Abstract
Current systemic treatments for metastatic uveal melanoma (UM) have not improved overall survival (OS). The fully human anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) monoclonal antibody, ipilimumab, improved OS of patients with advanced cutaneous melanoma in a phase 3 trial; however, UM patients were excluded. The aim of this subanalysis, performed by the ipilimumab-ocular melanoma expanded access program (I-OMEAP) study group, was to assess the activity and safety of ipilimumab in patients with UM in a setting similar to daily clinical practice. Patients participating in a multicenter expanded access program (EAP) received induction treatment with ipilimumab 10 mg/kg. Maintenance doses were administered in patients who experienced clinical benefit or at physicians’ discretion. Tumor assessment was evaluated per modified World Health Organization criteria at baseline, Week 12, Week 24, and Week 36. Adverse events (AEs) and immune-related AEs (irAEs) were collected according to Common Terminology Criteria for Adverse Events version 3.0. Thirteen pretreated patients with metastatic UM were treated at 6 European institutions. All patients received at least one dose of ipilimumab. Overall, no objective responses were observed; however, two patients had stable disease (SD), with a third patient achieving SD after initial progressive disease. Median OS as of July 1, 2011, was 36 weeks (range 2–172+ weeks). No grade 3/4 AEs of non-immune origin were reported. Three patients (23%) experienced grade 3 irAEs (1 thrombocytopenia, 1 diarrhea, and 1 alanine/aspartate aminotransferase elevation) that resolved with steroid therapy. The results indicate UM is a potential indication for ipilimumab treatment that should be further investigated in clinical trials.
Keywords: Uveal melanoma, Ipilimumab, Cytotoxic T-lymphocyte antigen-4, Melanoma, Immunotherapy
Introduction
Uveal melanoma (UM) arises from the vascular layers of the eye (iris, ciliary body, and choroid) and has a mean age-adjusted incidence of 4.3 per million in the United States, similar to that reported in European countries. Although comprising only 3% of all melanoma cases, UM is the most common type of intraocular tumor [1].
Primary UM is managed with enucleation or eye-conserving treatments such as photocoagulation, brachytherapy, radiotherapy, or local resection [2, 3]. Overall, the 5-year relative survival rate for patients with UM is approximately 69% [4]. However, 35–50% of patients develop metastatic disease within 10 years [2, 5] and the outcome is poor for these patients. Median survival after diagnosis of metastasis varies between 2 months and 15 months [6–11]. Predictive factors for recurrence include the location and size of the primary tumor [12–14], cell type and mitotic activity [13, 14], microcirculation architecture [15], lymphocytic infiltration [16], and cytogenetic abnormalities such as monosomy 3 and additional 8q material [17].
The most predominant site of metastases from UM is the liver [6]. Metastatic liver involvement accounts for the poor outcome of patients with UM [10, 18]. Among 145 consecutive patients with metastases from UM, liver involvement was documented in more than 90% of cases [8]. Metastases to lung, bone, and skin are also frequent in more advanced disease [19]. This pattern of dissemination is different from cutaneous melanoma, which more typically metastasizes to lung and lymph nodes [20].
Few studies have looked specifically at patients with metastatic UM. Treatment for metastatic disease includes surgery, hepatic intra-arterial chemoembolization [6], systemic chemotherapies such as dacarbazine, fotemustine, temozolomide, cisplatin, gemcitabine, and treosulfan [6, 7, 21] and combination chemotherapy regimens such as BOLD (bleomycin, vincristine, lomustine, and dacarbazine), with or without immunotherapy [9, 22]. Although prolonged survival has been reported following surgical resection of metastatic sites [23], there is no compelling evidence that any therapy improves overall survival for patients with metastatic UM [24], and efficacious therapies affording meaningful clinical benefits in first- and later-line settings are urgently required.
A recent antitumor strategy involves augmenting cell-mediated immunity by interrupting the inhibitory pathways involved in T-cell activation [25]. The discovery that cytotoxic T-lymphocyte antigen-4 (CTLA-4) functions as a negative regulator of activated T-cells led to the hypothesis that targeting this molecule could potentiate T-cell activation, resulting in a more effective antitumor immune response [25]. Ipilimumab (Bristol-Myers Squibb, New York, NY, USA), a fully human anti-CTLA-4 monoclonal antibody, improved overall survival in patients with previously treated metastatic cutaneous melanoma in a recent phase 3 trial [26], and data were consistent with results of phase 2 studies [27, 28]. Ipilimumab is generally well tolerated [26, 29–33], with the majority of adverse events (AEs) being consistent with the proposed mechanism of action. Class-specific immune-related AEs (irAEs) are generally well characterized, manageable, and most frequently affect the gastrointestinal tract and skin. The majority resolve spontaneously or following appropriate medical therapy, treatment interruption, or withdrawal [30, 32, 34, 35].
Although previous ipilimumab clinical trials excluded patients with UM, an expanded access program (EAP) provided the opportunity to treat patients affected by metastatic UM with ipilimumab in a setting closely mirroring daily clinical practice [36, 37]. Here, we report the first known experience of using ipilimumab in patients with metastatic UM from six European medical institutions.
Patients and treatment
Patient eligibility
Patients with life-threatening, unresectable metastatic UM, with or without brain metastasis, no prior treatment with ipilimumab, and whose physicians requested compassionate use of ipilimumab, were eligible for analysis. Patients were included if they had failed or could not tolerate previous systemic or loco-regional therapies, if no alternative drug or therapy was available, or if their physician believed that, based upon the available benefit-to-risk data, it was appropriate to administer ipilimumab. The EAP was approved by the local independent ethics committees, and all patients provided a signed informed consent form.
Treatment design
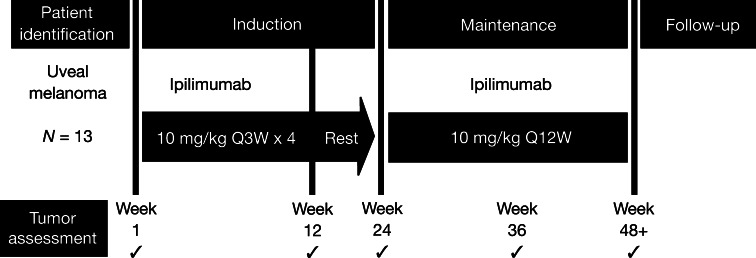
The treatment design is outlined in Fig. 1. Ipilimumab 10 mg/kg was administered intravenously over 90 min to patients with metastatic UM at Weeks 1, 4, 7, and 10 in the induction phase. At Week 24, patients considered by the treating physician to be obtaining clinical benefit, either because of apparent tumor stability or continued shrinkage and/or late response, or at the physicians’ discretion could receive further treatment with ipilimumab every 12 weeks in the maintenance phase.
Fig. 1.
Treatment schedule of ipilimumab dosing and tumor assessments
Tumor assessment was evaluated per modified World Health Organization (mWHO) criteria at baseline, Week 12, Week 24, and every 12 weeks thereafter. Clinical response was defined as complete response (CR; observed disappearance of all index lesions), partial response (PR; ≥50% decrease from baseline in the sum of the product of the diameters of defined index lesions), progressive disease (PD; 25% increase from baseline in the smallest recorded sum of the product of defined index lesions and/or appearance of any new lesions), or stable disease (SD; CR or PR criteria not met in the absence of PD).
All patients who received ipilimumab in the EAP were monitored and assessed for safety. AEs and irAEs were graded according to Common Terminology Criteria for Adverse Events version 3.0 (NCI CTCAE 3.0).
Results
Patient characteristics
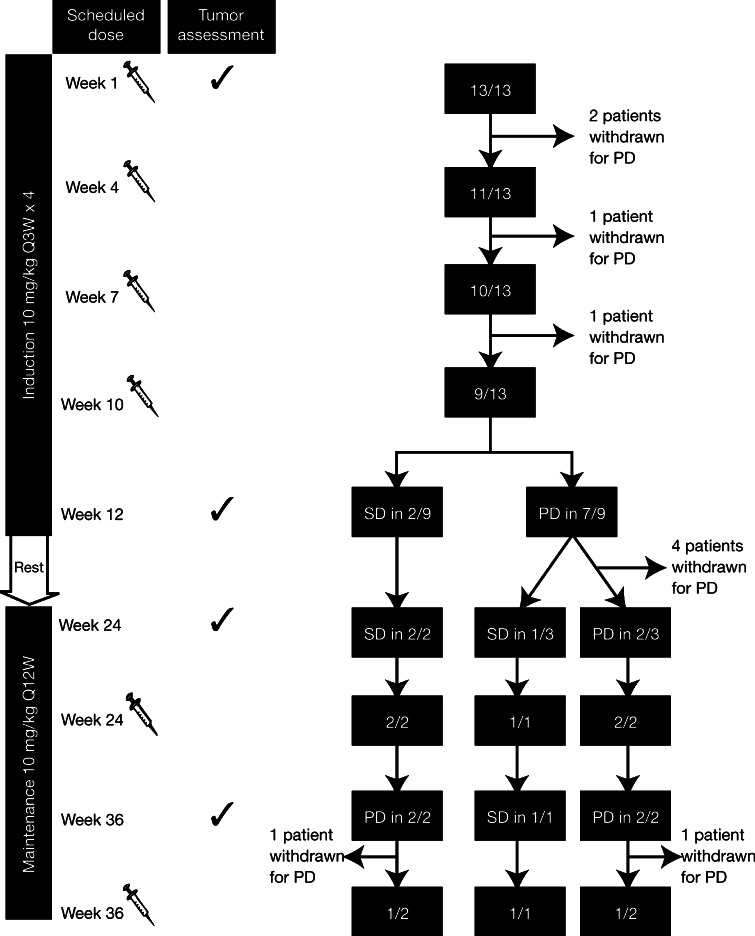
Thirteen patients with metastatic UM were treated at 6 European institutions: University Hospital of Siena, Siena; National Institute for Cancer Research, Genoa; European Institute of Oncology, Milan; Istituto Oncologico Veneto-IRCCS, Padua; Scientific Institute of Romagna for the Study and Treatment of Cancer, Meldola-Forlì (Italy); and Northern Centre for Cancer Care at Newcastle upon Tyne (United Kingdom) as part of the ipilimumab EAP. Baseline patient characteristics are provided in Table 1. All patients had metastatic disease at baseline, a median of 2 (range 1–4) previous systemic therapies and received at least 1 ipilimumab dose. The induction phase was not completed in four patients because of rapid disease progression. Nine patients (69%) completed the induction phase, undergoing tumor evaluation at baseline and at Week 12, and 5 of these patients entered the maintenance phase (Fig. 2).
Table 1.
Baseline patient characteristics (n = 13*)
| Characteristics | |
|---|---|
| Age, years, median (range) | 57 (30–76) |
| Sex, male, n (%) | 8 (62) |
| Disease stage, n (%) | |
| IV | 13 (100) |
| ECOG performance status, n (%) | |
| 0 | 9 (69) |
| 1 | 4 (31) |
| Metastatic sites, n (%) | |
| Liver | 13* (100) |
| Brain | 1 (7) |
| LDH >2× ULN, n (%) | 3 (23) |
| Number of prior systemic therapies, median (range) | 2 (1–4) |
| Chemotherapy, n (%) | 12 (92) |
| Immunotherapy, n (%) | 3 (23) |
| Chemo-immunotherapy, n (%) | 3 (23) |
ECOG Eastern cooperative oncology group, LDH lactate dehydrogenase, ULN upper limit of normal
* Evidence in 12, history in 1
Fig. 2.
Disease status of patients participating in the expanded access program. Ipilimumab was administered on Weeks 1, 4, 7, 10, 24, and 36 and tumor assessments performed on Weeks 1, 12, 24, and 36. Three patients with uveal melanoma were treated for more than 36 weeks (1 with stable disease [SD] and 2 with progressive disease [PD])
Clinical response
No objective tumor responses were observed. Of the nine patients who completed the induction phase, two patients had SD and seven had PD according to mWHO criteria. Both patients with SD at Week 12 remained stable until Week 36 (Fig. 2). Of the seven patients with PD at Week 12, four were withdrawn before Week 24; therefore, three patients with PD entered the maintenance phase. Two patients were maintained on ipilimumab, despite evidence of PD (Fig. 2). The third patient with PD at Week 12 stabilized at Week 24 and remained stable at the time of manuscript preparation (Fig. 3).
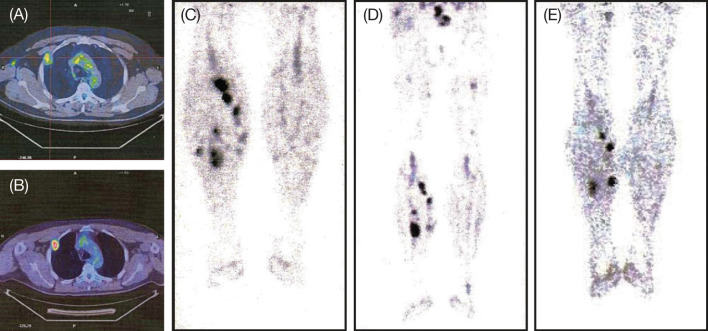
Fig. 3.
Position emission tomography (PET) scans of thorax metastasis in a 47-year-old male patient prior to treatment (a) and immediately before the fourth maintenance dose of ipilimumab (b); Fig. 3c–e show subcutaneous soft tissue lesions of the lower legs prior to treatment (c), immediately before the fourth maintenance dose of ipilimumab (d), and immediately before the eighth maintenance dose of ipilimumab at Week 108 (e)
The patient with PD followed by disease stabilization is a 47-year-old male. He had history of a single metastatic liver lesion that had undergone surgical resection and PD in soft tissues and lymph nodes after four lines of chemo-immunotherapy (interferon-alpha, dacarbazine plus thymosin α1, fotemustine, and paclitaxel). This patient had a steady decline in the volume of subcutaneous tumor lesions and new lesions appeared that subsequently shrank (Fig. 3c–e). Interestingly, asymptomatic hypothyroidism resulting from autoimmune thyroiditis was diagnosed at the time of disease stabilization (Week 24). The patient underwent hormonal replacement therapy with levothyroxine and maintenance therapy with ipilimumab is ongoing.
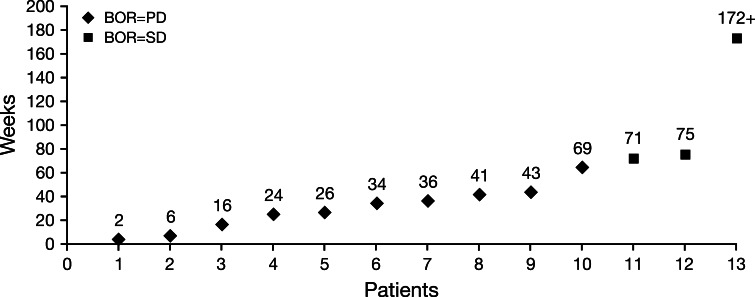
As of July 1, 2011, median overall survival for all 13 patients was 36 weeks (range 2–172+ weeks) (Fig. 4).
Fig. 4.
Survival outcomes of individual patients participating in the expanded access program. Among 13 treated patients, three had best overall response (BOR) of stable disease (SD) and 10 had progressive disease (PD). Median overall survival for all patients was 36 weeks (range 2–172+ weeks; including one patient with ongoing SD at the time of manuscript preparation)
Safety and tolerability
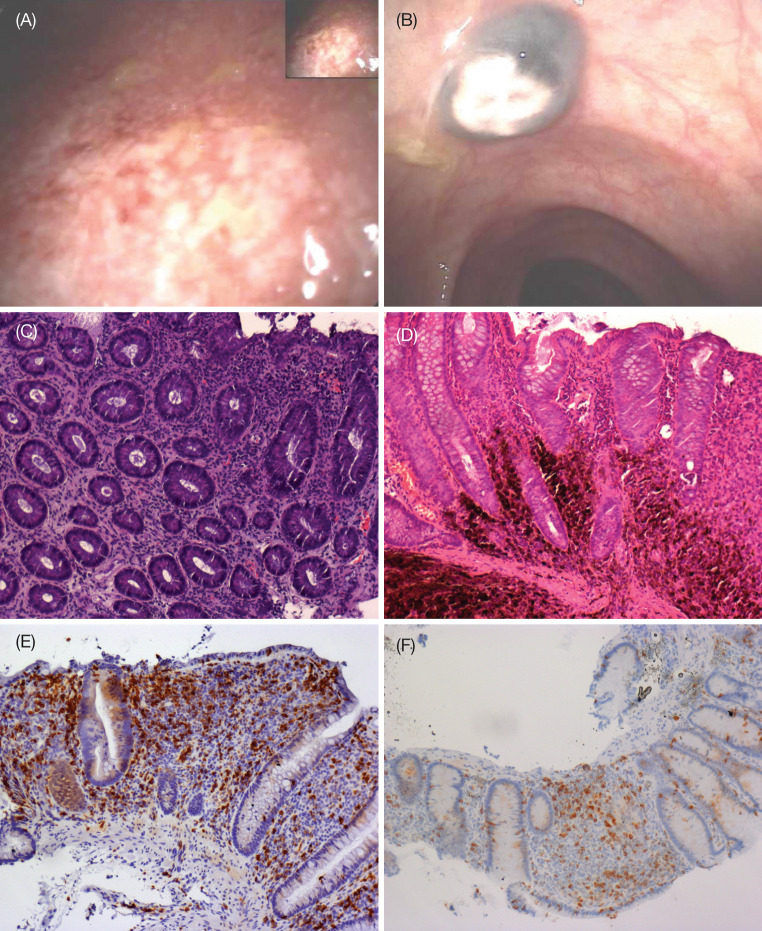
No patient prematurely discontinued treatment due to AEs and no grade 3/4 AEs of non-immune origin were reported. Three patients (23%) experienced grade 3 irAEs (one thrombocytopenia, one diarrhea, and one alanine aminotransferase/aspartate aminotransferase elevation) that resolved after steroid therapy. A 62-year-old male with liver, bone, subcutaneous tissue, and lung metastases after first-line chemotherapy with dacarbazine developed grade 3 diarrhea after the first dose of ipilimumab. Colonoscopic images from this patient indicated ulcerative colitis, micro-abscesses and evidence of colic, UM metastasis (Fig. 5a, b). Biopsy samples showed chronic, superficial, and productive inflammation (Fig. 5c). Steroid therapy was undertaken, and complete remission of gastrointestinal symptoms was obtained after 4 weeks (Fig. 5d). Although he only received one induction dose of ipilimumab, this patient survived for 30 weeks.
Fig. 5.
Colonoscopic images of a grade 3 ulcerative colitis with b evidence of colic uveal melanoma metastasis. Hematoxylin and eosin staining of biopsies demonstrates c autoimmune colitis with inflammatory cells, erosion of the tonaca propria and loss of gland goblet cells, and d metastatic deposits. Immunohistochemical labeling indicates e most of the inflammatory cells are of the CD8+ phenotype and f there is a decrease in CD8+ T-cells following steroid therapy. All images were captured at ×100 magnification
Grade 1/2 AEs and irAEs were not considered clinically relevant in this analysis, but detailed information is provided in Table 2.
Table 2.
AEs and irAEs occurring during treatment with ipilimumab
| All grades, n | Grade 3*, n | |
|---|---|---|
| AEs/irAEs leading to discontinuation | 0 | 0 |
| AEs | 10 | 0 |
| irAEs | ||
| Skin | 5 | 0 |
| Gastrointestinal | 4 | 1 |
| Liver | 1 | 1 |
| Endocrine | 1 | 0 |
| Hematologic | 1 | 1 |
| Other | 6 | 0 |
| Total | 18 | 3 |
AEs adverse events, irAEs immune-related adverse events
* There were no grade 4 AEs or irAEs
Discussion
Standard systemic chemotherapy for metastatic UM fails to provide meaningful or durable clinical benefit, and the addition of immunotherapy has not been shown to improve response rates or survival [24]. Although uveal and cutaneous melanomas are biologically different, the immune system seems to be involved in the progression of both diseases [14, 22, 38].
Clinical studies in patients with metastatic cutaneous melanoma show that ipilimumab treatment results in significant clinical activity and improved overall survival [26, 27]. To our knowledge, this is the first reported experience of patients with UM being treated with an anti-CTLA-4 antibody [36]. Because of the small number of patients involved, caution must be exercised in extrapolating from this experience; however, the observations described herein support prior studies of anti-CTLA-4 monoclonal antibodies in cutaneous melanoma and are promising with respect to results reported for the treatment of metastatic UM to date.
A characteristic finding in clinical trials of ipilimumab in patients with metastatic cutaneous melanoma is the durability of response and prolonged SD. A recent analysis of 6 clinical trials showed SD was the best overall outcome in 84/356 patients treated with ipilimumab, was durable, lasting ≥24 weeks in 23 patients (27%) and was ongoing in 33 patients at the time of analysis. There was also evidence of SD evolving into objective responses with time [39]. Response rates in larger trials of patients with metastatic UM treated with standard chemotherapy regimens ranged from 0 to 36% with a median survival of 2–15 months after diagnosis of metastasis [6–11, 21]. In view of this poor prognosis, prolonged SD should be considered a meaningful clinical outcome. In this analysis, 2/13 (15%) pretreated patients with metastatic UM had SD at Week 12 and remained stable for 36 weeks after the first dose of ipilimumab.
Some patients with metastatic cutaneous melanoma treated with ipilimumab experience objective responses or durable SD after initial PD or the emergence of new lesions. This may be because T-cell infiltration or inflammation occurring in baseline melanoma in response to therapy is misinterpreted as PD or because of the time needed for the immune system to recognize and control melanoma lesions [28, 37]. As a result, substantial PD, which may not be reflected in patients’ performance status and laboratory tests, should be confirmed before considering a new therapy [37]. In our experience, one patient experienced SD at Week 24 after initial PD at Week 12, as per mWHO criteria. This patient remained in SD and was alive at the time of the analysis, 172 weeks after the first dose of ipilimumab. There is limited evidence relating to the continuation of ipilimumab therapy beyond Week 24 in patients with confirmed PD who had neither an objective response nor SD in previous tumor assessments. In our analysis, 2/13 (15%) pretreated patients with metastatic UM with PD were maintained on ipilimumab treatment at the investigator’s discretion beyond Week 24. Interestingly, despite PD, both the patients experienced prolonged survival of 41 and 64 weeks, respectively. Observations such as these, even from a limited number of patients, indicate that pretreated patients with metastatic UM may benefit from ipilimumab treatment. The results also suggest, however, that mWHO criteria alone are not sufficient to evaluate treatment responses. Systematic criteria, designated immune-related response criteria (irRC), were therefore defined in an attempt to capture additional response patterns observed with immune therapy [40]. According to irRC, PD is recorded only after a 25% increase from baseline in total tumor burden has occurred twice and at least 4 weeks apart. WHO criteria, however, consider any new measurable lesion to indicate PD [40].
IrAEs in patients treated with ipilimumab have been well characterized and are generally mild to moderate in severity. It is critical that grade 3/4 irAEs, which are commonly gastrointestinal (diarrhea and colitis), are treated quickly with high-dose steroids because they have the potential to become life-threatening [34, 37]. In this analysis, one patient developed grade 3 diarrhea, which completely resolved after 4 weeks of steroid therapy. Colonoscopic images and histopathology were reminiscent of immune-related colitis.
Findings from preclinical and clinical trials indicate that treating irAEs with corticosteroids does not affect the efficacy of anti-CTLA-4-mediated tumor inhibition [34, 41], and in our experience, there were no reports of ipilimumab failure after steroid therapy.
The relationship between the onset of irAEs and clinical benefit in patients treated with ipilimumab is a matter of debate [37, 42]. We observed the onset of autoimmune thyroiditis concomitant to disease stabilization at Week 24 in a patient with prior PD at Week 12; however, caution must be used if extrapolating from a single clinical history.
Overall, the observations from this EAP indicate that ipilimumab may have clinical activity in pretreated patients with metastatic UM. Further investigation within prospective clinical trials is required to establish the optimal application for this anti-CTLA-4 antibody, considering that no standard first-line therapy currently exists for patients with metastatic UM.
Acknowledgments
The expanded access program was sponsored by Bristol-Myers Squibb. Editorial assistance was provided by StemScientific, funded by Bristol-Myers Squibb. The authors thank Dr Giorgio Frosini and Dr Mario Marini from the Gastroenterology Unit of the University Hospital of Siena for providing the colonoscopic pictures. They also thank the nurses (Angela Iacovelli, Sergio Speranza, Massimo Resti, and Marilena Piccinelli) and data managers (Giovanni Amato and Eliana Pittiglio) at the Medical Oncology and Immunotherapy Unit of the University Hospital of Siena for their contribution to the data collection.
Footnotes
This study was conducted for the ipilimumab-ocular melanoma expanded access program (I-OMEAP) study group.
References
- 1.Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:956–961. doi: 10.1016/S0161-6420(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 2.Diener-West M, Earle JD, Fine SL, Hawkins BS, Moy CS, Reynolds SM, Schachat AP, Straatsma BR. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969–982. doi: 10.1001/archopht.119.7.969. [DOI] [PubMed] [Google Scholar]
- 3.Shields JA, Shields CL, Donoso LA. Management of posterior uveal melanoma. Surv Ophthalmol. 1991;36:161–195. doi: 10.1016/0039-6257(91)90001-V. [DOI] [PubMed] [Google Scholar]
- 4.Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, Lutz JM, Paci E. Survival in patients with uveal melanoma in Europe. Arch Ophthalmol. 2008;126:1413–1418. doi: 10.1001/archopht.126.10.1413. [DOI] [PubMed] [Google Scholar]
- 5.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, Hawkins BS, Hayman JA, Jaiyesimi I, Jampol LM, Kirkwood JM, Koh WJ, Robertson DM, Shaw JM, Straatsma BR, Thoma J. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative ocular melanoma study group report no. 26. Arch Ophthalmol. 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 6.Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, Papadopoulos N, Benjamin RS. Treatment of uveal melanoma metastatic to the liver: a review of the MD Anderson cancer center experience and prognostic factors. Cancer. 1995;76:1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::AID-CNCR2820760925>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty LE, Unger JM, Liu PY, Mertens WC, Sondak VK. Metastatic melanoma from intraocular primary tumors: the Southwest oncology group experience in phase II advanced melanoma clinical trials. Am J Clin Oncol. 1998;21:568–572. doi: 10.1097/00000421-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gragoudas ES, Egan KM, Seddon JM, Glynn RJ, Walsh SM, Finn SM, Munzenrider JE, Spar MD. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–389. doi: 10.1016/s0161-6420(91)32285-1. [DOI] [PubMed] [Google Scholar]
- 9.Kivela T, Suciu S, Hansson J, Kruit WH, Vuoristo MS, Kloke O, Gore M, Hahka-Kemppinen M, Parvinen LM, Kumpulainen E, Humblet Y, Pyrhonen S. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer. 2003;39:1115–1120. doi: 10.1016/S0959-8049(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 10.Peters S, Voelter V, Zografos L, Pampallona S, Popescu R, Gillet M, Bosshard W, Fiorentini G, Lotem M, Weitzen R, Keilholz U, Humblet Y, Piperno-Neumann S, Stupp R, Leyvraz S. Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: experience in 101 patients. Ann Oncol. 2006;17:578–583. doi: 10.1093/annonc/mdl009. [DOI] [PubMed] [Google Scholar]
- 11.Queirolo P, Acquati M. Medical treatment of uveal melanoma. Tumori. 2007;93:27–30. [PubMed] [Google Scholar]
- 12.Diener-West M, Hawkins BS, Markowitz JA, Schachat AP. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol. 1992;110:245–250. doi: 10.1001/archopht.1992.01080140101036. [DOI] [PubMed] [Google Scholar]
- 13.McLean MJ, Foster WD, Zimmerman LE. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch Ophthalmol. 1977;95:48–58. doi: 10.1001/archopht.1977.04450010050004. [DOI] [PubMed] [Google Scholar]
- 14.Seddon JM, Albert DM, Lavin PT, Robinson N. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol. 1983;101:1894–1899. doi: 10.1001/archopht.1983.01040020896012. [DOI] [PubMed] [Google Scholar]
- 15.Rummelt V, Folberg R, Woolson RF, Hwang T, Pe’er J. Relation between the microcirculation architecture and the aggressive behavior of ciliary body melanomas. Ophthalmology. 1995;102:844–851. doi: 10.1016/s0161-6420(95)30947-5. [DOI] [PubMed] [Google Scholar]
- 16.de la Cruz PO, Jr, Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990;65:112–115. doi: 10.1002/1097-0142(19900101)65:1<112::AID-CNCR2820650123>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.White VA, Chambers JD, Courtright PD, Chang WY, Horsman DE. Correlation of cytogenetic abnormalities with the outcome of patients with uveal melanoma. Cancer. 1998;83:354–359. doi: 10.1002/(SICI)1097-0142(19980715)83:2<354::AID-CNCR20>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Eskelin S, Pyrhonen S, Hahka-Kemppinen M, Tuomaala S, Kivela T. A prognostic model and staging for metastatic uveal melanoma. Cancer. 2003;97:465–475. doi: 10.1002/cncr.11113. [DOI] [PubMed] [Google Scholar]
- 19.Einhorn LH, Burgess MA, Gottlieb JA. Metastatic patterns of choroidal melanoma. Cancer. 1974;34:1001–1004. doi: 10.1002/1097-0142(197410)34:4<1001::AID-CNCR2820340406>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 20.Albert DM, Ryan LM, Borden EC. Metastatic ocular and cutaneous melanoma: a comparison of patient characteristics and prognosis. Arch Ophthalmol. 1996;114:107–108. doi: 10.1001/archopht.1996.01100130103030. [DOI] [PubMed] [Google Scholar]
- 21.Schmittel A, Schmidt-Hieber M, Martus P, Bechrakis NE, Schuster R, Siehl JM, Foerster MH, Thiel E, Keilholz U. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann Oncol. 2006;17:1826–1829. doi: 10.1093/annonc/mdl309. [DOI] [PubMed] [Google Scholar]
- 22.Nathan FE, Berd D, Sato T, Shield JA, Shields CL, De Potter P, Mastrangelo MJ. BOLD+interferon in the treatment of metastatic uveal melanoma: first report of active systemic therapy. J Exp Clin Cancer Res. 1997;16:201–208. [PubMed] [Google Scholar]
- 23.Frenkel S, Nir I, Hendler K, Lotem M, Eid A, Jurim O, Pe’er J. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009;93:1042–1046. doi: 10.1136/bjo.2008.153684. [DOI] [PubMed] [Google Scholar]
- 24.Augsburger JJ, Correa ZM, Shaikh AH. Quality of evidence about effectiveness of treatments for metastatic uveal melanoma. Trans Am Ophthalmol Soc. 2008;106:128–135. [PMC free article] [PubMed] [Google Scholar]
- 25.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–2627. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 26.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, Verschraegen C, Humphrey R, Ibrahim R, de Pril V, Hoos A, Wolchok JD. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 28.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbe C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 29.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischkoff SA, Hersh E, Weber J, Powderly J, Khan K, Pavlick A, Samlowski W, O’Day SJ, Nichol G, Yellin M. Durable responses and long-term progression-free survival observed in a phase II study of MDX-010 alone or in combination with dacarbazine (DTIC) in metastatic melanoma [abstract] J Clin Oncol. 2005;23(16 suppl):7525. [Google Scholar]
- 31.Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Hughes M, Yellin MJ, Haworth LR, Levy C, Allen T, Mavroukakis SA, Attia P, Rosenberg SA. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29:455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribas A. Anti-CTLA4 antibody clinical trials in melanoma. Update Cancer Ther. 2007;2:133–139. doi: 10.1016/j.uct.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 34.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Danielli R, Queirolo P, Testori A, Plummer R, Razi E, Chiaron-Sileni V, Calabro L, Di Giacomo AM, Ridolfi R, Maio M. Ipilimumab in pretreated metastatic uveal melanoma patients: safety and clinical efficacy [abstract] Eur J Cancer Suppl. 2009;7:9315. doi: 10.1016/S1359-6349(09)71959-7. [DOI] [Google Scholar]
- 37.Di Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli M, Altomonte M, Maio M. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetter CS, Lieb W, Brocker EB, Becker JC. Loss of nonclassical MHC molecules MIC-A/B expression during progression of uveal melanoma. Br J Cancer. 2004;91:1495–1499. doi: 10.1038/sj.bjc.6602123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamid O, Urba WJ, Yellin M, Nichol G, Weber J, Hersh E, Tchekmedyian S, Hodi FS, Weber RW, O’Day SJ. Ipilimumab (MDX-010) in patients with stage III/IV melanoma: kinetics and duration of response [abstract] Eur J Cancer Suppl. 2007;5:7005. doi: 10.1016/S1359-6349(07)71459-3. [DOI] [Google Scholar]
- 40.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 41.Grob JJ, Hamid O, Wolchok JD, Maio M, Neyns B, Thomas L, DePril V, Ibrahim R, O’Day SJ, Lebbé C. Antitumor responses to ipilimumab in advanced melanoma are not affected by systemic corticosteroids used to manage immune-related adverse events (irAEs) [abstract] Eur J Cancer Suppl. 2009;7:9312. doi: 10.1016/S1359-6349(09)71956-1. [DOI] [Google Scholar]
- 42.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]