Abstract
A disintegrin and metalloproteinase 17 (ADAM17) is significantly upregulated not only in malignant cells but also in the pro-inflammatory microenvironment of breast cancer. There, ADAM17 is critically involved in the processing of tumor-promoting proteins. Therefore, ADAM17 appears to be an attractive therapeutic target to address not only tumor cells but also the tumor-promoting environment. In a previous study, we generated a monoclonal anti-ADAM17 antibody (A300E). Although showing no complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity, the antibody was rapidly internalized by ADAM17-expressing cells and was able to transport a conjugated toxin into target cells. As a result, doxorubicin-coupled A300E or Pseudomonas exotoxin A-loaded A300E was able to kill ADAM17-expressing cells. This effect was strictly dependent on the presence of ADAM17 on the surface of target cells. As a proof of principle, both immunotoxins killed MDA-MB-231 breast cancer cells in an ADAM17-dependent manner. These data suggest that the use of anti-ADAM17 monoclonal antibodies as a carrier might be a promising new strategy for selective anti-cancer drug delivery.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1346-x) contains supplementary material, which is available to authorized users.
Keywords: ADAM17, Monoclonal antibody, Doxorubicin, Pseudomonas exotoxin A
Introduction
ADAM17 (a disintegrin and metalloproteinase 17) 17, also known as tumor necrosis factor-alpha-converting enzyme (TACE), has been originally identified as a membrane-bound protease responsible for the cleavage of the type II transmembrane protein tumor necrosis factor-alpha (TNFα) [1]. Up to now, more than 70 substrates have been identified, including protransforming growth factor-α, heparin-binding epidermal growth factor, and proamphiregulin [2]. ADAM17 is implicated in many pathophysiological processes, including inflammation and cancer, where it processes growth factors to sustain inflammation and tumor growth by limited proteolysis. An increased ADAM17 cell surface expression has been found in various types of malignancies such as human primary colon carcinoma [3], lung cancer [4], and other types of cancers [5, 6] and has been correlated with tumor progression [7]. Also breast cancer cells show an elevated expression of ADAM17, which significantly correlates with the grade of malignancy. Apart from the malignant cells, ADAM17 is upregulated in tumor-supporting cells of the microenvironment [8], thereby promoting the malignant phenotype of this disease [5, 9, 10]. Based on these observations, ADAM17 might be a promising candidate for targeting breast cancer.
The potency of anti-cancer drugs is dramatically enhanced when targeted by tumor-selective antibodies to the tumor cells. Doxorubicin (Dox), an anti-cancer drug, is used to treat different cancers including bladder, breast, head and neck cancer as well as hematological malignancies. Since its systemic application is often associated with severe cardio-toxicity, there is a need for targeted application. Griffiths et al. [11] showed that a single dose of a Dox-anti-CD74 conjugate cured most animals challenged with human B-cell Burkitt’s lymphoma cells, whereas animals in control groups were not cured. Similarly, a Dox-coupled anti-RON antibody was more effective in killing hypoxic human colon cells as compared to free Dox [12]. Another approach, already in a preclinical study, used the efficiency of mAb drug delivery by a fusion protein consisting of a mouse Fc-binding antibody fragment and a truncated version of Pseudomonas exotoxin A (ETA′), an inhibitor of protein biosynthesis. This fusion protein is similarly designed as the recently described α-kappa-ETA′ that specifically binds to human antibodies by forming non-covalently linked antibody exotoxin A conjugates [13].
The extracellular part of ADAM17 consists of a pro-domain, catalytic domain, disintegrin domain and membrane-proximal domain followed by a transmembrane- and intracellular-region. The generated antibody A300E was targeted against the membrane-proximal domain, which is involved in multimerization and substrate recognition [14, 15].
The aim of this study was to examine the feasibility of anti-ADAM17 antibodies, conjugated with anti-cancer drugs to target cells with an elevated ADAM17 expression such as malignant and non-malignant cells involved in the progression of breast cancer.
Materials and methods
Antibody production
The anti-ADAM17 mAbs A300E and E20-19 were produced as described [16]. In short, hybridoma cells expressing the mAbs were cultivated in serum-free medium (ISF1, Biochrom, Germany) and the mAbs were purified via HiTrap Protein G high performance (HP) (GE Healthcare Freiburg, Germany). The purity of the mAbs preparations was verified by SDS–PAGE, and the subclass was determined using a mouse isotyping kit (Pierce, USA) according to the manufacturer’s protocol. The α-murine Fc-ETA′ is a fusion protein of an Fc-binding antibody fragment and a truncated version of pseudomonas exotoxin A, which was produced in analogy to α-kappa-ETA′ [13].
Cell lines and culture
CHO cells stably expressing the disintegrin, the membrane-proximal domain and the transmembrane region of human ADAM17 (CHO–C23) were generated by selection of transfected cells with G418 (500 μg/ml). The expression was verified by Western blotting using the A300 mAb [16]. Cell lines (OH1 from Prof. Dr. Udo Schumacher, UKE Hamburg, Germany; Karpas 299 and L540 from German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany; NCI-H292 from Dr. Anja Baumgart, TU Munich, Germany; and MDA-MB-231) were cultivated in RPMI-1640 medium supplemented with 10 % (v/v) FCS (PAA Laboratories, Germany) at 37 °C and 5 % (v/v) CO2 in a humidified incubator. CHO cells were cultivated in Ham’s F-12 containing 10 % (v/v) FCS (PAA Laboratories, Germany). Human NK cells with stable expression of CD16 (CD16 176V/F NK-92.05) were a kind gift of Prof. Dr. Kerry S. Campbell (Fox Chase Cancer Center Philadelphia, PA, USA).
Fluorescence-activated cell sorting
Cells were washed twice with FACS solution (PBS, 0.05 % (w/v) NaN3) and resuspended in staining buffer (PBS, 5 % (v/v) FCS, 0.05 % (w/v) NaN3); 106 cells were stained for 1 h at 4 °C with 2 μg/ml of anti-ADAM17 mAbs and washed twice with FACS solution. Then, the cell pellet was suspended in 100 μl staining buffer containing 2 μl of allophycocyanin (APC)-conjugated goat-anti-mouse Ig (Jackson ImmunoResearch Laboratories, USA). Again, cells were incubated for 1 h at 4 °C and washed twice. The samples were analyzed on a FACScan flow cytometer (BD FACSCanto™ Biosciences, USA). Cell populations of interest were gated and analyzed using BD FACSDiva software.
Western blotting
MDA-MB-231 and human fresh isolated PBMC cells were lysed with 250 μl of lysis buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1 % Triton X-100 and “Complete” protease inhibitor mixture; Roche applied science, Germany). After 30 min incubation at 4 °C, the cell lysate was centrifuged for 10 min at 14,000 rpm. The supernatant was collected and the protein concentration was determined using a BCA protein assay kit (Pierce, USA). Thirty μg protein of cell lysate was separated on 10 % polyacrylamide gel for 65 min. After transfer, the PVDF membrane (GE Healthcare Bio-Sciences, Sweden) was blocked with 5 % of milk powder (Carl Roth, Germany) in TBS-T (10 mM Tris–HCl, pH 7.6, 150 mM NaCl, 0.1 % Tween-20) for 2 h and then incubated with anti-ADAM17 monoclonal antibody A300 [16] diluted at 2 μg/ml in 1 % milk powder. Blots were then incubated for 1 h with anti-mouse IgG POD conjugate diluted at 1:1,000 in 1 % milk powder. After washing, the bands were visualized by chemiluminescence using an ECL kit (GE Healthcare Bio-Sciences, Sweden).
Complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC)
CDC and ADCC were measured by a calcein release assay [17]. This assay shows the same sensitivity as the traditional Cr51 assay [18]. MDA-MB-231 cells were seeded into flat-bottomed 96-well plates (104 cells per well) and left to adhere overnight at 37 °C. Then, Calcein-AM (5 μM in HBSS; Invitrogen, Germany) was incorporated by 1 h incubation at 37 °C. After washing, cells were incubated with the indicated mAbs at 37 °C for 30 min. For the CDC assay, 1:4 diluted rabbit complement MA (Cedarlane Laboratories, USA) was added to each well and incubated at 37 °C for additional 60 min. For the ADCC, the assay, CD16.NK-92 cells were added as indicated and incubated at 37 °C for 6 h. BB7.2 (mouse IgG2b, anti-HLA-A2) and isotype control (mouse IgG1, Biozol, Germany) were used as positive and negative control, respectively. For both assays, the maximal calcein release was determined by cell lysis (addition of 10 μl of 10 % (v/v) Triton X 100). Fifty μl cell supernatant from each well was transferred to a new 96-well plate, and fluorescence (485/528 nm) was measured on a fluorescence microplate reader (Bio-Tek, Germany). The percentage of specific lysis of cancer cells by antibody-CDC or -NK ADCC was calculated as previously described [17].
ELISA
To determine the concentration of anti-ADAM17 mAbs, the wells of a microtiter plate (Nunc, Denmark) were coated with 100 μl of human purified disintegrin and membrane-proximal domain at 10 μg/ml in coating buffer (0.5 M carbonate–bicarbonate buffer, pH 9.6). After overnight incubation at 4 °C, the plates were washed with washing solution (PBS, 0.05 % (v/v) Tween 20; Carl Roth, Germany) and blocked with 1 % (w/v) casein (Carl Roth, Germany) in PBS for 1 h at room temperature. After washing, 50 μl of antibody solution was added as indicated and incubated for 1 h at room temperature. PBS served as control. Plates were washed and incubated with anti-mouse IgG–POD conjugate (1:3,000 in PBS; SouthernBiotech, USA). After washing, wells were incubated with 100 μl of BM blue POD substrate (Roche, Germany) in the dark at room temperature for 10 min. The reaction was stopped by adding 50 μl of 0.8 M H2SO4. The optical density (OD) was measured at 450/540 nm on a plate reader (TECAN, Switzerland).
Preparation of PBMC
PBMCs were isolated from healthy donors by Ficoll density gradient centrifugation using standard procedures. After centrifugation, cells were washed with PBS and prepared for FACS staining and Western blotting as described above.
Confocal microscopy
MDA-MB-231 cells were seeded on cover slips and cultivated overnight at 37 °C. Cells were treated with 10 μg/ml of Dox-coupled antibody (A300E–Dox) and the isotype control antibody (BH1–Dox) or with 5 μg/ml of free Dox for 1 h at 37 °C. After washing with PBS, cells were fixed with 4 % (w/v) paraformaldehyde for 10 min at room temperature. Cells were stained with 1 μM DAPI and mounted with Mowiol/Dabco (Calbiochem/Sigma Aldrich, Perth, WA). Slides were analyzed with a FV1000 confocal microscope with a 60× lens (UPLSAPO 60× O NA: 1.35). Image acquisition was performed with FV10-ASW (version 3.0, Olympus Europa GmbH, Germany). Signals were detected by sequential scanning in two channels. With channel 1, Alexa Fluor 594 was detected by Ex 559 nm (8 % laser intensity) and Em 585–635 nm (PMT 680V). DAPI signals were collected in channel 2 centered on Ex 405 nm (3 % laser intensity) and Em 435–485 nm.
Downregulation of kinetic studies
MDA-MB-231 cells were incubated with 10 μg/ml A300E or E20-19 for 30 min on ice and washed with ice-cold RPMI medium. Cells were resuspended in growth medium and subdivided in two aliquots. One was kept at 4 °C and the other was incubated at 37 °C. At the indicated time points, the cells were incubated with APC-conjugated goat-anti-mouse Ig (Jackson ImmunoResearch Laboratories, USA) for 1 h at 4 °C. After washing, the samples were analyzed with a FACScan flow cytometer (BD FACSCanto™ Biosciences, USA). Percentage of mAb remaining on the cell surface was calculated as previously described [19].
Preparation of doxorubicin immunoconjugates
Antibodies were conjugated to Dox as described previously [11, 20]. Briefly, the cross-linker SMCC hydrazide (4-[N-maleimidomethyl] cyclohexane-1 carboxylhydrazide, Appolo Scientific, UK) was incubated with an equimolar amount of Dox (Sigma, Germany) for 30 min at 50 °C. The Dox-SMCC intermediate was isolated on a C-18 reverse phase high-performance liquid chromatography column and lyophilized. The mAb was partially reduced with a 40-fold molar excess of DTT. The reduced mAb was purified on NAP-10 spin column (GE Healthcare, Freiburg, Germany) in sodium acetate buffer (50 mM, pH 5.3) containing 2 mM EDTA. For conjugation, partially reduced mAb was mixed with the Dox–SMCC and kept on ice for 15 min. After purification on a NAP-10 spin column, the amount of Dox linked to the mAb was determined by UV–visible absorbance of the conjugate at 280 and 496 nm, as previously described [21].
Determination of cytotoxic effects of exotoxin A-based immunotoxins
As described previously, cells were seeded at 5 × 103 cells per 100 μl in 96-well plates [13]. α-Fc-ETA′ fusion protein was added at the indicated concentration in the presence or absence of antibodies. α-Fc-ETA′ is a fusion protein consisting of a mouse Fc-binding antibody fragment and a truncated version of Pseudomonas exotoxin A forming non-covalent antibody/exotoxin A conjugates. After 3 days of treatment, vital cell mass was measured using the titer blue reagent (Promega, Germany) according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed with Student’s t-test (http://www.studentsttest.com) from duplicate of three independent experiments. Values of p < 0.05 were considered significant.
Results
ADAM17 cell surface expression
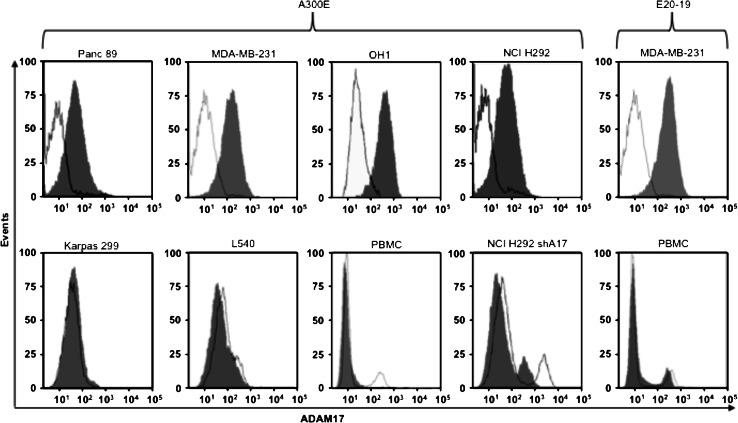
In order to investigated the expression of ADAM17 on the cell surface of various cancer cell lines by FACS analysis, we used the murine mAb A300E, which recognizes the membrane-proximal domain of human ADAM17 [14]. Strong ADAM17 cell surface expression was detectable in solid tumor cell lines, such as Panc89 (pancreatic cancer), MDA-MB-231 (breast cancer), OH1 (small cell lung carcinoma) and NCI-H292 (non-small lung cancer) (Fig. 1). In contrast, no ADAM17 cell surface expression was detectable in cell lines derived from hematological neoplasias such as Karpas 299 (T cell non-Hodgkin lymphoma) and L540 (Hodgkin lymphoma) and on primary human peripheral blood mononuclear cells (PBMCs). NCI-H292 shA17 cells, stably transfected with an ADAM17 shRNA [22], were used as a negative control. For the FACS analysis, the setting parameters of cell treated with shRNNA are different from that of wild-type NCI-H292 cells. Similar results were also obtained with a second anti-ADAM17 monoclonal antibody (E20-19) (Fig. 1). With both monoclonal antibodies (A300E and E20-19), we could not detect cell surface expression of ADAM17 on PBMCs. However, Western blot (Fig. S1) and literature data [23, 24] indicated that PBMCs contain intracellular ADAM17.
Fig. 1.
Binding of anti-ADAM17 antibody to tumor cells. FACS analysis of indicated cells incubated with anti-ADAM17 (A300E, E20-19; gray area) or isotype-matched control (white area); detection was by an allophycocyanin (APC)-conjugated anti-mouse antibody
Direct effect of the monoclonal antibody A300E on ADAM17-expressing cancer cells
Since A300E targets the membrane-proximal domain of ADAM17, which is implicated in substrate recognition [4], we looked whether it inhibits substrate shedding. The shedding of TNFα and IL1-RII, both known to be ADAM17 substrates, was not inhibited (data not shown). However, since ADAM17 has more than 70 different substrates [25] and many of the cleavage products influence cell proliferation and migration [26], MDA-MB-231 cells were treated for 3 days with A300E or an isotype control (both 10 μg/ml). No significant differences in cell proliferation or migration were detectable (data not shown). Therefore, we conclude that A300E does not interfere with the substrate recognition of ADAM17.
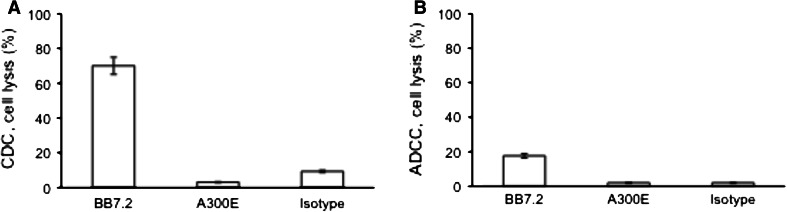
We tested the complement-dependent cytotoxic activity (CDC) of uncoupled A300E on MDA-MB-231 cells. A mouse IgG2b anti-HLA-A2 class I mAb (BB7.2) was used as a positive control. Whereas BB7.2 showed 70 % killing, the cytotoxic activity of A300E and the isotype control was very low (Fig. 2a).
Fig. 2.
Influence of uncoupled anti-ADAM17 on tumor cells. a Complement-dependent cytotoxicity (CDC) activity of A300E (10 μg/ml) on the viability of MDA-MB-231 cells using rabbit complement. b Antibody-dependent cellular cytotoxicity (ADCC) activity of A300E (10 μg/ml) on MDA-MB-231 cells using CD16 stable transfected NK92 cells as effectors cells at E:T ratio of 20:1. The mAb BB7.2 and isotype control were used as positive and negative control, respectively. Cell viability was determined by titer blue assay. One hundred percent cell lysis was determined by treating cell with 1 % Triton X-100. Error bars represent standard deviation of three independent experiments
The ability of A300E to induce lysis of MDA-MB-231 cells by antibody-dependent cellular cytotoxicity (ADCC) was tested using CD16 stably transfected NK cells (CD16 NK-92) as effector cells [27]. The CD16 NK-92 cells showed no cytotoxic effect on MDA-MB-231 cells in the presence of A300E (10 μg/ml) at an effector : target ratio of 20:1 (Fig. 2b). A similar result was obtained with freshly isolated mouse NK cells (data not shown). Thus, the results demonstrate that A300E does not mediate specific lysis of cancer cells through an CDC or ADCC mechanisms.
Downregulation of ADAM17
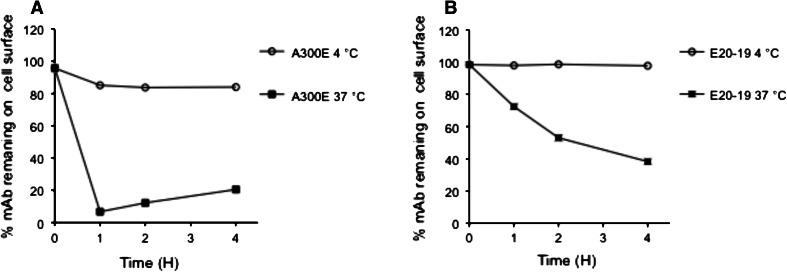
The presence of ADAM17 on the cell surface of MDA-MB-231 cells was studied by FACS analysis. Cells were treated for 1 h at 4 °C with two different anti-ADAM17 mAbs, A300E (Fig. 3a) and E20-19 (Fig. 3b), and then incubated at either 4 or 37 °C up to 4 h. Antibody E20-19 reacts with the membrane-proximal domain of ADAM17 and is generated as described in materials and methods. FACS analysis at different time points revealed that at 4 °C, the amount of A300E bound to the cell surface was stable even after 4 h incubation. In contrast, after 1 h incubation at 37 °C, the majority of antibody A300E on cell surface was lost. Antibody E20-19 showed similar result but with a different kinetic (Fig. 3). The results indicate that ADAM17 was downregulated and could be used as a marker to deliver a toxin into a target cell.
Fig. 3.
Downregulation of ADAM17. Breast carcinoma cells (MDA-MB-231) were treated for 1 h with mAb A300E (a) or mAb E20-19 (b) at 4 °C. After washing, cells were incubated for designated times either at 37 °C (filled square) or at 4 °C (filled circle). Cell surface-bound antibodies were detected by FACS analysis using an allophycocyanin-conjugated anti-mouse antibody. % mAb remaining on cell surface corresponding to 0 h incubation time was set arbitrary to 100 %
ADAM17-specific toxicity of the A300E–doxorubicin immunotoxin conjugate
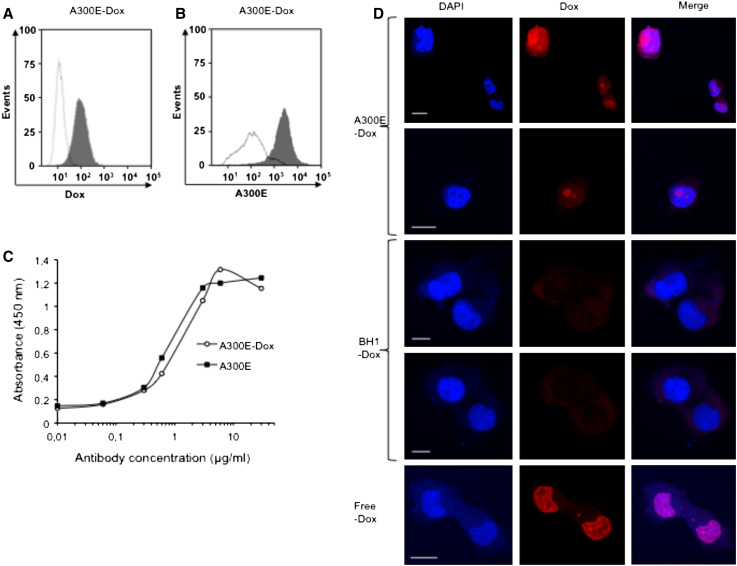
The A300E–Dox and the mouse isotype control antibody BH1 (BH1–Dox)-conjugate were prepared as described in materials and methods. The molar ratio of Dox–A300E and BH1 was 8.9:1. To ensure that A300E–Dox was internalized like the unconjugated antibody, we tested the ADAM17-specific toxicity of A300E–Dox. Specific binding of A300E–Dox to MDA-MB-231 cells was analyzed by flow cytometry, either by the Percp channel, which detects Dox (Fig. 4a) or by using APC-conjugated goat-anti-mouse Ig (Fig. 4b). FACS analysis revealed that A300E–Dox still recognizes ADAM17 on the cell surface. In addition, A300E–Dox and A300E showed almost the same binding affinity in an ELISA (Fig. 4c). Therefore, the conjugation of A300E–Dox does not affect the antigen-binding properties of this antibody.
Fig. 4.
Binding and internalization of A300E–Dox immunotoxin conjugate. FACS analyses of breast carcinoma cells (MDA-MB-231) treated with either A300E–Dox (gray areas) or isotype-matched control BH1–Dox (white areas) and detected either directly (a) with PerCP-A channel, which detects accumulated doxorubicin, or indirectly (b) by using allophycocyanin-conjugated anti-mouse antibody (APC). c ELISA: 96-well plate was coated with the membrane-proximal domain of ADAM17 and blocked for 1 h with 1 % casein. Indicated concentrations of mAb A300E (filled square) or IT A300E–Dox (filled circle) were added and the plate was incubated with anti-mouse-HRP antibody. After washing, substrate solution of BM blue was added and the absorbencies were measured at 450 nm. d Immunofluorescence microscopy of MDA-MB-231 was performed using A300E–Dox and an isotype control (BH1–Dox) as a negative controls. Cells treated with free Dox were used as positive control. Dox (red) was detected by emission fluorescence of Alexa Fluor 594. The cell nuclei were stained with DAPI (blue). White scale bars indicate 15 μm
A300E–Dox is internalized and doxorubicin localized in the nucleus
The internalization of anti-ADAM17 antibodies was confirmed by confocal microscopy using A300E–Dox. Cells treated with A300E–Dox showed red nuclei, indicating that the A300E–Dox was internalized and Dox is localized in the nuclei (Fig. 4d). In contrast, cells treated with BH1–Dox conjugate did not show any red staining of the nuclei (Fig. 4d). Cells treated with free Dox were used as positive control. Thus, A300E delivers Dox in a specific manner to ADAM17-positive cells.
A300E–Dox conjugate inhibits cell proliferation of ADAM17-expressing cells
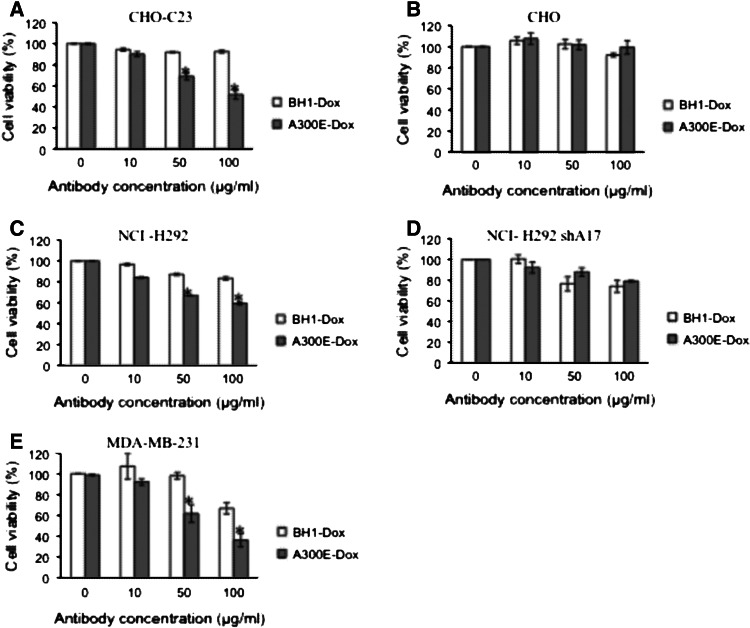
The influence of A300E–Dox on cell viability was determined by comparing CHO cells transfected with an ADAM17 variant lacking the catalytic domain (CHO–C23) and wild-type CHO cells. Whereas CHO–C23 cells were killed by A300E–Dox in a dose-dependent manner (Fig. 5a), reaching significant growth inhibition at 50 μg/ml (IC50 at 100 μg/ml), untransfected CHO cells were not significantly affected (Fig. 5b). In both cases, BH1-Dox was used as a control and exhibited only a minor effect on CHO–C23 cells (Fig. 5a, b). Interestingly, at an A300E concentration of 100 μg/ml, which corresponds to 3 μg/ml Dox, 50 % of the CHO–C23 was killed, whereas at a concentration of 3 μg/ml free Dox, more than 55 % of the cells was killed (data not shown). Nevertheless, the advantage of A300E–Dox is the expected specific delivery of the drug to ADAM17-expressing tumor cells.
Fig. 5.
In vitro toxicity of A300E–Dox immunotoxin conjugate. Indicated target cells were incubated for 2 h with increasing concentrations of A300E–Dox (gray column) or isotype BH1-Dox (white column) and washed twice with medium. After 96 h, cell viability was measured by using titer blue. Target cells are: a CHO cell line stably expressing the disintegrin and membrane-proximal domain followed by the transmembrane region of human ADAM17 (CHO–C23), b wild-type CHO, c non-small lung cancer cells (NCI-H292), d ADAM17 knockdown NCI-H292 cells (NCI-H292 shA17) and e breast carcinoma cells (MDA-MB-2321). Error bars represent standard deviation of three independent experiments. *Statistically significant difference compared to isotype control treated cells (p < 0.05)
To further demonstrate the ADAM17-dependent specificity of killing, we used wild-type NCI-H292 and ADAM17 knockdown NCI-H292 (NCI-H292-shA17) cells. After treatment with 100 μg/ml of A300E–Dox, the number of living wild-type NCI-H292 cells was significantly (p < 0.05) reduced in comparison with the number of cells treated with BH1-Dox as a control (90 vs 60 %) (Fig. 5c). The ADAM17-dependent specificity of killing was further proven by comparing A300E–Dox and BH–Dox treatment of NCI-H292-shA17 cells. No significant difference in cell viability was observed between these two Dox-conjugates (Fig. 5d).
Furthermore, we used the human breast cancer cell line MDA-MB-231 to examine the killing of A300E–Dox. In contrast to the BH–Dox control, the A300E–Dox mediated strong anti-tumor activity on this cell line (Fig. 5e). An A300E–Dox concentration of 50 μg/ml caused 40 % killing of MDA-MB-231 cells, whereas treatment with the BH1–Dox control mAb did not reduce the viable cell number.
A300E-dependent growth inhibition of ADAM17-expressing tumor cells by α-Fc-ETA′
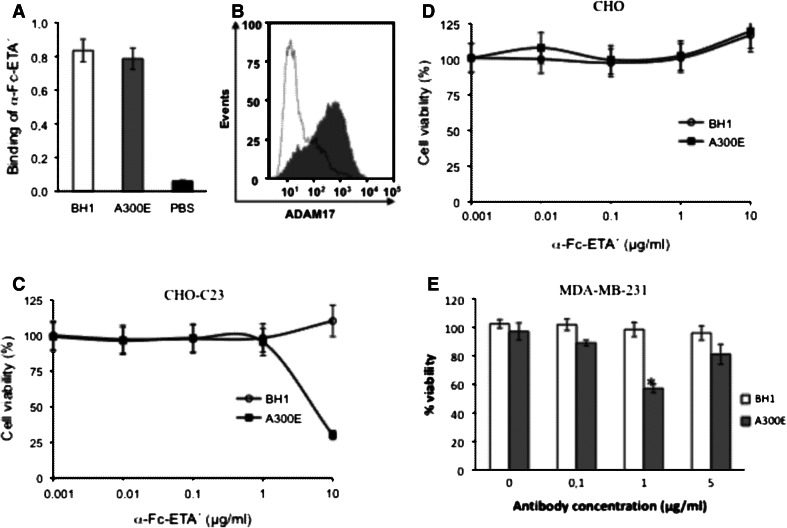
To further extend the use of A300E as a carrier for anti-cancer drugs, we investigated whether A300E was able to mediate an ADAM17-dependent toxin delivery (α-Fc-ETA′) to cancer cells. We performed these experiments using the recombinant α-Fc-ETA′ as toxin. α-Fc-ETA′ is a fusion protein consisting of an anti-mouse Fc-binding antibody fragment and a truncated version of Pseudomonas exotoxin A (EDTA′) forming non-covalent antibody/exotoxin A conjugates. In a first step, we tested the binding of α-Fc-ETA′ to A300E by ELISA. As shown in Fig. 6a, α-Fc-16ETA′ binds with a similar affinity to A300E and BH1. Furthermore, FACS data demonstrate that α-Fc-16ETA′ binds solely to CHO–C23 cells opsonized with A300E (Fig. 6b). Since α-Fc-ETA′ binds to both, A300E and BH1, only A300E was able to load CHO–C23 cells with the toxin α-Fc-ETA′.
Fig. 6.
Inhibition of cell viability by an anti-ADAM17/α-Fc-ETA′ toxin conjugate. a, b Binding of the mAb A300E to the fusion-toxin α-Fc-16-ETA′. a ELISA: 96-well plate was coated with the mAb A300E (gray column) or BH1 (white column) at 10 μg/ml and blocked for 1 h with 1 % casein. PBS (black column) was used as negative control. α-Fc-16-ETA′ (5 μg/ml) was added and the plate was incubated with rabbit anti-Pseudomonas exotoxin A. Finally, the plate was incubated with an anti-rabbit-HRP antiserum. After washing, substrate solution of BM blue was added and the absorbencies were measured at 450 nm. b FACS analyses of CHO cell line stably expressing the disintegrin and membrane-proximal domain followed by the transmembrane region of human ADAM17 (CHO–C23) treated with A300E (gray areas) or BH1 (white areas). After washing, α-Fc-ETA′ was added and then cells were incubated with rabbit anti-Pseudomonas exotoxin A polyclonal antibodies. Finally, cells were detected with FITC-conjugated anti-rabbit antibody. c–e Inhibition of cell viability by immunotoxin conjugate. Cell viability of c CHO cells stably transfected with a truncated ADAM17 (CHO–C23) or d wild-type CHO cells. Cells were incubated with either 5 μg/ml of A300E (filled square) or isotype-matched control BH1 (filled circle) in the presence of the indicated α-Fc-ETA′ concentrations. e Breast carcinoma cells (MDA-MB-231) were incubated with the indicated concentration of A300E (gray column) or BH1 (white column) in the presence of α-Fc-ETA′ (5 μg/ml). After 72 h, vital cell mass was determined by titer blue assay. Error bars represent standard deviation of three independent experiments. *Statistically significant difference compared to isotype control treated cells (p < 0.05)
To investigate A300E-dependent growth inhibition of ADAM17-expressing cells, we performed cell viability assays. CHO and CHO–C23 cells were treated with 10 μg/ml of A300E or BH1 and incubated with increasing concentrations of α-Fc-ETA′. As shown in Fig. 6c, A300E induced strong reduction in cell viability of CHO–C23 cells in comparison with the BH1 control, which did not bind to the target cells. Wild-type CHO cells remained unaffected (Fig. 6d).
In addition, we tested the growth inhibition mediated by A300E/α-Fc-ETA′ on MDA-MB-231 cells. Cells were treated with increasing A300E concentrations and a constant concentration of α-Fc-ETA′ (5 μg/ml). Here, 1 μg/ml of A300E induced killing of more than 40 % of the cells, whereas the same concentration of BH1 did not mediate any significant effect (Fig. 6e). Interestingly, there exists an optimal concentration of the antibody to yield a maximal growth inhibition effect and this effect is declining at higher concentrations (5 μg/ml), probably indicating that unfavorable antibody/toxin ratios do not result in efficient conjugate formation.
Discussion
Selective targeting of cancer cells is the key to overcome the side effects of conventional chemotherapy. Several targets including ADAM17 have been proposed as specific malignancy markers. In fact, many studies have shown that ADAM17 is overexpressed on the surface of cancer cells [6, 7] and that it is responsible for the shedding of more than 70 different substrates including several ligands of the epidermal growth factor receptor that is involved in tumor progression [2]. Based on these observations, ADAM17 arises as a potential marker for immunotherapy of human cancer [6]. Since the ADAM17-specific antibody A300E is efficiently internalized, we tested whether it is suitable for delivering a toxin to cancer cells by using two different approaches. In the first approach, we covalently coupled A300E–Dox via SMCC as cross-linker. Secondly, we extended this approach by using A300E in combination with α-Fc-ETA′, a fusion protein consisting of a mouse Fc-binding antibody fragment and a truncated version of the Pseudomonas exotoxin A.
Dox-conjugated mAbs have been demonstrated to be efficient in vitro as well as in tumor-animal models [28–30]. In fact, IMMU-110, an anti-CD74 mAb conjugated to Dox, showed at very low doses a remarkable activity in vitro and in human xenograft models of non-Hodgkin’s lymphoma or multiple myeloma [21]. Moreover, Inoh et al. [30] reported that an anti-Midkin antibody conjugated to Dox significantly inhibits the growth of HepG2 cells in comparison with a Dox-conjugated control IgG. In addition, a Dox-conjugated anti-envelope HIV antibody was shown to eliminate HIV-infected cells, thereby protecting mice challenged with HIV [20]. With a similar strategy, an anti-CD30 mAb conjugated to monomethyl auristatin E, an inhibitor of tubulin polymerization, was successfully used in a phase 1 clinical trial to treat patients with relapsed CD30-positive lymphomas [31, 32].
Immunotoxins (IT) consisting of an antibody or an antibody fragment and Pseudomonas exotoxin A (ETA) are highly active in killing of target cells with relatively low antigen density [33, 34]. The IT bind to and are internalized by the target cells, and the ETA translocates to the cytosol where it inhibits protein biosynthesis and induces apoptosis. Due to the extreme cytotoxicity of ETA, only a few molecules are sufficient to kill cells [33]. In order to prevent unspecific binding of ETA to normal cells, a truncated version (ETA′) was developed. The anti-tumor activity of mAb-ETA′ constructs was demonstrated in several tumor models. In fact, anti-CD30-ETA′ is a potent IT against a Hodgkin-derived cell line [35]. Moreover, moxetumomab pasudotox, a recombinant IT, containing a Fv fragment of anti-CD22 fused to ETA′, has anti-tumor activity in murine xenograft studies [36] and successfully passed the phase I trial [37]. Recently, it was shown that the anti-EGFR antibody Zalutumumab in combination with anti-kappa-ETA′, a fusion protein consisting of a human kappa-binding antibody fragment and a truncated version of Pseudomonas exotoxin A, was highly effective in killing the human epithelial carcinoma A431 cell line [13]. Our results also indicate a significant growth inhibition of the breast cancer cell line MDA-MB-231 treated with nano-molar concentrations of the anti-ADAM17 antibody A300E in combination with α-Fc-ETA′. By comparing the cytotoxicity mediated by A300E–Dox and A300E/α-Fc-ETA′ toward the breast cancer cell line MDA-MB-231, we observed that A300E/α-Fc-ETA′ is about 50 times more potent than A300E–Dox, reflecting the high killing activity of ETA′. Therefore, mAb A300E may serve as a new carrier for the delivery of toxins into breast carcinoma cells for the following two reasons: first, because ADAM17 is selectively upregulated on the cell surface of carcinoma cells and on cells in the tumor-promoting microenvironment. The second reason is that the A300E is efficiently internalized upon binding to ADAM17.
However, unwanted binding of immunotoxins to endothelial cells may provide a problem in clinical studies because it often causes the so-called vascular leak syndrome, which is associated with edema, dyspnea, tachycardia and myalgia. The side effects are dose-dependent and limit the clinical use of immunotoxins [38]. Currently, we are optimizing the antibody formulation to minimize such effects on endothelial cells.
Thus, an immunoconjugated anti-ADAM17 antibody, like A300E, may represent a new challenge for the development of a potential new drug against human breast adenocarcinoma and other ADAM17-overexpressing malignant diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Holger Kalthoff (UKSH Kiel, Germany) for providing the MDA-MB-231 breast cancer cell line and Panc89 pancreatic cancer cell line. This study has been supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (SFB 877, A6, Z3) and the cluster of excellence “Inflammation at Interfaces.” Kosuke Yamamoto was supported by a postdoctoral fellowship from the German Academic Exchange Service (DAAD).
Conflict of interest
The authors declare they have no conflict of interest.
References
- 1.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 2.Scheller J, Chalaris A, Garbers C, et al. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8):380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Blanchot-Jossic F, Jarry A, Masson D, et al. Up-regulated expression of ADAM17 in human colon carcinoma: co-expression with EGFR in neoplastic and endothelial cells. J Pathol. 2005;207(2):156–163. doi: 10.1002/path.1814. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra A, Postma DS, Noordhoek JA, et al. Expression of ADAMs (“a disintegrin and metalloprotease”) in the human lung. Virchows Arch. 2009;454(4):441–449. doi: 10.1007/s00428-009-0748-4. [DOI] [PubMed] [Google Scholar]
- 5.Zheng X, Jiang F, Katakowski M, et al. ADAM17 promotes glioma cell malignant phenotype. Mol Carcinog. 2011;51(2):150–164. doi: 10.1002/mc.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu K, Liao M, Liu B, et al. ADAM-17 over-expression in gallbladder carcinoma correlates with poor prognosis of patients. Med Oncol (Northwood, Lond, Engl) 2011;28(2):475–480. doi: 10.1007/s12032-010-9481-8. [DOI] [PubMed] [Google Scholar]
- 7.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45(2):146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narita D, Seclaman E, Ilina R, et al. ADAM12 and ADAM17 gene expression in laser-capture microdissected and non-microdissected breast tumors. Pathol Oncol Res. 2011;17(2):375–385. doi: 10.1007/s12253-010-9336-9. [DOI] [PubMed] [Google Scholar]
- 9.McGowan PM, McKiernan E, Bolster F, et al. ADAM-17 predicts adverse outcome in patients with breast cancer. Ann Oncol. 2008;19(6):1075–1081. doi: 10.1093/annonc/mdm609. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Jiang F, Katakowski M, et al. ADAM17 promotes breast cancer cell malignant phenotype through EGFR–PI3K–AKT activation. Cancer Biol Ther. 2009;8(11):1045–1054. doi: 10.4161/cbt.8.11.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths GL, Mattes MJ, Stein R, et al. Cure of SCID mice bearing human B-lymphoma xenografts by an anti-CD74 antibody-anthracycline drug conjugate. Clin Cancer Res. 2003;9(17):6567–6571. [PubMed] [Google Scholar]
- 12.Guin S, Ma Q, Padhye S, et al. Targeting acute hypoxic cancer cells by doxorubicin-immunoliposomes directed by monoclonal antibodies specific to RON receptor tyrosine kinase. Cancer Chemother Pharmacol. 2011;67(5):1073–1083. doi: 10.1007/s00280-010-1408-8. [DOI] [PubMed] [Google Scholar]
- 13.Kellner C, Bleeker WK, Lammerts van Bueren JJ, et al. Human kappa light chain targeted Pseudomonas exotoxin A—identifying human antibodies and Fab fragments with favorable characteristics for antibody-drug conjugate development. J Immunol Methods. 2011;371(1–2):122–133. doi: 10.1016/j.jim.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzen I, Trad A, Grotzinger J. Multimerisation of A disintegrin and metalloprotease protein-17 (ADAM17) is mediated by its EGF-like domain. Biochem Biophys Res Commun. 2011;415(2):330–336. doi: 10.1016/j.bbrc.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzen I, Lokau J, Düsterhöft S, et al. The membrane-proximal domain of A disintegrin and metalloprotease 17 (ADAM17) is responsible for recognition of the interleukin-6 receptor and interleukin-1 receptor II. FEBS Lett. 2012;586(8):1093–1100. doi: 10.1016/j.febslet.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Trad A, Hedemann N, Shomali M, et al. Development of sandwich ELISA for detection and quantification of human and murine a disintegrin and metalloproteinase17. J Immunol Methods. 2011;371(1–2):91–96. doi: 10.1016/j.jim.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z, Li J. Natural killer cells are crucial for the efficacy of Icon (factor VII/human IgG1 Fc) immunotherapy in human tongue cancer. BMC Immunol. 2010;11:49. doi: 10.1186/1471-2172-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai YT, Dillon M, Song W, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112(4):1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber HP, Kung-Sutherland M, Stone I, et al. Potent antitumor activity of the anti-CD19 auristatin antibody drug conjugate hBU12-vcMMAE against rituximab-sensitive and -resistant lymphomas. Blood. 2009;113(18):4352–4361. doi: 10.1182/blood-2008-09-179143. [DOI] [PubMed] [Google Scholar]
- 20.Johansson S, Goldenberg DM, Griffiths GL, et al. Elimination of HIV-1 infection by treatment with a doxorubicin-conjugated anti-envelope antibody. AIDS (Lond, Engl) 2006;20(15):1911–1915. doi: 10.1097/01.aids.0000247111.58961.60. [DOI] [PubMed] [Google Scholar]
- 21.Sapra P, Stein R, Pickett J, et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11(14):5257–5264. doi: 10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
- 22.Baumgart A, Seidl S, Vlachou P, et al. ADAM17 regulates epidermal growth factor receptor expression through the activation of Notch1 in non-small cell lung cancer. Cancer Res. 2010;70(13):5368–5378. doi: 10.1158/0008-5472.CAN-09-3763. [DOI] [PubMed] [Google Scholar]
- 23.Satoh M, Iwasaka J, Nakamura M, et al. Increased expression of tumor necrosis factor-α converting enzyme and tumor necrosis factor-α in peripheral blood mononuclear cells in patients with advanced congestive heart failure. Eur J Heart Fail. 2004;6(7):869–875. doi: 10.1016/j.ejheart.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Shimoda Y, Satoh M, Nakamura M, et al. Activated tumour necrosis factor-alpha shedding process is associated with in-hospital complication in patients with acute myocardial infarction. Clin Sci. 2005;108(4):339–347. doi: 10.1042/CS20040229. [DOI] [PubMed] [Google Scholar]
- 25.Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des. 2009;15(20):2319–2335. doi: 10.2174/138161209788682398. [DOI] [PubMed] [Google Scholar]
- 26.Maretzky T, Evers A, Zhou W, et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun. 2011;2:229. doi: 10.1038/ncomms1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binyamin L, Alpaugh RK, Hughes TL, et al. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180(9):6392–6401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleh MN, Sugarman S, Murray J, et al. Phase I trial of the anti-Lewis Y drug immunoconjugate BR96-doxorubicin in patients with lewis Y-expressing epithelial tumors. J Clin Oncol. 2000;18(11):2282–2292. doi: 10.1200/JCO.2000.18.11.2282. [DOI] [PubMed] [Google Scholar]
- 29.Muldoon LL, Neuwelt EA. BR96–DOX immunoconjugate targeting of chemotherapy in brain tumor models. J Neurooncol. 2003;65(1):49–62. doi: 10.1023/A:1026234130830. [DOI] [PubMed] [Google Scholar]
- 30.Inoh K, Muramatsu H, Torii S, et al. Doxorubicin-conjugated anti-midkine monoclonal antibody as a potential anti-tumor drug. Jpn J Clin Oncol. 2006;36(4):207–211. doi: 10.1093/jjco/hyl004. [DOI] [PubMed] [Google Scholar]
- 31.Gualberto A. Brentuximab vedotin (SGN-35), an antibody-drug conjugate for the treatment of CD30-positive malignancies. Expert Opin Investig Drugs. 2012;21(2):205–216. doi: 10.1517/13543784.2011.641532. [DOI] [PubMed] [Google Scholar]
- 32.Fanale MA, Forero-Torres A, Rosenblatt JD, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18(1):248–255. doi: 10.1158/1078-0432.CCR-11-1425. [DOI] [PubMed] [Google Scholar]
- 33.Kreitman RJ, Pastan I. Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin Cancer Res. 2011;17(20):6398–6405. doi: 10.1158/1078-0432.CCR-11-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreitman RJ, Pastan I. Accumulation of a recombinant immunotoxin in a tumor in vivo: fewer than 1,000 molecules per cell are sufficient for complete responses. Cancer Res. 1998;58(5):968–975. [PubMed] [Google Scholar]
- 35.Klimka A, Barth S, Matthey B, et al. An anti-CD30 single-chain Fv selected by phage display and fused to Pseudomonas exotoxin A (Ki-4(scFv)-ETA′) is a potent immunotoxin against a Hodgkin-derived cell line. Br J Cancer. 1999;80(8):1214–1222. doi: 10.1038/sj.bjc.6690488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alderson RF, Kreitman RJ, Chen T, et al. CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin Cancer Res. 2009;15(3):832–839. doi: 10.1158/1078-0432.CCR-08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreitman RJ, Tallman MS, Robak T, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30(15):1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastan I, Hassan R, FitzGerald DJ, et al. Immunotoxin treatment of cancer*. Annu Rev Med. 2007;58(1):221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.