Abstract
The production of soluble major histocompatibility complex class I–related chain A (MICA) is thought to antagonize NKG2D-mediated immunosurveillance. Interleukin-1β (IL-1β) is elevated in patients with chronic hepatitis C (CH), and this might contribute to the escape of hepatocellular carcinoma (HCC) cells from innate immunity. In this study, we investigated the immunoregulatory role of IL-1β in the production of soluble MICA of HCC cells. First, we investigated the correlation between the serum IL-1β levels and soluble MICA in CH patients. Serum IL-1β levels were associated with soluble MICA levels in CH patients. The serum IL-1β levels of CH patients with the HCC occurrence were significantly higher than those of CH patients without HCC. We next examined the MICA production of IL-1β-treated HCC cells. Addition of IL-1β resulted in significant increase in the production of soluble MICA in HepG2 and PLC/PRF/5 cells, human HCC cells. But soluble MICA was not detected in both non-treated and IL-1β-treated normal hepatocytes. Addition of IL-1β did not increase the expressions of membrane-bound MICA on HCC cells. These were observed similarly in various cancer cells including a gastric cancer (MKN1), two colon cancers (HCT116 and HT29) and a cervical cancer (HeLa). Addition of IL-1β also increased the expression of a disintegrin and metalloproteinase (ADAM)9 in HCC cells, and the knockdown of ADAM9 in IL-1β-treated HCC cells resulted in the decrease in the production of soluble MICA of HCC cells. These findings indicate that IL-1β might enhance the production of soluble MICA by activating ADAM9 in human HCC.
Keywords: IL-1β, Hepatocellular carcinoma, Soluble MICA, ADAM9
Introduction
Interleukin-1β (IL-1β) is a proinflammatory cytokine with multiple biological effects [1]. Serum levels of IL-1β are elevated in patients infected with hepatitis C virus (HCV), suggesting the role of IL-1β in the inflammation of liver [2–4]. Several polymorphisms of the IL-1 gene have been reported to affect IL-1β production [5, 6]. A number of clinical studies suggested that polymorphisms of IL-1β gene are associated with diverse disease including cancer [5, 7]. IL-1β gene polymorphisms have also been reported to be associated with HCC in HCV- or HBV-infected patients [8–10]. While genetic studies have suggested an important role for IL-1β in cancer, direct evidence that IL-1β contributes to the pathogenesis of cancer has been lacking. Recently, Tu et al. [11] reported that stomach-specific expression of IL-1β in transgenic mice leads to spontaneous gastric inflammation and cancer that correlates with myeloid-derived suppressor cells to the stomach. However, no studies have been published on the direct effect of IL-1β on the HCC cells in patients infected with HCV.
MHC class I–related chain A (MICA), a ligand for NKG2D, is rarely expressed on normal cells, but frequently on tumor cells [12, 13]. The engagement of MICA and NKG2D strongly activates NK cells enhancing their cytolytic activity and cytokine production [14]. Thus, the MICA-NKG2D pathway is an important mechanism by which the host immune system recognizes and kills transformed cells [15]. In addition to those membrane-bound forms, MICA molecules are cleaved proteolytically from tumor cells and appear as soluble forms in the sera of patients with malignancy including HCC [16–18]. The release of soluble MICA/B from tumor cells is thought to antagonize NKG2D-mediated immunosurveillance. We previously demonstrated that a disintegrin and metalloproteinase (ADAM)9 protease plays essential roles in the shedding of MICA molecules on HCC cells [19]. However, the mechanism of regulating the production of soluble MICA in HCC cells remains to be elucidated.
In this study, we investigated the immunoregulatory role of IL-1β in the production of soluble MICA from HCC cells. Of importance is the discovery that the serum IL-1β levels in chronic hepatitis patients with the HCC occurrence were significantly higher than those without HCC occurrence and that IL-1β enhances the production of soluble MICA via activating ADAM9 in human HCC cells. The present study sheds light on previously unrecognized immunological effects of IL-1β on HCC cells.
Materials and methods
HCC cell lines and normal hepatocyte cultures
HepG2 and PLC/PRF/5, human HCC cell lines, were purchased from American Type Culture Collection (Rockville, MD) and were cultured with Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (GIBCO/Life Technologies, Grand Island, NY) in a humidified incubator at 5% CO2 and 37°C. 2 × 105 HepG2 and PLC/PRF/5 cells or normal hepatocytes (ScienCell Research Laboratories, Carlsbad, CA) were cultured in 6-well tissue culture plates for 48 h in the presence or absence of human interleukin-1β (IL-1β) (50 ng/ml, PeproTech EC, London, UK), and the HCC cells were harvested and subjected to evaluating the expression of membrane-bound MICA and ADAM9 and the production of soluble MICA.
Flow cytometry
For the detection of membrane-bound MICA, HCC cells were incubated with anti-MICA-specific Ab (Santa Cruz Biotechnology, Santa Cruz, CA) and stained with Goat F(ab′)2 fragment anti-mouse IgG(H + L) − PE (Beckman Coulter, Fullerton, CA) as a secondary reagent. Flow cytometric analysis was performed using a FACScan flow cytometer (Becton–Dickinson, San Jose, CA).
Western blotting
The total cellular protein was electrophoretically separated by sodium dodecyl sulfate-12% polyacrylamide gels and transferred onto PVDF membrane. The membrane was blocked in Tris-buffered saline–Tween containing 5% skim milk for 1 h and then probed with anti-ADAM9 mAb (R&D Systems, Minneapolis, MN) at 4°C overnight. Horseradish peroxidase-conjugated anti-rabbit Ab and SuperSignal West Pico System (Pierce, Rockford, IL) were used for the detection of blots.
Real-time reverse transcription (RT) PCR
Total RNA was isolated using RNeasy Mini Kit (Qiagen K.K., Tokyo, Japan) and was reverse transcribed using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster city, CA). The mRNA levels were evaluated using ABI PRISM 7900 Sequence Detection System (Applied Biosystems). Ready-to-use assays (Applied Biosystems) were used for the quantification of ADAM9 (Hs00177638_m1), MICA (Hs00792195_m1) and β-actin (Hs99999903_m1) mRNAs according to the manufacturer’s instructions. β-Actin mRNA from each sample was quantified as an endogenous control of internal RNA.
RNA silencing
The small interfering RNA (siRNA) method was used to knockdown ADAM9 as previously described [19]. At 24-h post-transfection, the cells were analyzed for specific depletion of the protein of ADAM9 by western blotting. The following siRNA were used: ADAM9, 5′-UGUCCAAACACAUUAAUCCCGCCUG-3′; an irrelevant siRNA as a control, 5′-UGUCGCACAAACACUUAACUCCCUG-3′.
ELISA
The sera from chronic hepatitis C patients (N = 24) with or without the occurrence of HCC were subjected to analysis of IL-1β and soluble MICA. Informed consent, under an Institutional Review Board–approved protocol, was obtained from all patients before sample acquisition. The sera and the supernatants of cultured HCC cells were harvested, and the levels of IL-1β and soluble MICA were determined by human IL-1β ELISA set II (BD Biosciences, San Diego, CA) and DuoSet MICA eELISA kit (R&D Systems, Minneapolis, MN) in accordance with the manufacturer’s instructions, respectively.
NK cell analysis
NK cells were isolated from human peripheral blood mononuclear cells by magnetic cell sorting using CD56 MicroBeads (Miltenyi Biotech, Auburn, CA) as previously described [19]. HepG2 and PLC/PRF/5 cells were treated with IL-1β (50 ng/ml) for 48 h. The cytolytic ability of NK cells against IL-1β-treated or non-treated HepG2 and PLC/PRF/5 cells was assessed by 4-hr 51Cr-releasing assay as previously described [19].
Statistics
All values were expressed as the mean and SD. The statistical significance of differences between the groups was determined by applying Student’s t test or two-sample t test with Welch correction after each group had been tested with equal variance and Fisher’s exact probability test. We defined statistical significance as p < 0.05.
Results
Serum IL-1β levels were associated with soluble MICA in chronic liver disease patients
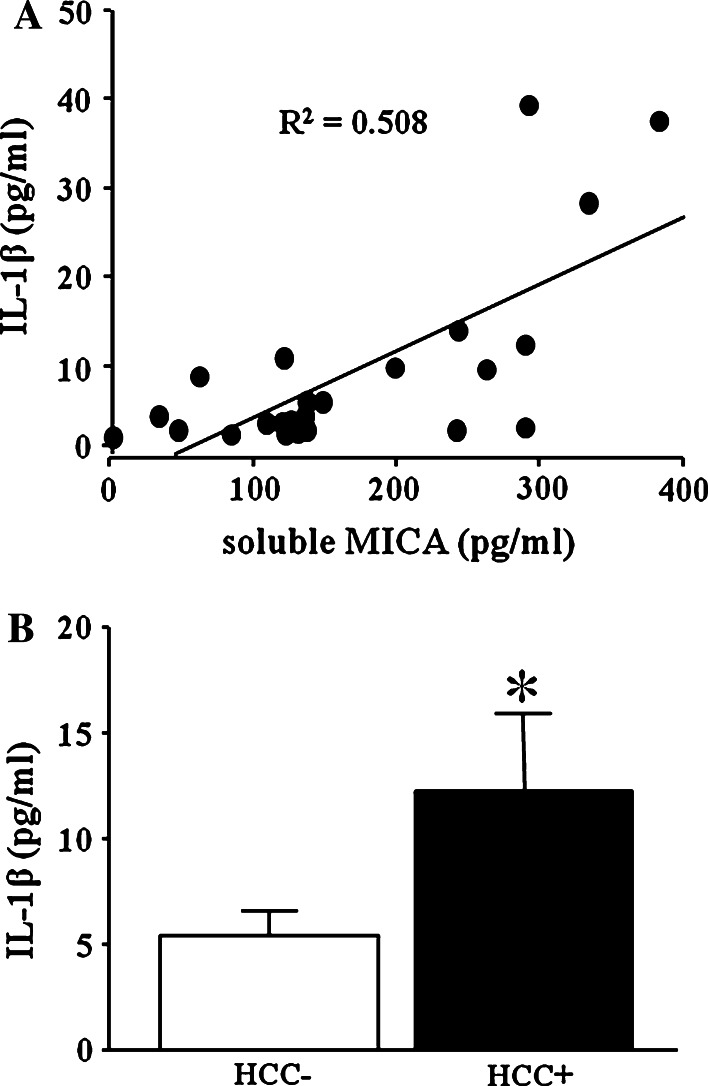
We first examined the IL-1β levels and soluble MICA levels of twenty-four chronic hepatitis C (CH) patients. Serum IL-1β levels in CH patients correlated with soluble MICA levels (Fig. 1a). We next examined the serum IL-1β levels of CH patients with or without the occurrence of HCC. We examined serum IL-1β levels of these 24 CH patients before HCC occurrence and followed these patients for 5 years. CH patients could be divided into two groups according to the occurrence of HCC (Table 1). As shown in Fig. 1b, the serum IL-1β levels of patients with the occurrence of HCC (n = 11) were significantly higher than those of patients without the occurrence of HCC (n = 13). These results suggested that the elevation of serum IL-1β levels might be associated with the occurrence of HCC in CH patients.
Fig. 1.
The correlation between serum IL-1β and soluble MICA in patients with chronic liver disease and serum IL-1β levels in chronic liver disease patients with or without the HCC occurrence. a Correlation between serum IL-1β levels and soluble MICA levels in patients with chronic liver disease (N = 24). The serum IL-1β and soluble MICA were evaluated by specific ELISA, respectively. b Serum IL-1β levels in chronic hepatitis patients with HCC occurrence (HCC+, N = 11) or without HCC occurrence (HCC−, N = 13) were evaluated by specific ELISA. All patients were HCV-RNA-positive. *p < 0.05
Table 1.
Clinical backgrounds
| HCC(+) | HCC(−) | |
|---|---|---|
| Number | 11 | 13 |
| Age | 61 ± 6 | 61 ± 8 |
| Gender (M/F) | 8/3 | 11/2 |
| Platelet (×104/μl) | 15 ± 5 | 14 ± 3 |
| ALT (IU/l) | 122 ± 109 | 89 ± 44 |
HCC(+) chronic hepatitis C patients with the occurrence of HCC, HCC(−) chronic hepatitis C patients without the occurrence of HCC, M male, F female, ALT alanine aminotransferase
IL-1β increases the production of soluble MICA from HCC cells, but not from normal hepatocytes
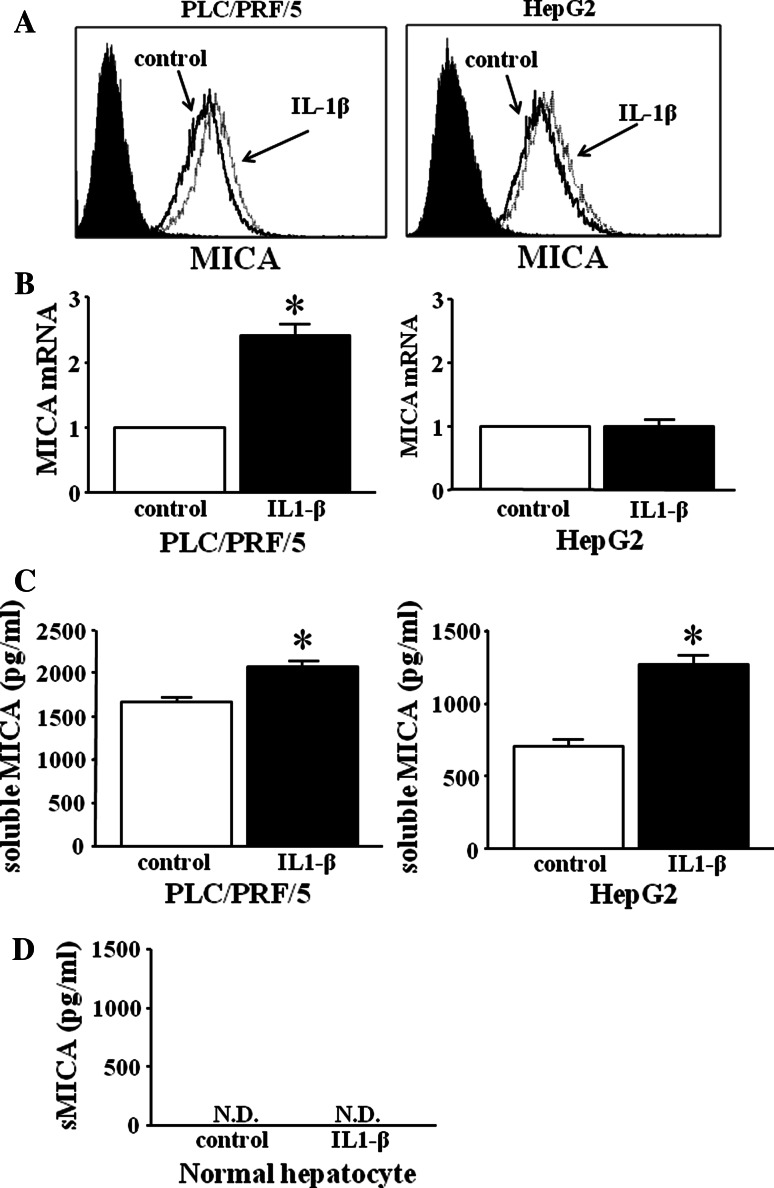
We examined whether IL-1β treatment could induce MICA expressions on HCC cells (PLC/PRF/5 cells and HepG2 cells). Both PLC/PRF/5 cells and HepG2 cells were cultured for 48 h with IL-1β (50 ng/ml) and then subjected to analysis of the expression of membrane-bound MICA and mRNA of MICA. The expression of membrane-bound MICA of IL-1β-treated HCC cells was similar to that of non-treated HCC cells (Fig. 2a). IL-1β treatment induced significant increase of mRNA of MICA in PLC/PRF/5 cells, but this did not in HepG2 cells (Fig. 2b). We next examined the production of soluble MICA in the supernatants of the IL-1β-treated HCC cells. IL-1β treatment resulted in the significant increase in the production of soluble MICA in both PLC/PRF/5 and HepG2 cells (Fig. 2c). These results demonstrated that the addition of IL-1β did not change the expression of membrane-bound MICA but resulted in significant increase in the production of soluble MICA in HCC cells. We also examined the effect of IL-1β on normal hepatocytes. As shown in Fig. 2d, normal hepatocytes did not produce soluble MICA and the addition of IL-1β did not result in its production. These results demonstrated that IL-1β could induce the increase in the production of soluble MICA only from HCC cells, but not from normal hepatocytes.
Fig. 2.
Expression of membrane-bound MICA and the production of soluble MICA in IL-1β-treated HCC cells and normal hepatocytes. Both PLC/PRF/5 cells and HepG2 cells were treated in the presence or absence of IL-1β (50 ng/ml) for 48 h. The expression of membrane-bound MICA (a) and mRNA expression of MICA (b) in IL-1β-treated or non-treated HCC cells were evaluated by flow cytometry or real-time RT-PCR, respectively. Black line histograms, MICA staining of non-treated cells; dotted line histograms, MICA staining of IL-1β-treated cells; shaded/black histograms, control IgG isotype Ab staining. Similar results were obtained from two independent experiments. *p < 0.05. c We examined the production of soluble MICA on IL-1β-treated or non-treated HCC cells by specific ELISA. *p < 0.05. d Normal hepatocytes were treated in the presence or absence of IL-1β (50 ng/ml) for 48 h. The production of soluble MICA on IL-1β-treated or non-treated normal hepatocytes was examined by specific ELISA. ND not detected
IL-1β treatment increases the production of soluble MICA from various cancer cells
We also examined IL-1β-dependent MICA regulation on another cancer cells including a gastric cancer cell line (MKN1), colon cancer cell lines (HCT116, HT29) and a cervical cancer cell line (HeLa). The expressions of membrane-bound MICA on these cells did not change by the addition of IL-1β in all cancer cells. Interestingly, the addition of IL-1β resulted in significant increase in the production of soluble MICA in all cancer cells (Fig. 3). These results demonstrated that IL-1β could induce the increase in the production of soluble MICA not only from HCC cells but also from various cancer cells.
Fig. 3.
Soluble MICA production of IL-1β-treated various cancer cells. Various cancer cells including MKN1, HCT116, HT29 and HeLa cells were treated in the presence or absence of IL-1β (50 ng/ml) for 48 h. Soluble MICA production of IL-1β-treated or non-treated various cancer cells was evaluated by specific ELISA (right panel). sMICA, soluble MICA. We also examined the expression of membrane-bound MICA on IL-1β-treated or non-treated various cancer cells by flow cytometry (left panel). Black line histograms, MICA staining of non-treated cells; Gray line histograms, MICA staining of IL-1β-treated cells; shaded/black histograms, control IgG isotype Ab staining. Similar results were obtained from two independent experiments. *p < 0.05
ADAM9 activated by IL-1β plays important roles in the production of soluble MICA from HCC cells
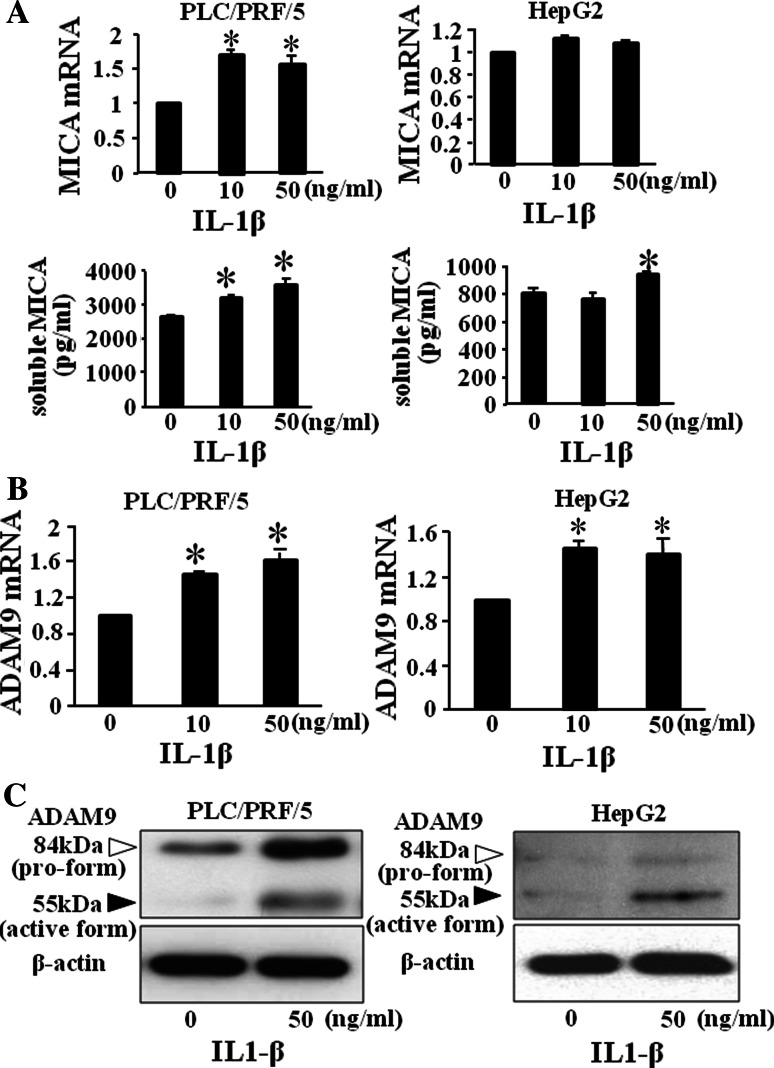
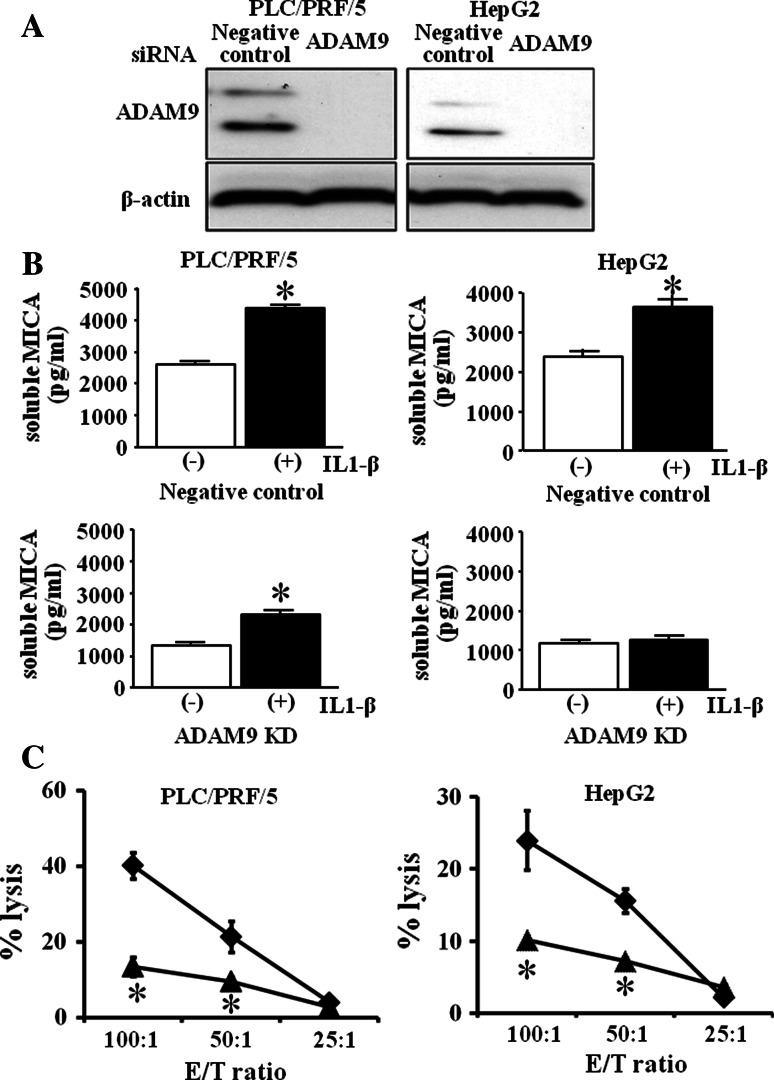
We next examined the mRNA of MICA and the production of soluble MICA in HCC cells treated with various doses of IL-1β. As shown in Fig. 4a, mRNA expression of MICA in IL-1β-treated PLC/PRF/5 cells significantly increased but that in HepG2 cells did not. The production of soluble MICA in IL-1β-treated PLC/PRF/5 cells significantly increased in a dose-dependent manner, and the production of soluble MICA significantly increased in 50 ng/ml IL-1β-treated HepG2 cells. Recently, members of the metzincin superfamily, such as ADAM proteins, have been reported to play essential roles in the proteolytic release of the ectodomain of transmembranous proteins, including MICA, from the cell surface [17, 20]. We previously reported that ADAM9 plays essential roles in MICA shedding in human HCC cells and that the activation of ADAM9 protease resulted in up-regulation of the production of soluble MICA from human HCC cells [19]. So we examined the involvement of ADAM9 in the up-regulation of soluble MICA production in IL-1β-treated HCC cells. As shown in Fig. 4b, mRNA levels of ADAM9 in IL-1β-treated PLC/PRF/5 cells significantly increased in a dose-dependent manner. mRNA of ADAM9 in IL-1β-treated HepG2 cells significantly increased in 10 ng/ml and 50 ng/ml IL-1β-treated HepG2 cells. The ADAM9 protein expression including both pro-form and active form also increased in IL-1β-treated HCC cells (Fig. 4c). To confirm the involvement of ADAM9 in IL-1β-treated HCC cells, we examined the soluble MICA production in IL-1β-treated ADAM9-knockdown (ADAM9KD) HCC cells. Both PLC/PRF/5 and HepG2 cells were transfected with ADAM9-siRNA or an irrelevant siRNA as a control. The expression of ADAM9 was clearly suppressed in PLC/PRF/5 cells and HepG2 cells at protein levels (Fig. 5a). In both PLC/PRF/5 and HepG2 cells transfected with control siRNA, the productions of soluble MICA in IL-1β-treated cells were significantly higher than those in non-treated HCC cells. In contrast, the production of soluble MICA in IL-1β-treated ADAM9KD-HepG2 cells was similar to that in non-treated ADAM9KD-HepG2 cells (Fig. 5b). The production of soluble MICA in IL-1β-treated ADAM9KD-PLC/PRF/5 cells also tended to decrease compared with that in non-treated ADAM9KD-PLC/PRF/5 cells (Fig. 5b). The decrease in soluble MICA production in ADAM9KD cells was different between PLC/PRF/5 cells and HepG2 cells. However, these results suggested at least that the increase in ADAM9 expression by IL-1β resulted in the increase in soluble MICA levels in IL-1β-treated HCC cells.
Fig. 4.
IL-1β increased the ADAM9 expression of HCC cells and the production of soluble MICA. PLC/PRF/5 and HepG2 cells were cultured with 0, 10 and 50 ng/ml IL-1β for 48 h. a The production of soluble MICA from IL-1β-treated HCC cells was examined by specific ELISA, and mRNA levels of MICA of IL-1β-treated HCC cells were examined by real-time PCR. b, c mRNA and protein expression of ADAM9 by real-time RT-PCR (b) and western blotting (c), respectively. Representative results are shown. Similar results were obtained from 3 independent experiments. *p < 0.05
Fig. 5.
The production of soluble MICA of ADAM9KD-HCC cells and the cytolytic activity against IL-1β-treated HCC cells. a Both HCC cells (PLC/PRF/5 and HepG2) were transfected with ADAM9 siRNA (ADAM9KD) or an irrelevant siRNA (negative control), and at 24-h post-transfection, the protein expression of ADAM9 was examined by western blotting. b Both HCC cells were cultured with (+) or without (−) 50 ng/ml IL-1β for 48 h. Soluble MICA production from ADAM9KD-HCC cells or negative control-HCC cells was evaluated by specific ELISA. *p < 0.05. Similar results were obtained from 3 independent experiments. c Both PLC/PRF/5 and HepG2 cells were cultured with or without IL-1β (50 ng/ml) for 48 h. The cytolytic activities of NK cells against IL-1β-treated or non-treated PLC/PRF/5 and HepG2 cells were evaluated by 51Cr-releasing assay. Non-treated cells (filled diamond), IL-1β-treated cells (filled triangle). Representative results are shown. Similar results were obtained from three independent experiments. *p < 0.05 versus the cytolytic activity of non-treated cells. Similar results were obtained from 3 independent experiments
IL-1β-treated HCC cells are resistant to the cytolytic activity of NK cells
We next examined whether IL-1β could modify the NK sensitivity of human HCC cells. The cytolytic activities of NK cells against IL-1β-treated PLC/PRF/5 and IL-1β-treated HepG2 cells were lower than those against non-treated HCC cells (Fig. 5c). These results demonstrated that IL-1β treatment resulted in the increased resistance of HCC cells to NK cells.
Discussion
The liver contains a large compartment of innate immune cells (NK cells and NKT cells) and acquired immune cells (T cells) [21, 22]. Recent study has demonstrated that innate immune system via NKG2D signal, expressing on NK cells, might play critical roles in tumor surveillance [23]. However, the escape mechanism of HCC cells from NK cells remains unclear. We previously demonstrated that membrane-bound MICA, activating molecule of NK cells, on HCC cells plays essential roles in the NK sensitivity of HCC cells [13, 24] and that the serum soluble MICA increase along the progression of chronic liver disease [18]. The production of soluble MICA in HCC patients is the highest compared with chronic hepatitis or liver cirrhosis patients without HCC [18]. These results suggest that unknown factors may accelerate the cleavage of MICA in HCC cells. IL-1β is produced mainly by local immune cells including activated Kupffer cells [25]. Because IL-1β increased in CH or LC patients [26–28], we focus on the possible role of IL-1β in the escape mechanism of HCC cells from NK cells.
Inflammatory cytokines including IL-1β and IL-6 increased in CH or LC patients [26–28], suggesting that both IL-1β and IL-6 might play roles in the HCC development. Recently high serum IL-6 level was an independent risk factor for HCC development in both chronic hepatitis C and B patients [29, 30], which suggested the possible roles of IL-6 in HCC development. However, the IL-1β levels in chronic liver disease, premalignant conditions, have been little reported. In this study, we demonstrated that serum IL-1β levels in chronic hepatitis C patients with HCC occurrence were significantly higher than those without HCC occurrence and that serum IL-1β levels correlated with soluble MICA which could inhibit NK activity. These results suggested that elevated IL-1β in CH patients might support the survival of HCC cells by changing local immunological environment.
MICA shedding is thought to be the principle mechanism by which tumor cells escape from NKG2D-mediated immunosurveillance [16]. In this study, we demonstrated that addition of IL-1β resulted in the increase in soluble MICA production from HCC cells. Interestingly, IL-1β treatment also resulted in the increase of soluble MICA in various cancer cells. Addition of other IL-1 family cytokines such as IL-1α, Il-18 and IL-33 did not result in the increase in soluble MICA production from both PLC/PRF/5 and HepG2 cells (Kohga, unpublished data). In addition to IL-1β, serum IL-6 and TNF-α are elevated in HCC patients. We compared IL-1β with IL-6 and TNF-α in the ability of the production of soluble MICA from HCC cells. IL-1β could increase the production of soluble MICA from HCC cells, but both IL-6 and TNF-α could not in PLC/PRF/5 cells and HepG2 cells. No synergistic effects of the combination of IL-1β, IL-6 and TNF-α were observed (Kohga, unpublished data). These results demonstrated that only IL-1β could induce the increase in the production of soluble MICA from HCC cells, suggesting that IL-1β might play an important role in the progression of HCC.
IL-1β treatment resulted in the increase in soluble MICA production but not the increase of mRNA in HepG2 cells. The production of soluble MICA depended on both the production of mRNA and the shedding of ADAM9. We previously demonstrated that ADAM9 plays an essential role in the shedding of MICA in HCC cells [19]. In the present study, we demonstrated that IL-1β treatment resulted in the increase in ADAM9 expression in HepG2 cells and that ADAM9 knockdown by siRNA resulted in the decrease in the production of soluble MICA from IL-1β-treated HepG2 cells. Our results suggested at least that the increase in ADAM9 might resulted in the increase in the shedding of soluble MICA in the IL-1β-treated HCC cells.
Recent studies have identified various metalloproteinases responsible for MICA/B cleavage in various cancers [31]. We previously found that ADAM9 plays critical roles in the shedding of MICA in human HCC. ADAM9 was directly associated with decreasing the expression of membrane-bound MICA and increasing the production of soluble MICA in human HCC [19]. Thus, it would be interesting to examine the activity of ADAM9 in IL-1β-treated HCC cells to understand how IL-1β regulates the production of soluble MICA from HCC cells. We demonstrated that IL-1β treatment could increase the mRNA and protein expression of ADAM9 in HCC cells and that ADAM9 knockdown in HCC cells resulted in decreasing of the soluble MICA production. These results suggested that ADAM9 played an important role in the increase in soluble MICA production from IL-1β-treated HCC cells. Both ADAMs and ADAMs with thrombospondin motifs (ADAMTS) are proteinases closely related to matrix metalloproteinases (MMPs). Structure of ADAMs and ADAMTS is highly conserved and involves metalloproteinase and disintegrin domains endowing them with features of both proteinases and adhesion molecules [32]. Several ADAMTSs including ADAMTS1 and ADAMTS9 were activated by IL-1β via NFATc1 transcription factor in chondrosarcoma [33, 34]. Although IL-1β may regulate such transcription factors in HCC cells, the detail mechanism of the activation of ADAM9 by IL-1β remains unclear. The concentration of IL-1β in our in vitro study was high compared with the serum IL-1β concentration level. However, the local IL-1β concentration in the liver tissues still remains unknown and may differ from the serum IL-1β concentration. Our in vitro study at least demonstrated that IL-1β could enhance the production of soluble MICA via up-regulating the expressions of ADAM9 in HCC cells, which might support the possible role of IL-1β in the survival of HCC cells.
Cai et al. [35] demonstrated that the numbers of CD56+ NK cells reduced in HCC tissues compared with healthy donors and CD56+ NK cells in HCC patients displayed impairments in cytotoxicity and IFN-γ production. This suggests that immunological microenvironment in liver tissues of CH patients might be favorable for the survival of HCC cells. We demonstrated that serum IL-1β levels correlated with soluble MICA in CH patients, which is consistent with our in vitro data. This suggests that the chronic elevation of IL-1β in CH patients might impair the function of NK cells by accelerating the production of soluble MICA. We also demonstrated that IL-1β treatment resulted in the inhibition of the cytolytic activity of NK cells against HCC cells. Intrahepatic activated macrophages and plasma cells could produce IL-1β inducing the inflammatory process in chronic liver disease [36]. If we could control the production of IL-1β with new reagents, it might be possible to develop a new therapeutic strategy against HCC. IL-1β receptor antagonist (IL-1RA) has been reported to apply clinically to the treatment of rheumatoid arthritis [37]. We believe the future clinical application of IL-1RA in HCC treatment as a new agent.
In spite of recent progress in the understanding of HCC, there remains to be unknown mechanism of the escape of HCC cells from innate immunity. We have shown here that ADAM9 was directly associated with increasing the production of soluble MICA in IL-1β-treated human HCC. These findings might indicate that IL-1β contributes to the survival of HCC cells by inhibiting innate immunity.
Acknowledgments
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a Grant-in-Aid for Research on Hepatitis and BSE from the Ministry of Health, Labour and Welfare of Japan.
Abbreviations
- IL
Interleukin
- HCC
Hepatocellular carcinoma
- MICA
Major histocompatibility complex class I–related chain A
- ADAM9
A disintegrin and metalloproteinase 9
Footnotes
Keisuke Kohga and Tomohide Tatsumi have equally contributed to this work and share the first authorship.
References
- 1.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 2.Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughhan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–765. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 3.Powell EE, Edwards-Smith CJ, Hay JL, Clouston AD, Crawford DH, Shorthouse C, Purdie DM, Jonsson JR. Host genetic factors influence disease progression in chronic hepatitis C. Hepatology. 2000;31:828–833. doi: 10.1053/he.2000.6253. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 5.Bidwell J, Keen L, Gallagher G, et al. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 1999;1:3–19. doi: 10.1038/sj.gene.6363645. [DOI] [PubMed] [Google Scholar]
- 6.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 7.Howell WM, Calder PC, Grimble RF. Gene polymorphism, inflammatory disease and cancer. Proc Nutr Soc. 2002;61:447–456. doi: 10.1079/PNS2002186. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Kato N, Hoshida Y, et al. Interleukin-1β gene polymorphism associated with hepatocellular carcinoma in hepatitis C virus infection. Hepatology. 2003;37:65–71. doi: 10.1053/jhep.2003.50017. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Furuta T, Suzuki S, Orito E, Yeo AE, Hirashima N, Sugauchi F, Ueda R, Mizokami M. Impact of interleukin-1β genetic polymorphism on the development of hepatitis C virus-related hepatocellular carcinoma in Japan. J Infec Dis. 2003;187:1822–1825. doi: 10.1086/375248. [DOI] [PubMed] [Google Scholar]
- 10.Hirankarn N, Kimkong I, Kummee P, Tangkijvanich P, Poovorawan Y. Interleukin-1β gene polymorphism associated with hepatocellular carcinoma in hepatitis B virus infection. World J Gastroenterol. 2006;12:776–779. doi: 10.3748/wjg.v12.i5.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδT cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jinushi M, Takehara T, Tatsumi T, et al. Expression of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acids. Int J Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara K, Lanier LL. NKG2D in NK and T cell-mediated immunity. J Clin Immunol. 2005;25:534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- 15.Caudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16:333–343. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Groh V, Wu J, Yee C, Spies T. Tumor-derived soluble MIC ligands impair expression of NKG2D and T cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 17.Salih HR, Rammensee HG, Steinle A. Downregulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 18.Kohga K, Takehara T, Tatsumi T, et al. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver disease and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci. 2008;99:1643–1649. doi: 10.1111/j.1349-7006.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohga K, Takehara T, Tatsumi T, Ishida H, Miyagi T, Hosui A, Hayashi N. Sorafenib inhibits the shedding of MICA on hepatocellular carcinoma cell by downregulating ADAM9. Hepatology. 2010;51:1264–1273. doi: 10.1002/hep.23456. [DOI] [PubMed] [Google Scholar]
- 20.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant disease. Int J Cancer. 2006;118:684–687. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- 21.Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 22.Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gatsroenterology. 2001;120:250–260. doi: 10.1053/gast.2001.20947. [DOI] [PubMed] [Google Scholar]
- 23.Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohga K, Takehara T, Tatsumi T, Miyagi T, Ishida H, Ohkawa K, Kanto T, Hiramatsu N, Hayashi N. Anti-cancer chemotherapy inhibits MICA ectodomain shedding by downregulating ADAM10 expression in hepatocellular carcinoma. Cancer Res. 2009;69:8050–8057. doi: 10.1158/0008-5472.CAN-09-0789. [DOI] [PubMed] [Google Scholar]
- 25.Oyanagi Y, Takahashi T, Matsui S, Takahashi S, Boku S, Takahashi K, Furukawa K, Arai F, Asakura H. Enhanced expression of interleukin-6 in chronic hepatitis C. Liver. 1999;19:464–472. doi: 10.1111/j.1478-3231.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 26.Lapinski TW. The levels of IL-1β, IL-4 and IL-6 in the serum and the liver tissue of chronic HCV-infected patients. Arch Immunol Ther Exp. 2001;49:311–316. [PubMed] [Google Scholar]
- 27.Bortolami M, Kotsafti A, Cardin R, Farinati F. Fas/FasL system, IL-1β expression and apoptosis in chronic HBV and HCV liver disease. J Viral Hepat. 2008;15:515–522. doi: 10.1111/j.1365-2893.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 28.Migita K, Abiru S, Maeda Y, et al. Serum levels of interleukin-6 and its soluble receptors in patients with hepatitis C virus infection. Human Immunol. 2005;67:27–32. doi: 10.1016/j.humimm.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa H, Maeda S, Yoshida H, et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender difference. Int J Cancer. 2009;125:2264–2269. doi: 10.1002/ijc.24720. [DOI] [PubMed] [Google Scholar]
- 30.Wong VW, Yu J, Cheng AS, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- 31.Waldhauer I, Goehlsdorf D, Gieseke F, et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 32.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 33.Yaykasli KO, Oohashi T, Hirohata S, Hatipoglu OF, Inagawa K, Demircan K, Ninomiya Y. ADAMTS9 activation by interleukin 1 beta via NFATc1 in OUMS-27 chondrosarcoma cells and in human chondrocytes. Mol Cell Biochem. 2009;323:69–79. doi: 10.1007/s11010-008-9965-4. [DOI] [PubMed] [Google Scholar]
- 34.Kalinski T, Krueger S, Sel S, Wemer K, Ropke M, Roessner A. ADAMTS1 is regulated by interleukin-1beta, not by hypoxia, in chondrosarcoma. Hum Pathol. 2007;38:86–94. doi: 10.1016/j.humpath.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Cai L, Zhang Z, Zhou L, et al. Functional impairment in circulating and intrahepatic NK cell and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Hassan G, Moreno S, Massimi M, Di Biagio P, Stefanini S. Interleukin-1-producing plasma cells in close contact with hepatocytes in patients with chronic active hepatitis. J Hepatol. 1997;27:6–17. doi: 10.1016/S0168-8278(97)80273-5. [DOI] [PubMed] [Google Scholar]
- 37.Gabby C, Lamacchia C, Palmer G. IL-1 pathway in inflammation and human disease. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]