Abstract
Background
NY-ESO-1 protein formulated in ISCOMATRIX™ results in CD4+, CD8+ T cell and antibody-mediated immunity. We evaluated persistence of immunity, relapse-free survival and tumour antigen expression upon relapse in patients vaccinated in an earlier trial.
Methods
Immunity was measured in 28 patients with resected NY-ESO-1-expressing tumours (melanoma 25, breast 3) 252–1,155 days (median = 681) after vaccination. In the earlier vaccination, trial patients received NY-ESO-1 with ISCOMATRIX™ adjuvant at three protein doses 10 μg, 30 μg or 100 μg (n = 14); 100 μg NY-ESO-1 protein (n = 8) or placebo (n = 6), together with 1 μg of intradermal (ID) NY-ESO-1 protein twice for DTH skin testing. Immune responses assessed in the current study included antibody titres, circulating NY-ESO-1-specific T cells and DTH reactivity 2 days after DTH skin testing with NY-ESO-1 protein (1 μg) or peptides (10 μg). Relapse-free survival was determined for 42 melanoma patients. On relapse NY-ESO-1 and HLA, class I was assessed by immunohistochemistry in 17.
Results
Persisting anti-NY-ESO-1 immunity was detected in 10/14 recipients who had previously received vaccine with ISCOMATRIX™ adjuvant. In contrast, immunity only persisted in 3/14 who received 100 μg un-adjuvanted NY-ESO-1 protein (3/8) or 2 μg DTH protein (0/6) P = 0.02. Hence, persisting NY-ESO-1 immunity was associated with prior adjuvant. Tumour NY-ESO-1 or HLA class I was downregulated in participants who relapsed suggesting immunoediting had occurred.
Conclusion
Immunoediting suggests that a signal of anti-tumour activity was observed in high-risk resected melanoma patients vaccinated with NY-ESO-1/ISCOMATRIX™. This was associated with measurable persisting immunity in the majority of vaccinated subjects tested. A prospective randomised trial has been undertaken to confirm these results.
Keywords: NY-ESO-1, Melanoma, Cancer vaccine, Immunotherapy, Immune monitoring, Immunoediting
Introduction
NY-ESO-1 is a cancer-testis (CT) antigen that has attracted particular attention as a candidate for cancer immunotherapy [1–5]. It is expressed by a wide variety of human cancer types including melanoma and is frequently the target of spontaneous immunity in cancer-bearing patients. Furthermore, normal tissue expression is restricted to testicular germ cells, oogonia and placenta, all of which are in immune-privileged sites [2, 3, 6]. These characteristics suggested that NY-ESO-1-based vaccines would be immunogenic and that the resulting immune responses are unlikely to target normal tissues.
Various HLA class I- and II-restricted epitopes have been identified along the NY-ESO-1 protein with a ‘hot spot’ occurring around the central region of the protein from amino acids 80–110 [7, 8]. Many additional epitopes recognised by CD4 T cells in the context of HLA class II have also been identified [8, 9, 10, 11, 12, 13, 14, 15, 16].
We have previously reported results of a placebo-controlled vaccine trial LUD99-008 (initial trial) testing the safety and immunogenicity of full-length NY-ESO-1 protein formulated with the saponin-based adjuvant ISCOMATRIX™ adjuvant (CSL Limited, Australia) [7, 17]. This study assessed responses in 46 evaluable participants with fully resected NY-ESO-1 positive tumours. NY-ESO-1 protein dose was tested in three cohorts 10 μg/dose (cohort A), 30 μg/dose (cohort B) and 100 μg/dose (cohort C). Each participant received three doses of vaccine intramuscularly at monthly intervals. Additional control cohorts received NY-ESO-1 protein without ISCOMATRIX™ adjuvant (cohort D) or placebo (normal saline for injection) (P). All participants in this study also received 1 μg NY-ESO-1 protein intradermally (ID) to test delayed-type hypersensitivity (DTH) responses prior to and after completing the course of vaccinations, at day 86.
The vaccine was found to be safe and immunologically potent [7, 8]. The majority of vaccinated participants achieved high-titre antibody responses, strong DTH skin reactions and circulating CD8+ and CD4+ T cells specific for a broad range of NY-ESO-1 epitopes. Weaker immune responses were observed in those who received NY-ESO-1 protein without ISCOMATRIX™ adjuvant, in cohort D or in cohort P as part of the DTH skin testing regimen [7].
Participants in the initial vaccination trial (LUD99-008) had fully resected tumours but were at high risk of disease recurrence. There was concern that immune responses generated during that trial may not be sustained long-term without booster vaccinations. The principal objective of the follow-up trial (LUD01-017) reported here was to characterise the persistence of NY-ESO-1-specific immune responses several years after NY-ESO-1/ISCOMATRIX™ vaccination. This included antibody responses, CD4+ and CD8+ T cell responses and DTH skin testing against the NY-ESO-1 protein. Additionally, peptides were included in the DTH testing regimen in order to evaluate epitope-specific responses following vaccination with a full-length antigen. The objective was to provide an insight into antigen-specific immunity using an alternative source of antigen as a specificity control (for example by eliminating responses to contaminants in the protein preparation). Administration of these peptides to all patients including HLA-A2-ve patients was undertaken since these served as a negative control population for peptide specificity.
During the course of this trial, we gained the impression that participants who had received adjuvanted vaccine relapsed at a lower frequency than those who received placebo or NY-ESO-1 protein alone. An unplanned analysis of relapse-free survival (RFS) was therefore performed to assess this. We also examined antigen expression in tumours resected from patients who relapsed. These data provide an indication of potential clinical activity and are also reported here.
Materials and methods
Participant population
All study subjects participated in the earlier vaccine trial LUD 99-008 ‘initial trial’ [7]. Participants eligible for the ‘follow-up’ trial (LUD01-017) to assess the persistence of NY-ESO-1 immunity fulfilled inclusion criteria including an expected survival of at least one month, adequate major organ function and written informed consent. Patients were not required to be disease free at entry into the follow-up trial and those who had relapsed between the two trials were eligible to participate if this was deemed to be an acceptable clinical option by the treating physician. Exclusion criteria included concomitant immunosuppressive therapy, immunodeficiency or HIV; other serious illnesses; metastatic disease to the central nervous system, unless treated and stable; recent chemotherapy, radiation therapy or immunotherapy; participation in another clinical trial involving another investigational agent within 4 weeks of enrolment; pregnancy or lactation. The trial protocol was approved by the Ludwig Institute for Cancer Research (LICR) Protocol Review Committee and the Austin Health Human Research Ethics Committee. The study was independently monitored by Kendle, Australia, and was performed prior to the requirement for trial registration at an ICMJE-compliant registry. Participants from the initial trial LUD99-008 who were unable to participate in LUD01-017 were included in analyses of survival and tumour antigen expression.
Trial design
The ‘follow-up’ trial LUD01-017 (Evaluation of NY-ESO-1 immunity in patients who have previously been vaccinated with NY-ESO-1 protein) was an open-label single-arm study. All participants were assigned sequentially to the skin DTH testing regimen. One microgram of NY-ESO-1 protein and 10 μg of each peptide were administered intradermally (ID) on a single occasion at separate sites 10 cm apart for the first eight participants (1, 6, 7, 20, 21, 24, 28, 50). As only one peptide DTH reaction was observed, the protocol was amended so that the 1 μg NY-ESO-1 protein was administered 4 weeks prior to the peptides to act as a potential boost for the remaining 20 patients on this trial.
Protein and peptides for DTH skin testing
Class I HLA-A2 restricted peptides were NY-ESO-1157–167 (SLLMWITQCFL); NY-ESO-1157–165 (SLLMWITQC) and melanoma antigen gp100280–288 (YLEPGPVTA). Class II HLA-DP4 restricted peptide was NY-ESO-1157–170 (SLLMWITQCFLPVF). All were manufactured by Multiple Peptide Systems (MPS, San Diego, CA) to GMP specifications and were characterised by quantitative amino acid analysis and mass spectrometry by M-Scan Limited, England (DP4) or Auspep Pty. Ltd., Melbourne, Australia. Biological safety testing was conducted by BioReliance Inc., Rockville, Maryland, USA. All passed tests for sterility/endotoxin detection and bacteriostatic/fungistatic activity.
NY-ESO-1 protein was produced by bacterial fermentation at CSL Limited, Melbourne, Australia, and purified and formulated in conjunction with LICR, Melbourne, Australia [18]. The final NY-ESO-1 protein concentration was 0.3 mg/mL in 4 M urea and 50 mM glycine; 150 mM Sodium Chloride, 100 mM Phosphate buffer, pH 6.5.
Immunologic assays
Serology
NY-ESO-1-specific antibodies were measured by ELISA using a validated assay as previously described [7]. Blood was drawn at baseline (day 0) and two or 6 weeks after 1 μg NY-ESO-1 ID protein administration. Sera from the initial study were re-assessed concurrently to enable direct comparisons of antibody titre.
DTH skin tests
DTH responses (induration and erythema) were assessed 2 days after ID injection. To establish a baseline for NY-ESO-1 peptide reactivity, a series of controls were assessed in 15 NY-ESO-1/ISCOMATRIX™ vaccine-naïve participants from two other Ludwig Institute-sponsored trials, LUD2002-003 n = 9 (unpublished) and the ‘initial trial’ LUD99-008 placebo cohort n = 6. Induration following ID injection of NY-ESO-1157–165, NY-ESO-1157–167 or NY-ESO-1157–170, peptides were 0 mm (mean + 2SD) in these 15 subjects.
DTH responses in the initial LUD99-008 trial were based on an examination of blinded data. Pre-existing responses were defined as a baseline induration of >5 mm. A positive response to vaccination was recorded if the second DTH measurement was >5 mm and at least double the baseline reading. Thus, in the LUD01-017 trial, a positive persisting DTH response to NY-ESO-1 was defined as induration >0 mm following peptide and >5 mm following NY-ESO-1 protein challenge. To reduce variability, the administration of skin testing reagents and interpretation of responses were limited to a small number of people. Some reactive injection sites were biopsied for histopathology, and toxicity was documented according to National Cancer Institute Common Toxicity Criteria version 2.0.
T cell assays
Flow cytometry assessed IFNγ production by T cells (intracellular cytokine stain; ICS) stimulated by a series of overlapping peptides as previously described [7]. For the ICS assay, T cells were expanded over a 10–14 day period using the appropriate NY-ESO-1 18-mer peptides. Peripheral blood mononuclear cells (5 × 106) were pulsed with 10 μM 18-mer peptides in pools of three 18-mers for 1 h at 37°C in 200 μL RPMI +10% FCS (RF10). An hour later, RF10 + 25 IU IL-2 was added. Cultures were fed and/or split every 2–3 days. An internal control (EBV-specific T cells) for T cell expansion efficiency was assessed in parallel. Positive controls for the ICS assays were newly thawed T cells of known specificity. T cells cultured without stimulating peptide served as negative controls. CD8+ and CD4+ T cell responses to undefined T cell epitopes was assessed by ICS using NY-ESO-1 13-mer peptides overlapping by 11 amino acids. A response was determined to be positive if more than 0.01% of T cells producing IFNγ could be identified as a discrete population by flow cytometry. 18-mer peptides were individually synthesised, and 13-mers were synthesised as cleaved pin-peptides (Chiron Mimotopes, Victoria, Australia).
Relapse-free survival (RFS)
Survival data for the participants from the initial trial LUD99-008 were collected with specific ethical approval and data monitored for accuracy by Kendle, Australia. The RFS analysis was restricted to the 42/46 vaccine recipients with a diagnosis of melanoma [7]. RFS was calculated from the time of entry into the initial trial LUD99-008. Patients without progression were censored at the date they were last known to be disease free.
Immunohistochemistry
Immunohistochemistry for NY-ESO-1 and S100 was performed as previously described [19]. The HC-10 antibody (kindly provided by Brian Tait, Victorian Transplantation and Immunogenetics Service, Melbourne, Australia) preferentially recognises beta-2-microglobulin-free HLA-A, -B, -C heavy chain and was used at a concentration of 0.004 mg/L. Antigen expression was categorised on the basis of the proportion of cells staining positively as previously described [19]. All samples were scored in a blinded fashion by a single dedicated pathologist (DW).
Statistical considerations
The study population was drawn from participants in the ‘initial trial’ LUD99-008 who were available, willing to participate and eligible for the ‘follow-up trial’ LUD01-017. The relationship between prior vaccination and persistent immunity was determined using Fisher’s exact test (two-tailed), as was the relationship between vaccination and subsequent tumour antigen (NY-ESO-1 &/or HLA class I) downregulation. As analysis of survival and RFS, data were not defined prospectively in either clinical protocol these are presented descriptively without statistical analysis. The results of a formal randomised phase II NY-ESO-1/ISCOMATRIX™ vaccination trial (Clinical Trial No.: LUD2003-009) will be reported in due course.
Results
Participants
Twenty-eight of 46 participants from the initial LUD99-008 trial were recruited into the follow-up LUD01-017 trial to assess persistence of NY-ESO-1 immunity. Of the remaining 18, eight had died, two were on corticosteroids, two were geographically inaccessible and six declined. The demographics of the study population are shown in Table 1. Of these, half (14) had previously received vaccine formulated with ISCOMATRIX™ adjuvant and 14 had received either NY-ESO-1 protein alone without adjuvant or placebo. All had previously also received 1 μg of intradermal (ID) NY-ESO-1 protein on two separate occasions for DTH skin testing. At the completion of the earlier trial, those participants who did not receive ISCOMATRIX™ adjuvant had little or no NY-ESO-1 immunity [7]. Of the 28 participants who were studied for persistent immunity in the follow-up trial, seven relapsed in the interval between the two studies although five were rendered disease free by surgery (pts 13, 30, 33, 44, 49). The remaining two patients had evident disease at study entry that did not require other treatment at the time (pts 1 and 51). A further two participants later relapsed at the time of RFS analysis.
Table 1.
Participant characteristics/demographics upon recruitment to the LUD01-017 study
| Characteristics/Demographics | N | % | |
|---|---|---|---|
| Number of subjects entered | Total | 28 | |
| Gender | Male | 15 | 54 |
| Female | 13 | 46 | |
| Age | Median (range) | 55 (34–79) | |
| No. of days since NY-ESO-1 protein exposure in LUD99-008 trial | Median (range) | 680 (252–1,155) | |
| Performance status | 100% | 24 | 86 |
| 90% | 3 | 11 | |
| ND | 1 | 4 | |
| Previous therapies | Surgery | 28 | 100 |
| Radiotherapy | 9 | 32 | |
| Systemic therapy | 9 | 32 | |
| Tumour stage at LUD99-008 study entry | I | 2 | 7 |
| II | 6 | 21 | |
| III | 13 | 46 | |
| IV | 7 | 25 | |
| Persistent or recurrent tumour between study LUD99-008 and LUD2001-017 | Progressive stage (relapsed)a | 7 | 25 |
| Stable stage (no relapse)b | 21 | 75 | |
| HLA-A2+ | 16 | 57 | |
| Treatment arm (LUD99-008) | A: vaccine dose 10 μg | 3 | 11 |
| B: vaccine dose 30 μg | 2 | 7 | |
| C: vaccine dose 100 μg | 9 | 32 | |
| D: NY-ESO-1 protein 100 μg without ISCOMATRIX™ adjuvant | 8 | 29 | |
| Placebo | 6 | 21 | |
| Primary diagnosis | Breast | 3 | 11 |
| Melanoma | 25 | 89 | |
aProgressive stage: subject had relapse/s between LUD99-008 and LUD01-017
bStable stage: subject had no relapse between LUD99-008 and LUD01-017
In order to evaluate relapse after vaccination, all 46 melanoma patients from the earlier trial were assessed for RFS and, when available, their tumours were biopsied after relapse in order to assess antigen expression.
Safety
There were no serious adverse events (AE) reported. Low-grade AE were injection site pain and itch, headache, sweating, fine tremor, sinus tachycardia, asymptomatic lymphopenia and leukopenia (grade 1) and insomnia (grade 2). No grade 3 or 4 events were observed.
Immunity
In order to assess persistent immunity, antibody, DTH and T cell assays were performed. The interval between the two trials ranged from 336 to 1,179 days.
Antibody to NY-ESO-1
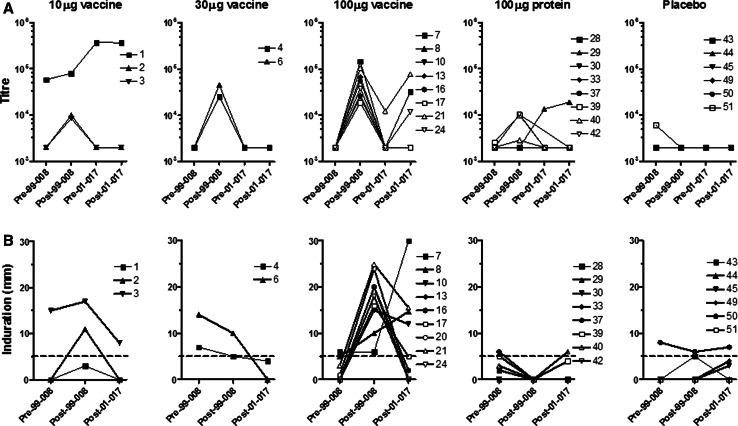
Sera were collected 2 weeks after a 1 μg ID challenge of NY-ESO-1 protein. Figure 1a shows antibody titres from the 23/28 participants tested. Five of these had detectable anti-NY-ESO-1 antibody; three were seropositive at the time of entry into the follow-up trial (pts 1, 21, 29) and in the other two, a response was boosted following antigen challenge (pts 7, 24). Both of these had previous vaccine-induced antibody following the protein/ISCOMATRIX™ adjuvant combination in the initial trial.
Fig. 1.
Antibody titre and skin DTH in NY-ESO-1 vaccine cohorts. a Antibody titres of individual participants grouped according to dose cohort in the initial vaccination trial (LUD99-008 or 99-008). Y-axis shows reciprocal antibody titre with 2,000 set as the baseline for this assay. Patients received three vaccinations or placebo on the LUD99-008 trial. Pre- and post-vaccination titres are indicated by Pre-99-008 and Post-99-008; Pre-01-017 indicates baseline antibody titre on LUD01-017 trial; Post-01-017 titre assessed 2 or 6 weeks following 1 μg ID NY-ESO-1 protein challenge. b Skin DTH induration measured two days after ID injection of 1 μg of NY-ESO-1 protein. DTH responses for individual participants are grouped according to dose cohort in the initial LUD99-008 vaccination trial. Pre- and post-vaccination DTH size are indicated by Pre-99-008 and Post-99-008; Post-01-017 indicates DTH reaction two days after 1 μg NY-ESO-1 protein challenge on LUD01-017 trial. DTH values below the dotted line (<5 mm) were classified as negative responses
DTH skin reactions to NY-ESO-1 protein
Figure 1b and Table 2 show the cutaneous responses to 1 μg ID NY-ESO-1 protein at three time points; (1) prior to vaccination on the initial trial, (2) at the completion of vaccination on that initial protocol and (3) following re-challenge 252–1,155 days later in the current trial. In most, skin reaction diameters declined between the two studies, from 8.1 ± 1.7 mm to 4.3 ± 1.3 mm. Seven positive DTH reactions to NY-ESO-1 protein were recorded in this study, four from vaccine recipients of cohort C and three from patients from other cohorts. Interestingly, all these three had positive skin tests prior to the initial trial suggesting pre-existing spontaneous NY-ESO-1 immunity. Twelve participants had persistently negative skin test reactions. There was no apparent relationship between a persistent antibody and a skin response to NY-ESO-1.
Table 2.
DTH responses (mm) to NY-ESO-1 protein and peptide challenge
| Patient no. | Cohort | HLA-A2+ | NY-ESO-1157–165 peptideb DTH (mm) | NY-ESO-1157–167 peptideb DTH (mm) | NY-ESO-1 protein DTH (mm) |
|---|---|---|---|---|---|
| 1a | A | + | − | − | − |
| 2 | A | + | 4 | − | − |
| 3 | A | + | 4 | 3 | 8c |
| 4 | B | + | − | − | 4c |
| 6a | B | + | − | − | −c |
| 7a | C | + | − | − | 30c |
| 8 | C | − | − | 15 | |
| 10 | C | − | − | 12 | |
| 13 | C | − | − | − | |
| 16 | C | − | − | 2 | |
| 17 | C | + | 3 | − | 5 |
| 20a | C | + | − | − | − |
| 21a | C | + | 3 | 4 | 15 |
| 24a | C | − | − | − | |
| 28a | D | − | − | − | |
| 29 | D | − | − | − | |
| 30 | D | + | − | – | 4 |
| 33 | D | + | 3 | 3 | − |
| 37 | D | − | − | 0c | |
| 39 | D | + | 1 | − | 4 |
| 40 | D | 4 | − | 6 | |
| 42 | D | + | − | − | − |
| 43 | P | − | − | − | |
| 44 | P | + | − | − | − |
| 45 | P | 3 | − | 3 | |
| 49 | P | + | − | − | − |
| 50a | P | − | − | 7c | |
| 51 | P | + | − | 4 | − |
aPatients received NY-ESO-1 protein challenge simultaneously with NY-ESO-1 peptides prior to LUD01-017 protocol amendment. For all other patients, peptide DTH responses were assessed 4 weeks following 1 μg NY-ESO-1 protein challenge and DTH skin testing
bNY-ESO-1157-165 and NY-ESO-1157-167 peptides are HLA-A2 restricted
cPre-existing NY-ESO-1 DTH response detected at commencement of LUD99-008 vaccination trial
Skin reactions to NY-ESO-1 peptides
Table 2 summarises the DTH skin reactions against NY-ESO-1 recorded in this trial. In addition, no DTH skin reactions were recorded to the control gp100280–288 peptide and only a single reaction was recorded for the HLA-DP4 restricted NY-ESO-1157–170 peptide (pt 45).
In general, peptide-specific responses were modest and correlated poorly with responses to protein, suggesting that cellular immune responses to these epitopes were unlikely to have contributed significantly to the inflammation induced by the protein preparation. Taken together with the assays for circulating T cell responses (Tables 2 and 3), we concluded that skin testing with these peptides did not give a reliable indication of anti-NY-ESO-1 T cell immunity.
Table 3.
Peripheral blood T cell responses to NY-ESO-1 peptides detected prior to and following the vaccination LUD99-008 and follow-up LUD01-017 trials
| Patient No. | Cohort | Peptide response prior to vaccine | LUD99-008 CD4 peptide | LUD01-017 CD4 peptide | LUD99-008 CD8 peptide | LUD01-017 CD8 peptide |
|---|---|---|---|---|---|---|
| 1 | A | 108–120 | 108–120 | |||
| 122–134 | 122–134 | |||||
| 157–165 | 157–165 | 157–165 | ||||
| 157–174 | 157–174 | |||||
| 3 | A | 87–99 | 87–99 | |||
| 6 | B | 157–174 | ||||
| 7 | C | 87–99 | 87–99 | |||
| 128–140 | 128–140 | 128–140 | 128–140 | |||
| 157–174 | 157–174 | 157–165 | 157–165 | |||
| 8 | C | 85–97 | 85–97 | 85–97 | ||
| 157–170 | 157–170 | 157–170 | ||||
| 10 | C | 85–96 | 85–96 | 85–96 | ||
| 85–102 | 85–102 | |||||
| 118–130 | 118–130 | 118–130 | ||||
| 120–132 | 120–132 | 120–132 | ||||
| 157–174 | 157–174 | |||||
| 16 | C | 121–138 | ||||
| 126–134 | 126–134 | |||||
| 17 | C | 85–102 | 85–102 | |||
| 21 | C | 60–72 | 60–72 | |||
| 82–94 | 82–94 | |||||
| 103–120 | 103–120 | |||||
| 24 | C | 86–98 | 86–98 | 86–98 | ||
| 33 | D | 157–165 | ||||
| 37 | D | 121–138 | 121–138 | |||
| 39 | D | 21–33 | 21–33 | 21–33 | ||
| 40–52 | 40–52 | 40–52 | ||||
| 157–165 | 157–165 | |||||
| 42 | D | 127–144 | 127–144 | 127–144 | ||
Cellular immune responses to NY-ESO-1
T cell responses against various NY-ESO-1 peptide epitopes were evaluated in 26/28 participants and for most previously cryopreserved cells from the earlier trial were available for side-by-side comparison. Table 3 summarises the responses that were seen in those 14 patients who had persisting T cell immunity. Most of these received ISCOMATRIX™ adjuvant in cohort C.
Relationship of long-term immunity with vaccine cohort
Table 4 summarises immunity before and after each trial. We were particularly interested to relate immune persistence to the initial vaccine dose and whether or not ISCOMATRIX™ adjuvant affected this. There was a clear association between persistent immunity and ISCOMATRIX™ adjuvant administered in either cohort A, B or C. In contrast, those who received protein without adjuvant, either at the 100 μg dose (cohort D) or as ID protein only for DTH testing (1 μg on 2 occasions; placebo cohort), rarely had persisting responses, P = 0.02.
Table 4.
Summary of NY-ESO-1 immunity in patients enroled in LUD99-008 and LUD01-017 studies
| Patient number | Treatment cohort | Persistent or recurrent tumour | Immunity prior to vaccination | Induced immunity LUD99-008 | Persisting immunity LUD01-017 |
|---|---|---|---|---|---|
| 1a | A | Yesb | Y | Y | Y |
| 2 | A | No | N | Y | N |
| 3 | A | No | Y | Y | Y |
| 4 | B | No | Y | Y | N |
| 6a | B | No | Y | Y | Y |
| 7a | C | No | Y | Y | Y |
| 8 | C | No | Y | Y | Y |
| 10 | C | No | Y | Y | Y |
| 13 | C | Yes | N | Y | N |
| 16 | C | No | N | Y | Y |
| 17 | C | No | N | Y | Y |
| 20a | C | No | N | Y | N |
| 21a | C | No | N | Y | Y |
| 24a | C | No | Y | Y | Y |
| Subtotal | C | 2/14 | 8/14 | 14/14 | 10/14 |
| 28a | D | No | N | N | N |
| 29 | D | No | N | N | Nc |
| 30 | D | Yes | N | N | N |
| 33 | D | Yes | N | N | N |
| 37 | D | No | Y | Y | Y |
| 39 | D | No | Y | Y | Y |
| 40 | D | No | N | Y | N |
| 42 | D | No | Y | Y | Y |
| 43 | P | No | N | N | N |
| 44 | P | Yes | N | N | N |
| 45 | P | No | N | N | N |
| 49 | P | Yes | N | N | N |
| 50a | P | No | Y | N | N |
| 51 | P | Yesb | Y | N | N |
| Subtotal | P | 5/14 | 5/14 | 4/14 | 3/14 |
Immunity defined as a positive DTH, antibody or T cell response. Vaccination trial = LUD99-008; persisting immunity assessed in LUD01-017 trial
aReceived intradermal NY-ESO-1 protein simultaneously with NY-ESO-1 peptides prior to protocol amendment
bMetastatic disease detected at end LUD99-008 vaccination trial
cSpontaneous antibody arose between both two trials, no response to vaccine in LUD99-008
Persistence of vaccine-induced immunity in cohort A, B and C vs. cohort D & P P = 0.02
Clinical outcomes
During the course of follow-up, we gained a strong clinical impression receiving ISCOMATRIX™ adjuvant in the initial LUD99-008 vaccination trial may have influenced the likelihood of relapse. All melanoma patients on this trial were evaluated to assess this. Median follow-up was 1,430 days, 5/19 patients relapsed in the ISCOMATRIX™ adjuvant-receiving cohorts A, B and C; whereas 13/23 relapsed from cohorts D (7/16) and P (6/7). A retrospective assessment of prognostic factors (age, sex, primary lesion thickness, time since diagnosis, stage at study entry, number of recurrences prior to entry, time since last resection prior to study entry) did not reveal any apparent differences between the groups.
Emergence of antigen downregulation tumour phenotype—‘immunoediting’
On relapse, we were able to analyse 26 biopsies from 17 patients with melanoma who were recruited onto the initial LUD99-008 vaccination trial. Because these patients had relapsed, a proportion was unable to participate in the follow-up LUD 01-017 trial. NY-ESO-1 and HLA class I heavy chain were assessed by immunohistochemistry. Patients comprised six from cohorts A, B and C all of whom received vaccine with ISCOMATRIX™ adjuvant, and 11 patients from cohorts D and P who received NY-ESO-1 protein alone or placebo. In 6/6 samples where patients received NY-ESO-1/ISCOMATRIX™, adjuvant downregulation of either HLA class I (n = 1) or NY-ESO-1 protein (n = 5) expression was demonstrated by immunohistochemistry (Table 5). Examples of four such patients are shown in Fig. 2. In contrast, evidence of antigen downregulation was only seen in 5 of the 11 patients from the cohorts that received no adjuvant (cohort D or P) (P = 0.043). This suggests that the adjuvanted vaccine was effective at inducing selective pressure on the tumour phenotype.
Table 5.
Immunohistochemistry of metastatic tumours removed from participants of the LUD99-008 vaccination trial at relapse
| Patient ID | Cohort | Antigen expression pre-vaccination on LUD99-008 | Antigen expression post-vaccination LUD99-008 | Antigen downregulation | |||
|---|---|---|---|---|---|---|---|
| NY-ESO-1 (%) | Class I (%) | Days to relapse* | NY-ESO-1 (%) | Class I (%) | Yes/No | ||
| 1 | A | >76 | >76 | 804 | >76 | <5 | Yes |
| 2 | A | 26–50 | 26–50 | 1,912 | 6–25 | >75 | Yes |
| 5 | B | 26–50 | <5 | 1,294 | Negative | 26–50 | Yes |
| 9 | C | 6–25 | 26–50 | 958 | Negative | 51–75 | Yes |
| 1,013 | <5 | >76 | Yes | ||||
| 1,281 | Negative | 6–25 | Yes | ||||
| 12 | C | 26–50 | 100 | 360 | <5 | 51–75 | Yes |
| 510 | 6–25 | 6–25 | Yes | ||||
| 541 | 6–25 | 6–25 | Yes | ||||
| 13 | C | 6–25 | 6–25 | 943 | <5 | >76 | Yes |
| 6/6 patients | |||||||
| 27 | D | <5 | 26–50 | 510 | <5 | 26–50 | No |
| 690 | <5 | 51–75 | No | ||||
| 28 | D | <5 | 51–75 | 619 | 26–50 | 26–50 | No |
| 29 | D | 6–25 | >76 | 958 | >76 | >76 | No |
| 30 | D | 26–50 | >76 | 210 | Negative | 51–75 | Yes |
| 32 | D | 6–25 | <5 | 73 | 51–75 | 6–25 | No |
| 79 | 6–25 | 6–25 | No | ||||
| 119 | 26–50 | 26–50 | No | ||||
| 33 | D | 6–25 | <5 | 340 | 6–25 | <5 | No |
| 1,111 | Negative | 51–75 | Yes | ||||
| 36 | D | >76 | <5 | 1,091 | >76 | <5 | No |
| 38 | D | <5 | <5 | 246 | <5 | >76 | No |
| 51 | P | <5 | >76 | 502 | <5 | >76 | No |
| 44 | P | 26–50 | <5 | 409 | Negative | >76 | Yes |
| 1,502 | Negative | 51–75 | Yes | ||||
| 49 | P | 26–50 | 100 | 214 | Negative | >75 | Yes |
| 5/11 patients | |||||||
*Multiple days refer to biopsies undertaken on repeated occasions
Fig. 2.
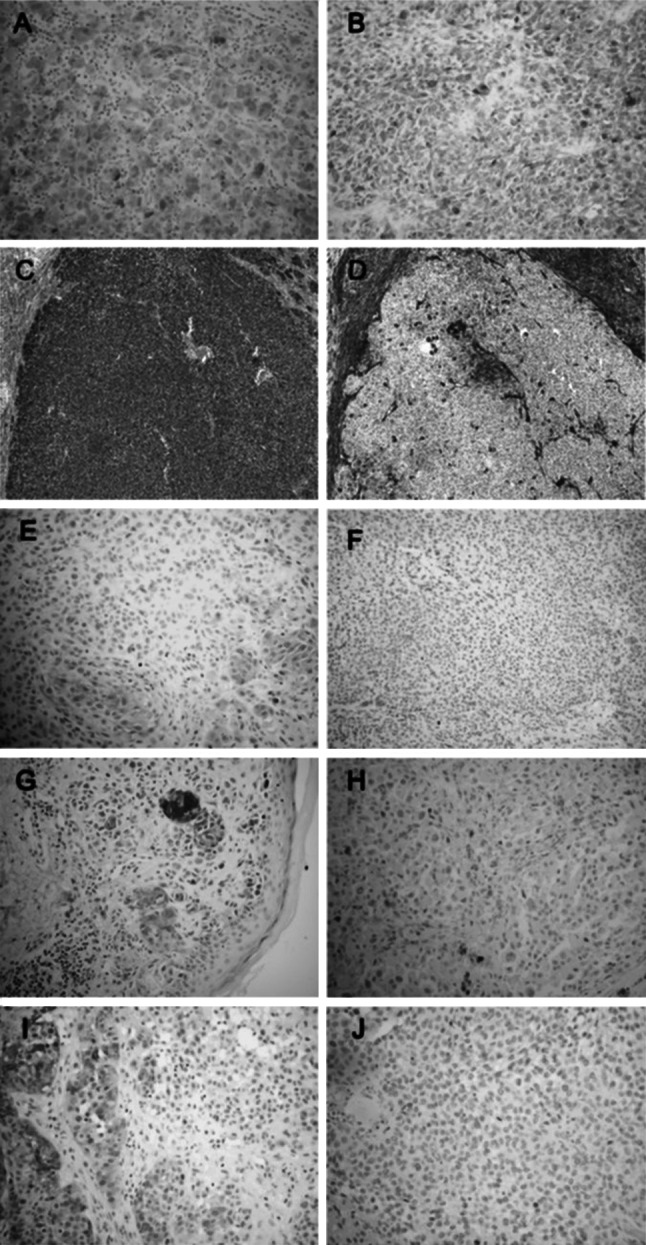
Antigen and HLA expression prior to vaccination and after relapse; a Participant 1 (cohort A): IHC for NY-ESO-1 in a subcutaneous nodule pre-vaccine (LUD99-008) showing homogeneous NY-ESO-1 expression and a ‘brisk’ lymphocytic infiltrate. b Participant 1 metastectomy from liver 420 days after vaccination demonstrates persisting NY-ESO-1 and loss of TILs. c Participant 1 melanoma antigen S100 and d HLA class I (HC-10) indicating loss of class I from liver metastasis. e Participant 12 (cohort C): pre-vaccination and f post-vaccination showing loss of NY-ESO-1 expression in relapse sample 122 days post-vaccine. g Participant 9 (cohort C): pre-vaccination and h post-vaccination showing loss of NY-ESO-1 expression in relapse sample 750 days post-vaccine. i Participant 13 (cohort C): pre-vaccination and j post-vaccine showing loss of NY-ESO-1 expression in relapse sample 919 days post-vaccine
Discussion
There is growing recognition of the dynamic response between immunity and cancer, described recently in three stages; ‘Elimination’, ‘Equilibrium’ and ‘Escape’ [20]. Unless total elimination is achieved, effective anticancer immunotherapy will require persistence of an immune response in order to sustain the equilibrium of remission status. It is therefore critical to evaluate the quality and longevity of any induced immune response against cancer antigens.
We sought to evaluate persistent immunity and clinical outcomes in a cohort of patients who had previously been vaccinated against the cancer antigen NY-ESO-1. That initial study was a phase I trial; so, this analysis is constrained by small numbers and by comprising patients treated at a variety of dose levels. Additionally, the current trial could only be performed with surviving patients, thereby introducing a bias in the study population. Nonetheless, it has the advantage of using an antigen system that can induce robust cellular and humoral immunity, so even with small numbers, hypothesis-generating observations could be made. These serve as the basis for future prospective trials.
The patients examined in this study had been vaccinated years earlier on a protocol that included NY-ESO-1 with or without ISCOMATRIX™ adjuvant. We show here that NY-ESO-1-specific immunity persisted long-term, especially in those patients who received the adjuvant. Immunity was boosted following re-challenge with antigen and at the highest dose of vaccine (100ug) with adjuvant), there was a signal of improved relapse-free survival. Finally, downregulation of NY-ESO-1 or HLA class I suggested that immune evasion might be induced, thereby providing a mechanism for relapse in some.
Very little is known about the persistence of vaccine-induced immunity following immunisation with full-length protein cancer antigens. We observed T cell responses in 14/26 patients following NY-ESO-1 re-challenge administered between 252 and 1,155 days from the last exogenous NY-ESO-1 exposure. These responses were characterised by readily detectable CD4+ and CD8+ lymphocyte responses elicited following in vitro re-stimulation with peptides. In most cases, the responses detected had the same specificities as those elicited during initial vaccination (Table 3). In contrast, cutaneous responses and antibody titres were either attenuated or lost in the period between NY-ESO-1 administered in the initial and follow-up studies (Fig. 1a, b).
Atanackovic et al. [21] reported a phase II trial in which recombinant MAGE-A3 protein was administered with or without adjuvant AS02B to 18 lung cancer patients after tumour resection. The presence of the adjuvant was essential for the development of humoral and cellular responses against selected epitopes. In 14 patients that still had no evidence of disease up to 3 years after vaccination, four additional doses of MAGE-A3 protein with adjuvant AS02B were administered. After just one boost injection, six of seven patients originally vaccinated with protein plus adjuvant re-attained the anti-MAGE A3 antibody titres that were comparable to those that were achieved after the first course of vaccination. All seven patients subsequently developed even greater antibody responses. Furthermore, booster vaccination widened the spectrum of CD4+ and CD8+ T cells against various new and known MAGE-A3 epitopes. The quality of the response reported here probably highlights the value of the higher vaccination and boosting dose of antigen (300 μg) although there may also be differences due to differing characteristics of the two adjuvant and antigen systems. Nonetheless, both studies do establish the importance of the adjuvant during initial vaccination in order to achieve persistent memory and recall responses.
Other investigators have reported long-term follow-up after peptide vaccination. Chiong et al. described persistence CD8+ T cells specific for a gp100 epitope 36 months after vaccination in five melanoma patients with minimal residual disease who remained free of disease for more than 4.5 years [22]. It is likely that these responses were vaccine-induced; however, baseline values were not reported, so some may have arisen spontaneously. Another report in patients with epithelial ovarian cancer vaccinated with NY-ESO-1 peptides characterised the longevity of CD8+ and CD4+ responses [23]. Patients were selected on the basis of being disease free at least six months post-vaccination and all had demonstrable vaccine-induced responses at early time points. CD4+ T cells were detected in 5/5 patients six and 12 months following peptide (NY-ESO-1157–170) administration. However, only two patients had detectable CD8+ T cells at six and 12 months (K Odunsi, personal communication).
Although previous trials have used skin testing to assess immunity to tumour antigens [23–28], the peptides were generally both used for treatment and for monitoring responses. In contrast, our study used synthetic peptides for the assessment epitope-specific reactions following vaccination with a full-length antigen. Despite modifying the protocol in an attempt to boost immunity, skin reactions to the HLA-A2-restricted peptide were only seen in 6/16 HLA-A2+ve patients and to the DP4 peptide in 1/22 DP4+ve patients. Furthermore, some responses were inconsistent with expectations since two HLA-A2-ve patients responded to the HLA-A2-restricted NY-ESO-1157–165 peptides (pts 40, 45) and some HLA-A2+ve patients responded to NY-ESO-1157–165 but not NY-ESO-1157–167 peptide (pts 2, 17, 39). Since NY-ESO-1157–165 lies within the longer NY-ESO-1157–167 peptide, we expected patients to respond to both and this was not observed. So, skin testing with these peptides does not appear to provide a reliable indication of anti-NY-ESO-1 T cell immunity.
The cellular immune responses elicited in each of the trials are shown in Table 3. These assays, which rely on in vitro re-stimulation, were not quantitative, and responses have been recorded as present or undetectable. It is clear that those epitope-specific responses that dominated the first time around were the same as those that were seen years later. Although this persistence can likely be attributed to the effect of the earlier vaccine, we cannot exclude micro-metastatic disease serving as a source of antigen to sustain immunity. Indeed, this is suggested by the appearance of spontaneous antibody between vaccination and re-challenge exposures in one participant.
Persisting immunity can only be considered helpful it delays or prevents disease relapse. We therefore sought to relate the immunity to clinical endpoints. Two measures of anti-cancer immunity were assessed; tumour recurrence and phenotype. Relapses did appear reduced in those subjects who received the vaccine. Nonetheless, they were not prevented altogether; so, we sought evidence for tumour immunoediting either through downregulation of NY-ESO-1 or HLA class I. The demonstration of this (Fig. 2 and Table 5) suggests that the persisting immune response did apply selective pressure capable of altering the tumour phenotype. While it is clear that there was not an absolute loss of antigen, IHC does not reflects tumour ‘visibility’ from the perspective of T cell and it is not been technically possible to determine the presence of antigen-specific HLA-peptide complexes on the surface of cells. Nonetheless, in each case, ISCOMATRIX™ adjuvant appeared to enhance the effect. In recent studies, we have shown that ISCOMATRIX™ assists cross presentation of NY-ESO-1 to generate T cell responses against epitopes that are targets for immune recognition on cancer cells as well as dendritic cells [29, 30]. This may prove important, since differing or alternative antigen-processing pathways can be accessed in professional antigen-presenting cells (APC) and tumours [31]. For effective immunity to occur, antigen processing by APC needs to generate the same epitopes that are presented on tumour target cells. Immunoediting suggests that this is occurring in the case of this vaccine.
To confirm the observations reported here, a randomised placebo-controlled trial of NY-ESO-1 ISCOMATRIX™ adjuvant in patients with stage III and resected stage IV melanoma has been initiated (LUD2003-009, clinicaltrials.gov identifier NCT00199901). Results from that trial will be reported separately.
Acknowledgments
The investigators are members of the Cancer Vaccine Collaborative. We acknowledge the invaluable assistance of the staff of the Ludwig Institute for Cancer Research, Melbourne, Australia, our research nurses and clinical fellows. Support for clinical trial LUD01-017 was generously provided by the Cancer Vaccine Collaborative and Cancer Research Institute, NY. IDD was supported by an Australian National Health and Medical Research (NHMRC) Council Career Development Award and currently by a Victorian Cancer Agency Clinician Researcher Fellowship and an honorary NH&MRC Practitioner Fellowship. WC was supported by an International Senior Research Fellowship from the Wellcome Trust (066646/Z/01/Z). JC is an NHMRC Practitioner Fellow. Operational Infrastructure Support Program Funding of the Victorian Government and NHMRC Independent Research Institutes Infrastructure Support Scheme. We wish to thank Zdenka Bolcevic and Jodie Palmer for assistance with writing and preparing tables and figures for this publication.
Conflict of interest
JC, IDD and WC hold a patent in relation to an NY-ESO-1 vaccine. JC has received research support from GSK, which has licensed CT antigens from LICR.
References
- 1.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94(5):1714–1718. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholaou T, Ebert L, Davis I, Robson N, Klein O, Maraskovsky E, Chen W, Cebon J. Directions in the immune targeting of cancer; lessons learned from the cancer testis antigen NY-ESO-1. Immunol Cell Biol. 2006;84(3):303. doi: 10.1111/j.1440-1711.2006.01446.x. [DOI] [PubMed] [Google Scholar]
- 3.Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 4.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cebon J, Knights A, Ebert L, Jackson H, Chen W. Evaluation of cellular immune responses in cancer vaccine recipients: lessons from NY-ESO-1. Expert Rev Vaccin. 2010;9(6):617–629. doi: 10.1586/erv.10.58. [DOI] [PubMed] [Google Scholar]
- 7.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4+ and CD8+ T cell responses in humans. Proc Natl Acad Sci USA. 2004;101(29):10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Jackson H, Parente P, Luke T, Rizkalla M, Tai TY, Zhu HC, Mifsud NA, Dimopoulos N, Masterman KA, Hopkins W, Goldie H, Maraskovsky E, Green S, Miloradovic L, McCluskey J, Old LJ, Davis ID, Cebon J, Chen W. Immunodominant CD4+ responses identified in a patient vaccinated with full-length NY-ESO-1 formulated with ISCOMATRIX adjuvant. Proc Natl Acad Sci USA. 2004;101(25):9363–9368. doi: 10.1073/pnas.0403271101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gnjatic S, Atanackovic D, Jager E, Matsuo M, Selvakumar A, Altorki NK, Maki RG, Dupont B, Ritter G, Chen YT, Knuth A, Old LJ. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci USA. 2003;100(15):8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S, Castelli F, Land S, Bennouna J, Logan T, Kirkwood JM. NY-ESO-1 119–143 is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor- reactive CD4+ T cells. Cancer Res. 2002;62(1):213–218. [PubMed] [Google Scholar]
- 11.Zarour HM, Storkus WJ, Brusic V, Williams E, Kirkwood JM. NY-ESO-1 encodes DRB1*0401-restricted epitopes recognized by melanoma- reactive CD4+ T cells. Cancer Res. 2000;60(17):4946–4952. [PubMed] [Google Scholar]
- 12.Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY- ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO- 1 antibody production. Proc Natl Acad Sci USA. 2001;98(7):3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng G, Touloukian CE, Wang X, Restifo NP, Rosenberg SA, Wang RF. Identification of CD4+ T cell epitopes from NY-ESO-1 presented by HLA- DR molecules. J Immunol. 2000;165(2):1153–1159. doi: 10.4049/jimmunol.165.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann F, Wagner C, Kubuschok B, Stevanovic S, Rammensee HG, Pfreundschuh M. Identification of an antigenic peptide derived from the cancer-testis antigen NY-ESO-1 binding to a broad range of HLA-DR subtypes. Cancer Immunol Immunother. 2004;53(7):589–599. doi: 10.1007/s00262-003-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandic M, Castelli F, Janjic B, Almunia C, Andrade P, Gillet D, Brusic V, Kirkwood JM, Maillere B, Zarour HM. One NY-ESO-1-derived epitope that promiscuously binds to multiple HLA-DR and HLA-DP4 molecules and stimulates autologous CD4+ T cells from patients with NY-ESO-1-expressing melanoma. J Immunol. 2005;174(3):1751–1759. doi: 10.4049/jimmunol.174.3.1751. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Immunity peptide database (2010) Table 2. Shared tumor-specific antigens. Acad Cancer Immunol. http://www.cancerimmunity.org/peptidedatabase/tumorspecific.htm
- 17.Barr IG, Mitchell GF. ISCOMs (immunostimulating complexes): the first decade. Immunol Cell Biol. 1996;74(1):8–25. doi: 10.1038/icb.1996.2. [DOI] [PubMed] [Google Scholar]
- 18.Murphy R, Green S, Ritter G, Cohen L, Ryan D, Woods W, Rubira M, Cebon J, Davis ID, Sjolander A, Kypridis A, Kalnins H, McNamara M, Moloney MB, Ackland J, Cartwright G, Rood J, Dumsday G, Healey K, Maher D, Maraskovsky E, Chen YT, Hoffman EW, Old LJ, Scott AM. Recombinant NY-ESO-1 cancer antigen: production and purification under cGMP conditions. Prep Biochem Biotechnol. 2005;35(2):119–134. doi: 10.1081/PB-200054732. [DOI] [PubMed] [Google Scholar]
- 19.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12(3 Pt 1):764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 20.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 21.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA. 2008;105(5):1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiong B, Wong R, Lee P, Delto J, Scotland R, Lau R, Weber J. Characterization of long-term effector-memory T-cell responses in patients with resected high-risk melanoma receiving a melanoma peptide vaccine. J Immunother. 2004;27(5):368–379. doi: 10.1097/00002371-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman EW, Pan L, Ritter G, Villella J, Thomas B, Rodabaugh K, Lele S, Shrikant P, Old LJ, Gnjatic S. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci USA. 2007;104(31):12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bender A, Karbach J, Neumann A, Jager D, Al-Batran SE, Atmaca A, Weidmann E, Biskamp M, Gnjatic S, Pan L, Hoffman E, Old LJ, Knuth A, Jager E. LUD 00–009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun. 2007;7:16. [PMC free article] [PubMed] [Google Scholar]
- 25.Davis ID, Chen Q, Morris L, Quirk J, Stanley M, Tavarnesi ML, Parente P, Cavicchiolo T, Hopkins W, Jackson H, Dimopoulos N, Tai TY, MacGregor D, Browning J, Svobodova S, Caron D, Maraskovsky E, Old LJ, Chen W, Cebon J. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. J Immunother. 2006;29(5):499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Jackson H, Shackleton M, Parente P, Hopkins W, Sturrock S, MacGregor D, Maraskovsky E, Tai TY, Dimopoulos N, Masterman KA, Luke T, Davis ID, Chen W, Cebon J. Characterization of antigen-specific CD8+ T lymphocyte responses in skin and peripheral blood following intradermal peptide vaccination. Cancer Immun. 2005;5:5. [PubMed] [Google Scholar]
- 27.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, Chen Q, Parente P, Jefford M, Masterman KA, Caron D, Chen W, Maraskovsky E, Cebon J. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 28.Jager E, Gnjatic S, Nagata Y, Stockert E, Jager D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, Hoffman E, Arand M, Old LJ, Knuth A. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers [In Process Citation] Proc Natl Acad Sci USA. 2000;97(22):12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robson NC, McAlpine T, Knights AJ, Schnurr M, Shin A, Chen W, Maraskovsky E, Cebon J. Processing and cross presentation of individual HLA-A, -B or -C epitopes from NY-ESO-1 or a HLA-A epitope for Melan-A differ according to the mode of antigen delivery. Blood. 2010;116(2):218–225. doi: 10.1182/blood-2009-10-249458. [DOI] [PubMed] [Google Scholar]
- 30.Schnurr M, Orban M, Robson NC, Shin A, Braley H, Airey D, Cebon J, Maraskovsky E, Endres S. ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II. J Immunol. 2009;182(3):1253–1259. doi: 10.4049/jimmunol.182.3.1253. [DOI] [PubMed] [Google Scholar]
- 31.Dannull J, Lesher DT, Holzknecht R, Qi W, Hanna G, Seigler H, Tyler DS, Pruitt SK. Immunoproteasome down-modulation enhances the ability of dendritic cells to stimulate antitumor immunity. Blood. 2007;110(13):4341–4350. doi: 10.1182/blood-2007-04-083188. [DOI] [PubMed] [Google Scholar]