Abstract
The inhibitory immune checkpoint PD-L1/PD1 promotes the alternative splicing of the FKBP5 gene, resulting in increased expression of its variant 4 in the peripheral blood mononuclear cells of melanoma patients. The variant 4 transcript is translated into the truncated FKBP51s protein. Given the importance of co-inhibitory signalling in tumour immune escape, here we tested the potential for using FKBP51s expression to predict immunotherapy outcomes. To do this, we immunophenotyped PBMCs from 118 melanoma patients and 77 age- and sex-matched healthy controls. Blood samples were collected before patients underwent ipilimumab treatment. In 64 of the 118 patients, FKBP51s expression was also assessed in regulatory T cells (Tregs). We found that each PBMC subset analysed contained an FKBP51spos fraction, and that this fraction was greater in the melanoma patients than healthy controls. In CD4 T lymphocytes, the FKBP51sneg fraction was significantly impaired. Tregs count was increased in melanoma patients, which is in line with previous studies. Also, by analyses of FKBP51s in Tregs, we identified a subgroup of ipilimumab nonresponder patients (p = 0.002). In conclusion, FKBP51s-based immunophenotyping of melanoma patients revealed several profiles related to a negative immune regulatory control and identified an unknown Treg subset. These findings are likely to be useful in the selection of the patients that are candidate for immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2004-0) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Immunophenotype, Ipilimumab, Tregs, FKBP5
Introduction
Immune checkpoint targeting therapy is a new frontier in cancer therapy. The most frequently used immune checkpoint targeting therapy is monoclonal antibody-based targeting of the programmed cell death 1 protein (PD1)/programmed cell death ligand 1 (PD-L1) immune checkpoint or cytotoxic T lymphocyte antigen-4 (CTLA4). The likelihood of response to immunotherapy differs strongly across tumour types. However, even in those cancer types that respond (e.g., melanoma, renal cell carcinoma, nonsmall cell lung cancer), non-responsiveness is observed in a high proportion of patients [1]. Considering the high cost of such treatments and the possibility of immuno-related toxicities, biomarkers that predict the response to immunotherapy could help avoid unsuccessful and non-tolerated treatments.
The FK506 binding protein 51 (FKBP51) is abundantly expressed in melanoma cells [2, 3] and resident in lymphocytes [4]. FKBP51 belongs to a multifunctional class of proteins, called immunophilins, which are highly conserved across species [5, 6]. An important property of immunophilins is their peptidyl-prolyl cis–trans isomerase activity (PPIase), which catalyses the reversible cis to trans and trans to cis interconversion of a peptide bond preceding internal proline residues in a polypeptide substrate [6]. This enzymatic activity is inhibited by drug-ligand binding (e.g., FK506 and rapamycin) [5, 6]. Because of its role as a rotamase, FKBP51 is important for multiple cellular processes [7]. Recent studies have shown that FKBP51 plays a role in the control of the NF-κB [8] pathway, as well as in responses to TGF-β [9], a cytokine with immunosuppressive function.
Work from our lab has recently shown that, in melanoma cells and lymphocytes, engagement of PD-L1 with its receptor PD-1 bidirectionally induces transcription of a spliced isoform (variant 4) of FKBP51 that encodes FKBP51s, a protein shorter than the canonical one [10]. Increased expression of FKBP51s was measured by quantitative PCR in PBMCs of a study population that included 99 primary and 25 metastatic melanoma patients [10].
Given the importance of PD-L1/PD1 in tumour immune escape and the high FKBP51s transcript levels in melanoma patient blood samples [10], here, we aimed to measure the expression of FKBP51s, as a molecular sensor of the PD-L1/PD1 interaction. To do this, we used flow cytometry to immunophenotype PBMCs. FKBP51s expression was measured in peripheral blood T lymphocytes subsets (CD3/CD4, CD3/CD8, CD25pos, and PD-L1pos) and CD14 monocytes from a cohort of 118 patients and 77 age- and sex-matched healthy controls. Blood samples were collected before patients underwent ipilimumab treatment. Our results show that each PBMC subset analysed contained an FKBP51spos fraction, and that this was significantly increased in the melanoma patients compared to the healthy controls. We also noticed increases in the CD3/CD8 and PD-L1pos lymphocyte subsets in patients compared to controls. Differently, CD4 T lymphocytes, even if the total count was within the normal range, showed the fraction of FKBP51sneg significantly impaired. Because we noticed that a subset CD25/FKBP51spos resulted modulated by ipilimumab [11] and a study by Simeone et al. found Regulatory T cell (Treg) count affected by ipilimumab [12], we also tested FKBP51s expression levels in Tregs (CD4/CD25/FoxP3) in 64 of the 118 patients. The Treg count in the peripheral blood of patients was increased compared to controls, which is in line with previous studies [13, 14]. Interestingly, the FKBP51spos Tregs count defined a subgroup in which most (92.6%) of the patients did not respond to ipilimumab treatment.
Materials and methods
Peripheral blood mononuclear cells
PBMCs were isolated from the heparinized blood of 118 patients with advanced melanoma and 77 age- and sex-matched healthy donors. Blood samples were obtained from the National Cancer Institute G. Pascale Foundation, as part of the routine management for patients with melanoma, following informed consent. The study was approved by the Pascale Foundation Ethics Committee (Protocol no. 80/15) and conducted in accordance with the ethical principles of the Declaration of Helsinki. Clinical information and the results of the study were handled by authorized personnel only. In compliance with patients’ rights, patient identity was kept confidential. Eighty-seven patients were nonresponders (NR) and 31 responders (R) to immunotherapy, according to immune-related response criteria [1].
Blood was collected from patients before initiating their systemic ipilimumab treatment. Briefly, 5 ml of blood was collected in sterile K3EDTA vacutainer blood collection tubes. PBMCs were separated by differential centrifugation through a Ficoll-Hypaque density gradient (Histopaque-1077®, Sigma Life Science, St Luis, MO, USA), washed, and resuspended in 5% FCS-RPMI 1640 (Biowest, Nuaillè, France). After the count, cells were processed for analysis by immunofluorescence.
Flow cytometry analysis
BD-Pharmingen Fc block (2.5 μg/106 cell) was used to minimize non-specific binding of immunoglobulins to Fc receptors, prior to flow cytometry staining. PBMCs were subjected to a multiple immunofluorescence staining. For this purpose, 5–10 μl (in accordance with concentration and manufacturer’s instruction) of mouse monoclonal antibody recognizing the typical cluster differentiation (CD) was added to 50 μl of PBMC suspension. Cells were incubated for 15 min in the dark at room temperature (20–25 °C). The following antibodies were used: anti-CD3-PerCP (OKT3 clone; eBioscience, San Diego, CA, USA); anti-CD3-PE (UCHT1 clone; BD Pharmingen); anti-CD14-Allophycocyanin (APC)-conjugated (TÜK4 clone; Miltenyi); anti-CD4-PerCP (VIT4 clone; Miltenyi); anti-CD8-PerCP (BW135/80 clone; Miltenyi); anti-CD25-PE (M-A251 clone (RUO); BD Pharmingen); and anti-PD-L1-PE (MIH1 clone; eBioscience). Next, 200 μl of a fixation/permeabilization buffer (BD-Pharmingen Cytofix/Cytoperm™ Kit, San Jose, CA, USA) was added to each tube and incubated for 20 min in the dark at 4 °C. After fixation and permeabilization, the cells were further incubated for intracytoplasmic staining with anti-FKBP51s, TGF-β (Santa Cruz Biotechnology, CA, USA) or phosphorylated mammalian target of rapamycin (p-mTOR) (Cell Signaling, Denver, MA). FKBP51s analysis was performed by direct immunofluorescence using anti-FKBP51s antibody [10] conjugated with 5-carboxyfluorescein (FAM). This was generated using an AnaTagTM 5-FAM Protein Labeling Kit (AnaSpec, Fremont, CA, USA), following the manufacturer’s instructions, and used at a concentration of 0.02 μg/ml. FAM-conjugated rabbit IgG was used as control antibody for FKBP51s immunofluorescence. TGF-β and p-mTOR were measured by indirect immunofluorescence, using the rabbit polyclonal anti-TGF-β antibody at a dilution of 1:100; TGF-β primary antibody was then recognized with a FITC-conjugated anti-rabbit antibody and p-mTOR with a PerCP-conjugated anti-rabbit antibody. To make the nuclei accessible, before staining with an anti-Foxp3-PE antibody (PCH101 clone; eBioscience), cell fixation and permeabilization was performed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience).
Lymphocyte and monocyte gating and subset counts are described in detail in Supplementary figures 1–4. The samples were analysed by a FACScan (Becton–Dickinson, BD), flow cytometer or C6 BDAccuri flow cytometer (BD), or combinations of these. CD4+ lymphocytes were sorted from PBMCs using a BD FACSAria™ (BD Biosciences, San Jose, CA, USA). The sorted population was >98% CD4+.
Immunoblot
Whole cell lysates were homogenized in modified radio immunoprecipitation assay (RIPA) buffer and assayed by immunoblot. The primary antibodies against FKBP51s, rabbit polyclonal [10], and FKBP51 (rabbit polyclonal; Novus Biologicals, Littleton, CO, USA) were used diluted 1:2500, γ-tubulin (mouse monoclonal; Sigma-Aldrich, St. Louis, MO, USA) was used diluted to 1:5000. Protein samples were then separated by SDS-PAGE.
Statistical analyses
Student’s t test was used to analyse the differences between means of values. p values ≤0.05 was considered statistically significant. Chi square tests were used in intergroup comparisons of categorical variables, which were expressed as numbers and percentages. p values ≤0.05 was considered statistically significant. Multivariate logistic regression analyses were done using SPSS (version 16). A heat map clustering method was used to obtain the heat maps with the R heat map package (v1.0.8), which uses Euclidean distances for the hierarchical clustering of sample features. OS was calculated by the Kaplan–Meier method to generate survival curves, which were compared using a log-rank test. Patients were dichotomized into two groups based on their heat map clustering: clusters 1 and 2 and cluster 3.
Results
Peripheral blood lymphocyte subsets express high levels of FKBP51s
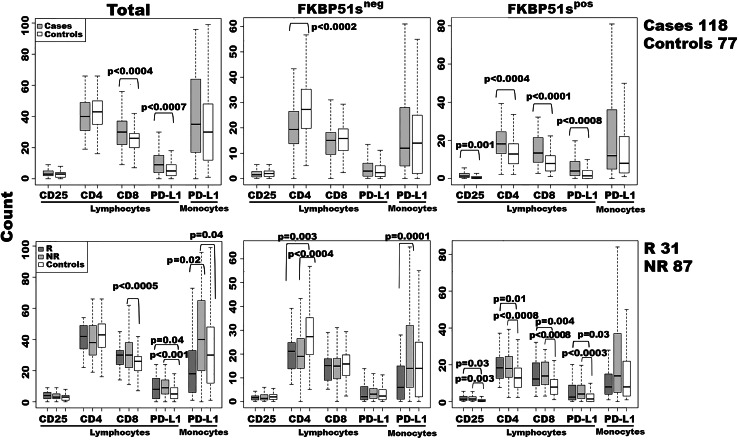
We have previously shown that the inhibitory checkpoint PD-L1/PD1 bidirectionally stimulates an alternative splicing of the FKBP5 gene [10]. The FKBP51s transcript level is significantly increased in melanoma patients [10]. Thus, we tested the immunophenotype of a sample of 118 advanced melanoma patients, using an anti-FKBP51s antibody in a multiparametric analysis. By this approach, we aimed to assess the impact of an enhanced co-inhibitory receptor signalling on PBMC subsets in melanoma patients. In the period between January 2014 and April 2016, 118 patients, that were undergoing ipilimumab treatment, were enrolled for this study. The patient characteristics are given in Supplementary Table 1. All peripheral blood samples were obtained from patients after written informed consent and before receiving their first dose of ipilimumab. According to immune-related response criteria, 87 (73.7%) of these patients were nonresponders (NR) and 31 (26.3%) responders (R) to immunotherapy [1]. Details on flow cytometry analysis are given in Supplementary figures 1a, 2, and 3. An immunoblot confirmed that the anti-FKBP51s antibody was specific for the spliced FKBP51 isoform (Supplementary figure 1b), in accordance with a previous study [10]. Figure 1 shows the immunophenotyping results. We detected an increase in CD3/CD8 and PD-L1pos lymphocytes in the patient group compared to the controls. The same increase was also measured in the NR subgroup, whereas, R patients showed an increase in PD-L1pos but not CD3/CD8 lymphocytes, when compared to the controls. The number of PD-L1pos monocytes was also increased in the NR patients compared to R patients and the controls. Analysis of FKBP51s showed that the proportion of FKBP51sneg CD4 T cells was decreased in both the R and NR patients compared to the controls. The proportion of FKBP51spos cells, within each lymphocyte subset analysed, was significantly increased in the patient group compared to the controls, independently of R and NR condition.
Fig. 1.
Immunophenotyping of patients and healthy controls. Each subset was measured within a lymphocyte- or monocyte-gate, as described in Supplementary figures 1–3. Whole subset or FKBP51sneg and FKBP51spos sub-components were measured and represented as a boxplot. Moreover, cases are represented either as a whole or in R and NR groups. The p values of statistical difference tests are shown
Regulatory T cells of melanoma patients express increased levels of FKBP51s
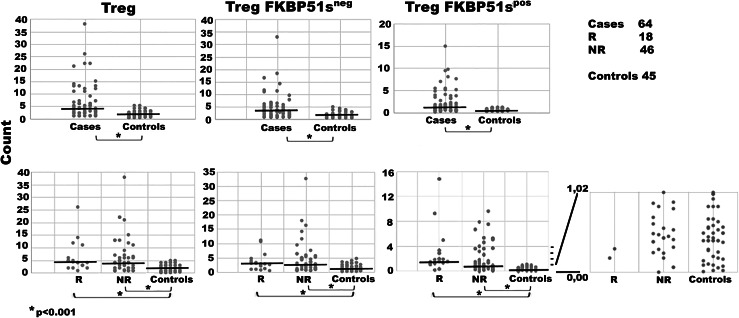
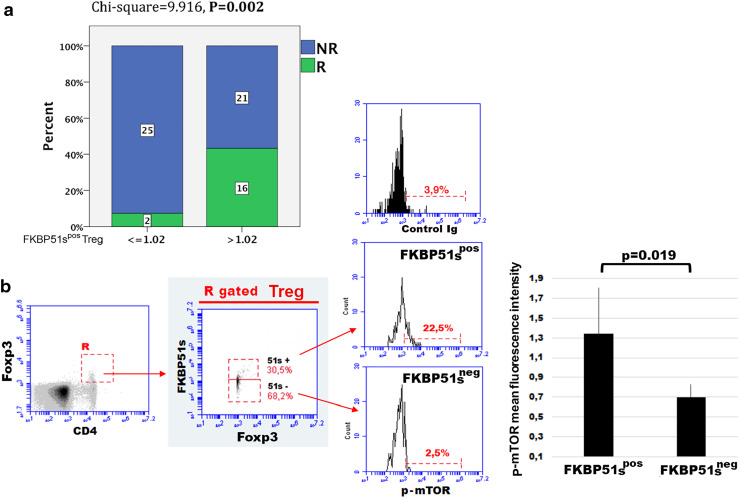
Tregs are an inhibitory subset of CD4/CD25 T cells that maintain peripheral tolerance, accumulate in tumour tissues and the peripheral blood of melanoma patients, and contribute to immune evasion [13, 14]. Here, we attempted to investigate the expression of FKBP51s in Tregs. For details on gating strategy and flow cytometry analysis, see Supplementary figure 4. Similar to effector T cells, also Tregs of the patient group showed an increased expression of FKBP51s compared to the controls (Fig. 2). However, the FKBP51sneg Treg subset resulted also increased in patients when compared to the controls (Fig. 2). In 27 (42%) of 64 tested patients, the Treg/FKBP51spos fraction remained within the range measured for healthy donors (min. value, 0.00; max. value, 1.02). Twenty-five (92.6%) of these 27 patients were NR to ipilimumab. These results suggest that a low frequency of FKBP51spos Treg is associated with unresponsiveness to ipilimumab (Chi square = 9.916, p = 0.002) (Fig. 3a). To address whether the two Treg subsets (FKBP51spos and FKBP51sneg) had some functional differences, we measured the levels of p-mTOR, the serine-threonine kinase that programs the suppressive function of Tregs [15]. Flow-cytometry analysis of five samples showed that FKBP51spos Tregs expressed more p-mTOR than FKBP51sneg Tregs (p = 0.019) (Fig. 3b). This suggests that FKBP51s marks a fraction of Tregs with enhanced suppressor function. We next performed a multiple logistic regression analysis to correct the significant p value obtained by the univariate analysis. Logistic regression confirmed that FKBP51s expression in Tregs is associated with the probability of response to immunotherapy (Table 1). Moreover, statistical analysis also suggested that serum lactate dehydrogenase level influences immunotherapy outcome, as previously reported [16].
Fig. 2.
Treg counts of patients and healthy controls. Details of the cell counts are shown in Supplementary figure 4. The FKBP51sTreg values (scale minimum value 0.00; maximum value 1.02) are shown enlarged and in greater detail
Fig. 3.
FKBP51s expression in Tregs can predict immunotherapy response and is associated with high p-mTOR levels. a Distribution of R and NR according to FKBP51s Treg count and multivariate analysis. b p-mTOR expression in Tregs. Once placed a gate on the Foxp3/CD4 double positive cell, FKBP51spos and FKBP51sneg Treg subsets were selected as shown in the middle histogram. Then, p-mTOR levels were measured in gated Treg subsets (see “Materials and methods” for details)
Table 1.
Multivariate analysis of prognostic factors
| Marker | p value | ORs | CI |
|---|---|---|---|
| FKBP51sTreg (≤1.02 vs. >1.02) | 0.014 | 14.26 | 1.73–117.56 |
| BRAF (WT vs. MUT) | 0.608 | 0.59 | 0.079–4.24 |
| Age at diagnosis | 0.483 | 0.97 | 0.914–1.04 |
| Sex | 0.054 | 0.13 | 0.026–1.03 |
| LDH (in the rangea vs. higher) | 0.003 | 0.02 | 0.002–0.27 |
Statistically significant p values highlighted in bold
OR odds ratio, CI confidence interval
a190–480 U/L
Expression pattern of FKBP51s identifies distinct immune profiles of melanoma patients
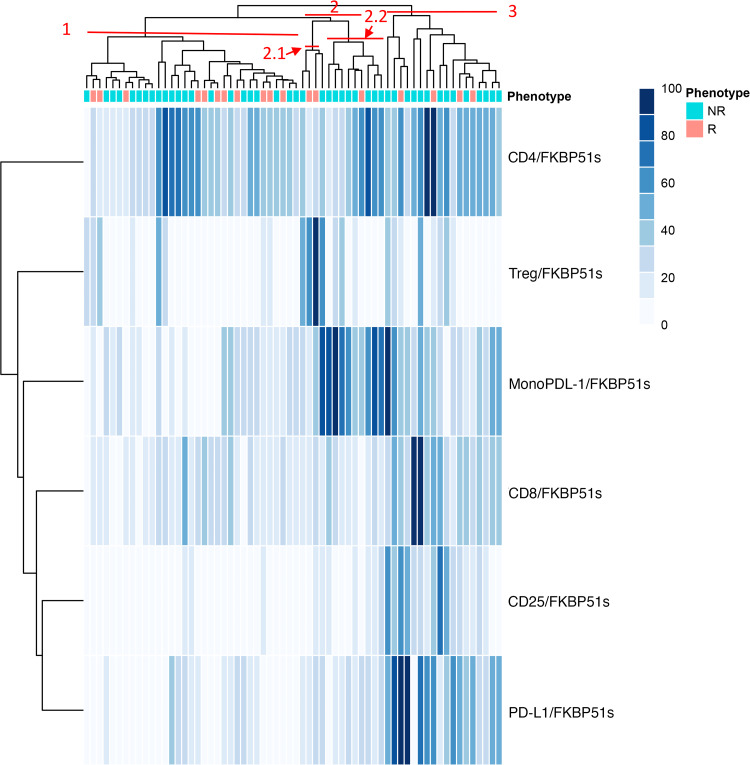
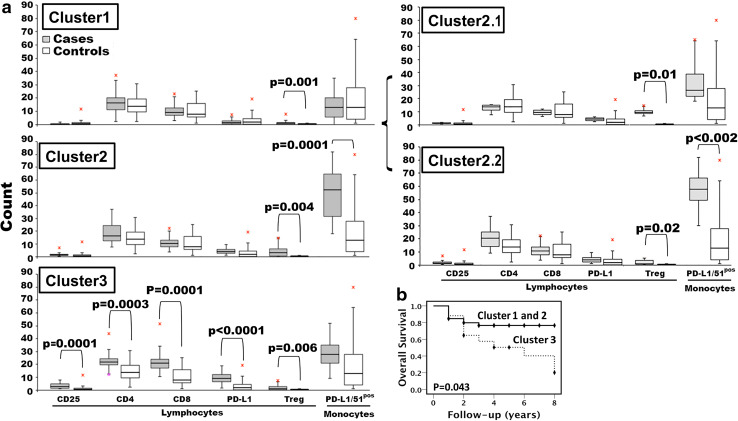
We generated a 2D hierarchical partitioning of data (resembling a heat map) for visualization of FKBP51s expression profiles in PBMC subsets of patients. Values were normalized and assigned a score from zero to 100 (100 was the maximum value measured for each specific subset, within the study population). The hierarchical clustering revealed three main clusters: C1 (33 patients, 51.5%), C2 (14 patients, 22%), and C3 (17 patients, 26.5%), which are highlighted in red in Fig. 4. By comparing immunophenotypes of the three clusters with controls (Fig. 5a), the FKBP51spos Treg subset appeared globally increased in all clusters. In C1, values of effector T cells and monocytes were not different from values of the control group. C2 showed a significant increase in FKBP51spos PD-L1 monocytes compared to healthy controls. More specifically, FKBP51spos PD-L1 monocytes clustered in one of the two sub-clusters within C2, labelled C2.2 (Figs. 4, 5a). C3 showed a significant increase in FKBP51s expression in all T lymphocyte subsets analysed, with monocytes being apparently unaffected (Fig. 5a). In this latter cluster, the prevalence of activation (CD25) and co-inhibitory (PD-L1) markers, together with the expansion of FKBP51spos effector T cells, might reflect a condition of chronic lymphocyte stimulation, as it occurs in some advanced melanoma patients, contributing to T cell exhaustion [17]. The observation that patients included in C3 had worse survival than patients in C1 and C2 (p = 0.043) (Fig. 5b) supports such a hypothesis. Supplementary Table 2 shows the distribution of R and NR patients in the map clusters. None of C2.2 patients classified as high (FKBP51spos Treg >1.02%) responded to ipilimumab (Chi square = 5.46; p = 0.019). Although the sample is small, this result, together with the expansion of a PD-L1/FKBP51s/CD14 subset, is consistent with the hypothesis that response to ipilimumab is prevented by accessory cells exerting a negative immune regulatory control. In support of such a hypothesis, TGF-β expression levels in CD14-monocytes from C2.2 patients resulted increased compared to levels in monocytes from C1 patients (Supplementary figure 5).
Fig. 4.
Hierarchical clustering of immune phenotypes. A heat map representing low-to-high expression levels with a white-to-blue colour scale. For each antigen, data were scaled to one hundred prior to being analysed and plotted
Fig. 5.
Patient immune profiles as defined by clustering. a Values measured for each cluster are reported as box plots. The p values of statistical difference tests are shown. b Kaplan–Meier curves for OS rates. OS rates were compared between cluster 1 and cluster 2 combined versus cluster 3
Sixteen of the ipilimumab NR patients received nivolumab. Supplementary Table 3 shows the cluster distribution and FKBP51sTreg status (high or low) of the NR and R (to nivolumab) patients. Only 1 of the 9 low FKBP51sTreg patients responded to nivolumab, compared to 5 of the 7 high FKBP51sTreg patients (Chi square = 6.11; p = 0.013). Although the sample size is small, this result is consistent with the association between low FKBP51sTreg count and NR. Interestingly, 3 of the 4 patients clustering in C2.2 responded to nivolumab, suggesting that this immune profile might benefit from double-checkpoint targeting immunotherapy.
Discussion
It is generally accepted that successful immunotherapies mostly depend on T cells, but the characteristics of highly effective T cells remain largely unknown [18]. Many biomarker studies of anti-CTLA4 therapies have focused on the diversity, phenotype, and function of PBLs before and after therapy [19, 20]. A rise in the absolute lymphocyte count in peripheral blood correlates with a higher rate of response to ipilimumab [21], while the soluble CD25 level increases in sera of patients resistant to ipilimumab [22]. Here, we propose FKBP51s, a spliced isoform induced by co-inhibitory immune receptor signalling [10], as a potential molecular sensor of the immunosuppression status of patients with advanced melanoma. Indeed, we found that expression of this protein was increased in all lymphocyte subsets analysed, and was associated with an expansion of PD-L1pos lymphocytes, consistent with the notion of enhanced inhibitory immune checkpoint-signalling in melanoma patients [23]. The basal FKBP51s level measured in PBMCs of normal donors might be accounted for by the physiological triggering of a co-inhibitory signalling, thus enabling proper self-tolerance and immune surveillance functioning. Interestingly, the increase in FKBP51spos CD4 T cells apparently occurred at the expense of the FKBP51sneg CD4 T cell types. Even if the total count of CD3/CD4 was unaffected, the proportion of effector CD4 T cells that, virtually, have not received inhibitory signals (i.e., FKBP51sneg), remained restrained. This finding suggests a reduced number of functional CD4 T cells in melanoma patients which might reflect impaired helper T cell function, as previously reported [23]. In accordance with the notion that PD-L1/PD1 signalling is exploited by Tregs [24], FKBP51s was also found to be increased in this lymphocyte subset. Interestingly, FKBP51s marks those Tregs that, given their high levels of p-mTOR, should have an enhanced suppressor capability [15]. Our results identify this Treg subset as a factor associated with response to immunotherapy. More precisely, our study suggests that when FKBP51sTreg count is within the normal range (which is the case in about 40% of advanced melanoma patients) unresponsiveness to immunotherapy is likely. Our Tregs FKBP51spos and FKBP51sneg findings are consistent with the notion that diverse subsets of immunosuppressive regulatory T cells exist and play critical roles in maintaining immune homeostasis and self-tolerance [25]. It is worth noting that a successful immunotherapy can counteract the immunosuppressive tendencies in the tumour microenvironment, thus reinforcing immune surveillance and restraining tumour progression. The efficacy of anti-CTLA4 treatment in those melanoma patients with increased FKBP51sTreg levels suggests a contribution of this T cell subset to the immunosuppressive melanoma microenvironment. Also, the unsuccessful outcome of anti-CTLA4 treatment in patients with low FKBP51sTreg levels is consistent with the hypothesis that ipilimumab can target this Treg subset.
The immunophenotyping approach used here also allowed us to identify immune profiles that will be useful during the prognostic stratification of patients. The expansion of an FKBP51spos population in effector T cells may reflect a condition of reduced immune performance that can occur in melanoma patients and apparently impact on overall survival. Finally, our approach seems to have been able to identify those immune conditions in which an excess of inhibitory accessory cells prevents response to anti-CTLA-4 treatment.
Here, we identify FKBP51s as a candidate functional marker for Tregs that is predictive of immunotherapy response. This should now be confirmed with a larger sample. Additionally, further investigation of FKBP51s-based immune profiles will likely lead to results that could help in the development of multiple immune checkpoint-targeted therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Cardiovascular Service for supporting our research. We also thank Prof. Tommaso Russo (Dept. Molecular Medicine and Medical Biotechnology, Federico II University of Naples) for helpful discussion and advice.
Abbreviations
- APC
Allophycocyanin
- C
Cluster
- FAM
5-Carboxyfluorescein
- FKBP
FK506 binding protein
- mTOR
Mammalian target of rapamycin
- NR
Nonresponder
- p-mTOR
Phosphorylated-mTOR
- R
Responder
- Treg
Regulatory T cell
Compliance with ethical standards
Conflict of interest
Simona Romano, Anna D’Angelillo, and Maria Fiammetta Romano have intellectual property rights (Patent No. 1 419 465, RM2013A000406, 11/7/2013 “A tumor biomarker, in particular of melanoma”). Paolo Antonio Ascierto has received research grants from Bristol-Myers Squibb, Roche-Genentech, and Array and has had a consultant/advisory role for Bristol-Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Amgen, Array, Merck, and Pierre-Fabre. The other authors declare no conflict of interest.
Contributor Information
Paolo A. Ascierto, Email: paolo.ascierto@gmail.com
Maria Fiammetta Romano, Email: mariafiammetta.romano@unina.it.
References
- 1.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 2.Romano MF, Avellino R, Petrella A, Bisogni R, Romano S, Venuta S. Rapamycin inhibits doxorubicin-induced NF-kappaB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer. 2004;40:2829–2836. doi: 10.1016/j.ejca.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Romano S, D’Angelillo A, Pacelli R, Staibano S, De Luna E, Bisogni R, et al. Role of FK506 binding protein 51 [FKBP51] in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 2010;17:145–157. doi: 10.1038/cdd.2009.115. [DOI] [PubMed] [Google Scholar]
- 4.Baughman G, Wiederrecht GJ, Faith Campbell N, Martin MM, Bourgeois S. FKBP51, a novel T-cell specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15:4395–4402. doi: 10.1128/MCB.15.8.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dornan J, Taylor P, Walkinshaw MD. Structures of immunophilins and their ligand complexes. Curr Top Med Chem. 2003;3:1392–1409. doi: 10.2174/1568026033451899. [DOI] [PubMed] [Google Scholar]
- 6.Fischer G, Aumüller T. Regulation of peptide bond cis/trans isomerization by enzyme catalysis and its implication in physiological processes. Rev Physiol Biochem Pharmacol. 2003;148:105–150. doi: 10.1007/s10254-003-0011-3. [DOI] [PubMed] [Google Scholar]
- 7.Romano S, D’Angelillo A, Romano MF. Pleiotropic roles in cancer biology for multifaceted proteins FKBPs. Biochim Biophys Acta. 2015;1850:2061–2068. doi: 10.1016/j.bbagen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Romano S, Xiao Y, Nakaya M, D’Angelillo A, Chang M, Jin J, et al. FKBP51 employs both scaffold and isomerase functions to promote NF-κB activation in melanoma. Nucleic Acids Res. 2015;43:6983–6993. doi: 10.1093/nar/gkv615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romano S, D’Angelillo A, D’Arrigo P, Staibano S, Greco A, Brunetti A, et al. FKBP51 increases the tumour promoter potential of TGF-beta. Clin Transl Med. 2014;3:1. doi: 10.1186/2001-1326-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romano S, D’Angelillo A, Staibano S, Simeone E, D’Arrigo P, Ascierto PA, et al. Immunomodulatory pathways regulate expression of a spliced FKBP51 isoform in lymphocytes of melanoma patients. Pigment Cell Melanoma Res. 2015;28:442–452. doi: 10.1111/pcmr.12378. [DOI] [PubMed] [Google Scholar]
- 11.Romano MF, D’Angelillo A, Ascierto PA, Simeone E, Staibano S, D’Arrigo P, et al. Expansion of a lymphocyte subset expressing a spliced FKBP51 isoform in melanoma patients. J Clin Oncol. 2015 doi: 10.1111/pcmr.12378. [DOI] [PubMed] [Google Scholar]
- 12.Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caracò C, Curvietto M, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63:675–683. doi: 10.1007/s00262-014-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 14.Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22:2908–2918. doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert L, Harview C, Emerson R, Wang X, Mok S, Homet B, et al. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology. 2014;3:e29244. doi: 10.4161/onci.29244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannani D, Vétizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25:208–224. doi: 10.1038/cr.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.