tumor cell injection and subsequent treatment (TC combination, untreated control and single-agent comparison is shown in the upper panel, and TC combination, untreated control and other combination treatments in the lower panel); difference in percentage of mice with regressing tumor between TC and all the other treatments is statistically significant: p < 0.005; right panels: mean tumor volume from the same samples; cumulative results of two to three independent experiments are shown; the total number of mice is indicated in the right panels. Mice with regressing tumor became completely tumor free in 15–20 days and remained as such for the whole observation period, at least 80–100 days. b Representative ventral visions of 3-D rendering volumes from ultrasound images of tumors at the time of second (+10 days) treatment and their follow-up to day 40. All mice were injected intra-prostate on day 0 with 2 × 106 T1525p cells, and 10 days later, the tumor injected locally with the aid of ultrasound-guide with the indicated agents in combination or not. Examples from two TC-treated mice and from one mouse treated with control adenoviral vector + CpG + IL-10R mAb and one treated with AdCCL16 + IL-10R mAb. Red zone indicates the tumor volume
Triple-combination treatment induces leukocyte infiltration and massive necrosis at tumor site
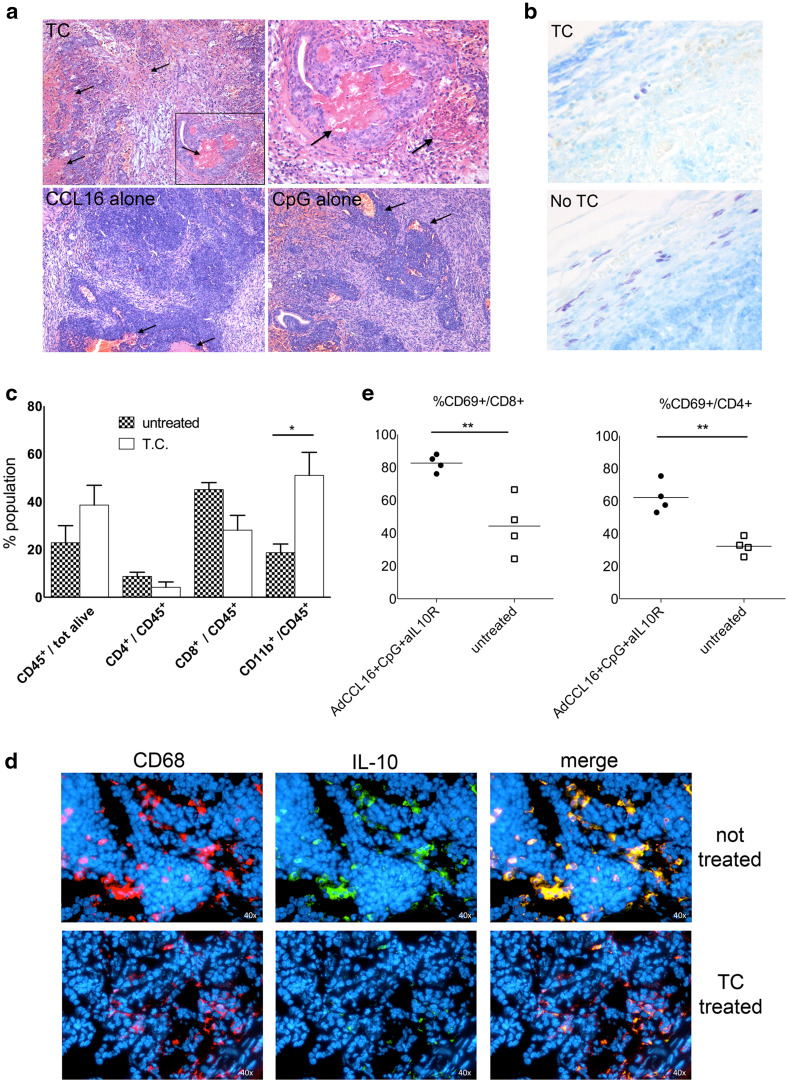
In order to investigate the mechanisms responsible for tumor rejection after TC local treatment, we firstly analyzed histologically tumor samples at early time points after the first treatment (48 h). Hematoxylin/eosin staining highlighted stromal rearrangement and massive hemorrhagic necrosis occurring in TC-treated tumors (Fig. 2a) that are absent in untreated tumors (not shown) and very limited after single- or dual-agent treatments (Fig. 2a). Additional evidence of stromal reshuffle and tumor shrinkage came from observing the complete disappearance of mast cells (Fig. 2b) that normally surround the prostate adenocarcinoma in response to tumor-produced stem cell factor [8]. Since CCL16 chemokine is chemotactic for both monocytes and lymphocytes, we next evaluated leukocyte infiltration at tumor site at the same early time point after TC treatment and found a general increase in CD45+ cells in treated tumors, with significant higher number of CD11b+ cells (Fig. 2c). To investigate the phenotype of infiltrating monocytic cells, we performed immunofluorescence analysis of IL-10 expression as marker of a M2/pro-tumoral phenotype: whereas in untreated tumors we observed a very nice colocalization of CD68-stained monocytic cells and IL-10 expression, in TC-treated tumors CD68+-infiltrating cells only rarely colocalize with IL-10 staining that is overall much less evident (Fig. 2d), suggesting a skewing toward a less pro-tumorigenic and immunosuppressive microenvironment.
Fig. 2.
Leukocyte recruitment and massive necrosis characterize regressing tumors soon after combinatory treatment. a Histological analysis of tumor site at early time point after first treatment (48 h); hematoxylin/eosin staining for triple-combination treatment and single-agent treatment; black arrows indicate areas of necrosis; upper right panel shows an enlargement of image on the left (magnification ×10 and ×40 for the inset). b Representative toluidine staining of tumor samples at 48 h after first triple-combination treatment (upper panel) and untreated tumors (lower panel) showing mast cell infiltration (magnification ×40). c FACS analysis of leukocyte population in tumor samples at 48 h after first treatment of triple-combination therapy in comparison with untreated tumors. Mean of four tumors per group is shown; the experiment repeated twice. Antibodies for CD45, CD4, CD8 and CD11b have been used, and gating on CD45+ cells has been done to evaluate the percentage of the different leukocyte populations. d Representative double immunofluorescence staining of tumors 48 h after first triple-combination treatment (lower panels) and untreated tumors (upper panels) showing colocalization of CD68+ cells with IL-10 expression. Single-channel and merged images are shown. e FACS analysis of the activation state of CD8 and CD4 T cells in tumor samples at 48 h after first treatment of triple-combination therapy in comparison with untreated tumors. Percentage of CD69+ cells is done gating cells on CD8 and CD4 cells. For each analysis, at least four mice per group have been analyzed, and the experiment repeated three times
No significant numeric difference was detected for both CD8 and CD4 T cells (Fig. 2b); however, when lymphocytes were analyzed for the expression of CD69, a marker of T cell activation, we observed a significant increase in CD69+ cells in both CD8 and CD4 cells in TC-treated tumors (Fig. 2e), suggesting the activation of an adaptive immune response.
CD8 T cells are required for efficient tumor rejection
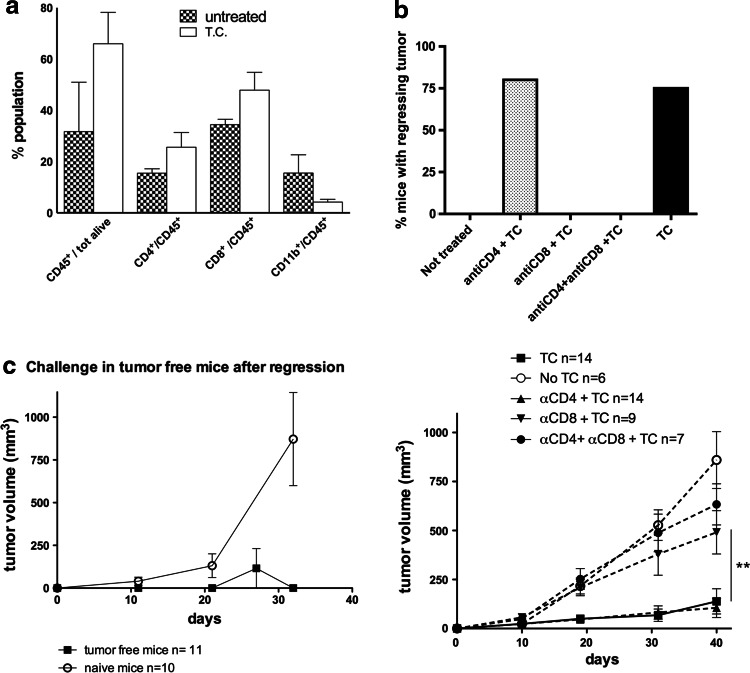
The analysis of leukocyte infiltration at later time points, 5 days after the second treatment, confirmed the higher number of overall CD45+ leukocytes and highlighted an increase, although not statistically significant, in both CD8 and CD4 T cells (Fig. 3a). To test whether adaptive immunity was required for complete tumor rejection, CD4 and/or CD8 T cells were depleted before therapeutic treatment. CD8 but not CD4 T cell depletion impaired tumor rejection: none of nine CD8 T cell-depleted mice showed regressing tumor after TC treatment, whereas 11 out of 14 CD4 T cell-depleted mice became tumor free (Fig. 3b). In addition, mice rendered tumor free by TC therapy, but not naïve mice, rejected a subsequent challenge with a tumorigenic dose of T1525p cells given subcutaneously (Fig. 3c), indicating the development of systemic immunity.
Fig. 3.
CD8 T lymphocytes are required for efficient therapeutic effect of triple-combination treatment. a FACS analysis of leukocyte population infiltrating tumor samples from triple-combination treatment in comparison with untreated tumors at 5 days after second therapeutic treatment. Mean of four tumors per group is shown; the experiment repeated twice. Antibodies for CD45, CD4, CD8 and CD11b have been used, and gating on CD45+ cells has been done to evaluate the percentage of the different leukocyte populations. b Upper panel percentage of tumor-free mice after intra-prostatic T1525p tumor cell injection and subsequent triple-combination treatment in immune-competent mice in comparison with CD4, CD8 or CD8 + CD4 T cell-depleted mice. T cell depletion has been performed prior to first therapeutic treatment and efficient depletion (>95 %) evaluated by FACS analysis with CD4- and CD8-specific antibodies; difference in percentage of mice with regressing tumor between TC and all the other treatment is statistically significant (p < 0.005), but for CD4-depleted mice. Mice with regressing tumor became completely tumor free in 15–20 days and remained as such for the whole observation period, at least 80–100 days. Lower panel mean tumor volume from the same samples; cumulative results of two independent experiments are shown, and the total number of mice is indicated in the figure. c growth of T1525p tumors injected in tumor-free mice after triple-combinatory therapy and in naïve mice as controls; mean tumor volume is shown; cumulative results of three independent experiments are shown, and the total number of mice is indicated in the figure
Triple-combination treatment induces the expansion of tumor antigen-specific cytotoxic CD8+ T cells
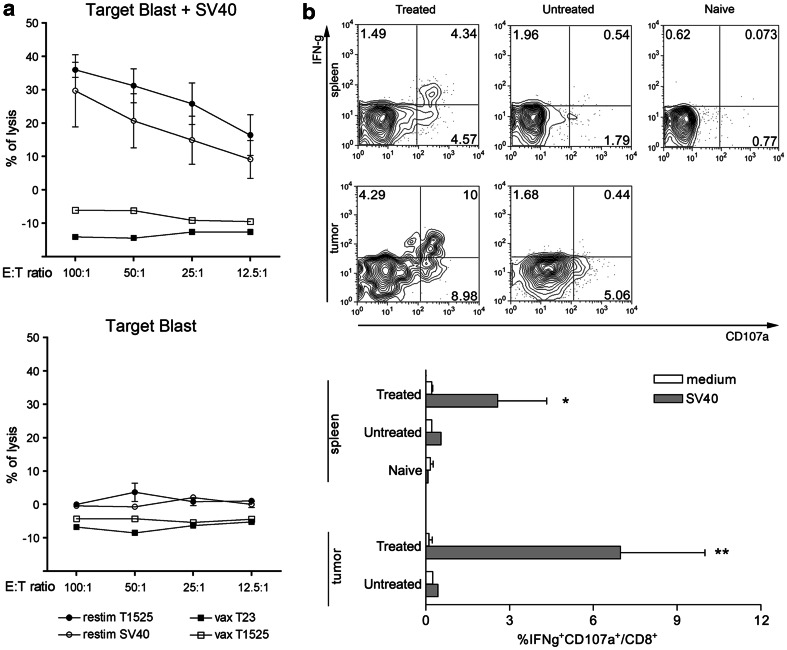
In the presence of memory response detected using the transplantation assay, we tested whether cytotoxic T cells directed against the known SV40 Tag immunodominant antigen peptide IV were involved. Splenocytes from mice showing regressing tumor, because of treatment with the triple combination, were isolated 5 days after the end of treatment and tested in vitro for lytic activity against SV40 Tag peptide-pulsed blast cells upon restimulation in vitro with T1525p cells or SV40 Tag peptide. As shown in Fig. 4a, splenocytes from TC-treated mice with regressing tumor were able to lyse SV40 Tag peptide-pulsed blast cells. Of note, the immunogenicity of regressing tumors was not matched by repeated immunizations with irradiated SV40 Tag-expressing T1525p or T23 prostate tumor cells that indeed were not able to mount any cytotoxic T cell response. In vitro cytotoxic activity of splenocytes from TC-treated mice correlated with in vivo expansion of CD107a+ cells producing IFNγ isolated from both the tumor microenvironment and the spleen of mice with regressing tumor (Fig. 4b).
Fig. 4.
CCL16/CpG + anti-IL-10R mAb combination treatment induces CD8 cytotoxic T cells. a In vitro cytotoxic assay of splenocytes from tumor-free mice after triple-combination treatment. In vitro restimulation has been done for 5 days with either T1525p cells (filled circle) or SV40 Tag IV peptide (open circle) and tested for CTL activity against 51Cr-labeled syngeneic blast cells pulsed (upper panel) or not (lower panel) with SV40 Tag IV peptide. As controls, splenocytes from mice immunized three times with irradiated T1525p cells (open square) or the unrelated TRAMP tumor cell line T23 (filled square). Splenocytes from a pool of three mice per group have been used, and the experiment repeated twice. b FACS analysis of IFNγ production by CD8 T cells obtained from spleen and tumors of treated and untreated mice 5 days after the end of therapy. Ex vivo CD8 T cells are then induced in vitro to degranulate in response to a short stimulation with SV40 peptide. Upper panel representative plots showing the concomitant expression of IFNγ and CD107a; lower panel shows histograms of the percentage of IFNγ−producing CD107a+ CD8+ T cells. Mean percentage from three mice is shown; experiment has been repeated twice; naïve splenocytes have been used as controls
Discussion
In the previous works we have demonstrated that a combination of immunostimulatory treatments consisting of the CCL16 chemokine and CpG oligonucleotides given intra-lesionally, together with systemic IL-10 inhibition via IL-10R-blocking antibody, is very effective in inducing tumor rejection through the concerted action of innate and adaptive immune response. Indeed, this combination can redirect tumor-infiltrating macrophages from M2 to M1 phenotype and restore tumor-associated DC function toward CTL response [11]. Here, we have extended the application of this approach to visceral tumors taking advantage of ultrasound-guided injection of the therapeutic treatment to reach tumor masses not easily accessible as located in deep organs and demonstrated the feasibility and efficacy of such strategy to induce local tumor rejection and systemic immunity also in this setting.
Chemokines, because of their ability to recruit different leukocytic populations and of their immune-modulatory properties, have been widely considered for the development of immunotherapeutic approaches. However, such molecules, when used as single agent, are unable to exert a curative effect because of their inability to overcome the immunosuppression that develops both locally at the tumor site and systemically [15]. From these observations, it comes the rationale of associating chemokines with other immunostimulatory molecules in order to revert the effect of tumor-induced immunosuppression. In the specific case of CCL16, we have previously shown that its local delivery through adenoviral vectors is able to recruit at tumor site F4/80+ macrophages and CD11c+ dendritic cells (DC) that, however, are characterized by the production of IL-10, indicating that although CCL16 is chemotactic for macrophages and DC, these newly infiltrating cells acquire the character of tumor-resident leukocytes [11]. The addition of local delivery of CpG and the systemic treatment with anti-IL-10R antibody are able to change their phenotype toward a lower production of IL-10 and enhanced production of IL-12. Similarly, in the present study, we show that the triple-combination treatment is able to reduce IL-10 production by infiltrating monocytes/macrophages rendering the tumor microenvironment more permissive for the development of a tumor-specific adaptive immune response. Indeed, both CD8 and CD4 T cells at the tumor site show an activated phenotype few days after the therapeutic treatment, and CD8 T cells isolated from the spleen of mice with regressing tumor are capable of tumor antigen-specific cytotoxic activity and secretion of IFNγ. As for our previous models of breast and colon carcinomas [11], also in the case of orthotopic prostate cancer, the efficacy of the combinatory treatment relies, after the initial innate response, upon the development of tumor-specific adaptive immune responses. Indeed, if mice are depleted of CD8 T cells before the administration of the therapy, the antitumor effect is impaired and after an initial slowdown of tumor growth probably due to the early hemorrhagic necrosis, tumor masses grow similarly to untreated control tumors.
The efficiency of combinatory local delivery of different molecules that, similarly to ours, can act synergistically to activate or redirect several antitumor immune effectors has recently been showed also by a paper from Levy’s group in which the local injection at the tumor site of CpG combined with two antibodies (anti-CTLA-4 and OX-40) was able to eradicate a subcutaneous tumor and induce systemic immunity [16]. Whereas local therapy of subcutaneous tumor masses is easily practicable, much less feasible is the in situ treatment of visceral tumors, located in deep, not easily accessible, organs. The subcutaneous pad is a surrogate for many organs where tumors commonly arise and therefore poorly represents the tissue microenvironment that develops together with the incipient tumor and their reciprocal interactions. Here, we have applied in vivo imaging and ultrasound-guided access to orthotopic prostate tumors to demonstrate the feasibility and therapeutic efficiency of local injection of selected immunostimulatory molecules for tumors located in deep organs.
Acknowledgments
This work has been supported by Italian Association for Cancer Research (AIRC) IG Project 10137 and Fondazione Italo Monzino Prostate Program.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 3.Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, et al. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:894–907. doi: 10.4161/onci.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melero I, Gabari I, Tirapu I, Arina A, Mazzolini G, et al. Anti-ICAM-2 monoclonal antibody synergizes with intratumor gene transfer of interleukin-12 inhibiting activation-induced T-cell death. Clin Cancer Res. 2003;9:3546–3554. [PubMed] [Google Scholar]
- 5.Palmer K, Hitt M, Emtage PC, Gyorffy S, Gauldie J. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther. 2001;8:282–290. doi: 10.1038/sj.gt.3301386. [DOI] [PubMed] [Google Scholar]
- 6.Reay J, Kim SH, Lockhart E, Kolls J, Robbins PD. Adenoviral-mediated, intratumor gene transfer of interleukin 23 induces a therapeutic antitumor response. Cancer Gene Ther. 2009;16:776–785. doi: 10.1038/cgt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guiducci C, Di Carlo E, Parenza M, Hitt M, Giovarelli M, et al. Intralesional injection of adenovirus encoding CC chemokine ligand 16 inhibits mammary tumor growth and prevents metastatic-induced death after surgical removal of the treated primary tumor. J Immunol. 2004;172:4026–4036. doi: 10.4049/jimmunol.172.7.4026. [DOI] [PubMed] [Google Scholar]
- 8.Pittoni P, Tripodo C, Piconese S, Mauri G, Parenza M, et al. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res. 2011;71:5987–5997. doi: 10.1158/0008-5472.CAN-11-1637. [DOI] [PubMed] [Google Scholar]
- 9.Rigamonti N, Bellone M. Prostate cancer, tumor immunity and a renewed sense of optimism in immunotherapy. Cancer Immunol Immunother. 2012;61:453–468. doi: 10.1007/s00262-012-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison T, Graham F, Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977;77:319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- 11.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 12.Tevethia SS, Lewis M, Tanaka Y, Milici J, Knowles B, et al. Dissection of H-2Db-restricted cytotoxic T-lymphocyte epitopes on simian virus 40 T antigen by the use of synthetic peptides and H-2Dbm mutants. J Virol. 1990;64:1192–1200. doi: 10.1128/jvi.64.3.1192-1200.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, et al. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125–133. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/S0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 15.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 16.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]