Abstract
Background
Low doses of the demethylating agent decitabine have been shown to enhance the sensitivity of tumors to immune effector cells and molecules through upregulation of tumor antigen presentation and apoptotic pathways. Effects on host immune effector and suppressor responses have not been well characterized.
Methods
Mice bearing B16 melanoma were treated with low-dose decitabine, cytokine, interleukin-2 (IL-2), toll-like receptor 9 agonist ODN1826, and/or a viral vectored vaccine targeting the melanoma antigen Trp2. Lymphoid and myeloid effector and suppressor cells were examined both systemically and intratumorally with functional, flow cytometric, and polymerase chain reaction–based assays.
Results
Enhancement of tumor growth delay was observed when decitabine was applied sequentially but not concurrently with IL-2. In contrast, complete responses and prolonged survival were observed when decitabine was applied with ODN1826 as therapy and with ODN1826 as a Trp2 vaccine adjuvant. Decitabine decreased natural killer and antigen-specific cellular immune responses when administered concurrently with IL-2 and with ODN1826; the Th1-associated transcription factor Tbet also decreased. T regulatory cells were not affected. When applied concurrently with ODN1826, decitabine increased macrophage cytotoxicity, M1 polarization, and dendritic cell activation. Myeloid-derived suppressor cells were reduced.
Conclusion
Low-dose decitabine promotes both anti- and pro-tumor host immune responses to immunotherapeutics in melanoma-bearing mice. Macrophage effector and dendritic cell activation increase, and myeloid suppressor cells decrease. Lymphoid effector responses, however, can be inhibited.
Keywords: Hypomethylation, Regulatory T cells, Myeloid suppressor cells, Immunotherapy
Introduction
Activation of cytolytic lymphocytes is a cornerstone of the treatment for malignant melanoma. Immunotherapy, however, is only partially effective. Most patients do not respond with tumor regression. Both natural killer (NK) cells and cytolytic T lymphocytes (CTL) release proteases and engage death receptors that result in tumor apoptosis. CTL require the expression of specific antigens within the context of class I major histocompatibility complex (MHC) on the target cell for activity. Down-regulation of apoptotic pathways and down-regulation of MHC class I and tumor antigen expression have been implicated in immunotherapy resistance. In melanoma, suppression of these pathways can result from the epigenetic repression of gene transcription by methylation and/or histone modification [1, 2]. Demethylating agents and histone deacetylase inhibitors have been shown to reverse this repression with resultant enhancement of sensitivity to cytolytic lymphocytes and immune effector molecules [3].
Modifying gene expression with the demethylating agent decitabine can be achieved with low doses that are less cytotoxic, and there has been interest in applying decitabine with cancer immunotherapy. A phase I clinical trial in which decitabine was combined with interleukin-(IL-)2 has been reported [4]. Clinical trials of decitabine and interferon (IFN-) α are in progress [5]. The combined antitumor effects of decitabine and immunotherapeutics in mouse solid tumor models, however, have been modest. In B16 melanoma, the antitumor effects of the concurrent administration of decitabine and IL-12 were limited to tumor growth delay [6]. In contrast, in the L1210 leukemia model, the majority of mice treated with the combination of decitabine and IL-12 manifested prolonged survival. Pretreatment with decitabine led to upregulated neoantigen expression of a DNA vaccine encoding calreticulin linked to papillomavirus E7 antigen and enhanced vaccine-induced E7-specific CD8+ T cells. Only a minority of mice, however, manifested prolonged survival when challenged with the TC-1 lung tumor line transduced to express E7 [7].
Although chromatin-modifying drugs can sensitize tumors to immune effector cells and molecules, not only cytotoxicity but also alterations in immune effectors can be induced that may limit their antitumor activity. In addition, cells implicated in the suppression of antitumor immunity can be promoted and also contribute to tumor immune escape. Decitabine has been shown to inhibit T-cell proliferation and activation in response to mitogens [8], induce inhibitory receptors on NK cells [9], decrease the production of antitumor cytokines such as IFN-γ and tumor necrosis factor-α (TNF-α) [8], and increase immune suppressing regulatory T (Treg) cells [10, 11]. The Treg-cell-promoting effects of decitabine have been exploited to prevent graft-versus-host disease in bone marrow transplantation models [8, 12]. Histone deacetylase inhibitors have been shown to inhibit NK cytotoxicity [13, 14], induce tumor-promoting cytokines such as transforming growth factor (TGF) β and IL-10; [15] promote Treg cells [16], and alter the activation of macrophages and dendritic cells (DC) to stimulate T helper (Th) 2 and not Th1 effector cells [17–20].
Little has been reported regarding the effects of demethylating agents on the host response to cancer immunotherapeutics. We examined in mouse B16 melanoma the effects of low-dose decitabine on the host antitumor and suppressor immune response to IL-2, a cytokine approved to treat patients with metastatic melanoma. We also examined the effects on the host response to a Toll-like receptor (TLR) 9 agonist, CpG oligodeoxynucleotide (ODN), formulations of which are being tested in patients with melanoma as a vaccine adjuvant and as systemic therapy [21, 22]. In contrast to IL-2, which functions directly to activate lymphocyte effector mechanisms, TLR9 agonists activate macrophage and DCs to prime these cells for induction of antitumor immunity [23]. Whereas decitabine had potentially deleterious effects on host lymphoid responses, potentially beneficial effects on host myeloid response were observed. Furthermore, significant antitumor activity was observed when decitabine was combined with the TLR9 agonist.
Materials and methods
Reagents
Decitabine was purchased from Tocris (Ellisville, MO); CpG ODN1826, from The Midland Certified Reagent Company, Inc. (Midland, TX); and IL-2, human recombinant, from Novartis Pharmaceuticals Corporation (East Hanover, NJ). A recombinant adeno-associated virus (AAV) −2 encoding mouse dopachrome tautomerase gene, DCT, also known as TYRP2, which encodes tyrosine-related protein 2 (Trp2), was constructed as previously described [24].
Cell lines and animals
B16 mouse melanoma cells (B16.F10) and RAW 264.7 mouse macrophage cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in Dulbecco’s modified essential medium (DMEM) with 10% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin (Mediatech, Herndon, VA). The cultures were grown at 37°C in 5% CO2 to confluence, passaged by treatment with 0.05% trypsin in EDTA at 37°C, and washed in media before being centrifuged at 200g for 10 min to form a pellet. Female C57BL/6 mice, 4–6 weeks of age, were obtained from Taconic Farms (Hudson, NY) and fed with commercial diet and water ad libitum. The animal use and care protocol was approved by the Institutional Animal Use and Care Committee.
Tumor model
Tumors were established by injecting 2 × 105 B16 cells in 100 μl of serum-free DMEM subcutaneously (s.c.) into a flank. Mice were treated with decitabine at 0.2 mg/kg, ODN1826 at 50 μg, and IL-2 at 180,000 IU in 0.5 mL three times per week. AAV-DCT immunizations were by a single s.c. injection of 1010 rAAV-DCT in 50 μl normal saline. All treatments were injected peritumorally anticipating the collection of tumor-draining lymph nodes. Tumor size was measured bidimensionally with calipers every 2–3 days, and tumor volume calculated by the formula (length × width2)/2. Mice were euthanized when tumors reached the size of 2,000 mm3. Mice were also euthanized to obtain tumor, lymph nodes, and spleens.
Flow cytometry
Splenocytes were washed twice in PBS—1% bovine serum albumin plus 0.05% sodium azide and stained for 30 min on ice with phycoerythrin or fluorescein isothiocyanate–conjugated mAb specific to B220, CD8, CD11b, CD11c, CD86, Gr1 and NK1.1 (BD Biosciences, San Jose, CA) and CD4, CD25, and FoxP3 (eBioscience Inc., San Diego, CA). Appropriate isotype controls were used in all experiments. After incubation, cells were washed and fixed with 2% paraformaldehyde. All samples were analyzed using an EPICS Altra flow cytometer (Beckman Coulter, Fullerton, CA).
Cytokine production
Single-cell suspensions of splenocytes were cultured in the presence of B16 cell freeze–thaw lysates, 20 μg/ml purified H2-Kb Trp2 peptide (180–188), or, as a control, a H2-Kb mesothelin peptide (351–358) (Lerner Research Institute Molecular Biotechnology Core, Cleveland, OH). After 3 days, culture supernatants were collected and assayed for IFN-γ by ELISA (R&D Systems) and for IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-17A, IL-10 using BD Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 Cytokine Kit (San Jose, CA) according to the manufacturer’s instructions. Results are expressed as Trp2-specific (production in response to Trp2 peptide—production in response to control peptide).
DC separation
CD11c+ DC from resected lymph nodes were removed by magnetic activated cell sorting (MACS; Mitenyi Biotec, Auburn, CA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Dissected tissues were placed in RNA Later (Ambion, Austin, TX) and stored at 4°C. RNA was then extracted with RNeasy and stored at −80°C. An ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA) and prestandardized primers and TaqMan probes for mouse arginase (Arg1), CD206 (Mrc1), CXCL10, forkhead box P3 (Foxp3), Gata-3, IFN-γ, IL-1β, IL-6 IL-4, IL-10, IL-12, IL-17, receptor-related orphan receptor γt (Rorc), T-bet (Tbx21), TGF-β1, and TNF-α were used. Glyceraldehyde-3-phosphate dehydrogenase expression was used as the endogenous control (Applied Biosystems). The reverse transcription and PCR was accomplished using a one-step protocol and TaqMan Universal Master Mix (Applied Biosystems) according to the recommendations of the manufacturer. Cycle threshold (Ct) values were determined, and the relative number of copies of mRNA (RQ) was calculated using the ΔΔCt method (Relative Quantitation of Gene Expression, User Bulletin #2, ABI Prism 7700 Sequence Detection System, Applied Biosystems). To account for recognized variation related to specimen collection, processing, and testing, only differences in RQ of >0.5 log were considered significant [25].
Lymphocyte cytotoxicity
Splenocyte effectors were pretreated with IL-2 at 400 MIU for 3 days, washed with culture medium, and then used for examination of cytotoxic activity against 1 × 104 target YAC-1 cells in the same culture medium at various effector/target (E/T) ratios ranging from 2.5/1 to 20/1 in 96-well round-bottom microtiter plates (Becton–Dickinson, Franklin Lakes, NJ). The microtiter plates were centrifuged at 200g for 5 min to facilitate contact between effector and target cells, followed by incubation at 37°C for 4 h in 5% CO2/air. After incubation, cytotoxicity was assayed with CytoTox-Glo™ Cytotoxicity Assay (Promega, Madison, WI) according to the recommendations of the manufacture. Percentage cytotoxicity was calculated using the following formula: [1 − (RLUE+T − RLUE)/RLUT] × 100, where RLUE+T = luminescence of wells with both effector and target cells; RLUE, wells with effector cells alone; and RLUT, wells with targets cells alone.
Macrophage cytotoxicity
Peritoneal exudate cells were collected by lavage of the peritoneal cavity with chilled Dulbecco’s phosphate-buffered saline (PBS; Gibco BRL). After centrifugation, cells were plated in plastic Petri dishes in culture medium, incubated for 1.5 h at 37°C in 5% CO2, and washed gently three times with warm culture medium to remove non-adherent cells. Adherent cells with morphological characteristics of macrophages were harvested by forced rinsing with cold Dulbecco’s PBS. Following one washing, macrophages were resuspended in culture medium, counted, and dispensed into wells of a 96-well flat-bottomed microtiter plate at a concentration of 2.5 × 104 cells in 0.1 ml per well. Then, 5 × 103 B16 melanoma cells in 0.1-ml culture medium were added to each well (within 2 h) at varying E:T ratios and cells were cultured for 3 days. Cytotoxicity was assayed and calculated as above.
Statistical analysis
Means and standard errors of the mean (SEM) for each set of tumor measurements were calculated and represented as y-axis error bars on each graph. Tumor volume data were analyzed using ANOVA. Means and standard deviations (SD) were calculated for cell frequencies, cytokine response, and cytotoxicity assays. These parameters were analyzed with two-sided Student’s t test. P < 0.05 was considered statistically significant. All analyses were 2-sided.
Results
Concurrent decitabine enhances the antitumor activity of ODN1826 but not of IL-2
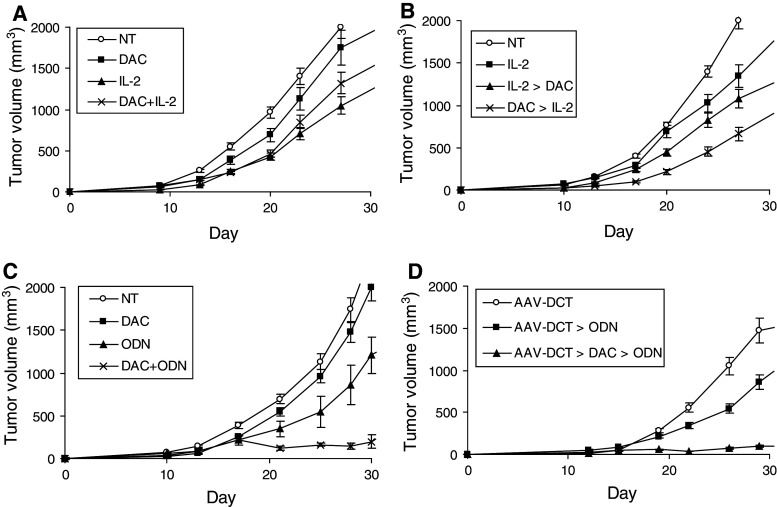
The effects of low-dose decitabine on B16 melanoma tumor growth when administered concurrently with IL-2 were tested first. IL-2 alone delayed tumor growth (P < 0.003) compared to mice not receiving treatment. This dose and schedule of decitabine did not significantly modulate tumor growth and, when added to IL-2, abrogated the antitumor activity observed with IL-2 (P < 0.003; Fig. 1a). Antagonism was confirmed in a follow-up experiment in which mice were treated with IL-2 alone and IL-2 and decitabine (n = 7 mice per group, P < 0.003; data not shown). Pretreatment with decitabine had been effectively applied to enhance tumor antigen presentation and sensitivity to immune effectors, and decitabine was then administered after and before IL-2. Administering decitabine after (P < 0.03) as well as before (P < 0.02) IL-2 improved antitumor activity significantly over IL-2 alone (Fig. 1b). The antitumor effects with sequential decitabine-IL-2 were not dramatic and were limited to tumor growth delay. The effects on tumor growth of decitabine and the TLR9 agonist, ODN1826, were then examined. One of the genes upregulated in vivo in B16 melanoma with low-dose decitabine as applied is DCT, which encodes the melanoma-associated antigen, Trp2 [26]. Decitabine was also administered sequentially with immunization with AAV-DCT and ODN1826, a vaccine approach partially effective in B16 melanoma model [24]. ODN1826 alone delayed tumor growth (P < 0.001) compared to mice not receiving treatment. Decitabine improved the antitumor activity of ODN1826 both when applied concurrently as monotherapy (P < 0.0008; Fig. 1c) and sequentially as a vaccine adjuvant (P < 0.0005; Fig. 1d). Complete tumor responses and prolonged survival were observed. Vitiligo at the site of tumor inoculation was observed in mice (2/7) immunized sequentially with AAV-DCT, decitabine, and ODN1826.
Fig. 1.
Effects on tumor growth in vivo. B16 cells were implanted on day 1. a Starting on day 3, groups of mice were treated with decitabine (DAC) and/or IL-2 for 2 weeks. A group was not treated (NT). Data represent mean tumor volume ± SEM, n = 10 mice per group. b Starting on day 3, groups of mice were treated with decitabine for 1 week and then IL-2 for 1 week (DAC → IL-2); IL-2 for 1 week and then decitabine for 1 week (IL-2 → DAC); or IL-2 for 2 weeks (IL-2). A group was not treated (NT). Data represent mean tumor volume ± SEM, n = 7 mice per group. c Starting on day 3, groups of mice were treated with DAC and/or ODN1826 (ODN) for 2 weeks. Data represent mean tumor volume ± SEM, n = 10 mice per group. d One week before tumor inoculation, groups of mice were immunized with rAAV-DCT. Starting on day 3, groups of mice were treated with DAC for 1 week and ODN for 1 week (AAV-DCT → DAC → ODN) or with ODN (AAV-DCT → ODN) for 1 week starting on day 10. Data represent mean tumor volume ± SEM, n = 7 mice per group
Decitabine reduces innate and adaptive lymphoid effectors but not lymphoid suppressors
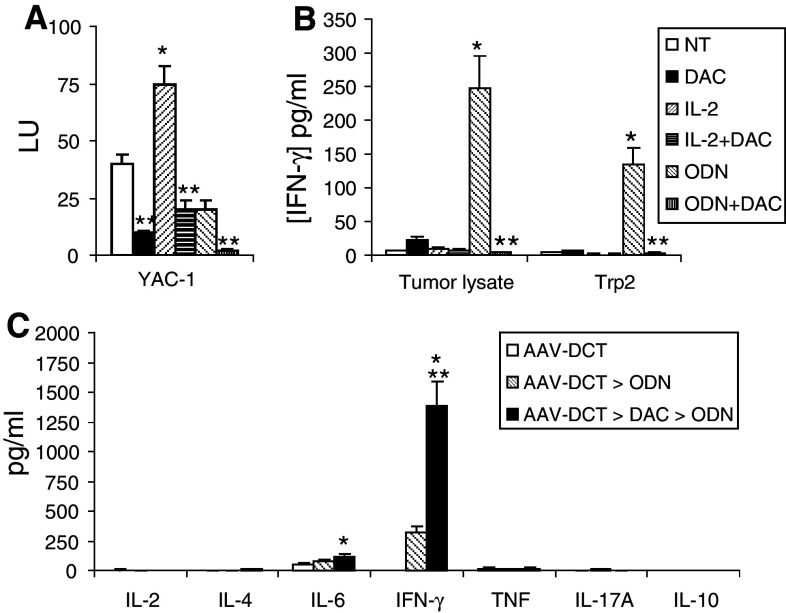
The effects of low-dose decitabine, either alone or combined with IL-2 or ODN1826, on host lymphoid responses were then examined to evaluate mechanisms by which antitumor activity was modulated in the immunotherapy models. Decitabine decreased the innate lymphoid effector response, as manifested by NK activity (Fig. 2a). Adaptive immune effectors were examined by testing the production of the Th1-associated IFN-γ by splenocytes in response to stimulation with B16 cell lysates and a melanoma-associated peptide corresponding to Trp2. A significant lysate and Trp2 response was enhanced by ODN1826 but not by IL-2; concurrent decitabine decreased this response (Fig. 2b). The adaptive immune response, in terms of Th1/Th2/Th17 bias, was compared in mice immunized sequentially with AAV-DCT, decitabine, and ODN-1826. A Th1-associated response to Trp2 was elicited and was enhanced in mice administered decitabine (Fig. 2c).
Fig. 2.
Effects on lymphoid effector activity in vivo. B16 cells were implanted on day 1. Beginning on day 10, mice were treated with decitabine (DAC), IL-2, and/or ODN1826 (ODN) for 2 weeks. A group of mice was not treated (NT). Splenocytes were collected on day 24 and a NK activity against YAC-1 target cells and b IFN-γ response to stimulation to B16 tumor lysates and Trp2 peptide were determined. Data represent mean ± SD, n = 4 mice per group. *P < 0.05 compared to NT; **P < 0.05 compared to without DAC. c One week before tumor inoculation, groups of mice were immunized with rAAV-DCT. Starting on day 3, a group of mice was treated with DAC for 1 week and ODN1826 for 1 week (AAV-DCT → DAC → ODN), or with ODN1826 for 1 week starting on day 10 (AAV-DCT → ODN). Splenocytes were collected on day 24. Th1/Th2/Th17 cytokine production in response to Trp2 peptide was assessed by CBA. Data represent mean ± SD, n = 4 mice per group. *P < 0.05 compared to AAV-DCT; **P < 0.05 compared to AAV-DCT → ODN
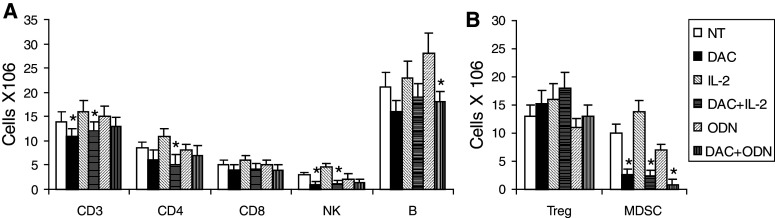
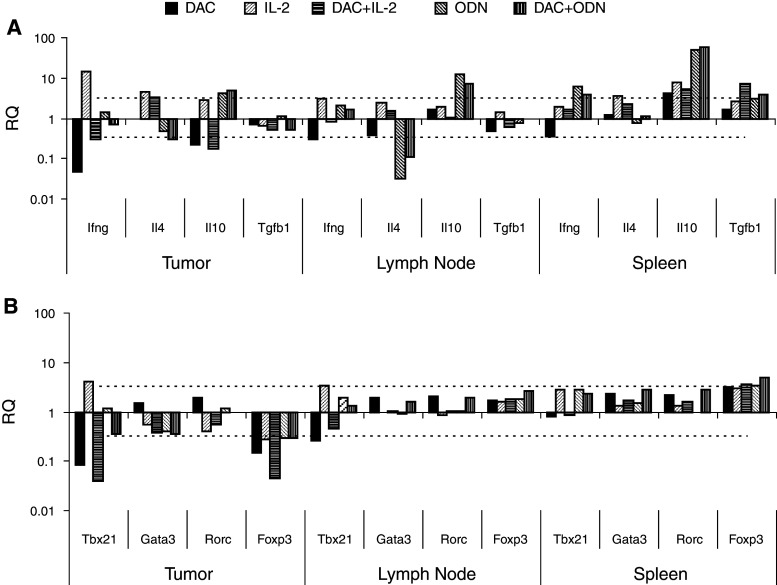
Systemic lymphoid effector and suppressor cells were examined by phenotyping splenocytes. Decitabine decreased NK1.1+ NK cells and CD3+ and CD4+ T cells. B200+ B cells, which increased with ODN1826, were also reduced (Fig. 3a). CD4+CD25+FoxP3+ Treg cells tended to increase, although increases did not reach the level of significance (Fig. 3b). A qRT-PCR-based method was also used to examine cytokines and transcription factors that characterize lymphoid responses intratumorally, in draining lymph nodes, and in spleen. The effects of decitabine were dependent on the tissue examined. Decitabine decreased the Th1-associated IFN-γ in all tissues. In tumor, the Th2-associated IL-4 and the Treg-cell-associated IL-10 decreased, while in lymph nodes and/or spleens these, along with TGF-β1, increased. Levels of IL-17 were at the limits of detection (Ct ≥ 37). The decreases in IFN-γ were paralleled with decreases in the Th1-associated transcription T-bet in tumor and lymph nodes (Fig. 4). Decitabine decreased intratumoral Foxp3, expression of which is highly restricted to Treg cells in mice; in spleens, Foxp3 expression increased. Significant changes were not observed in the Th2-associated Gata3 and the Th17-associated Rorc in any tissue.
Fig. 3.
Effects on splenocyte phenotype in vivo. B16 cells were implanted on day 1. Beginning on day 10, mice were treated with decitabine (DAC), IL-2, and/or ODN1826 (ODN) for 2 weeks. A group of mice was not treated (NT). Splenocytes were collected on day 24. a CD3+, CD4+, CD8+ T cells, NK1.1+ NK, and B220+ B cells were determined by flow cytometry. b CD4+CD25+FoxP3+ Treg cells and CD11b+Gr1+ MDSC were determined by flow cytometry. Data represent mean ± SD, n = 4 mice per group. *P < 0.05 compared to without DAC
Fig. 4.
Effects on lymphocyte markers in vivo. B16 cells were implanted s.c. on day 1. Beginning on day 10, mice were treated with decitabine (DAC), IL-2, and/or ODN1826 (ODN) for 2 weeks. A group of mice was not treated. Tumor, lymph nodes, and spleens were harvested on day 24. Mouse lymphokine (a) and lymphocyte transcription factors (b) were assessed by qRT-PCR and compared to the group not treated. Data represent mean RQ, n = 3 mice per group. Mean RQ changes >0.5 log (dotted line) were considered significant. * mean RQ changes >0.5 log compared to without DAC
Decitabine enhances myeloid effectors and reduces myeloid suppressors
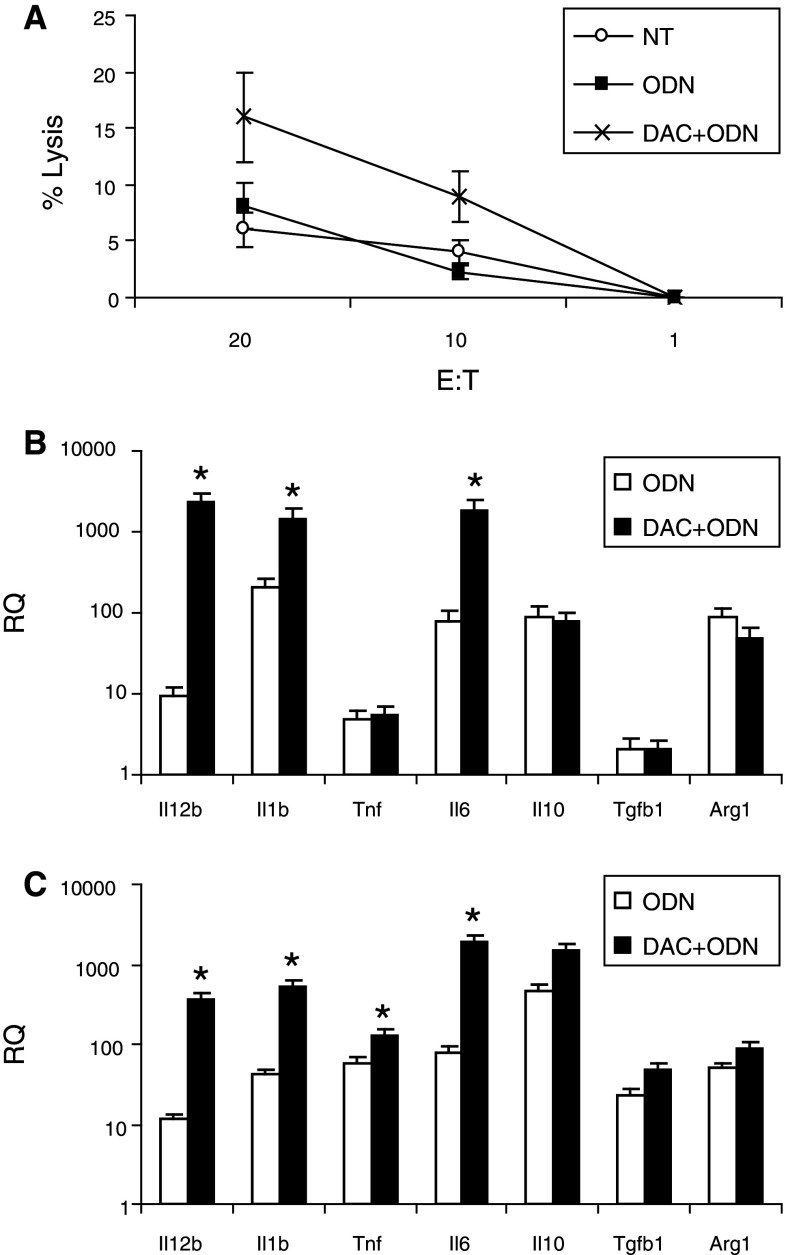
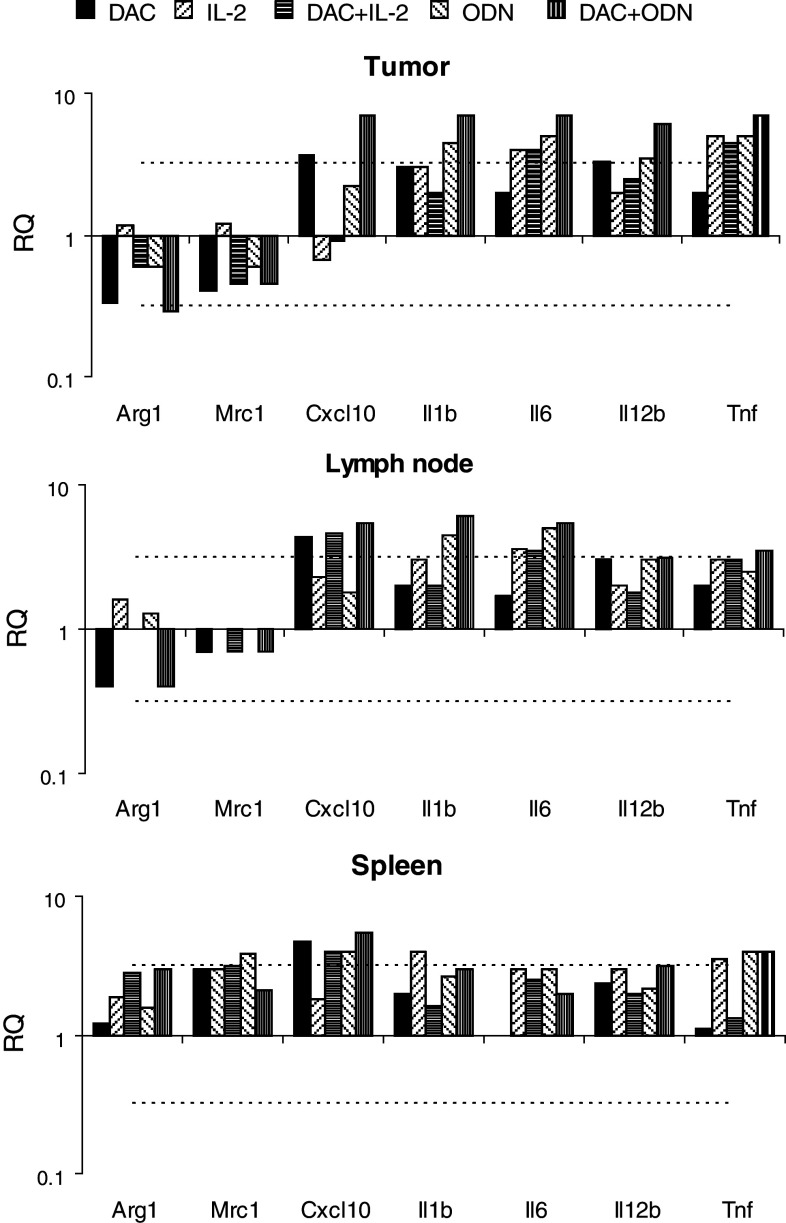
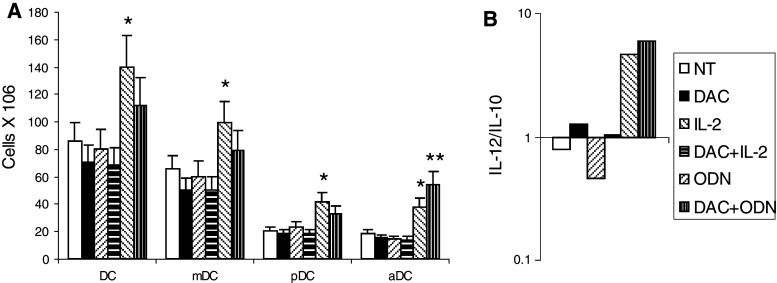
The effects of decitabine, alone and combined with IL-2 or ODN1826, on macrophage cytotoxicity was examined using peritoneal exudative macrophages collected from mice treated in vivo. An increase in macrophage cytotoxicity was observed in mice that were administered decitabine and ODN1826; other treatments in vivo had no measurable effect on macrophage cytotoxicity (Fig. 5a and data not shown). Markers of macrophage polarization were also evaluated in vitro using peritoneal exudative macrophages (Fig. 5b) and RAW cells, a macrophage cell line derived from C57Bl/6 mice (Fig. 5c). Compared to ODN1826 alone, which increased all markers, treatment with decitabine increased the production of the mRNA of IL-1β, IL-6, IL-12, and, in RAW cells, TNF-α, cytokines associated with M1 polarization. Macrophage polarization and myeloid suppressor cells were also evaluated in vivo within tumor, lymph nodes, and spleen using qRT-PCR (Fig. 6). In all tissues, CXCL10, and in tumor, IL-1 and IL-12, markers of antitumor M1 macrophages, increased. In tumor, arginase, a product of pro-tumor myeloid-derived suppressor cells (MDSC) and M2-polarized macrophages, decreased. The frequency of CD11b+Gr1+ MDSC among splenocytes also decreased in response to decitabine (Fig. 3b). DC were evaluated in lymph nodes draining injection sites. Small, though significant, decreases were observed in vivo in total CD11c+ DC, CD11c+CD11b+ mDC, and CD11c+B220+ pDC in mice that were administered decitabine. In contrast, small, though significant, increases in CD11c+CD86+-activated DC were observed in mice administered decitabine and ODN1826 compared to ODN1826 alone (Fig. 7a). CD11c+ DC were isolated from these lymph nodes. More IL-12 mRNA relative to IL-10 mRNA was expressed by these DC in response to the combination of decitabine and ODN182; the ratio of IL-12/IL-10 went from <1 without treatment to >1 with treatment (Fig. 7b).
Fig. 5.
Effects of decitabine on macrophage activation in vivo and in vitro. B16 cells were implanted on day 1. Mice were treated with decitabine, IL-2, and/or ODN1826 for 2 weeks. A group of mice was not treated. a Peritoneal macrophages were harvested on day 24 and tested for cytotoxicity versus B16 targets. Data represent mean ± SD, n = 3 mice per group. b Peritoneal macrophages and c RAW cells were cultured in vitro with 5 μM decitabine without or with 1 or 10 μg ODN1826. After 18 h RNA was isolated for QRT-PCR to assess RQ relative to unstimulated. Data represent mean RQ of ± SD, n = 3. * mean RQ changes >0.5 log compared to without DAC
Fig. 6.
Effects on myeloid regulatory factors in vivo. B16 cells were implanted on day 1. Beginning on day 10, mice were treated with decitabine (DAC), IL-2, and/or ODN1826 (DAC) for 2 weeks. A group of mice was not treated. Tumors, lymph nodes, and spleens were harvested on day 24. Mouse myeloid markers were assessed by qRT-PCR and compared to the group not treated. Data represent mean RQ, n = 4 mice per group. Mean RQ changes >0.5 log (dotted line) were considered significant. * mean RQ changes >0.5 log compared to without DAC
Fig. 7.
Effects on DC activation in vivo. Mice were treated with decitabine (DAC) and/or ODN1826 (ODN) for 1 week. A group of mice was not treated (NT). Regional lymph nodes were then collected. a CD11c+DC-, CD11c+BB20+ pDC-, CD11c+CD11b+ mDC- and CD11c+CD86+-activated DC (aDC) phenotypes were determined in lymph node by flow cytometry. Data represent mean cell numbers ± SD, n = 4 mice per group. *P < 0.05 compared to NT; **P < 0.05 compared to without DAC. b CD11c+ DC were isolated from lymph nodes by MACS and IL-12 and IL-10 mRNA expression was assessed by qRT-PCR. Data represent the mean IL-12/IL-10 ratio, n = 4 mice per group
Discussion
Demethylating agents can have both pro- and antitumor effects. They have been shown to increase tumor expression of components of both antigen presentation and apoptotic pathways, which functionally translates into enhanced susceptibility to killing by immune effector cells and molecules [27]. The effects of demethylating agents on immune effector cells and molecules have not been well characterized. Epigenetic mechanisms, including DNA methylation, are involved in regulating host immune responses, and demethylating agents have demonstrated immune effects that are potentially tumor-promoting. We focused on the effects of low-dose decitabine on the host response to cancer immunotherapeutics. Whereas decitabine had potentially deleterious effects on host lymphoid responses, potentially beneficial effects on the host myeloid responses were observed.
Low-dose decitabine increased macrophage cytotoxicity, intratumoral markers of macrophage M1 polarization and DC activation. CD11b+Gr1+ myeloid-derived suppressor cells decreased. Furthermore, the most dramatic antitumor effects were observed with the combination of decitabine and the TLR9 agonist. Macrophages are essential for antitumor effects against weakly immunogenic murine tumors produced by ODN1826 and other class B CpG-oligodeoxynucleotides [28]. Decitabine has been previously shown to have several effects on myeloid cells, including the promotion of the differentiation of monocyte-macrophages [29, 30]. It has recently been reported that in vitro exposure of freshly excised mouse tumors to decitabine results in selective elimination of tumor cells while enriching CD11b myeloid cells, which, when cultured with granulocyte macrophage colony stimulating factor, were differentiated into mature CD11c+ antigen-presenting cells that produced reduced amounts of immunosuppressive mediators [31]. The myeloid effects of decitabine observed do vary with those reported with histone deacetylase inhibitors, which have been shown to alter the activation of macrophages and DCs to promote Th2 and not Th1 responses [17–20], and to induce immune suppressive cytokines such as TGF-β [15].
Although chromatin-modifying drugs have been shown to enhance the sensitivity of tumor targets to NK cells, DNA methylation plays a central role in regulating NK cell activation [9]. As has been reported with histone deacetylase inhibitors, decitabine inhibited NK cytotoxicity [16]. Our results do vary from those in a recent report in which decitabine treatment in vitro enhanced NK cytotoxicity [32]. This study used human NK cells that had been isolated after culture with IL-2. It may be that once activated, immune cells are no longer susceptible to the suppressive effects of chromatin-modifying agents [33]. The enhanced antitumor activity we observed with sequential decitabine and IL-2 would support this. Epigenetic mechanisms play a central role in Th and Treg differentiation [16, 34]. We observed that decitabine decreased Th1-associated antitumor response. Significant decrease in Th1-associated transcription factor Tbet, which plays a key role in NK-mediated control of melanoma metastatic disease, was also observed [35].
We did not observe an increase in CD4+CD25+Foxp3+ Treg cells systemically as had been previously reported in other models [8, 10–12]. Our results vary with those reported in the RENCA renal cell carcinoma model in BALB/c mice in which a significant improvement in antitumor activity was observed with the combination of IL-2 and the histone deacetylase inhibitor, MS-275, and was associated with an increase in splenocyte cytotoxicity and a decrease in splenocyte Treg cells [36]. Foxp3 mRNA expression, however, did increase in spleens. Conversely, Foxp3 expression decreased intratumorally in responding tumors. Of note, changes in other factors, such as IL-10, also varied intratumorally compared to systemically. That intratumoral and systemic immune responses can vary is well recognized, as are tissue-specific effects of demethylating agents [37, 38]. Furthermore, in the absence of methylation analysis of treated immune cells, these effects could be just “off-target” effects of low-dose decitabine.
Low-dose decitabine can be effectively applied with IL-2 and a TLR9 agonist to enhance antitumor activity in melanoma-bearing mice. It should be noted that the treatments applied were not optimized to achieve antitumor activity, but rather to establish model systems to examine the host response. Clinical translation will require further study. There are important differences in mice and human host immune responses. In mice, TLR9 is broadly expressed on all major DC subtypes as well as in macrophages and B cells. In human, TLR9 is expressed by pDCs and B cells [39, 40]. To investigate host immune response, we used the B16F10 melanoma model and C57BL/6 mice. B16 tumors are characterized by a significant infiltration of macrophages that are M2 polarized, and there is evidence implicating the activation of macrophages without T cells or NK cells in tumor rejection in this model [41–44]. Furthermore, MDSC expansion has been reported to be higher in B16 melanoma than other tumor models [45]. C57Bl/6 mice have also been characterized by a Th1 bias and less Treg-cell expansion than other mice, including BALB/c [46]. Nonetheless, M2-macrophage polarization and MDSC populations have been identified in patients with melanoma [47, 48]. Furthermore, tumor responses have been observed in early phase clinical trials of CpG ODN in melanoma [22]. Combinations of low-dose decitabine and CpG ODN merit clinical evaluation.
Acknowledgments
This work was supported by National Institutes of Health grants R01CA118660.
References
- 1.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Re-expression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer. 2001;94:243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 2.Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordón-Cardó C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 3.Tomasi TB, Magner WJ, Khan AN. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–1184. doi: 10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gollob JA, Sciambi CJ, Peterson BL, Richmond T, Thoreson M, Moran K, Dressman HK, Jelinek J, Issa JP. Phase I trial of sequential low-dose 5-aza-2′-deoxycytidine plus high-dose intravenous bolus interleukin-2 in patients with melanoma or renal cell carcinoma. Clin Cancer Res. 2006;12:4619–4627. doi: 10.1158/1078-0432.CCR-06-0883. [DOI] [PubMed] [Google Scholar]
- 5.http://clinicaltrials.gov/ct2/show/NCT00791271
- 6.Kozar K, Kamiński R, Switaj T, Ołdak T, Machaj E, Wysocki PJ, Mackiewicz A, Lasek W, Jakóbisiak M, Gołab J. Interleukin 12-based immunotherapy improves the antitumor effectiveness of a low-dose 5-Aza-2′-deoxycitidine treatment in L1210 leukemia and B16F10 melanoma models in mice. Clin Cancer Res. 2003;9:3124–3133. [PubMed] [Google Scholar]
- 7.Lu D, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Treatment with demethylating agent, 5-aza-2′-deoxycytidine enhances therapeutic HPV DNA vaccine potency. Vaccine. 2009;27:4363–4369. doi: 10.1016/j.vaccine.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, Caballero-Velazquez T, Blanco B, Herrero-Sánchez C, García JL, Carrancio S, Hernández-Campo P, González FJ, Flores T, Ciudad L, Ballestar E, Del Cañizo C, San Miguel JF, Pérez-Simon JA. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–121. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 9.Santourlidis S, Trompeter HI, Weinhold S, Eisermann B, Meyer KL, Wernet P, Uhrberg M. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J Immunol. 2002;169:4253–4261. doi: 10.4049/jimmunol.169.8.4253. [DOI] [PubMed] [Google Scholar]
- 10.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+ CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, Piwnica-Worms DR, DiPersio JF. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogbomo H, Michaelis M, Kreuter J, Doerr HW, Cinatl J., Jr Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett. 2007;581:1317–1322. doi: 10.1016/j.febslet.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Kuick R, Hanash S, Richardson B. DNA methylation inhibition increases T cell KIR expression through effects on both promoter methylation and transcription factors. Clin Immunol. 2009;130:213–224. doi: 10.1016/j.clim.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira JM, Scheipers P, Sorensen P (2003) The histone deacetylase inhibitor trichostatin A modulates CD4+ T cell responses. BMC Cancer (http://www.biomedcentral.com/1471-2407/3/30) [DOI] [PMC free article] [PubMed]
- 16.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, Bromberg JS. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, Huang Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowska A, Jagodzinski PP. Effect of trichostatin A on CD4 surface density in peripheral blood T cells. Folia Histochem Cytobiol. 2006;44:259–262. [PubMed] [Google Scholar]
- 19.Guo L, Hu-Li J, Zhu J, Watson CJ, Difilippantonio MJ, Pannetier C, Paul WE. In TH2 cells the Il4 gene has a series of accessibility states associated with distinctive probabilities of IL-4 production. Proc Natl Acad Sci USA. 2002;99:10623–10628. doi: 10.1073/pnas.162360199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, Nguyen D, Wright KL, Atadja PW, Bhalla K, Pinilla-Ibarz J, Seto E, Sotomayor EM. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2008;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fourcade J, Kudela P, Andrade Filho PA, Janjic B, Land SR, Sander C, Krieg A, Donnenberg A, Shen H, Kirkwood JM, Zarour HM. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother. 2008;31:781–791. doi: 10.1097/CJI.0b013e318183af0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pashenkov M, Goëss G, Wagner C, Hörmann M, Jandl T, Moser A, Britten CM, Smolle J, Koller S, Mauch C, Tantcheva-Poor I, Grabbe S, Loquai C, Esser S, Franckson T, Schneeberger A, Haarmann C, Krieg AM, Stingl G, Wagner SN. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 23.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 24.Triozzi PL, Aldrich A, Ponnazhagan S. Regulation of the activity of an adeno-associated virus vector cancer vaccine administered with synthetic Toll-like receptor agonists. Vaccine. 2010;28:7837–7843. doi: 10.1016/j.vaccine.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 25.Press RD, Galderisi C, Yang R, Rempfer C, Willis SG, Mauro MJ, Druker BJ, Deininger MW. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. 2007;13:6136–6143. doi: 10.1158/1078-0432.CCR-07-1112. [DOI] [PubMed] [Google Scholar]
- 26.Alcazar O, Achberger S, Aldrich W, Hu Z, Negrotto S, Saunthararajah Y, Triozzi PL (2011) Epigenetic regulation of melanoma differentiation in vitro and in vivo. doi:10.1002/ijc.26320 [DOI] [PMC free article] [PubMed]
- 27.Rothhammer T, Bosserhoff AK. Epigenetic events in malignant melanoma. Pigment Cell Res. 2007;20:92–111. doi: 10.1111/j.1600-0749.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- 28.Buhtoiarov IN, Sondel PM, Eickhoff JC, Rakhmilevich AL. Macrophages are essential for antitumour effects against weakly immunogenic murine tumours induced by class B CpG-oligodeoxynucleotides. Immunology. 2007;120:412–423. doi: 10.1111/j.1365-2567.2006.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koschmieder S, Agrawal S, Radomska HS, Huettner CS, Tenen DG, Ottmann OG, Berdel WE, Serve HL, Müller-Tidow C. Decitabine and vitamin D3 differentially affect hematopoietic transcription factors to induce monocytic differentiation. Int J Oncol. 2007;30:349–355. [PubMed] [Google Scholar]
- 30.Laurenzana A, Petruccelli LA, Pettersson F, Figueroa ME, Melnick A, Baldwin AS, Paoletti F, Miller WH., Jr Inhibition of DNA methyltransferase activates tumor necrosis factor alpha-induced monocytic differentiation in acute myeloid leukemia cells. Cancer Res. 2009;69:55–64. doi: 10.1158/0008-5472.CAN-08-0245. [DOI] [PubMed] [Google Scholar]
- 31.Daurkin I, Eruslanov E, Vieweg J, Kusmartsev S. Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2′-deoxycytidine. Cancer Immunol Immunother. 2010;59:697–706. doi: 10.1007/s00262-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmiedel BJ, Arélin V, Gruenebach F, Krusch M, Schmidt SM, Salih HR. Azacytidine impairs NK cell reactivity while decitabine augments NK cell responsiveness toward stimulation. Int J Cancer. 2011;128:2911–2922. doi: 10.1002/ijc.25635. [DOI] [PubMed] [Google Scholar]
- 33.Schmudde M, Friebe E, Sonnemann J, Beck JF, Bröker BM. Histone deacetylase inhibitors prevent activation of tumour-reactive NK cells and T cells but do not interfere with their cytolytic effector functions. Cancer Lett. 2010;295:173–181. doi: 10.1016/j.canlet.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Moon C, Kim SH, Park KS, Choi BK, Lee HS, Park JB, Choi GS, Kwan JH, Joh JW, Kim SJ. Use of epigenetic modification to induce FOXP3 expression in naïve T cells. Transplant Proc. 2009;41:1848–1854. doi: 10.1016/j.transproceed.2009.02.101. [DOI] [PubMed] [Google Scholar]
- 35.Werneck MB, Lugo-Villarino G, Hwang ES, Cantor H, Glimcher LH. T-bet plays a key role in NK-mediated control of melanoma metastatic disease. J Immunol. 2008;180:8004–8010. [Google Scholar]
- 36.Kato Y, Yoshimura K, Shin T, Verheul H, Hammers H, Sanni TB, Salumbides BC, Van Erp K, Schulick R, Pili R. Synergistic in vivo antitumor effect of the histone deacetylase inhibitor MS-275 in combination with interleukin 2 in a murine model of renal cell carcinoma. Clin Cancer Res. 2007;13(15 Pt 1):4538–4546. doi: 10.1158/1078-0432.CCR-07-0014. [DOI] [PubMed] [Google Scholar]
- 37.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 38.Santoso B, Ortiz BD, Winoto A. Control of organ-specific demethylation by an element of the T-cell receptor-alpha locus control region. J Biol Chem. 2000;275:1952–1958. doi: 10.1074/jbc.275.3.1952. [DOI] [PubMed] [Google Scholar]
- 39.Rehli M. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 2002;23:375–378. doi: 10.1016/S1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;10:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 41.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 42.Clarke JH, Cha JY, Walsh MD, Gamboni-Robertson F, Banerjee A, Reznikov LL, Dinarello CA, Harken AH, McCarter MD. Melanoma inhibits macrophage activation by suppressing toll-like receptor 4 signaling. J Am Coll Surg. 2005;201:418–425. doi: 10.1016/j.jamcollsurg.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 43.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 44.Hara I, Nguyen H, Takechi Y, Gansbacher B, Chapman PB, Houghton AN. Rejection of mouse melanoma elicited by local secretion of interleukin-2: implicating macrophages without T cells or natural killer cells in tumor rejection. Int J Cancer. 1995;61:253–260. doi: 10.1002/ijc.2910610219. [DOI] [PubMed] [Google Scholar]
- 45.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melencio L, McKallip RJ, Guan H, Ramakrishnan R, Jain R, Nagarkatti PS, Nagarkatti M. Role of CD4(+)CD25(+) T regulatory cells in IL-2-induced vascular leak. Int Immunol. 2006;18:1461–1471. doi: 10.1093/intimm/dxl079. [DOI] [PubMed] [Google Scholar]
- 47.Piras F, Colombari R, Minerba L, Murtas D, Floris C, Maxia C, Corbu A, Perra MT, Sirigu P. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer. 2005;104:1246–1254. doi: 10.1002/cncr.21283. [DOI] [PubMed] [Google Scholar]
- 48.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]