Abstract
Chemotherapy combined with a tumor vaccine is an attractive approach in cancer therapy. This study was designed to investigate the optimal schedule and mechanisms of action of a novel GM-CSF (granulocyte–macrophage colony-stimulating factor) surface-modified tumor-cell vaccine in combination with paclitaxel in the treatment of mouse RM-1 prostate cancer. First, the anti-tumor efficiencies of various dosage of paclitaxel (4, 20, 40 mg/kg) in combination with the vaccine in different administration sequences were examined in the mouse RM-1 prostate cancer model. Then, the in vivo and in vitro effects of various dosage of paclitaxel on RM-1 cells, T cells, and DCs (dendritic cells) were evaluated. The results showed that: (a) the GM-CSF-surface-modified tumor-cell vaccine was more potent at inducing the uptake of tumor antigens by DCs than irradiated tumor cells plus free GM-CSF; (b) 4 mg/kg paclitaxel combined with the GM-CSF-surface-modified tumor-cell vaccine was the most effective at enhancing tumor regression in RM-1 prostate cancer mice when the vaccine was administrated 2 days after paclitaxel; and (c) administration of 4 mg/kg paclitaxel followed by the vaccine induced the highest degree of CD8+ T-cell infiltration in tumor tissue, suggesting that the induction of tumor-specific immune response had occurred. These findings suggested that the GM-CSF-surface-modified tumor-cell vaccine may have potential clinical benefit for patients with prostate cancer when it is combined with paclitaxel. Furthermore, the effect of immunochemotherapy depends on careful selection of paclitaxel dosage and the sequence of paclitaxel/vaccine administration.
Keywords: Cancer immunochemotherapy, Granulocyte–macrophage colony-stimulating factor, Vaccine, Paclitaxel
Introduction
Prostate cancer is currently one of the most commonly diagnosed malignancies in men around the world [1]. Typical treatments such as radical prostatectomy and local radiation therapy are generally used to treat prostate cancer, but 20–40% of the patients ultimately recur [2–4]. Androgen-deprivation therapy is the standard method of treatment for patients who have failed primary therapy, but most patients’ disease eventually progresses to castration-resistant prostate cancer (CRPC). Docetaxel is now considered first-line chemotherapy for patients with CRPC due to the fact docetaxel improves media survival, but management of CRPC after docetaxel has become challenging [5–7]. Recently, a second-line paclitaxel-based chemotherapy after docetaxel was well tolerated with favorable PSA response rates and survival in patients with metastatic CRPC, although paclitaxel has not been shown to improve overall survival in a large randomized study [8, 9].
The development of vaccine strategies designed to break tolerance of tumor to body and generate a sustained and potent immune response against prostate cancer represents a novel therapeutic approach. The whole-tumor-cell vaccine approach allows presentation of a wide range of antigens to the immune system. Recent preclinical and clinical studies have supported the viability of whole-tumor-cell vaccines in the treatment of prostate cancer [10–12]. Granulocyte–macrophage colony-stimulating factor (GM-CSF) has a variety of effects on the immune response, such as upregulating the expression of MHC II expression on macrophages, enhancing DC maturation, stimulating DC migration, and producing a localized inflammatory response [13]. Prostate GVAX is a whole-tumor-cell vaccine composed of two allogeneic prostate cancer cell lines (PC3 and LNCaP), genetically modified through adenoviral transfer to secrete GM-CSF and irradiated to prevent further cell division [14]. The advantage of this whole-cell-based approach is that the vaccine can be manufactured in large quantities and multiple tumor antigens can be targeted simultaneously. Two single-arm phase II studies in men with asymptomatic metastatic CRPC showed promising anti-tumor effects for prostate GVAX: one which revealed an overall survival of 26.2 months and the other demonstrating an overall survival ranging from 20.0 to 29.1 months depending on the dose. In both studies, the proportion of patients generating antibody responses to one or both cell lines increased with the dose of vaccine given, and no dose-limiting or autoimmune toxicities were observed [15–17]. However, the recent two phase III studies of prostate GVAX failed to show a survival benefit of GVAX or GVAX/docetaxel over standard docetaxel/prednisone in the patients with metastatic CRPC and thus were prematurely terminated. The mechanism for the failure of the large phase III trials remains unexplained.
As an autologous cellular immunotherapy, PROVENGE has been approved recently by the FDA for the treatment of asymptomatic or minimally symptomatic metastatic CRPC. It consists of patient’s own peripheral blood mononuclear cells, which have been ex vivo cultured with a recombinant human protein, PAP-GM-CSF, consisting of prostatic acid phosphatase (PAP), an antigen expressed in prostate cancer tissue, linked to GM-CSF. Clinical studies showed that PROVENGE extended the overall survival median to more than 4 months, while the precise mechanism of action is still unknown. It is believed that the PAP-GM-CSF-activated antigen-presenting cells (APCs) account for the immunological response.
There are several weaknesses for the genetic modification of whole-tumor-cell vaccines, such as a low gene transfer efficiency, low expression level and biosafety concerns associated with viral vector-mediated gene transfer [18]. In order to circumvent the limits, we previously described a novel platform for tumor vaccination, by utilizing the rapid and almost irreversible binding of streptavidin (SA) to any biotin-linked molecule and the ability of biotin to be incorporated easily into various biological materials. The bifunctional fusion protein SA-GM-CSF could be efficiently and durably immobilized on the surface of the biotinylated tumor cells, so that the resultant whole-tumor-cell vaccine could provide the similar anti-tumor activity as GM-CSF gene-transduced tumor vaccines, but avoid the gene transfer [19, 20].
Despite these advantages, the use of anti-cancer vaccines as monotherapy for patients with large tumor burdens has been shown to be either ineffective or have minimal clinical efficacy. Cytotoxic chemotherapy is the mainstay of treatment in patients with metastatic disease. Taxanes, including docetaxel and paclitaxel, which work by disrupting intracellular microtubular networks, have been used in a variety of malignancies, such as breast, prostate, and lung cancers. Recent studies have shown that they can modulate the immune response in mice and humans. Several preclinical studies have examined the use of vaccines in combination with a taxane (docetaxel or paclitaxel) in murine tumor models, showing that paclitaxel could modulate the immune response and combined therapy of paclitaxel and cancer immunotherapy improved cellular immunity to tumor, but the therapeutic effects varied in accordance with paclitaxel dose, combination sequence, tumor type, and vaccine applied [21–24]. Nevertheless, immunochemotherapy research is still new and underdeveloped, and studies on the best schedules and modalities of combination therapy are still accruing data [25–27]. To our knowledge, few studies on the combined therapy of paclitaxel and immunotherapy for CRPC have been previously reported. Therefore, paclitaxel was chosen as the chemotherapy in combination with our cancer vaccine to examine the synergistic anti-cancer effects in the murine prostate cancer model.
In this report, we used a novel GM-CSF-surface-modified tumor vaccine to identify whether paclitaxel can enhance vaccine efficacy in a mouse model of prostate cancer. Our findings suggest (a) the GM-CSF-surface-modified tumor vaccine was more potent at inducing the uptake of tumor antigens by DCs than irradiated tumor cells plus free GM-CSF; (b) low-dose paclitaxel administration enhanced vaccine-induced tumor regression; (c) the mechanisms of the paclitaxel-induced immune response varied by the dose that was administered.
Materials and methods
Animals
Male C57BL/6 mice, aged 6–8 weeks old, were purchased from the Laboratory Animal Center of Southern Medical University (Guangzhou, China) and housed in transparent plastic cages under pathogen-free conditions. Animals that weighed 18–22 g were used in the experiments, and all experiments were conformed to relevant laws and institutional guidelines.
Tumor-cell lines and reagents
The murine prostate cancer cell line RM-1, derived from the spontaneous prostate tumors [28], was cultured in DMEM plus 10% FCS (fetal calf serum), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. rmGM-CSF and rmIL-4 were purchased from BD Bioscience. Paclitaxel (Bristol-Myers Squibb) was diluted in PBS before injection. ConA (purchased from eBioscience) was diluted in sterile PBS and preserved at −20°C.
Preparation of GM-CSF-surface-modified RM-1 whole-cell vaccine
Preparation of GM-CSF-surface-modified RM-1 whole-cell vaccine was performed as previously described [18]. Briefly, RM-1 cells (2 × 107 cells/ml) were incubated with EZ-Link Sulfo-NHS-LC-Biotin (Pierce) at a final concentration of 0.5 mg/ml in PBS (phosphate-buffered saline) for 2 h at 4°C and then incubated for 30 min with 4 μg of 6× His-L-SA-GM-CSF fusion protein (prepared in our lab) in a total volume of 2 ml. After extensive washing, cells were resuspended in PBS and irradiated at a dose of 70 Gy. The biological activity of GM-CSF anchored on the RM-1 cell was examined as we previous described [16].
In vitro phagocytosis tests
RM-1 cells were labeled with PKH67 green fluorescent cell linker (Sigma–Aldrich) according to the manufacturer’s instructions. 1 × 107 cells were briefly washed in PBS and centrifuged, and the supernatant was carefully and completely aspirated. Cell pellet was suspended again in 1 ml Diluent C and mixed immediately with 1 ml 4 × 10−6 M PKH67 dye for 5 min at room temperature. The staining reaction was stopped by addition of an equal volume of serum. Cells were then extensively washed in complete medium. PKH67-labeled RM-1 cells were biotinylated as described preparation of vaccine, followed by incubation with or without SA-L-GM-CSF fusion protein for 30 min and irradiation at a dose of 70 Gy. Then, the cells were co-cultured with DCs at a ratio of 1:1 for 4 or 16 h at 37°C or 4°C (for controls). Irradiated RM-1 cells with or without free SA-L-GM-CSF (200 ng per 106 RM-1 cells, which was equal to the dose of 106 GM-CSF-surface-modified RM-1 cells that were used) were used as control. All cells were harvested, washed, and stained with CD11c-FITC antibody. Phagocytosis was assessed by FACS (fluorescence-activated cell sorting) analysis of double-positive cells.
In vitro RM-1 cell proliferation test
MTT assay was performed to test the cytotoxicity of paclitaxel on RM-1. RM-1 cells were seeded onto 96-well plates at a concentration 2 × 104 cells/well. After 24 h, twofold serial dilutions of paclitaxel (from 2 to 512 nM) were added to the medium, and the cells were cultured for a further 24, 48, and 72 h. The effects of inhibition were calculated as follows: cell inhibition (%) = the OD570 value of (control-test) × 100/the OD570 value of Control. The IC50 was calculated by Calcusyn software (Biosoft).
Test of cytotoxic effect induced by various doses paclitaxel in the in vivo model of RM-1 prostate cancer
Male C57BL/6 mice were induced with RM-1 cells (5 × 105 cells/mouse) on day 0. When the tumor diameter reached about 0.3–0.5 cm, a single intraperitoneal injection of different doses of paclitaxel (from 0 to 40 mg/kg) was administered. Mice were sacrificed at different time points from day 1 to day 7 after the drug administration, and a single-cell suspension was prepared from the tumor tissues. Propidium iodide (PI) staining was performed to detect the dead cells, and the percentage of PI-positive cells was measured by FACS. Moreover, the tumor areas were measured on day 7 after the indicated drug injection.
Experimental design in a mouse model of prostate cancer
A total of 5 × 105 RM-1 cells suspended in 100 μl of PBS were inoculated subcutaneously into the backs of C57BL/6 mice. Mice were then randomly divided into four groups: (a) control group (treated with PBS); (b) vaccine-only group—GM-CSF-modified irradiated RM-1 tumor-cell vaccines (1 × 106/100 μl) were given for two cycles via simultaneous subcutaneously injections (right and left hind limbs) using a 1-ml syringe and a 27-gauge needle on day 0 and day 14; (c) chemotherapy only—varying doses of paclitaxel (4, 20, and 40 mg/kg) were given via intraperitoneal injection on day 4 and day 18; and (d) combination therapy groups—for the pre-vaccine groups, varying doses of paclitaxel were given as chemotherapy on day 4 and day 18, and the vaccines were given via subcutaneous injections on day 6 and day 20 (2 days after chemotherapy), and for post-vaccine groups, the vaccine was given on day 0 and day 14 (4 days before chemotherapy). The time of chemotherapy administration was identical in all combination groups to avoid confounding the effect of chemotherapy on tumor burden decrease among groups. Tumor size was measured twice a week with calipers and recorded as the tumor area (mm2). Survival, defined as the time to either sacrifice or death, was also monitored. Mice were euthanized when their tumors reached an area of 400 mm2 or if the mice became moribund or cachectic. The therapeutic effect of the same treatment strategies as above was also assessed in an orthotopic mouse model of prostate cancer. Briefly, in male C57BL/6 mice, 1 × 104 RM-1 cells were inoculated via intraprostatic injection on day 0 to establish primary prostate tumors, as has been described previously with minor modification [28]. The same treatments as subcutaneous challenge groups were administered. All studies consisted of six mice per group. Experiments were independently repeated three times.
Mouse interferon gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assays
The ELISPOT IFN-γ assays (MABTECH) kit was used to detect the presence of IFN-γ on spleen cells that were responsive to RM-1 cells. Splenocytes from mice from various treatment groups (5 × 105 cells/well) were cultured for 24 h at 37°C in a 5% CO2 incubator in the complete medium alone or in the presence of irradiated RM-1 cells at a ratio of 10:1. After washing with PBS-Tween (0.05%), detection antibodies were added to each well. The plates were then incubated for 1 h at 37°C, and the washing steps were repeated. Afterward, streptavidin–peroxidase (Streptavidin-HRP, 5 μg/ml) was added to the individual wells, and the plates were washed five times with PBS-Tween and twice with PBS. A total of 100 μl of aminoethylcarbazole staining solution was added to each well to develop the spots. The reaction was stopped after 4–6 min with deionized water. The spots were counted by computer-assisted image analysis (ImmunoSpot Series 2 analyzer).
Immunohistochemistry
Tumor tissues from mice were snap-fixed in 10% neutral-buffered formalin (NBF) overnight and embedded in paraffin. Next, 5-μm-thick sections were cut from the paraffin-embedded tumor tissues and subsequently deparaffinized in xylene and rehydrated. Endogenous peroxidase activity was blocked using 0.3% H2O2 in methanol for 20 min. Before staining, the sections were heated in 0.01 M citrate buffer (pH 6.0) in a microwave oven at 97°C for 20 min for antigen retrieval. Then, the sections were stained with anti-mCD8+ antibody according to the protocol of the HRP detection HIC kit (Bioss) and counterstained with hematoxylin.
Lymphocyte proliferation assay
Single-cell suspensions of splenocytes were washed and resuspended in RPMI 1640 medium with 10% FCS at a concentration of 5 × 106/ml and seeded in quadruplicate into 96-well plates (100 μl/well). Another 100 μl of RPMI 1640 medium containing 2 μg/ml ConA and various concentrations of paclitaxel was added, and cells were cultured for 3 days at 37°C in a 5% CO2 humidified incubator. Cell proliferation was examined in triplicate by MTT. To evaluate the effect of paclitaxel on splenic lymphocytes in vivo, splenocytes were isolated from normal mice 2 days after intraperitoneal injection with the indicated concentrations of paclitaxel and seeded into 96-well plates at a density of 5 × 105 cells per well along with 200 μl complete medium supplemented with 2 μg/ml ConA. Three days after cell seeding, splenocyte proliferation was assessed by MTT.
Flow cytometry analysis of T-cell subsets after paclitaxel treatment
To determine the effect of paclitaxel on different T-cell subsets in mice, Mice received a single intraperitoneal injection of 4, 20, or 40 mg/kg of paclitaxel. Spleens were collected at the indicated time points. The total number of splenocytes was calculated and stained with FITC-labeled anti-CD4 and anti-CD8 antibodies as well as APC-labeled anti-CD25 antibodies (Biolegend). To enumerate the CD4+, CD25+, and FoxP3+ regulatory T-cell populations, cells were first stained with anti-CD4 and anti-CD25 antibodies, then fixed and permeabilized before they were stained with PE-labeled anti-FoxP3 antibody (Biolegend). After washing, cells were run on the FACScalibur flow cytometer and analyzed using Cellquest software (B–D bioscience).
Culture of murine bone marrow-derived DCs
Murine bone marrow-derived DC culture was performed as described previously [29], with minor modifications. Briefly, bone marrow cells (BMCs) from C57BL/6 mice were resuspended in RPMI-1640 complete medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, l-glutamine, 2-mercaptoethanol (50 μM, Sigma), and 10% heat-inactivated FCS. At day 0, BM cells were seeded onto 10-cm dishes at a concentration of 5 × 106 per dish in 10 ml of medium containing 20 ng/ml rmGM-CSF plus 10 ng/ml rmIL-4. On day 2, another 10 ml of complete medium containing the same concentration of cytokines was added to the plates. On day 4, half of the culture medium was replaced with fresh medium containing the same concentration of cytokines. Cells were further cultured until day 7.
In vitro inhibition assay of DCs
After bone marrow-derived DC precursors (0.5 × 106/ml) were cultured for 4 days, RM-1 tumor cells (0.5 × 104/ml) or 0.5 × 106/ml RM-1 cell culture supernatant with or without 20 nM of paclitaxel were added to dendritic cell cultures. Dendritic cells were harvested on day 7 and stained with appropriately diluted FITC-labeled anti-mouse CD80 and phycoerythrin-labeled CD11c and analyzed by FACS (Biolegend). The double-positive cells were considered to be mature DCs.
Annexin V binding assay
DCs that had been treated with indicated concentrations of paclitaxel for 3 days were double stained with FITC-conjugated Annexin V and PI (Bender Medsystems). Cells undergoing early and late apoptosis were Annexin V-positive/PI-negative and Annexin V-positive/PI-positive, respectively. The total apoptosis percentages of cells were calculated as the number of cells undergoing early apoptosis plus the number of cells undergoing late apoptosis.
Statistical analysis
Statistical analyses of tumor areas, FACS data, and survival were performed using one-way ANOVA (analysis of variance) and Kaplan–Meier analyses, respectively. All analyses were performed using SPSS version 17.0. All P values <0.05 were considered to be statistically significant.
Results
The biological activity of GM-CSF-modified RM-1 vaccine
Our results showed that the percent of cells that were found to carry the protein was (97.4 ± 0.8) %. The BMCs proliferation responses by the GM-CSF activity of the vaccine per 2 × 105 cells were evaluated by MTT. The ED50 had a dilution of 2.5 × 104, and the biological activity of GM-CSF in the GM-CSF-modified RM-1 vaccine was 2.5 × 104 U/2 × 106 vaccine cells.
The surface-modified-GM-CSF vaccine enhanced the phagocytosis of tumor vaccine by DCs
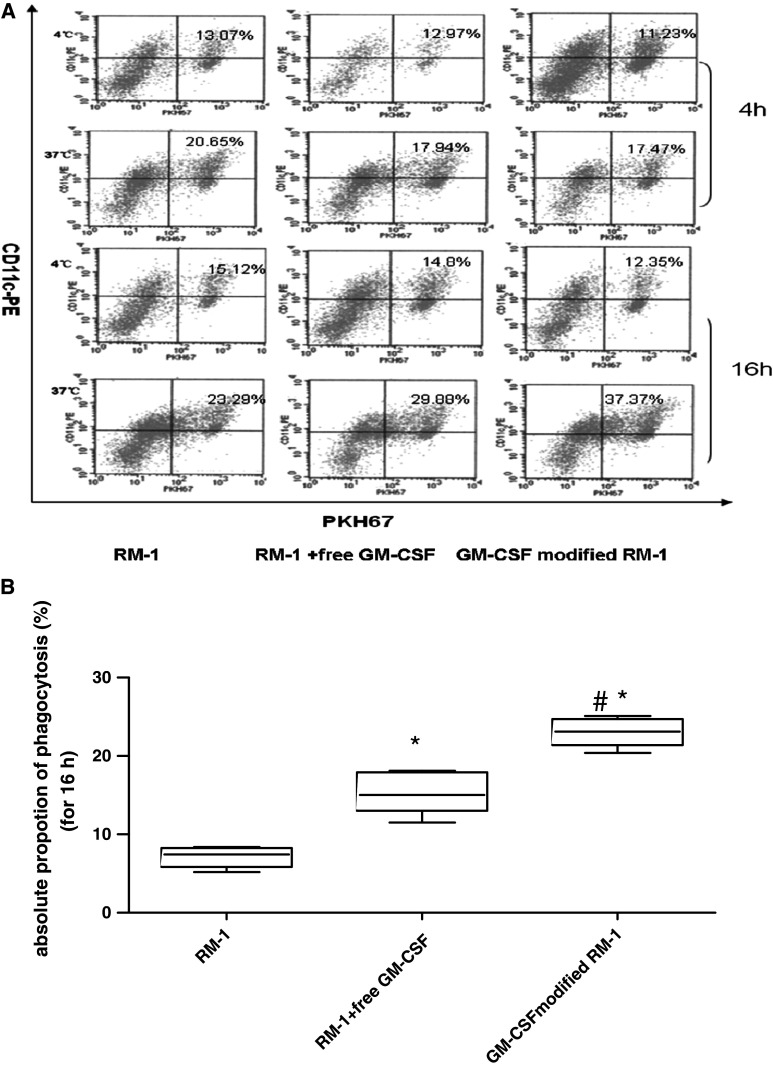
Our results showed that the proportion of DCs phagocytosis tumor cells was higher in the presence of free GM-CSF than irradiated RM-1 cells only. Moreover, GM-CSF-surface-modified tumor cells were engulfed by DCs with higher efficiency than tumor cells plus the same concentration of free GM-CSF (Fig. 1a and b). This result suggested that the surface-modified-GM-CSF cell vaccine was more potent at inducing the uptake of tumor antigens by DCs than free GM-CSF.
Fig. 1.
Enhancement of the phagocytosis of surface-modified GM-CSF tumor vaccine by DCs. DCs were co-cultured with PKH67-labeled cells such as irradiatied-RM-1, irradiatied-RM-1 plus GM-CSF or membrane-modified GM-CSF tumor vaccine for 4 h or 16 h at a ratio of 1:1 at 4°C (for control) or 37°C. At the end of incubation, all of cells were harvested, washed, and stained with CD11c-FITC antibody. Phagocytosis was assessed by FACS analysis of double-positive cells. a Typical data from a representative experiment (n = 5); b Absolute phagocytosis rate was calculated after 16 h co-culture. (Absolute phagocytosis rate = phagocytosis rate in 37°C-phagocytosis rate in 4°C. Signal *indicated P < 0.05 in comparison with RM-1 group, #indicated P < 0.05 in comparison with RM-1 plus free GM-CSF
Cytotoxic effect of paclitaxel in vitro and in vivo
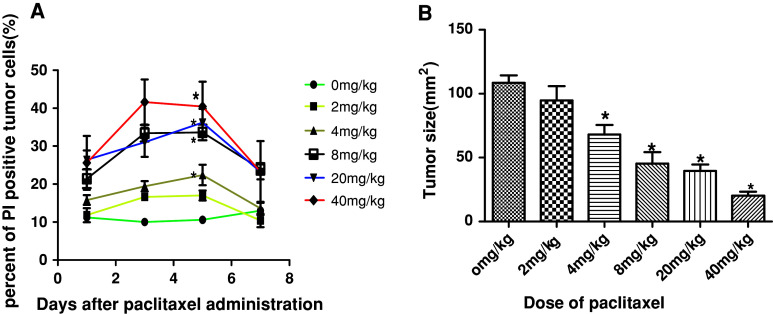
The MTT results showed that paclitaxel efficiently inhibited the proliferation of RM-1 cell in a dose- and time-dependent manner. The IC50 levels at 24, 48, and 72 h were 128.9, 45.6 and 17.5 nM, respectively. For the in vivo anti-cancer effect of paclitaxel, as shown in. 2A, the peak of PI-positive cells was appeared from day 3 to day 5 in all treatment groups. The maximum percentage of PI-positive cells in mice that received 2 mg/kg and 0 mg/kg of paclitaxel did not differ significantly (14.0 ± 3.5% and 10.6 ± 3.1%, respectively; P > 0.05). However, mice that received higher doses of paclitaxel (i.e., 4–40 mg/kg) had a significantly higher number of PI-positive cells (22.4–37.4%) than the mice in the 0 mg/kg group (P < 0.05).
Moreover, tumor-bearing (0.3–0.5 cm in diameter) mice were treated with various dose of paclitaxel followed by tumor size measurement as described in the “Materials and methods” section. As shown in Fig. 2b, in the 4 mg/kg or higher dose group, paclitaxel induced significant reduction in RM-1 prostate cancer. Thus, the 4 mg/kg dose level was considered the minimal dose of paclitaxel that was adequate to provide tumoricidal activity against RM-1 prostate cancer cells in vivo.
Fig. 2.
Tumoricidal activity of different doses of paclitaxel on RM-1 prostate cancer in vivo. RM-1 cells from tumor tissues of mice treatment with 0, 2, 4, 20, and 40 mg/kg paclitaxel when tumors reached 0.3–0.5 cm in diameter were stained by PI and the percents of PI positive were assayed by FACS at different time points from day 1 to day 7 after paclitaxel treatment (a) and the sizes of tumors determined by multiplying the two perpendicular diameters were measured with caliper on day 7 after paclitaxel injection (b). *indicated P < 0.05 in comparison with 0 mg/kg group. 5–6 mice per group
Paclitaxel enhanced vaccine-induced tumor regression dependent on the dose and sequence of paclitaxel administered
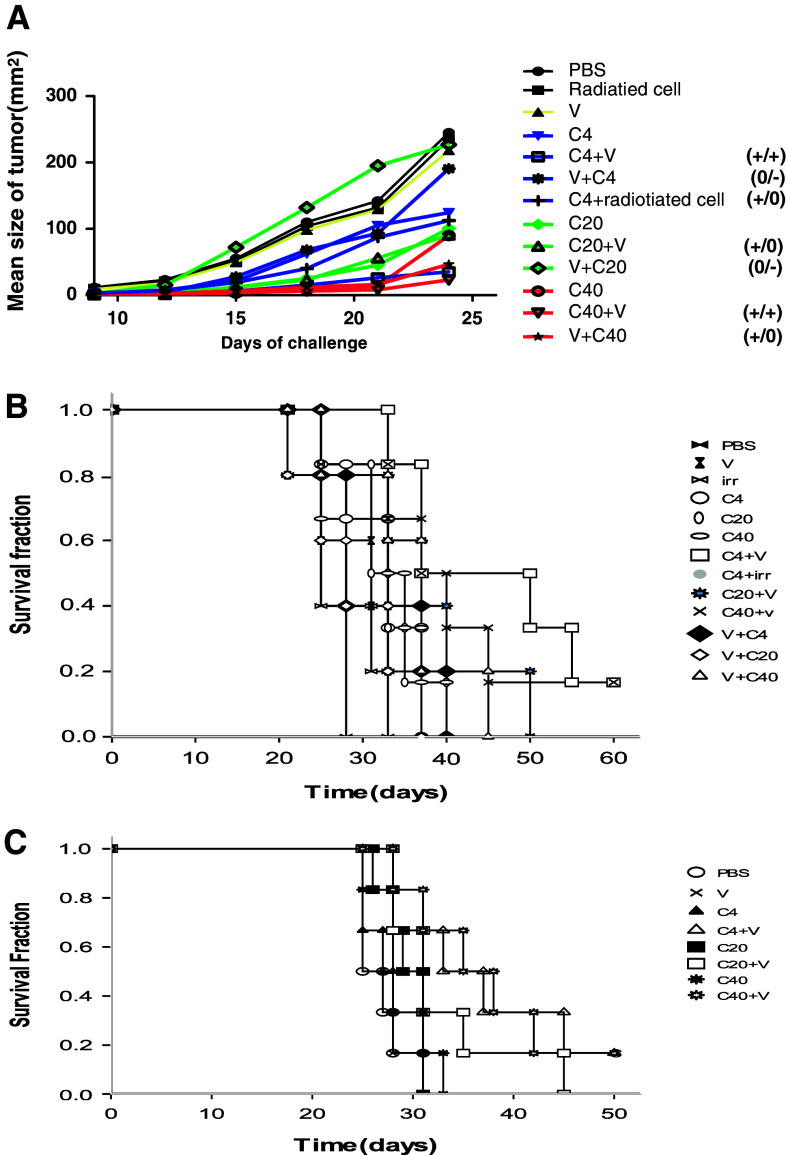
Chemoimmunotherapy efficacy was critically dependent on the dose of paclitaxel that was delivered as well as the sequence in which the two drugs were administered. In this research, tumor size over a period of 25 days is shown in Fig. 3a. For low-dose paclitaxel (4 mg/kg), the mean tumor size on day 24 was 35 ± 11.0 mm2 in the group in which the chemotherapy was delivered prior to the vaccine (C4+V), 124 ± 21.0 mm2 for the group that received chemotherapy alone (C4), 235.6 ± 35.5 mm2 for the group that received vaccine alone (V), 190.6 ± 16.4 mm2 for the group that received chemotherapy following the vaccine (V+C4), and 112.4 ± 19.2 mm2 for the group that received chemotherapy plus irradiated RM-1 cells (C4+irradiated RM-1 cells), respectively. The results showed that the C4+V group had a significant delay in tumor progression compared with the mice treated with chemotherapy alone, vaccine alone, V+C4, or C4+irradiated RM-1 cells (P < 0.05). There were no differences between the C4+irradiated RM-1 cell and C4 group (P = 1.000), indicating that the presence of surface-modified GM-CSF on the cell vaccine is critical to the synergetic effect of the combination therapy. In the groups that mice received 20 mg/kg paclitaxel, the mean tumor sizes (mm2) were 90 ± 24.9 for the group that received paclitaxel prior to the vaccine (C20+V), 100.5 ± 23.0 for the group that received paclitaxel alone (C20), and 226.8 ± 20.9 for the group that received paclitaxel following the vaccine (V+C20). Although C20+V is superior to vaccine alone (P < 0.001) and V+C20 (P = 0.000), it was not superior to C20 alone (P = 1.000). For the mice that received 40 mg/kg paclitaxel, the mean tumor size (mm2) was 22.8 ± 6.9 for the group that received chemotherapy prior to the vaccine (C40+V), 89.8 ± 26.9 for the group that received chemotherapy alone (C40), and 46.6 ± 18.7 for the group that received paclitaxel following the vaccine (V+C40). C40+V was superior to C40 (P < 0.001), but there were no differences identified between the C40+V and V+C40 (P = 0.887) groups or between the C40 and V+C40 (P = 0.124) groups. According to results above, paclitaxel administration prior to vaccination was superior to paclitaxel administration following the vaccine at paclitaxel doses of 4 and 20 mg/kg, but not when the dose reached 40 mg/kg, at which point no significant differences were identified between the administration sequences. Moreover, C4+V treatment and C40+V treatment were all superior to monotherapy (either C or V), but C20+V treatment was not superior to chemotherapy alone, even though it was superior to the vaccine alone.
Fig. 3.
Paclitaxel enhanced the anti-tumor effects of the GM-CSF-modified whole-tumor-cell vaccine in proper time and dosage. a C57BL/6 mice were challenged with 5 × 105 viable RM-1 cells via subcutaneous injection on the back on day 0 and received chemotherapy (C), vaccine (V), combination treatment such as chemotherapy 2 day before vaccine (C+V) or chemotherapy 4 days after vaccine (V+C), the doses of paclitaxel were various from 4 mg/mg (C4) to 40 mg/kg (C40). Tumors sizes were determined by multiplying the two perpendicular diameters. P value was determined by one-way ANONA analyses with SPSS version 17.0 according to the tumor sizes on day 24. Differences were considered significant when P < 0.05. +/+ indicated combination treatment group statistically superior (P < 0.05) to each treatment modality alone; +/0 indicated combination treatment group statistically superior (P, 0.05) to vaccine group but not chemotherapy group; 0/− indicated inhibition effect (no statistical difference between combination treatment group and vaccine group but statistically inferior (P < 0.05) to chemotherapy group); b Kaplan–Meier estimate of overall survival time for mice received various treatment as above was performed. Comparison with each treatment modality alone such as vaccine (V) or chemotherapy (c). There were 5–6 mice each group. C, survival time for mice receiving various treatments was also evaluated in orthotopic primary RM-1 cancer
The survival times of mice treated with the various therapies outlined above were also observed. As shown in Fig. 3b, all of the mice in the chemotherapy-only groups, but none of the mice in the vaccine-only groups, had longer survival times compared with the PBS group. Administration of C4+V significantly improved the survival compared with C4 or V (P < 0.05). However, there was no significant difference between C20+V and the relevant monotherapies (i.e., C20 or V) or between C40+V and the relevant monotherapies (C40 or V) (P > 0.05). Only C4+V did improve survival when compared with the relevant monotherapies. In addition, the locomotor activities of the mice in the C40 and C40+V groups were decreased compared with the C4 and C20 groups after the second dose of chemotherapy. This finding may have been associated with the toxicity of high doses of paclitaxel.
Paclitaxel had different effects on tumor size in different tissues [30]. To further evaluate the effect of the chemoimmunotherapy on intraprostatic cancer, an orthotopic mouse model of RM-1 prostate cancer model was established. These mice received similar treatments to the mice with subcutaneous tumors. As shown in Fig. 3c, C4+V and C40+V improved survival time compared with their relevant monotherapies (P < 0.05). C20+V was superior to vaccine alone, but not to chemotherapy alone. These results confirmed the synergetic effect that C4+V has in chemoimmunotherapy for the treatment of RM-1 cancer.
Paclitaxel enhanced vaccine-induced tumor-specific T-cell response in proper sequence and dose
To further explore the mechanism by which paclitaxel enhanced the efficacy of the vaccine, tumor-specific T cells from various groups were assayed after treatment by IFN-γ ELISPOT. As shown in Fig. 4a, 1 week after the last treatment (in mice with subcutaneous RM-1 tumors), all of mice who received paclitaxel prior to the vaccine had an increased number of tumor-specific T cells compared with the mice who received monotherapy. For the 4 and 20 mg/kg doses of paclitaxel, the synergetic effect disappeared when the drug was administrated after the vaccine. However, in mice that received the 40 mg/kg dose, paclitaxel administration after the vaccine also increased the number of tumor-specific T cells that were present. The presence of high numbers of CD8+ T cells among tumor-infiltrating lymphocytes is a strong indicator of a favorable clinical outcome [31]. To examine the degree of CD8+ T cells infiltration in the tumor tissues, immunohistochemistry was performed. As shown in Fig. 4c, CD8+ T-cell infiltration was observed in all mice that received a vaccine (alone or in combination), but C4+V induced the highest degree CD8+ T-cell infiltration of all of the groups.
Fig. 4.
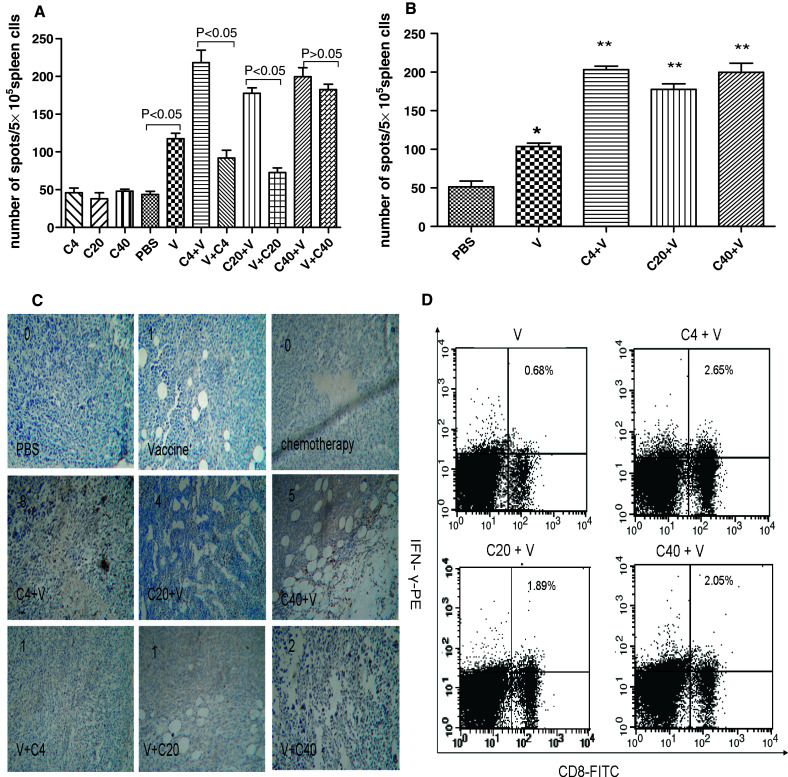
Tumor-specific immune responses of combination treatment were enhanced in proper drug doses and sequence of paclitaxel administration. Tumor-specific immune response was evaluated by IFN-γ ELISPOT as described in “Materials and methods”. a the numbers of spot were calculated in various treatment in subcutaneous implanting model. b the numbers of spot were calculated in various treatment in orthotopic primary model. *indicated P < 0.05 in comparison with PBS, **indicated P < 0.05 in comparison with V treatment group. There were 3 mice each group. c immunohistochemistry for CD8+ T cell of tumor tissues from mice receiving various treatments in subcutaneous implanting model was performed. The sections were scored three times for infiltrated areas, which were represented as a percentage of the total vital tumor tissue. D, CD8+, and intracellular IFN-γ+lymphocytes were assayed by FACS in mice receiving various treatments in orthotopic primary prostate cancer model
For the orthotopic RM-1 mice, similar results were obtained for the IFN-γ ELISPOT analysis (Fig. 4b). Because IFN-γ production by CD8+ T cells is representative of cytotoxic T-lymphocyte activity, intracellular IFN-γ+ CD8+ T cells were assayed by FACS. As shown in Fig. 4d, the proportion of IFN-γ+ CD8+ T cells was 0.68% in the V group, 2.65% in the C4+V group, 1.89% in the C20+V group, and 2.05% in the C40+V group. Combination treatment (chemotherapy followed by vaccination) clearly induced higher number of tumor-specific IFN-γ+ CD8+ T cells than others.
Inhibitory effect of paclitaxel on ConA-stimulated lymphoproliferation
As shown in Fig. 5a, in comparison with the non-treatment group, administration of paclitaxel at a lower concentration (31 nM) did not inhibit ConA-stimulated lymphoproliferation (P > 0.05), whereas administration of paclitaxel at higher concentrations (from 62 nM to 500 nM) inhibited lymphoproliferation (P < 0.05).
Fig. 5.
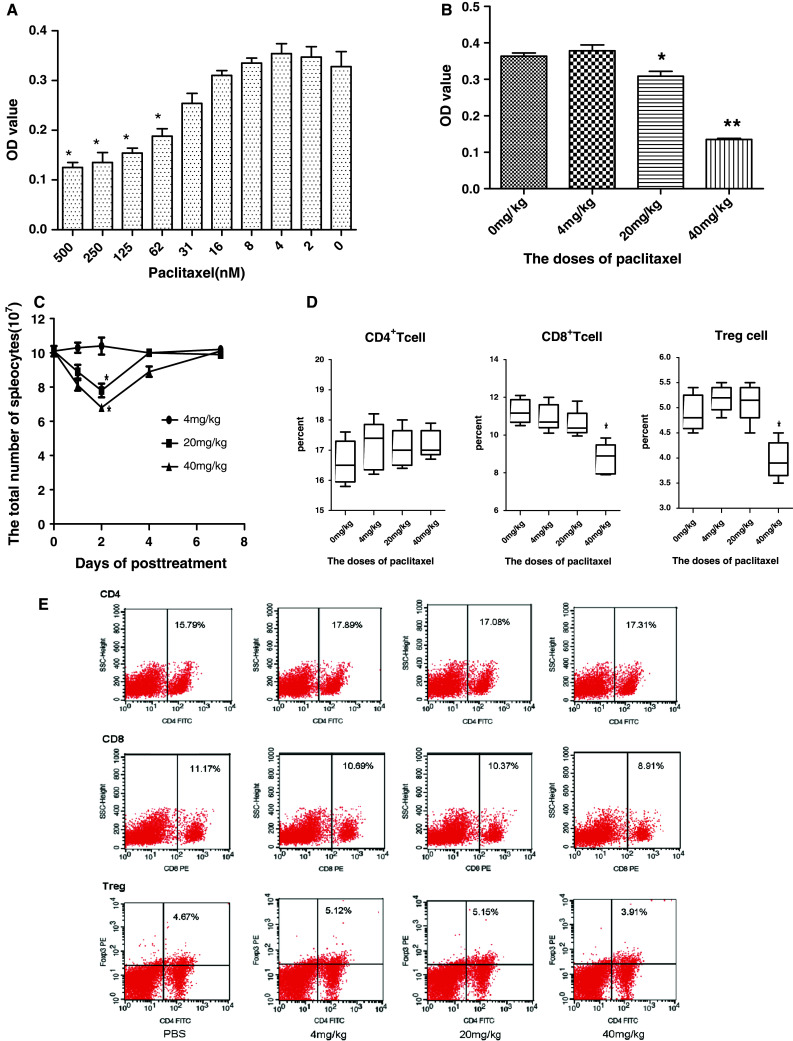
Effect of various doses of paclitaxel on T cells in vitro and in vivo. To evaluate the effects of various doses of paclitaxel on lymphocyte proliferation, spleen cells from naive C57BL/c mice were treated in quadruplicate with different concentrations of paclitaxel in presence of 2 µg/ml ConA for 3 days in 96-well flat-bottom tissue culture plates, proliferation responses were evaluated by MTT (a). Spleen cells were prepared from naive mice treated with paclitaxel at dose levels of 0, 4, 20 and 40 mg/kg for 2 days, and seeded into 96-well flat-bottom tissue culture plates containing 2 µg/ml ConA complete medium, proliferation responses were evaluated by MTT as above (b). Optical density (OD) at 570 nm was calculated as mean ± SE. To evaluate the effects of various doses of paclitaxel on spleen cells and T-cell subpopulations, naive C57BL/c mice were treated with various doses of paclitaxel, the total number of spleen cells were calculated from on day 0 to on day 7 after paclitaxel injection (c). T-cell subpopulations of spleen were assayed 2 days after a single treatment with various doses of paclitaxel (d). Typical dada from a representative experiment for D (n = 5) were presented (e). *P < 0.05 and **P < 0.01 compared with non-treatment control group
To further evaluate the effect of paclitaxel on lymphoproliferation in vivo, six- to eight-week-old naive male C57BL/6 mice were treated with different doses of paclitaxel [i.e., 4 mg/kg (the minimal dose of paclitaxel sufficient to induce tumoricidal activity), 20 mg/kg (normal treatment dose level reported by others [28, 32, 33]), and 40 mg/kg (high dose)]. After 48 h, single-cell suspensions of splenocytes were prepared, and ConA-stimulated lymphoproliferation was assayed as described above. As shown in Fig. 5b, paclitaxel did not inhibit lymphoproliferation at a dose of 4 mg/kg, whereas it did inhibit lymphoproliferation significantly at a dose of 20 mg/kg (P < 0.05) and 40 mg/kg (P < 0.01).
The results showed that the inhibitory effect of paclitaxel on T-cell proliferation was dose-dependent and that 4 mg/kg in vivo or 31 nM in vitro was the lowest dose that could inhibit T-cell proliferation.
Effect of paclitaxel on T-lymphocyte subpopulations
The percentage of T-cell subpopulations (CD4+ T cell, CD8+ T cell, and T-reg) in normal mice injected with various doses of paclitaxel were analyzed at indicated time points by FACS. The results showed that 4 mg/kg paclitaxel did not decrease the total number of splenocytes, but both 20 and 40 mg/kg of paclitaxel significantly decreased the total splenocytes that were present. The number of splenocytes was lowest on day 2 post-treatment (Fig. 5c). On day 2 post-treatment, there was no difference between groups in the proportion of CD4+ T cells that were present (P > 0.05), whereas the percentage of CD8+ T cells and regulatory T cells in 40 mg/kg group were both significantly reduced by about 20% compared with the 0–20 mg/kg groups (P < 0.05) (Fig. 5d and e),which did not reduce the proportion of CD8+ and regulatory T cells that were present in the spleen lymphocyte population. Following reduction in the total number of spleen lymphocytes by treatment with 20 and 40 mg/kg of paclitaxel, the total numbers of regulatory T cells, CD4+ T cells, and CD8+ T cells were decreased in comparison with mice treated with PBS. Moreover, a greater reduction in CD4+ T cells and CD8+ T cells was observed at the 20 mg/kg dose, whereas a greater reduction in regulatory T cells was observed in the 40 mg/kg group. As shown in Fig. 5c, the 4 mg/kg dose of paclitaxel did not impact either the total number of spleen cells that were present or the proportions or the total number of T-cell subpopulations that were present.
Altogether, the results suggest that the effect of paclitaxel on T cells depends on the dose that is administered. Low-dose paclitaxel (i.e., 4 mg/kg) did not affect T-cell proliferation, the number, or overall T cells that were present, or the proportion of specific T-cell subpopulations that were present. For normal-dose paclitaxel (i.e., 20 mg/kg), paclitaxel reduces all of the T-cell subpopulations, but has a larger impact on the CD4+ and CD8+ T-cell populations. For high-dose paclitaxel (i.e., 40 mg/kg), the regulatory T subpopulation is reduced to a larger degree than the CD4+ and CD8+ T-cell subpopulations.
Effect of paclitaxel on dendritic cells
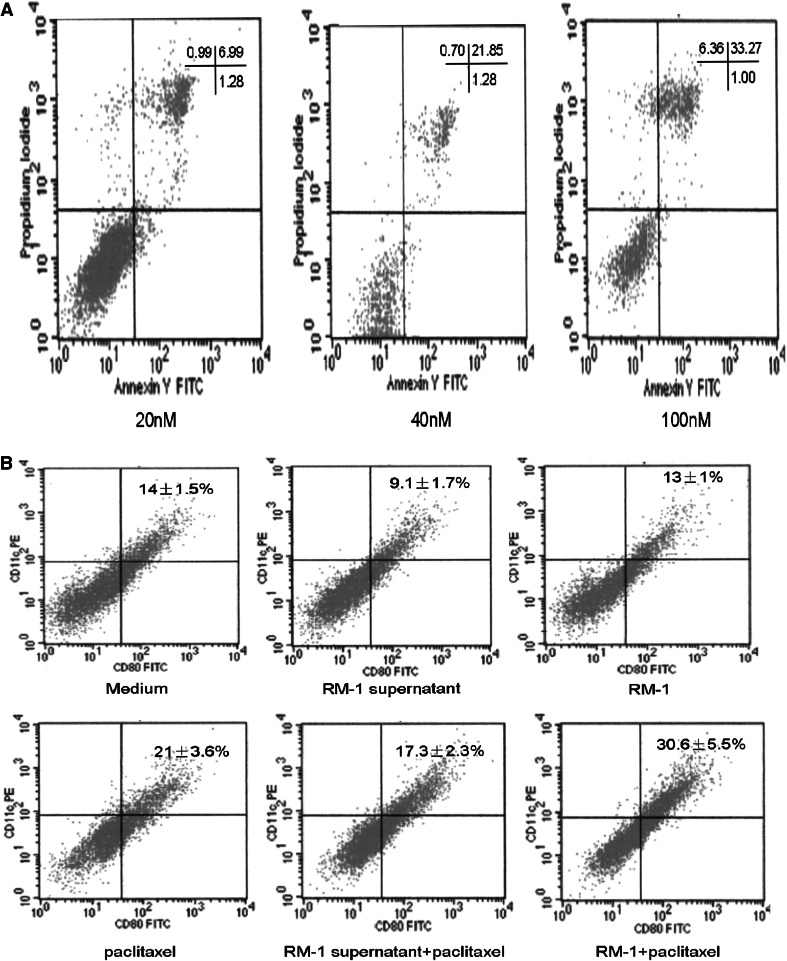
First, we examined the effect of paclitaxel on DC apoptosis. As shown in Fig. 6a, at a concentration of 20 nM for 3 days, which was close to the IC50 level for RM-1 s, 6.69% cells underwent apoptosis, whereas at concentrations of 40 and 100 nM, the proportion of apoptotic cells reached 21.85 and 33.27%, respectively.
Fig. 6.
The effect of various concentrations of paclitaxel on dendritic cell in vitro. To evaluate the effect of various concentrations paclitaxel on DCs, the cultured-7 days dendritic cells were exploded in various concentrations of paclitaxel for 3 days and the proportion of apoptosis cells were assayed by FACS (a). To evaluate the effect of 20 nM paclitaxel on the maturation of DCs in presence of tumor cell (or tumor cell cultured supernatant) or not, after bone marrow-derived dendritic cell precursors (0.5 × 106/mL) were cultured for 4 days, RM-1 tumor-cell (0.5 × 104/mL) or RM-1 cell (0.5 × 106/mL) cultured supernatant combination with or without 20 nM paclitaxel were added to dendritic cell cultures (b). Dendritic cells were harvested on day 7, and the proportion of DC maturation was assayed by FACS (n = 3)
To evaluate the effect of paclitaxel on the maturation of DCs, the presence of CD80 and CD11c on DCs was analyzed by FACS. As shown in Fig. 6b, in the control medium group, paclitaxel treatment increased the proportion of CD11c+ CD80+ cells from 14 ± 1.5% to 21 ± 3.6% (P < 0.05). In the RM-1 cell culture supernatant medium group, the maturation of DCs was inhibited (9.1 ± 1.7%), whereas paclitaxel treatment could eliminate the inhibitory effect of RM-1 culture supernatant on DC maturation. When paclitaxel was added to co-cultured DCs and RM-1 cells, the proportion of mature DCs increased from 13 ± 1% to 30.6 ± 5.5%, which was higher than the reduction seen with paclitaxel alone (P < 0.05).
These results suggest that paclitaxel promoted the maturation of DCs, whereas tumor cells could inhibit the maturation of DCs, perhaps by cell-secreted cytokines. Moreover, the direct contact between tumor cells and immature DCs in the presence of paclitaxel significantly promoted the maturation of DCs, probably by enhancing the phagocytosis of tumor cells by immature DCs.
Discussion
The successful treatment of advanced prostate cancer remains critically needed, and immunotherapy emerges as an efficient approach. In order to induce efficient anti-tumor cellular immunology, it is critical to ensure that the tumor antigens are efficiently taken up by DCs. Immature DCs and their precursors express high level of GM-CSF receptors [34]. So, as an important immune stimulatory molecule, GM-CSF has been mostly used in many immunotherapy schemes. For example, PROVENGE utilized PAP-GM-CSF fusion protein to activate DCs and then induce immune response to PAP. During ex vivo culture, the recombinant fusion protein PAP-GM-CSF is directed toward APCs (mainly DCs) through the conjunction of GM-CSF and their receptors on the surface of APCs. The APCs take up and process the recombinant target antigen into small peptides and then activate cellular immunity. Clinical studies showed that T-cell proliferative and γ-IFN ELISPOT responses to PAP-GM-CSF fusion protein were observed in the lymphocytes collected from peripheral blood of patients in the PROVENGE treatment group but not in controls and that PROVENGE extended the overall survival median to more than 4 months in a phase III trial and thus has been approved by FDA for the treatment of advanced prostate cancer. We have previously shown that this GM-CSF surface-modified whole-cell vaccines possessed significant anti-cancer activity against mouse melanoma and bladder cancer [19, 20]. In this study, we first evaluated the phagocytosis of the novel vaccine by DCs. The results clearly demonstrate that the GM-CSF surface-modified RM-1 cell was more effective than the tumor cell plus free GM-CSF at promoting phagocytosis by DCs, indicating that much more tumor antigens were taken up and processed. Direct contact between DCs and GM-CSF surface-modified tumor cells through the conjunction of GM-CSF and their receptors may enhance the phagocytosis process.
Compared with PROVENGE, the GM-CSF-surface-modified RM-1 cell vaccine has several characteristics. First, GM-CSF-surface-modified RM-1 cell vaccine used whole cancer cells instead only one tumor-associated antigen PAP. It is advantageous for the RM-1 cell vaccine to inspire immune response, as the whole cell expresses a large repertoire of antigens. However, the immune response triggered by the RM-1 cell vaccine may not be focused enough. In this study, immunization of mice with the vaccine alone was unable to successfully control the growth of tumor, a finding that is in agreement with previous data showing that vaccines alone failed to prevent the growth of established tumors [35]. Second, GM-CSF-surface-modified tumor vaccine does not need to collect the patient’s peripheral blood mononuclear cells for ex vivo culture. Third, the first stage of the immune response occurs in vivo for GM-CSF-surface-modified tumor vaccine, but in the case of PROVENGE, the initial response occurs during ex vivo culture.
Michaels and colleagues demonstrated that pretreatment with paclitaxel prior to a GM-CSF-secreting whole-cell vaccine enhanced anti-cancer immune response in a HER-2/neu-tolerized mouse model of breast cancer when paclitaxel doses varied from 20 to 30 mg/kg. But for the doses above 35 mg/kg, this synergic effect disappears [33]. However, others have shown that 36 mg/kg of paclitaxel enhanced the anti-tumor effects of the TLR9 agonist PF-3512676 [36]. Moreover, Chopra et al. demonstrated that administration of 2.25 mg/kg paclitaxel 2 days prior to the administration of DNA-based cell vaccine also enhanced the anti-cancer effect of the vaccine [37]. In addition, Eralp et al. [22] also reported that administration of the same dose of paclitaxel (25 mg/kg) 1 day before the vaccine enhanced the anti-cancer immune response when combined with VRP-neu, but not when combined with SINCP-neu. What led to the discrepancies between the results presented above? Was it related to the paclitaxel dose, the sequence of combination, the type of tumor, or the vaccine?
Taxanes such as docetaxel and paclitaxel were initially characterized as mitotic inhibitors, and their anti-neoplastic actions were attributed to their ability to suppress cellular division via microtubule stabilization. Less appreciated is the observation that taxanes induce other biological effects, especially in the immune system. For example, taxanes are immunostimulatory against neoplasms, supporting the idea that these agents suppress cancer through several mechanisms and not solely through inhibiting cell division. In addition, they may actually exert beneficial immunomodulatory effects through a variety of mechanisms, including cytokine production and T-cell infiltration of tumor cells [38]. It has been documented previously that the administration of some chemotherapeutic drugs at suboptimal doses may have an immunomodulatory effect [39].
To explore the proper combination of paclitaxel and the novel vaccine for the treatment of murine prostatic cancer, various doses of paclitaxel were studied. First, preparatory dose-finding experiments were performed. The results showed that 4 mg/kg paclitaxel was the minimal dose of paclitaxel that induced tumoricidal activity in RM-1 prostate cancer in vivo. This finding was in contrast to previous studies that demonstrated that 20 mg/kg dose was the proper dose [28, 32, 33]. Thus, we administered the minimal dose of paclitaxel (4 mg/kg), the normal treatment dose (20 mg/kg), and the high dose of paclitaxel (double of normal dose, 40 mg/kg) prior to or after administering the vaccine. In contrast to the observation of Machiels et al. [33], the synergetic effect of the administration of 20 mg/kg paclitaxel prior to the vaccine was not significant, whereas administration of 40 mg/kg of paclitaxel obviously enhanced the anti-tumor efficacy of the vaccine, regardless of whether it was given before or after the vaccine. The dissimilar outcomes that were observed may lead by the cycles of paclitaxel administration. Machiels et al. administered paclitaxel once, whereas in our study, paclitaxel was given twice at an interval of 2 weeks. It is possible that the fact that we administered more cycles of paclitaxel influenced the results, as Yu et al. [40] reported that administration of repeated cycles of paclitaxel has a different effect on the immune system than administration of a single dose. These results highlight the importance of treatment schedule for chemoimmunotherapy.
Previous studies highlighted the effectiveness of the pre-vaccine administration of paclitaxel to enhance the synergetic effect of chemoimmunotherapy [36, 41]. At the lower paclitaxel doses in our study (i.e., 4 and 20 mg/kg), we found that pre-vaccine administration was superior to post-vaccine administration. However, our results showed that there were no significant differences between pre- vs. post-vaccine administration of paclitaxel when the dose of paclitaxel reached 40 mg/kg. ELISPOT analysis demonstrated that the curative effect was associated with increasing of system tumor-specific INF-γ-secreting T cells. Although post-vaccine administration of the 4 and 20 mg/kg doses of paclitaxel impaired the number of tumor-specific IFN-γ-secreting T cells in comparison with pre-vaccine paclitaxel administration, the reduction in the number of IFN-γ-secreting T cells was similar among mice treated with pre-vaccine and post-vaccine paclitaxel at the 40 mg/kg dose.
The results suggest that different immunomodulatory mechanisms contribute to the anti-tumor effects exerted by different doses of paclitaxel. Whereas previous studies did not address this problem, the present study confirmed this hypothesis by evaluating the effect of paclitaxel on both T cells and DCs. The effect of paclitaxel on T cells was dependent on the concentration/dose of paclitaxel that was delivered in vitro and in vivo, respectively. Low-dose paclitaxel (4 mg/kg) not only did not suppress T-cell proliferation but also did not influence the proportion of T-cell subpopulations that were present. For the 20 mg/kg paclitaxel dose, the proportion of CD8+ T cells decreased significantly, whereas the proportion of regulatory T cells was not markedly suppressed. Previous studies found that paclitaxel enhanced the anti-tumor effect of chemoimmunotherapy by decreasing the number of regulatory T cells that were present [36, 42]. Our data showed that a decrease in the regulatory T-cell subpopulation is only induced in high doses of paclitaxel and that for low-dose paclitaxel (4 mg/kg), this is not found. Moreover, the effect of paclitaxel on DCs is also dose dependent. High concentrations of paclitaxel obviously induced the apoptosis of DCs, whereas low concentrations of paclitaxel not only did not induce the apoptosis of DCs but also actually induced the maturation of DCs and eliminated the inhibition of DCs maturation caused by the tumor. Recently, Shurin et al. [43] suggested that low-dose paclitaxel enhanced the phagocytosis of antigens by DCs. Our data suggest that different doses of paclitaxel enhance the anti-tumor effect by various immunomodulatory mechanisms. Low-dose (4 mg/kg) paclitaxel is potent enough to induce phagocytosis of tumor antigens by DCs and to enhance the maturation of DCs, which cause DCs to prime the anti-tumor immune response. At the same time, the effect that low-dose paclitaxel has on the function and proliferation on T cells is minimal. Therefore, low-dose paclitaxel mainly enhances the anti-tumor immune response at the priming time, which suggests that pre-vaccine paclitaxel administration (i.e., at the time of priming) is more potent than post-vaccine paclitaxel. High-dose (40 mg/kg) paclitaxel markedly impairs the function and proliferation of T cells, including the regulatory T-cell subpopulation. The impairment of the regulatory T-cell subpopulation may compensate for the immunosuppression that occurs from suppression of the other T-cell subpopulations. Regulatory T cells mainly influence the anti-tumor immune response at the level of T-cell activation, although they also play a role in priming by DCs, which suggests that post-vaccine paclitaxel administration is more appropriate when high-dose paclitaxel is given. On the other hand, the proliferation suppression of T cells is more obvious when paclitaxel is given after the vaccine than when it is given before the vaccine. Thus, it may not matter whether high-dose paclitaxel is given before or after the vaccine because it appears to have similar efficacy at both time points. For the 20 mg/kg dose, these two immunomodulatory mechanisms may be involved, but they do not all appear to play a significant role.
Thus, in this study, we have demonstrated for the first time that a GM-CSF-surface-modified whole-tumor vaccine is more prone to phagocytosis by DCs than whole-cell vaccine plus free GM-CSF. The combination of paclitaxel with the cancer vaccine enhanced the anti-tumor efficacy of the vaccine. However, the different doses of paclitaxel we used exerted different immunomodulatory mechanisms, which helped guide the optimal sequence of treatment for that paclitaxel dose. Our results indicate that in addition to drug, vaccine, and tumor type, drug dose may influence the success of chemoimmunotherapy.
The effect of this type of treatment may be enhanced if we select the proper treatment sequence for various drug/vaccine combinations based on the immunomodulatory mechanisms of each treatment.
Although docetaxel was approved for the treatment of metastatic castration-resistant prostate cancer in 2004, additional therapies are still required. Prostate cancer is often slow growing and expresses many tumor-associated antigens, making it a feasible target for immunotherapy. Several therapeutic cancer vaccines have been developed for prostate cancer, including antigen-presenting-cell-based, vector-based, and whole-tumor-cell vaccines. Initial trials demonstrated that vaccine approaches have limited toxicity. Clinical trials of targeted biologic therapies have demonstrated that patient selection is vital, and there is preliminary evidence that clinical parameters can be used to encompass metastatic prostate cancer patients who will more probably respond to vaccine treatment. In addition to appropriate patient selection, a successful clinical trial must have an appropriate primary endpoint as well. Three randomized, ‘placebo’-controlled studies in metastatic castration-resistant prostate cancer have suggested a clinically significant survival advantage in spite of a lack of improvement in time to progression, implying that overall survival is the ideal endpoint for such trials. Careful examination of data from completed immunotherapy clinical trials in prostate cancer has identified appropriate patient populations and endpoints. Those principles need to be applied to future trial design to properly evaluate prostate cancer vaccines.
Although PROVENGE has received FDA approval for patients with metastatic CRPC, challenges remain in identifying immunotherapy strategies that overcome immune tolerance, especially when disease burden is substantial. An emerging paradigm focuses on using immunotherapy together with checkpoint antagonists or in combination with conventional therapies in patients with early stage disease [47].
The administration of cytotoxic chemotherapy can result in bone marrow suppression and has been traditionally perceived to have a negative impact on immune function. Accordingly, the concurrent administration should be avoided in the combined therapy of cytotoxic chemotherapy and cancer immunotherapy [22, 24–27, 33, 37–39, 44–47]. However, as mentioned above, taxane-based chemotherapy may actually exert beneficial immunomodulatory effects through a variety of mechanisms, including cytokine production and T-cell infiltration of tumor cells [38, 39]. Therefore, it is critical to identify the optimal dose of the particular taxane (paclitaxel) in combination with cancer vaccine and the combination sequence in order to achieve the synergistic anti-cancer effects as much as possible. As the present study showed the low dose (4 mg/kg), but not the regular dose (20 mg/ml) or high dose (40 mg/ml), of paclitaxel in combination with the GM-CSF-surface-modified tumor-cell vaccine was the most effective at the induction of tumor regression in RM-1 prostate cancer mice when the vaccine was administrated 2 days after paclitaxel.
Taken together, we have clarified the dose, timing, and mechanism of action by which immunomodulatory paclitaxel can be translated to a clinical setting in the combined therapy with the GM-CSF-surface-modified tumor-cell vaccine for prostate cancer, especially for CRPC.
Acknowledgments
This work was supported in part by grants from Chinese National 863 plan (2006AA02Z4C4), the Natural Science Foundation of China (30928023 and 30971516), Zhejiang Provincial Major Research Program (2007C13020 and 2008C14082), the Natural Science Foundation of Zhejiang Province (R2080407 and Y2100925), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents, Science & Technology Innovation Program for College/University Students in Zhejiang Province (2009R413042 and 2010R413047) and Wenzhou Municipal Research Program (G20090142).
Footnotes
Q. He and J. Li contributed equally to this study.
Contributor Information
Zhiming Hu, Phone: +86-20-62789135, FAX: +86-20-61648553, Email: hzhiming99@126.com.
Jimin Gao, Phone: +86-577-86699341, FAX: +86-577-86689748, Email: jimingao@yahoo.com.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Coen JJ, Zietman AL, Thakral H, et al. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 3.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3, 478 consecutive patients:long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 4.Stamey TA, Yemoto CM, McNeal JE, et al. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163:1155–1160. doi: 10.1016/S0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 5.Calabro F, Sternberg CN. Current indications for chemotherapy in prostate cancer patients. Eur Urol. 2007;51:17–26. doi: 10.1016/j.eururo.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 8.Jeske S, Tagawa ST, Olowokure O et al (2010) Carboplatin plus paclitaxel therapy after docetaxel in men with metastatic castrate resistant prostate cancer. Urol Oncol [Epub ahead of print] [DOI] [PubMed]
- 9.Sella A, Yarom N, Zisman A, et al. Paclitaxel, estramustine and carboplatin combination chemotherapy after initial docetaxel-based chemotherapy in castration-resistant prostate cancer. Oncology-Basel. 2009;76:442–446. doi: 10.1159/000217264. [DOI] [PubMed] [Google Scholar]
- 10.Pandha H, Eaton J, Greenhalgh R, et al. Immunotherapy of murine prostate cancer using whole tumor cells killed ex vivo by herpes simplex viral thymidine kinase/ganciclovir suicide gene therapy. Cancer Gene Ther. 2005;12:572–578. doi: 10.1038/sj.cgt.7700836. [DOI] [PubMed] [Google Scholar]
- 11.Suckow MA, Wolter WR, Pollard M. Prevention of de novo prostate cancer by immunization with tumor-derived vaccines. Cancer Immunol Immunother. 2005;54:571–576. doi: 10.1007/s00262-004-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moody DB, Robinson JC, Ewing CM, et al. Interleukin-2 transfected prostate cancer cells generate a local antitumor effect in vivo. Prostate. 1994;24:244–251. doi: 10.1002/pros.2990240505. [DOI] [PubMed] [Google Scholar]
- 13.Eager R, Nemunaitis J. GM-CSF gene-transduced tumor vaccines. Mol Ther. 2005;12:18–27. doi: 10.1016/j.ymthe.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 15.Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 16.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 17.Simons JW, Carducci MA, Mikhak B, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 18.Moon C, Park JC, Chae YK, et al. Current status of experimental therapeutics for prostate cancer. Cancer Lett. 2008;266:116–134. doi: 10.1016/j.canlet.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, Huang S, Li M, et al. GM-CSF-surface-modified B16.F10 melanoma cell vaccine. Vaccine. 2006;24:5265–5268. doi: 10.1016/j.vaccine.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Hu Z, Tan W, Zhang L, et al. A novel immunotherapyfor superficial bladder cancer by intravesical immobilization of GM-CSF. J Cell Mol Med. 2010;14:1836–1844. doi: 10.1111/j.1582-4934.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prell RA, Gearin L, Simmons A, et al. The anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine is not inhibited by docetaxel administration. Cancer Immunol Immunother. 2006;55:1285–1293. doi: 10.1007/s00262-005-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eralp Y, Wang X, Wang JP, et al. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004;6:R275–R283. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machiels JP, Duck L, Honhon B, et al. Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadiOxCape study. Ann Oncol. 2005;16:1898–1905. doi: 10.1093/annonc/mdi406. [DOI] [PubMed] [Google Scholar]
- 24.Chopra A, Kim TS, O-Sullivan I, et al. Combined therapy of an established, highly aggressive breast cancer in mice with paclitaxel and a unique DNA-based cell vaccine. Int J Cancer. 2006;118:2888–2898. doi: 10.1002/ijc.21724. [DOI] [PubMed] [Google Scholar]
- 25.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noguchi M, Kakuma T, Uemura H, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 2010;59:1001–1009. doi: 10.1007/s00262-010-0822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang AL, Russell PJ. Paclitaxel suppresses the growth of primary prostate tumours (RM-1) and metastases in the lung in C57BL/6 mice. Cancer Lett. 2006;233:185–191. doi: 10.1016/j.canlet.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 30.El HN, Rubio N, Blanco J. Different effect of paclitaxel on primary tumor mass, tumor cell contents, and metastases for four experimental human prostate tumors expressing luciferase. Clin Cancer Res. 2005;11:1253–1258. [PubMed] [Google Scholar]
- 31.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8 +/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang GS, Lopez-Barcons L, Freeze BS, et al. Potentiation of taxol efficacy and by discodermolide in ovarian carcinoma xenograft-bearing mice. Clin Cancer Res. 2006;12:298–304. doi: 10.1158/1078-0432.CCR-05-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 34.Conti L, Gessani S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology. 2008;213:859–870. doi: 10.1016/j.imbio.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Bodey B, Jr, Bodey B, Siegel SE, et al. Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res. 2000;20:2665–2676. [PubMed] [Google Scholar]
- 36.Vicari AP, Luu R, Zhang NR, et al. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58:615–628. doi: 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galsky MD, Vogelzang NJ. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann Oncol. 2010;21:2135–2144. doi: 10.1093/annonc/mdq050. [DOI] [PubMed] [Google Scholar]
- 38.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2001;49:181–185. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zagozdzon R, Golab J. Immunomodulation by anticancer chemotherapy: more is not always better. Int J Oncol. 2001;18:417–424. doi: 10.3892/ijo.18.2.417. [DOI] [PubMed] [Google Scholar]
- 40.Yu B, Kusmartsev S, Cheng F, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–294. [PubMed] [Google Scholar]
- 41.Zhong H, Han B, Tourkova IL, et al. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13:5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Dermawan K, Jin M, et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129:219–229. doi: 10.1016/j.clim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J Immunother. 2008;31:491–499. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song CK, Han HD, Noh KH, et al. Chemotherapy enhances CD8+ T cell-mediated antitumor immunity induced by vaccination with vaccinia virus. Mol Ther. 2007;15:1558–1563. doi: 10.1038/sj.mt.6300221. [DOI] [PubMed] [Google Scholar]
- 45.Wada S, Yoshimura K, Hipkiss EL, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu Y, Wang LX, Yang G, et al. Efficacy of GM-CSF-producing tumor vaccine after docetaxel chemotherapy in mice bearing established Lewis lung carcinoma. J Immunother. 2006;29:367–380. doi: 10.1097/01.cji.0000199198.43587.ba. [DOI] [PubMed] [Google Scholar]
- 47.Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]