Abstract
Background
Toll-like receptors (TLRs) have garnered an extraordinary amount of interest in cancer research due to their role in tumor progression. By activating the production of several biological factors, TLRs induce type I interferons and other cytokines, which drive an inflammatory response and activate the adaptive immune system. The aim of this study was to investigate the expression and clinical relevance of TLR3, 4, and 9 in prostate cancer.
Methods
The expression levels of TLR3, TLR4, and TLR9 were analyzed on tumors from 133 patients with prostate cancer. The analyses were performed by immunohistochemistry on tissue arrays and real time-PCR.
Results
Cancerous cells showed high expression levels of TLRs compared with controls. Samples of carcinomas with recurrence exhibited a significant increase in the mRNA levels of TLR3, TLR4, and TLR9. In addition, the tumors that showed high TLR3 or TLR9 expression levels were significantly associated with higher probability of biochemical recurrence.
Conclusion
TLR expression is associated with prostate cancer with recurrence and the role of TLR receptors in the biology of malignancy merits study. Therapeutic strategies to boost or block TLRs may be of interest.
Keywords: Prostate carcinoma, Tissue array, Real time PCR, Prognosis, TLR, Tumor invasion
Introduction
Prostate cancer is a common cause of morbidity and mortality in men in the developed world. Several published autopsy series have demonstrated that up to one-third of men between 30 and 40 years of age harbor histological evidence of prostate carcinoma [1]. While several determinants such as age, ethnicity, and family history have been implicated in prostate cancer etiology, other risk factors remain elusive [2]. Although inflammation and cancer are two distinct processes, studies have demonstrated a common link involving cytokines and chemokines. This association was found to play a part in the promotion of angiogenesis, metastasis, and subversion of adaptive immunity [3–10]. High levels of proinflammatory cytokines were found in prostatic tissue samples and in semen of patients with chronic prostatitis [11] as well as in prostatic fluid collected from prostatectomy [12].
Toll-like receptors (TLRs) are considered a link between innate (non-specific) and adaptive (specific) immunity and contribute to the immune system’s capacity to efficiently combat pathogens [13]. As molecular sensors, TLRs detect pathogen-derived products and couple to different adapter proteins that trigger specific signaling pathways such as the IL1 receptor-associated kinase (IRAK) family and TBK-1. These adapters initiate pathways leading to the activation of their respective transcription factors, nuclear factor kappa B (NFκB) and interferon regulatory factor 3 (IRF3). Both NFκB and IRF3 induce the release of various immune and inflammatory cytokines such as tumor necrosis factor (TNF) and IL6, that have been shown to be excellent targets for inflammatory diseases [14]. Some interleukins also have the capacity to activate survival-related genes, such as IL4 in diffuse large B-cell lymphoma [15]. Although complex and efficient, the persistence of infection-fighting agents can be deleterious [16]. For example, they may produce mutagenic agents that react with DNA and cause mutations in proliferating epithelial and stromal cells [17]. Therefore, TLRs may represent a target in patients. Among the family of TLRs, the present study has included TLR3, TLR4, and TLR9 because they have been related to the pathogenesis of prostate cancer in previous studies. TLR3 is implicated in modulating tumorogenesis and the use of TLR3 agonists has been successful in prostate immune-based therapies [18–20]. Genetic variation in TLR4 and TLR9 has been associated with incidence in prostate carcinoma [21]. TLR9 is increased in the most poorly differentiated forms of prostate cancer and may promote IL8 through NFκB activation [22, 23]. In addition, TLR9 agonists stimulate prostate cancer invasion in vitro [24].
The purpose of the present study was to investigate the expression of TLR3, TLR4 and TLR9 in prostate cancer as well as its relation to biochemical recurrence. To address these questions, we analyzed the protein levels of TLR3, TLR4 and TLR9 by tissue arrays technology (TA) and immunohistochemistry and their mRNA levels by real time-PCR.
Materials and methods
Patients and tissues samples
Histological material was obtained from 133 patients with prostate carcinoma (aged 44–79 years) diagnosed between 1990 and 2007. We selected patients with prostate adenocarcinomas who had undergone radical prostatectomy. In cases of non-recurrence, patients had been followed-up for a minimum of 5 years. The exclusion criteria were: (1) metastatic disease at presentation, (2) prior history of any type of malignant tumor, (3) any type of neoadjuvant therapy, (4) development of a second primary cancer, and (5) absence of sufficient tissue in paraffin blocks. From a total of 158 patients fulfilling these criteria, we randomly selected a sample size of 133 patients, divided them into two different groups of similar size and stratified each group with regard to the development of biochemical recurrence, the key study variable. Biochemical recurrence was found in 47 patients. According to European Association of Urology (EAU), biochemical recurrence is defined as a PSA level greater than 0.2 ng/ml after radical prostatectomy with a subsequent increase in PSA. Patients and tumor characteristics are listed in Table 1. Tumors were staged according to the 1992 TNM classification [25]. Histological tumor grading was established according to the Gleason’ criteria. A single uropathologist assigned Gleason scores in the stained tissue array spots. Gleason score was graded in accordance to the criteria by Epstein et al. [26]. PSA serum levels were determined, pre and postoperatively, using the “Elecys” immune-assay tests (Roche Diagnostic GMbH, Mannheim, Germany). One month after surgical treatment, all patients were found to have undetectable PSA serum levels. Finally, all cases were evaluated for disease recurrence or survival status by clinical, radiologic, and biologic examinations every 6 months. The mean follow-up period was 62 months (range 6–144 months). Patients were treated according to approved guidelines at our institutions. The study adhered to national regulations and was approved by our institution’s Ethics and Investigation Committee.
Table 1.
Basal characteristics of 133 patients with prostate carcinoma
| Characteristics | No biochemical recurrence (n = 86) | Biochemical recurrence (n = 47) | p | ||
|---|---|---|---|---|---|
| N° (%) | N° (%) | ||||
| Age (year) | 0.25 | ||||
| <65 | 52 (39.5) | 64.87 ± 6.47 | 28 (40.4) | 66.21 ± 6.27 | |
| >65 | 34 (60.5) | 19 (59.6) | |||
| Tumor stage | <0.0001 | ||||
| T2 | 79 (91.9) | 27 (57.4) | |||
| T3–4 | 7 (8.1) | 20 (42.6) | |||
| Score gleason | <0.003 | ||||
| 2–4 | 14 (16.3) | 4 (8.5) | |||
| 5–6 | 50 (58.1) | 17 (36.2) | |||
| 7–10 | 22 (25.6) | 26 (55.3) | |||
| PSA (ng/ml)a | 0.051 | ||||
| <10 | 65 (75.6) | 28 (59.6) | |||
| >10 | 21 (24.4) | 19 (40.4) | |||
Mean age (±SD) in each group (with recurrence and with no recurrence) is shown
aPSA level before patients were operated
Tissue arrays and immunohistochemistry
All radical retropubic prostatectomy specimens were routinely fixed in 10% neutral buffered formalin and stored in paraffin at room temperature for a period of 4 months–5 years before further testing. Histopathological representative tumor areas were defined on hematoxylin and eosin-stained sections and marked on the slide. Tumor tissue array (TA) blocks were obtained by punching a tissue cylinder (core) with a diameter of 1.5 mm through a histological representative area of each ‘donor’ tumor block, which was then inserted into an empty ‘recipient’ tissue array paraffin block using a manual tissue arrayer (Beecher Instruments, Sun Prairie, Winconsin, USA) as described elsewhere [27]. Collection of tissue cores was carried out under highly controlled conditions. Two cores were employed for each case.
Four composite high-density TA blocks were designed, and serial 5-μm sections were consecutively cut with a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and transferred to adhesive-coated slides. One section from each TA block was stained with hematoxylin and eosin, and these slides were then reviewed to confirm that the sample was representative of the original tumor. Immunohistochemistry was done on these sections using a TechMate TM50 autostainer (Dako, Glostrup, Denmark). Antibodies for TLR3 (TLR3.7; sc-32232), TLR4 (H-80; sc-10741) and TLR9 (H-100; sc-25468) were obtained from Santa Cruz Biotechnology Inc. (California, USA). The dilution for each antibody was established based on negative and positive controls (1/10 for TLR3, 1/40 for TLR4, and 1/80 for TLR9).
Tissue sections were deparaffinized in xylene, and then rehydrated in graded concentrations of ethyl alcohol (100, 96, 80, and 70%) and water. To enhance antigen retrieval for some antibodies, TA sections were microwave-treated (H2800 Microwave Processor, EBSciences, East Granby, Connecticut, USA) in citrate buffer (Target Retrieval Solution, Dako) at 99°C for 16 min. Endogenous peroxidase activity was blocked by incubating the slides in peroxidase-blocking solution (Dako) for 5 min. The EnVision Detection Kit (Dako) was used as the staining detection system. Sections were counterstained with hematoxylin, dehydrated with ethanol, and permanently coverslipped.
Tissue arrays analysis
The location of immunoreactivity, percentage of stained cells, and intensity were determined for each antibody preparation. All cases were semiquantified for each protein-stained area. An image analysis system using the Olympus BX51 microscope and analysis soft (analySIS®, Soft imaging system, Münster, Alemania) was employed as follows: tumor sections were stained with antibodies according to the method explained above and counterstained with hematoxylin. There were different optical thresholds for both stains. Each core was scanned with a 400× power objective in two fields per core. Fields were selected on the basis of protein-stained areas. The computer program selected and traced a line around antibody-stained areas (red spots for higher optical thresholds). The remaining non-stained areas (hematoxylin-stained tissue with lower optical threshold) appear as a blue background. Each field has an area ratio of stained (red) versus non-stained areas (blue). A final area ratio was obtained after averaging two fields. To evaluate immunostaining intensity we used a numeric score ranging from 0 to 3, reflecting the intensity as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, intense staining. Using an excel spreadsheet, the mean score was obtained by multiplying the intensity score (I) by the percentage of stained cells [28] and the results were added together (total score: I × PC). This overall score was then averaged with the number of cores that were done for each patient. If there was no tumor in a particular core, then no score was given. In addition, for each tumor, the mean score of two core biopsies was calculated.
From a subset of ten randomly selected cases, whole-tissue sections from blocks for either tumor specimens were compared with the corresponding tissue array disks, insofar as TLR expression. The corresponding clinicopathological data were in line with the whole series. Each whole-tissue section was scanned with a 400× power lens in ten different fields. Fields were selected searching for the protein-stained areas, as described above.
Real time PCR
Total RNA was isolated from ten prostate carcinomas and ten benign pathologies (prostatic intraepithelial neoplasia and benign prostate hyperplasia) using the RNeasy Mini kit (Quiagen, Hilden, Germany), including DNase treatment. The integrity of the eluted total RNA was checked by agarose gel electrophoresis and the RNA concentration was determined spectrophotometrically. The ratio of absorbances at 260 and 280 nm, measured in a NanoDrop ND-1000 spectrophotometer (Thermo Scientific), was used as a parameter to quantify and evaluate the quality of the total RNA extracted (values ranging between 2.07 and 2.35). First strand cDNA was made using the High Capacity cDNA Reverse Transcrition kit (Applied Byosystems, Cheshire, UK) following the manufacturer’s instructions. The reverse transcription step was carried out using the following program: 25°C for 10 min, 37°C for 120 min and 85°C for 5 s. Expression levels of genes were assessed by real time PCR using ABI Prism 7900 HT thermocycler (Applied Biosystems, Cheshire, UK), 200 ng of cDNA and the Fast SYBR Green Master Mix (Applied Biosystems, Cheshire, UK) with the following cycling conditions: 95°C for 20 s, 40 cycles of 95°C for 1 s, and 60°C for 20 s. The primers used are described in Table 2. All real time PCR were performed in triplicate and the amplification signal from the target was normalized using β-actin control. SDS RQ Manager Program (Applied Biosystems) was used to analyze the results. PCR products were separated on 2% agarose gels containing ethidium bromide (0.5 μg/ml).
Table 2.
Real time PCR primers
| Gene product | Primer sequence (5′–3′) forward | Primer sequence (5′–3′) reverse |
|---|---|---|
| TLR3 | TAGCAGTCATCCAACAGAATCAT | AATCTTCTGAGTTGATTATGGGTAA |
| TLR4 | ACTCCCTCCAGGTTCTTGATTAC | CGGGAATAAAGTCTCTGTAGTGA |
| TLR9 | CTTCCCTGTAGCTGCTGTCC | CCTGCACCAGGAGAGACAG |
| IL1 | TAGTAGCAACCAACGGGAAG | CTCTTGAGTCATTGGCGAGT |
| IL4 | CCACGGACACAAGTGCGATAT | CGTAACAGACATCTTTGCTGCC |
| IL5 | AAAGGCAAACGCAGAACGTGT | CTCTTGGAGGTGCCTACGTGT |
| IL6 | GAACTCCTTCTCCACAAGCGCCTT | CAAAAGACCAGTGATGATTTTCACCAGG |
| IL10 | ATGCAGGACTTTAAGGGTTACTTGGGTT | ATTTCGGAGAGAGGTACAAACGAGGTTT |
| IL12 | TCGCGTTCACAAGCTCAAGT | CAAACCTGACCCACCCAAGA |
| IL17 | GTCTGGGCGCAGGTATGTGG | CACCGTGGAGACCCTGGAGGC |
| IL18 | CAGACAACTTTGGCCGACTTCA | ACACAAACCCTCCCCACCTAACT |
| IFNα | TGGCTGTGAAGAAATACTTCCG | TGTTTTCATGTTGGACCAGATG |
| IFNβ | TCTCCACGACAGCTCTTTCCA | ACACTGACAATTGCTGCTTCTTTG |
| IRF3 | GTTCTGTGTGGGGAGTCAT | CTGTTGGAAATGTGCAGGTC |
| CCL3 | CTTGCTGTCCTCCTCTGCAC | CTGTTGGAAATGTGCAGGTC |
| ICAM1 | AGGCCACCCCAGAGGACAAC | CCCATTATGACTGCGGCTGCTA |
| IRAK4 | CAGACTCTCTTGCTTGGATGGT | AGCTGACCCTGAGCAATCTT |
| Myd88 | TGGCACCTGTGTCTGGTCTA | ACATTCCTTGCTCTGCAGGT |
| NFκB | TCTCCCTGGTCACCAAGGAC | TCATAGAAGCCATCCCGGC |
| TNFα | TCTCGAACCCCGAGTGACAA | CCACTGGAGCTGCCCCTC |
| TRIF | CCTCCTCCTCCTCCTCATC | GCGTGGAGGATCACAAAGTT |
| β-actin | GGCACCCAGCACAATGAAG | CCGATCCACACGGAGTACTTG |
Data analysis and statistical methods
Differences in percentages were calculated with the Chi-square test. Immunostaining score values for each protein were expressed as median (range). A comparison of group immunostaining values was made with the Mann–Whitney or Kruskal–Wallis tests. For biochemical recurrence analysis we used the Cox univariate method. Cox proportional hazards regression was used to explore independent predictors of biochemical recurrence after radical prostatectomy in our series. A significance level value <0.1 was considered for the selection of terms to include in the multivariate model.
Results
In the present study, we investigated the expression levels of TLR3, TLR4, and TLR9 in tumors from 133 men diagnosed with prostate cancer. Tissue arrays presented minimal internal variance of score data between duplicate patient tissue cores, indicating strong agreement for each protein (r > 0.95 and p < 0.0001, for each protein). In the validation study of TMA cases and corresponding whole-tissue sections, there was complete concordance in terms of global expression as well as immunostaining intensity for each TLR. Highly significant correlations were also found in immunostaining scores (r > 0.90 and p < 0.0001, for each protein).
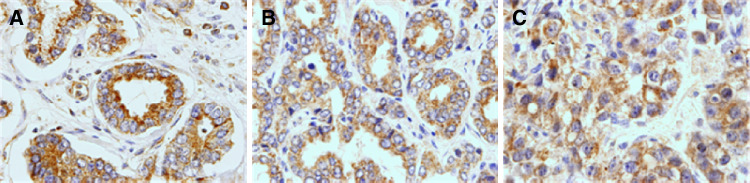
Figure 1 shows three examples of immunostaining for TLR3, TLR4, and TLR9 in prostate tumors. Positive staining was generally found in cancer cells but also in some stromal cells (fibroblast-like cells as well as in mononuclear inflammatory cells, MICs). TLR4 was localized in the cell surface while TLR3 and TLR9 showed an intracellular localization pattern. Table 3 summarizes the percentages of each TLR staining in each cellular type. In tumors, cancer cells exhibited high TLR expression: 75.9% for TLR3, 76.1% for TLR4, and 84.2% for TLR9 when compared to non cancerous cells.
Fig. 1.
a TLR4 positive staining in prostatic carcinoma (×400); b TLR3 positive staining in prostatic carcinoma (×400); c TLR9 positive staining in prostatic carcinoma (×400)
Table 3.
The expression of TLRs by the different cellular types in 133 prostate carcinomas
| Factor | Total cases | Tumor cells | Fibroblast-like cells | MICs |
|---|---|---|---|---|
| No. positive cases (%) | No. positive cases (%) | No. positive cases (%) | ||
| TLR3 | 112 | 85 (75.9) | 1 (0.9) | 0 (0) |
| TLR4 | 117 | 89 (76.1) | 5 (4.3) | 16 (13.7) |
| TLR9 | 101 | 85 (84.2) | 11 (10.9) | 1 (1) |
MICs mononuclear inflammatory cells
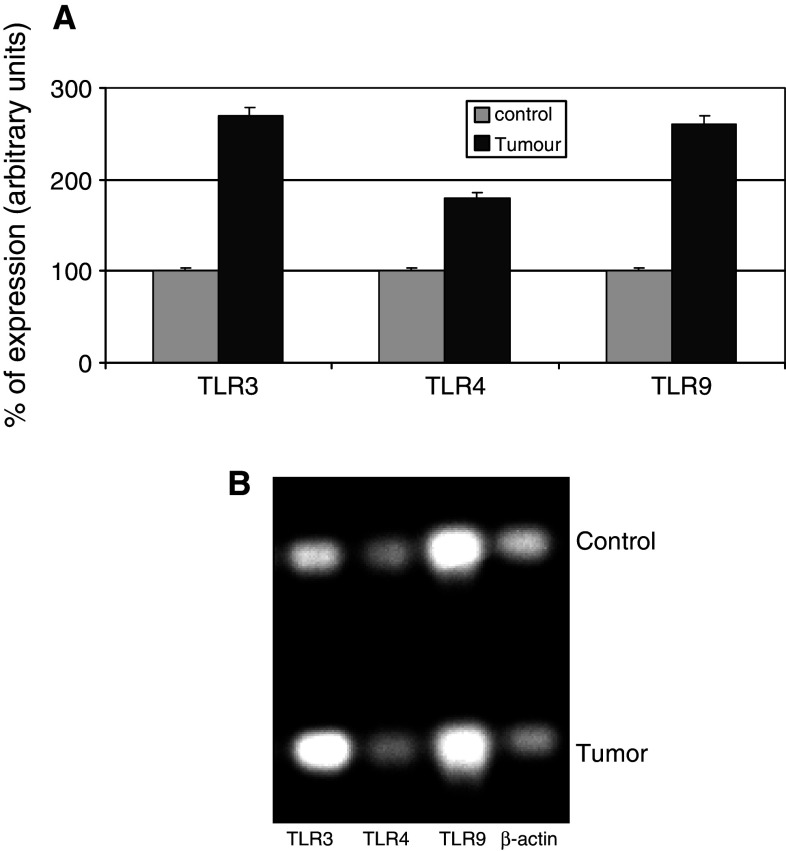
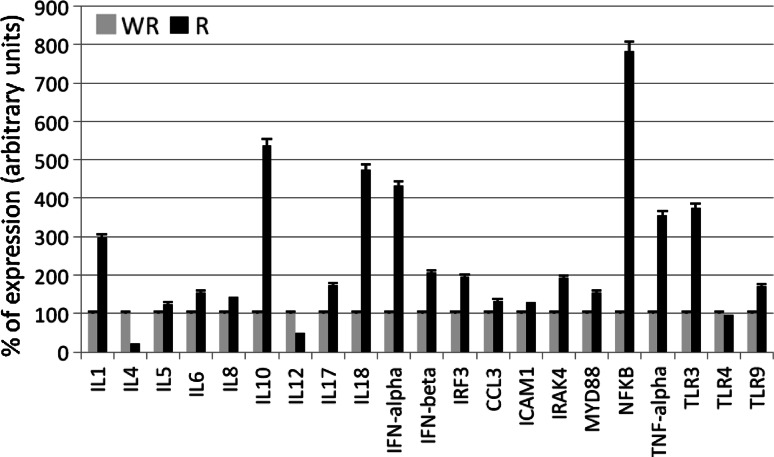
Figures 2 and 3 show the results of the real time-PCR. The percentage of TLR cDNA expression in samples obtained from prostate tumors and benign pathologies are compared in the upper panel whereas the electrophoresis analysis is shown in the lower panel. We found elevated TLR expression levels in carcinoma tissue relative to benign tissue. Figure 3 compared prostate tumors with and without recurrence in terms of interleukin, interferon, and some transcription factors related to inflammation. Samples of carcinomas with recurrence, expressing increased levels of TLR3 and TLR9, showed high levels of interleukins 1, 5, 6, 8, 10, 17 and 18, interferons IFNα, IFNβ, and IRF3 as well as mediators of signals related to inflammation CCL3, ICAM1, IRAK4, Myd88 and NFκB.
Fig. 2.
TLR3, 4, and 9 gene expression measured by semiquantitative real time PCR in prostate carcinomas and controls (benign pathologies). Upper panel (a), shows the percentage of TLR expression in control and tumor samples, and lower panel (b) shows the electrophoresis bands after real time PCR performed on equal amounts of cDNA from each sample. The housekeeping used was β-actin. Data represent the mean SD of three independent experiments
Fig. 3.
Percentage of expression of interleukins, interferons, and mediators of inflammation measured by semiquantitative real time PCR in prostate with (R) and without recurrence (WR). The housekeeping used was β-actin. Data represent the mean SD of three independent experiments
Immunostaining score values ranged widely for each TLR: TLR3 [median: 48.605 (range 0–152.29)], TLR4 [51.27 (0–280.06)], and TLR9 [52.42 (0–133.78)]. We also evaluated the possible relationship between the TLR expression and clinicopathological factors of prostate carcinomas including age, PSA level, tumor stage, and Gleason score, as summarized in Tables 4 and 5. TLR3 expression was significantly and positively associated with tumor stage and PSA level. A significant association between the TLR9 expression score and clustered Gleason score was also found (p = 0.005). Thus, patients with a TLR9 expression score equal to or greater than the median had an elevated Gleason score (over 7) with a significantly higher than expected frequency (Table 3).
Table 4.
Relationship between the expression of TLRs (score median) and tumor size and Gleason score in prostate carcinomas
| TLR3 score | TLR4 score | TLR9 score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <median n (%) |
≥median n (%) |
p | <median n (%) |
≥median n (%) |
p | <median n (%) |
≥median n (%) |
p | |
| Tumor stage | |||||||||
| T2 | 49 (87.5) | 45 (71.4) | 0.03 | 48 (82.8) | 43 (74.1) | 0.18 | 42 (82.4) | 39 (78) | 0.38 |
| T3–4 | 7 (12.5) | 16 (28.6) | 10 (17.2) | 15 (25.9) | 11 (17.6) | 11 (22) | |||
| Score gleason | |||||||||
| 2–7 (3 + 4) | 48 (85.7) | 33 (80.4) | 0.3 | 51 (87.9) | 45 (77.6) | 0.11 | 46 (90.2) | 38 (76) | 0.05 |
| 7 (4 + 3)–10 | 8 (14.3) | 11 (19.6) | 7 (12.1) | 13 (22.4) | 5 (9.8) | 12 (24) | |||
Table 5.
Relationship between the expression of TLRs and preoperative serum PSA levels in patients with prostate carcinoma
| TLR expression | N (%) | PSA serum levels (ng/ml) | p |
|---|---|---|---|
| Mean (range) | |||
| TLR3 ≥ median | 56 (42) | 10.78 (2.7–31) | 0.016 |
| TLR3 < median | 56 (42) | 8.01 (0.7–22.6) | |
| TLR4 ≥ median | 58 (43) | 9.72 (2.7–31) | 0.45 |
| TLR4 < median | 58 (43) | 8.88 (0.7–28.4) | |
| TLR9 ≥ median | 50 (37) | 9.7 (0.7–28.4) | 0.7 |
| TLR9 < median | 51 (38) | 9.22 (3.9–41) |
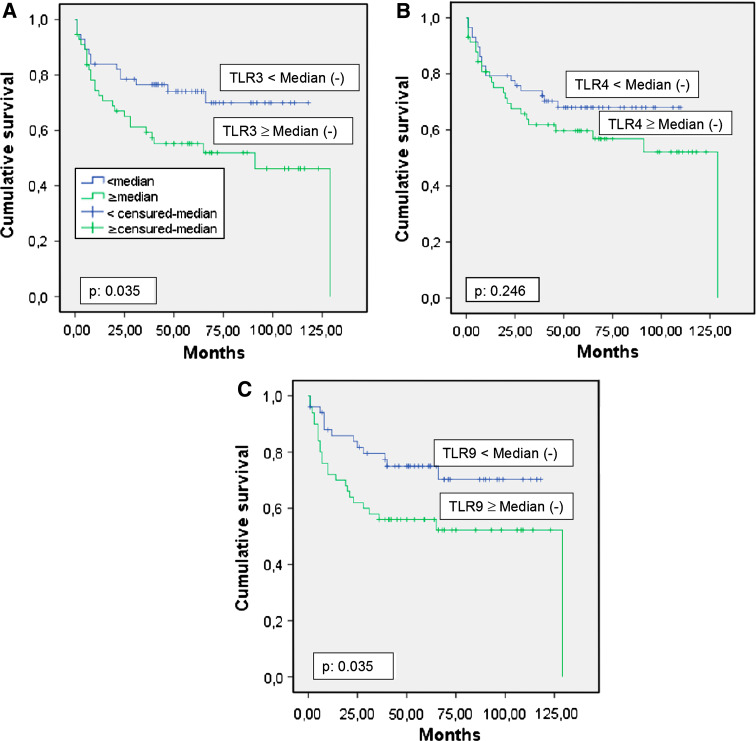
We also analyzed the possible relationship between global TLR expression and biochemical recurrence in prostate carcinomas (Fig. 4). Higher score values for TLR3 (p = 0.039) and TLR9 (p = 0.039) expression, but not TLR4 (p = 0.246), were significantly associated with a greater rate of tumor progression (Table 6). However, multivariate analysis with a Cox model demonstrated that only PSA level, tumor stage and Gleason score were significantly and independently associated with biochemical recurrence in patients with prostate carcinoma (Table 6).
Fig. 4.
a Probability of biochemical recurrence as function of TLR3 median (p 0.035), b probability of biochemical recurrence as function of TLR4 median (p 0.246), c probability of biochemical recurrence as function of TLR9 median (p 0.035)
Table 6.
Cox univariate (HR) and multivariate (RR) regression analysis of the relationship between TLR expression and biochemical recurrence, adjusted by stage (T2 vs. T3–4) and preoperative PSA level (≤10 vs. >10)
| Total cases | Events frequency | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | RR (95% CI) | p value | |||
| TLR3 (Score <median vs. >median) | 56/56 | 15/27 | 1.95 (1.03–3.6) | 0.039 | 1.2 (0.56–2.59) | 0.639 |
| TLR4 (Score <median vs. >median) | 58/58 | 18/25 | 1.43 (0.77–2.64) | 0.251 | 1.15 (0.55–2.4) | 0.7 |
| TLR9 (Score <median vs. >median) | 51/50 | 13/24 | 2.04 (1.03–4.02) | 0.039 | 1.67 (0.81–3.43) | 0.16 |
| PSA (≤10 vs. >10) | 93/40 | 28/19 | 1.87 (1.04–3.38) | 0.036 | 2.18 (1.05–4.54) | 0.037 |
| Tumor stage (T2 vs. T3–4) | 106/27 | 27/20 | 4.81 (2.67–8.69) | <0.001 | 2.89 (1.28–6.49) | 0.01 |
| Gleason score | 1.8 (1.4–2.4) | <0.001 | 1.67 (1.18–2.34) | 0.04 | ||
Gleason score was considered as a continuous variable
Discussion
To our knowledge, this is the first study that analyzes tumor expression as well as the prognostic significance of TLRs in prostate cancer. The results demonstrate an association between TLR3 and TLR9 expression and biochemical recurrence.
Elevated TLR expression has been described in different human tumors [14, 20, 29–37]. Mice deficient in such receptors were found to be protected from or develop less inducible tumors in experimental models [34, 35]. Cancer cells activated by TLR signals may release cytokines and chemokines that in turn may recruit immune cells and stimulate them to release further cytokines and chemokines. This process results in a cytokine profile that is associated with immune tolerance, cancer progression, and propagation of the tumor microenvironment [36]. Furthermore, chronic inflammatory conditions in selected organs increase the risk of cancer [38, 39]. Thus, some cytokines are implicated in the regulation of metalloproteases and their inhibitors [40, 41], some of which are involved in prostate cancer by degradation of extracellular matrix (ECM) [42]. Our assays demonstrated that tumor samples from patients with biochemical recurrence expressed high levels of TLR3 and TLR9. These tumors showed increased levels of cytokines IL1, IL10, IL17, IL18 and TNFα; interferons IFN-α, IFN-β, IRF3; mediators of immune response MyD88, IRAK4; and the transcription factor NFκB. Such factors are related to prostate cancer and play an important role in cell survival, proliferation, and angiogenesis [43–48]. Biological signals elicited from TLR-activated tumor cells might be a molecular link between inflammation and cancer.
Our results show a high expression of TLR3, TLR4, and TLR9 in prostate cancer cells but not benign prostate tissues in concordance with previous results published by Ilvesaro et al. [24]. Expression of TLR3 was also reported in prostate cancer cell lines LNCaP and PC3. However, the authors suggested that TLR3 plays a role in inhibiting cell cycle inducing apoptosis [20]. In addition, the use of TLR3 agonists has been successful in prostate immune-based therapies [18, 19]. Although it is unclear whether receptor activity is reactive or causal, our results showed associations between TLR3 tumor expression and clinical parameters of tumor aggressiveness. The implications for therapeutic interventions using TLR’s as targets could justify numerous creative strategies.
Recent studies have also shown high TLR9 expression in various normal epithelial and cancer cells, including breast, brain, gastric, lung, and prostate cancer cells [24, 49–53]. Our data support recent evidence that expression of TLR9 is increased in prostate cancer specimens [22], and also suggest that TLR9 may help to identify the population of men with prostatic cancer associated with worse prognosis. However, in the present study we found no association between TLR4 expression and outcome for patients with prostate cancer. It is noteworthy that TLRs are evolutionarily well-conserved transmembrane proteins that recognize microbe-derived molecular patterns. TLR4 is the receptor for bacterial lipopolysaccharide, and TLR3 and TLR9 subfamily are receptors for microbial RNA and DNA [54]. Further studies are needed to determine the influence of these recognized stimuli on prostate cancer prognosis.
Components of bacteria and viruses have been identified within pathological specimens of men with prostate cancer. There is evidence that the presence of pathogens in the urinary system may contribute to the malignant transformation of prostate epithelia through the activation of TLRs [32, 55, 56]. In the present study, no evidence of previous urinary infection was observed. Patients had no symptoms of chronic prostatitis and infectious agents were not demonstrated in prostate specimens. However, since the association between inflammatory infiltrates and proliferative epithelial atrophy in the prostate was first described, a growing body of histopathologic, molecular, and epidemiologic evidence indicates that inflammation plays a key role in the promotion of these neoplastic processes [57–59]. Nevertheless, until prostate carcinogenesis is better understood, the overall impact of inflammation on prostate cancer continues to be elusive [60]. In this context, the study of TLRs in prostate cancer could be particularly interesting.
One possible limitation of the present study is that the follow-up period may not have been long enough in our patients. It is known that more than 90% of biochemical recurrences occur within 5 years after radical prostatectomy [61]. In addition, while PSA recurrence universally antedates prostate cancer-specific mortality (PCSM), it is a limited surrogate end point due to its variable natural history. However, given the long time required to observe clinical events, most studies currently use PSA biochemical failure as the end point after radical prostatectomy. It is assumed that biochemical failure is a surrogate end point for the development of these clinical events. Despite the statistical significance in univariate analysis, TLR expressions were not independent variables of biochemical recurrence in the multivariate Cox analysis of this data set. TLR expression appears to be an important factor in the biological pathogenesis of prostate cancer; however, as a series of candidate independent prognostic factors these markers may be confounded by variables such as Gleason score. Further study of this association is warranted, and therapeutic strategies to boost or block these pathways may be relevant.
Acknowledgements
We gratefully thank Dr. Esperanza Fernández for helpful discussion and critical and careful reading. This work was supported by IPSEN PHARMA S.A. and FICYT (IB08-170).
Conflict of interest
The authors indicated no potential conflicts of interest.
References
- 1.Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In Vivo. 1994;8:439–443. [PubMed] [Google Scholar]
- 2.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 3.Khatami M. ‘Yin and Yang’ in inflammation: duality in innate immune cell function and tumorigenesis. Expert Opin Biol Ther. 2008;8:1461–1472. doi: 10.1517/14712598.8.10.1461. [DOI] [PubMed] [Google Scholar]
- 4.Ferrantini M, Capone I, Belardelli F. Dendritic cells and cytokines in immune rejection of cancer. Cytokine Growth Factor Rev. 2008;19:93–107. doi: 10.1016/j.cytogfr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Khatami M. Developmental phases of inflammation-induced massive lymphoid hyperplasia and extensive changes in epithelium in an experimental model of allergy: implications for a direct link between inflammation and carcinogenesis. Am J Ther. 2005;12:117–126. doi: 10.1097/01.mjt.0000143699.91156.21. [DOI] [PubMed] [Google Scholar]
- 6.Risques RA, Rabinovitch PS, Brentnall TA. Cancer surveillance in inflammatory bowel disease: new molecular approaches. Curr Opin Gastroenterol. 2006;22:382–390. doi: 10.1097/01.mog.0000231812.95525.a7. [DOI] [PubMed] [Google Scholar]
- 7.Smyth MJ, Trapani JA. Lymphocyte-mediated immunosurveillance of epithelial cancers? Trends Immunol. 2001;22:409–411. doi: 10.1016/S1471-4906(01)01977-9. [DOI] [PubMed] [Google Scholar]
- 8.Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1:779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley MA, Pett MR, Coleman N. HPV: from infection to cancer. Biochem Soc Trans. 2007;35:1456–1460. doi: 10.1042/BST0351456. [DOI] [PubMed] [Google Scholar]
- 10.Costantini S, Capone F, Guerriero E, Castello G. An approach for understanding the inflammation and cancer relationship. Immunol Lett. 2009;126:91–92. doi: 10.1016/j.imlet.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, Schaeffer AJ. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000;56:1025–1029. doi: 10.1016/S0090-4295(00)00844-X. [DOI] [PubMed] [Google Scholar]
- 12.Fujita K, Ewing CM, Sokoll LJ, Elliott DJ, Cunningham M, De Marzo AM, Isaacs WB, Pavlovich CP. Cytokine profiling of prostatic fluid from cancerous prostate glands identifies cytokines associated with extent of tumor and inflammation. Prostate. 2008;68:872–882. doi: 10.1002/pros.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lossos IS, Alizadeh AA, Rajapaksa R, Tibshirani R, Levy R. HGAL is a novel interleukin-4-inducible gene that strongly predicts survival in diffuse large B-cell lymphoma. Blood. 2003;101:433–440. doi: 10.1182/blood-2002-06-1931. [DOI] [PubMed] [Google Scholar]
- 16.Gohji K, Fujimoto N, Hara I, Fujii A, Gotoh A, Okada H, Arakawa S, Kitazawa S, Miyake H, Kamidono S. Serum matrix metalloproteinase-2 and its density in men with prostate cancer as a new predictor of disease extension. Int J Cancer. 1998;79:96–101. doi: 10.1002/(SICI)1097-0215(19980220)79:1<96::AID-IJC18>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Maeda H, Okamoto T, Akaike T. Human matrix metalloprotease activation by insults of bacterial infection involving proteases and free radicals. Biol Chem. 1998;379:193–200. doi: 10.1515/bchm.1998.379.2.193. [DOI] [PubMed] [Google Scholar]
- 18.Chin AI, Miyahira AK, Covarrubias A, Teague J, Guo B, Dempsey PW, Cheng G. Toll-like receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010;70:2595–2603. doi: 10.1158/0008-5472.CAN-09-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicodemus CF, Wang L, Lucas J, Varghese B, Berek JS. Toll-like receptor-3 as a target to enhance bioactivity of cancer immunotherapy. Am J Obstet Gynecol. 2010;608:e1–e8. doi: 10.1016/j.ajog.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Paone A, Starace D, Galli R, Padula F, De Cesaris P, Filippini A, Ziparo E, Riccioli A. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis. 2008;29:1334–1342. doi: 10.1093/carcin/bgn149. [DOI] [PubMed] [Google Scholar]
- 21.Zheng SL, Augustsson-Balter K, Chang B, Hedelin M, Li L, Adami HO, Bensen J, Li G, Johnasson JE, Turner AR. Sequence variants of toll-like receptor 4 are associated with prostate cancer risk: results from the CAncer Prostate in Sweden Study. Cancer Res. 2004;64:2918–2922. doi: 10.1158/0008-5472.CAN-03-3280. [DOI] [PubMed] [Google Scholar]
- 22.Vaisanen MR, Vaisanen T, Jukkola-Vuorinen A, Vuopala KS. Expression of toll-like receptor-9 is increased in poorly differentiated prostate tumors. Prostate. 2010;70:817–824. doi: 10.1002/pros.21115. [DOI] [PubMed] [Google Scholar]
- 23.Di JM, Pang J, Pu XY, Zhang Y, Liu XP, Fang YQ, Ruan XX, Gao X. Toll-like receptor 9 agonists promoter IL-8 and TGF-beta 1 production via activation of nuclear factor kappaB in PC-3 cells. Cancer Genet Cytogenet. 2009;192:60–67. doi: 10.1016/j.cancergencyto.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Ilvesaro JM, Merrell MA, Swain TM, Davidson J. Toll like receptor-9 agonists stimulate prostate cancer invasion in vitro. Prostate. 2007;67:774–781. doi: 10.1002/pros.20562. [DOI] [PubMed] [Google Scholar]
- 25.Flemming ID, Cooper IS, Hemson DE. The 1992 TNM classification. American Joint Committtee on Cancer Staging Manual. 5. Philadelphia: JB Lippincott; 1997. pp. 219–222. [Google Scholar]
- 26.(ISUP), TISoUP Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 27.Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, Gilks CB. Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol. 2002;117:723–728. doi: 10.1309/PEF8-GL6F-YWMC-AG56. [DOI] [PubMed] [Google Scholar]
- 28.Liu N, Lapcevich RK, Underhill CB, Han Z, Gao F, Swartz G, Plum SM, Zhang L, Green SJ. Metastatin: a hyaluronan-binding complex from cartilage that inhibits tumor growth. Cancer Res. 2001;61:1022–1028. [PubMed] [Google Scholar]
- 29.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 30.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 31.Wolska A, Lech-Maranda E, Robak T. Toll-like receptors and their role in carcinogenesis and anti-tumor treatment. Cell Mol Biol Lett. 2009;14:248–272. doi: 10.2478/s11658-008-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kundu SD, Lee C, Billips BK, Habermacher GM, Zhang Q, Liu V, Wong LY, Klumpp DJ, Thumbikat P. The toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate. 2008;68:223–229. doi: 10.1002/pros.20710. [DOI] [PubMed] [Google Scholar]
- 33.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 34.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato Y, Goto Y, Narita N, Hoon DS. Cancer cells expressing toll-like receptors and the tumor microenvironment. Cancer Microenviron. 2009;2:205–214. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stark JR, Wiklund F, Gronberg H, Schumacher F, Sinnott JA, Stampfer MJ, Mucci LA, Kraft P. Toll-like receptor signaling pathway variants and prostate cancer mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1859–1863. doi: 10.1158/1055-9965.EPI-08-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 39.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 40.Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993;59:329–341. doi: 10.1016/0163-7258(93)90074-N. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi H, Stearns V, Hayes DF. When is a tumor marker ready for prime time? A case study of c-erbB-2 as a predictive factor in breast cancer. J Clin Oncol. 2001;19:2334–2356. doi: 10.1200/JCO.2001.19.8.2334. [DOI] [PubMed] [Google Scholar]
- 42.Escaff S, Fernandez JM, Gonzalez LO, Suarez A, Gonzalez-Reyes S, Gonzalez JM, Vizoso FJ. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br J Cancer. 2010;102:922–929. doi: 10.1038/sj.bjc.6605569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saenz-Lopez P, Carretero R, Cozar JM, Romero JM, Canton J, Vilchez JR, Tallada M, Garrido F, Ruiz-Cabello F. Genetic polymorphisms of RANTES, IL1-A, MCP-1 and TNF-A genes in patients with prostate cancer. BMC Cancer. 2008;8:382. doi: 10.1186/1471-2407-8-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindmark F, Zheng SL, Wiklund F, Balter KA, Sun J, Chang B, Hedelin M, Clark J, Johansson JE, Meyers DA. Interleukin-1 receptor antagonist haplotype associated with prostate cancer risk. Br J Cancer. 2005;93:493–497. doi: 10.1038/sj.bjc.6602729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricote M, Garcia-Tunon I, Bethencourt FR, Fraile B, Paniagua R, Royuela M. Interleukin-1 (IL-1alpha and IL-1beta) and its receptors (IL-1RI, IL-1RII, and IL-1Ra) in prostate carcinoma. Cancer. 2004;100:1388–1396. doi: 10.1002/cncr.20142. [DOI] [PubMed] [Google Scholar]
- 46.Lebel-Binay S, Thiounn N, De Pinieux G, Vieillefond A, Debre B, Bonnefoy JY, Fridman WH, Pages F. IL-18 is produced by prostate cancer cells and secreted in response to interferons. Int J Cancer. 2003;106:827–835. doi: 10.1002/ijc.11285. [DOI] [PubMed] [Google Scholar]
- 47.Faupel-Badger JM, Kidd LC, Albanes D, Virtamo J, Woodson K, Tangrea JA. Association of IL-10 polymorphisms with prostate cancer risk and grade of disease. Cancer Causes Control. 2008;19:119–124. doi: 10.1007/s10552-007-9077-6. [DOI] [PubMed] [Google Scholar]
- 48.Zabaleta J, Su LJ, Lin HY, Sierra RA, Hall MC, Sartor AO, Clark PE, Hu JJ, Ochoa AC. Cytokine genetic polymorphisms and prostate cancer aggressiveness. Carcinogenesis. 2009;30:1358–1362. doi: 10.1093/carcin/bgp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J Biol Chem. 2004;279(18):19008–19017. doi: 10.1074/jbc.M311618200. [DOI] [PubMed] [Google Scholar]
- 50.Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, Bals R. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004;173:1219–1223. doi: 10.4049/jimmunol.173.2.1219. [DOI] [PubMed] [Google Scholar]
- 51.Schmausser B, Andrulis M, Endrich S, Muller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295:179–185. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Droemann D, Albrecht D, Gerdes J, Ulmer AJ, Branscheid D, Vollmer E, Dalhoff K, Zabel P, Goldmann T. Human lung cancer cells express functionally active Toll-like receptor 9. Respir Res. 2005;6:1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa T, Gehrs B, Rosenthal E, Chen D, Shackley B, Harris KW, Selander KS. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res. 2006;4:437–447. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 54.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/S0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 55.Nickel JC, Moon T. Chronic bacterial prostatitis: an evolving clinical enigma. Urology. 2005;66:2–8. doi: 10.1016/j.urology.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 56.Tanner MA, Shoskes D, Shahed A, Pace NR. Prevalence of corynebacterial 16S rRNA sequences in patients with bacterial and “nonbacterial” prostatitis. J Clin Microbiol. 1999;37:1863–1870. doi: 10.1128/jcm.37.6.1863-1870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Nelson WG, De Marzo AM, Deweese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–S11. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- 59.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 60.Stock D, Groome PA, Siemens DR. Inflammation and prostate cancer: a future target for prevention and therapy? Urol Clin North Am. 2008;35:117–130. doi: 10.1016/j.ucl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101–105. doi: 10.1016/S0022-5347(05)67457-5. [DOI] [PubMed] [Google Scholar]