Abstract
The anti-ErbB2 antibody trastuzumab has currently been approved for ErbB2-positive gastric cancer. Despite the effectiveness of trastuzumab, resistance is common. Thus, there is an urgent need to overcome trastuzumab resistance. Here, we obtain a trastuzumab-resistant cell line, which is derived from the human gastric cancer NCI-N87 cell line, by modeling the development of acquired resistance in patients. Our data show that combining trastuzumab and cetuximab leads to a significant decrease in EGFR/ErbB2 heterodimers and signaling compared with either antibody alone, and the combination results in greater antitumor activity against the trastuzumab-resistant NCI-N87 cell line, both in vitro and in vivo, suggesting that a combined EGFR/ErbB2 inhibition may overcome trastuzumab resistance.
Keywords: ErbB2, EGFR, Trastuzumab, Cetuximab, NCI-N87, Trastuzumab-resistant
Introduction
Gastric cancer is one of the most prevalent malignant tumors worldwide, and it has the second highest mortality among cancers [1]. Overexpression of the human epidermal growth factor receptor-2 (ErbB2 or HER2) is frequently found in gastric cancer [2] and is more often associated with poor prognosis [3, 4]. Trastuzumab (Herceptin), a humanized monoclonal antibody directed against the dimerization interfaces in domain IV of ErbB2 [5], has currently been approved for clinical use in patients with ErbB2-positive metastatic gastric and gastro-esophageal junction cancer. Despite the effectiveness of trastuzumab, the majority of trastuzumab-responsive patients develop resistance after continuous treatment [6]. Thus, novel therapeutic approaches are needed to overcome trastuzumab resistance.
Recent studies have highlighted that prolonged treatment with trastuzumab may induce tumor cells to reprogram themselves by overexpressing various receptor tyrosine kinases (RTKs) to develop alternative compensatory pathways to sustain cell proliferation, ultimately leading to trastuzumab resistance [7–9]. Thus, novel therapeutic approaches that target the multiple altered signaling pathways leading to resistance are warranted. The epidermal growth factor receptor (EGFR) is frequently expressed in human gastric cancer [10]. EGFR forms homodimers or heterodimers with both ligand-free and ligand-bound forms of ErbB2 and triggers potent mechanisms of cell proliferation and survival [5]. Cetuximab (Erbitux™), an anti-EGFR chimeric antibody whose epitope lies on domain II of EGFR [11], has proven useful in the treatment of carcinomas that co-express EGFR and ErbB2 [12–14]. A recent study has shown that combinatorial treatment with cetuximab and trastuzumab effectively suppressed the in vitro and in vivo growth of the ErbB2-positive human gastric cancer cell line NCI-N87, which has a high level of ErbB2 and a moderate level of EGFR expression [12]. However, the antitumor activity of trastuzumab plus cetuximab in trastuzumab-resistant gastric cancer has not yet been reported.
In this study, we obtained a trastuzumab-resistant sub-line of NCI-N87 by modeling the development of acquired resistance in patients. We investigated the antitumor effect of trastuzumab in combination with the cetuximab on the trastuzumab-resistant gastric cancer cell line, as well as its mechanism of action. Our data indicate that combinatorial treatment with cetuximab plus trastuzumab renders a comprehensive blockade of EGFR/ErbB2 heterodimers and signaling, and the combination could be a clinically useful strategy for overcoming trastuzumab resistance.
Materials and methods
Cell lines and antibodies
The human gastric cancer cell line NCI-N87 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were maintained in RPMI 1640 (Sigma-Aldrich) supplemented with 10 % fetal bovine serum. The NCI-N87 TR subline with acquired trastuzumab resistance was generated as described previously. Briefly, parental cells were continuously exposed to trastuzumab (10 μg/mL) for more than 6 months. Surviving cells from each plate were then pooled together and tested for response to trastuzumab, as described below. The anti-HER2 humanized antibody trastuzumab was purchased from Roche, and the anti-EGFR chimeric antibody cetuximab was from Merck.
Cell proliferation assay
Cells (5 × 103/well) were seeded in 96-well plates and incubated with control IgG (10 μg/mL), trastuzumab (10 μg/mL), cetuximab (10 μg/mL), or trastuzumab plus cetuximab (10 μg/mL each), followed by either addition of EGF (5 nM) or no addition of EGF. After 5 days, cell proliferation was determined by the CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS assay) kit (Promega, Madison, WI). The percentage of cell growth inhibition was calculated according to the following formula: inhibition (%) = [(A492mAb-untreated cells − A492mAb-treated cells)/(A492mAb-untreated cells − A492culture medium)] × 100.
Real-time quantitative PCR
Total RNA was isolated from cells using the TRIzol reagent (Invitrogen) and was reverse transcribed with the PrimeScript RT reagent kit (Takara, Dalian, China). The real-time quantitative PCR was performed using the SYBR Premix Ex Tag kit (Takara) on a 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control to normalize expression levels. The primers used were as follows: EGF: (F) 5′-TGG ATG TGC TTG ATA AGC GG-3′, (R) 5′-ACC ATG TCC TTT CCA GTG TGT -3′; HB-EGF: (F) 5′-ATC GTG GGG CTT CTC ATG TTT-3′, (R) 5′-TTA GTC ATG CCC AAC TTC ACT TT-3′;TGFα: (F) 5′-AGG TCC GAA AAC ACT GTG AGT-3′, (R) 5′-AGC AAG CGG TTC TTC CCT TC-3′. Relative expression levels of target genes = 2−△Ct (△△Ct = △△Ct (experimental group) − △△Ct (control group)).
Immunoblotting
Cells were incubated with antibodies in serum-free medium for 1 h at 37 °C. The cells were then treated with EGF (5 nM) or were left untreated for 15 min. After collection on ice and washing with PBS, the cells were lysed in SDS lysis buffer, and the cell lysates were subjected to SDS-PAGE and immunoblotted with antibodies against EGFR (sc-03; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-EGFR-Tyr1068 (2236; Cell Signaling, Danvers, MA), ErbB2 (sc-7301; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-ErbB2-Tyr1221/1222 (2243; Cell Signaling, Danvers, MA), ErbB3 (4754; Cell Signaling, Danvers, MA), phospho-ErbB3-Tyr1289 (4791; Cell Signaling, Danvers, MA), AKT (9272; Cell Signaling, Danvers, MA), phospho-AKT-Ser473 (4060; Cell Signaling, Danvers, MA), p44/42 MAPK (9102; Cell Signaling, Danvers, MA) or phospho-p44/42 MAPK-Thr202/Tyr204 (9106; Cell Signaling, Danvers, MA).
Immunoprecipitation
To detect the ligand-independent ErbB2-containing heterodimers, the cells were cross-linked using a reversible chemical crosslinking procedure [15]. Briefly, cells were incubated with the indicated antibodies for 2 h at 37 °C. After washing twice with ice-cold HEPES/NaCl buffer (50 mM HEPES (pH 7.2), 150 mM NaCl), the cells were incubated with 2 mM 3,3′-dithiobis[sulfosuccinimidylpropionate] (DTSSP; Thermo Scientific, Rockford, IL) dissolved in HEPES/NaCl buffer for 1 h at 4 °C. The cells were then washed three times with ice-cold 25 mM Tris (pH 7.1), 150 mM NaCl and lysed in NP-40 lysis buffer supplemented with protease and phosphatase inhibitors. For co-immunoprecipitation experiments, we incubated the total cell lysate with an agarose-conjugated anti-ErbB2 monoclonal antibody (sc-7301AC; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C. The precipitated proteins were subjected to SDS-PAGE, followed by Western blot analysis. The formation of EGF-induced, ErbB2-containing heterodimers was assayed by the method described above, with slight modifications. Briefly, the cells were starved overnight in growth medium without serum and then incubated with the indicated antibodies for 2 h at 37 °C. Recombinant human EGF (R&D Systems, Minneapolis, MN), which is a ligand of EGFR, was added at a final concentration of 5 nM for 15 min of incubation, and then, the cells were washed three times and lysed in NP-40 lysis buffer. The co-immunoprecipitation experiments were then performed as previously described.
Tumor xenograft studies
Five-week-old female BALB/c nude mice were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). All animals were treated in accordance with the Institutional Animal Care and Use Committee. Each mouse was inoculated subcutaneously in the right flank with either 5 × 106 cells per mouse of human gastric cancer cell lines NCI-N87 or NCI-N87 TR in a 1:1 PBS:matrigel suspension (BD matrigel; BD Biosciences, San Jose, CA). When tumor volumes reached an average of approximately 100–150 mm3, the mice were randomly divided into groups with an even distribution of tumor sizes (8 mice/group). Treatments consisted of twice weekly intravenous injection of different antibodies (10 mg/kg) for six consecutive weeks. Control mice were given vehicle (IgG) alone. Tumors were measured with digital calipers, and tumor volumes were calculated by the following formula: volume = length × (width)2/2.
Results
EGFR is over-expressed and highly activated in the trastuzumab-resistant NCI-N87 cell line
We modeled the development of acquired resistance in patients by treating the ErbB2-overexpressing gastric cancer cells line NCI-N87 with 10 μg/mL of trastuzumab for 6 months to obtain the trastuzumab-resistant subline, NCI-N87TR. Compared with the parental line, NCI-N87TR was significantly more resistant to trastuzumab treatment in vitro and in vivo (Fig. 1a, b). Meanwhile, we found that the amounts of EGFR and EGFR/ErbB2 heterodimers were notably up-regulated in the NCI-N87TR cells compared with the parental cells (Fig. 1c). Consistent with this, western blotting also indicated that the NCI-N87TR cell line showed a marked increase in EGFR and MAPK phosphorylation (Fig. 1d). In addition, the NCI-N87TR cells also expressed higher levels of EGF than the parental cells (Fig. 1e). The unchanged ErbB3 and AKT phosphorylation in NCI-N87TR indicates that the acquired trastuzumab resistance was not due to PI3 K pathway alterations (Fig. 1c, d). These results indicate that the up-regulated EGFR expression and EGFR/ErbB2 signaling pathways may contribute to the acquired resistance mechanisms.
Fig. 1.
Characterization of the trastuzumab-resistant gastric cancer cell line NCI-N87TR. a MTS assay evaluating cell proliferation of NCI-N87 and NCI-N87TR cell lines upon treatment with trastuzumab. Error bars, SD. ***P < 0.0001. The data are representative of three independent experiments. b Tumor volume of NCI-N87 and NCI-N87TR xenografts after treatment with 10 mg/kg of control IgG or trastuzumab. The data are shown as the mean ± SEM. ***P < 0.0001. c Co-immunoprecipitation assay detecting ligand-independent ErbB2/EGFR and ErbB2/ErbB3 heterodimerization in the NCI-N87 and NCI-N87TR cell lines. The data are representative of three independent experiments. d Immunoblots comparing major cell signaling changes between the NCI-N87 and NCI-N87TR cell lines. The data are representative of three independent experiments. e Real-time quantitative PCR analysis of expression of ErbB ligands. The data are shown as the mean ± SD. All quantitative data were generated from a minimum of three replicates
Combining trastuzumab and cetuximab effectively inhibits the formation of EGFR/ErbB2 heterodimers and activation in the trastuzumab-resistant NCI-N87 cell line
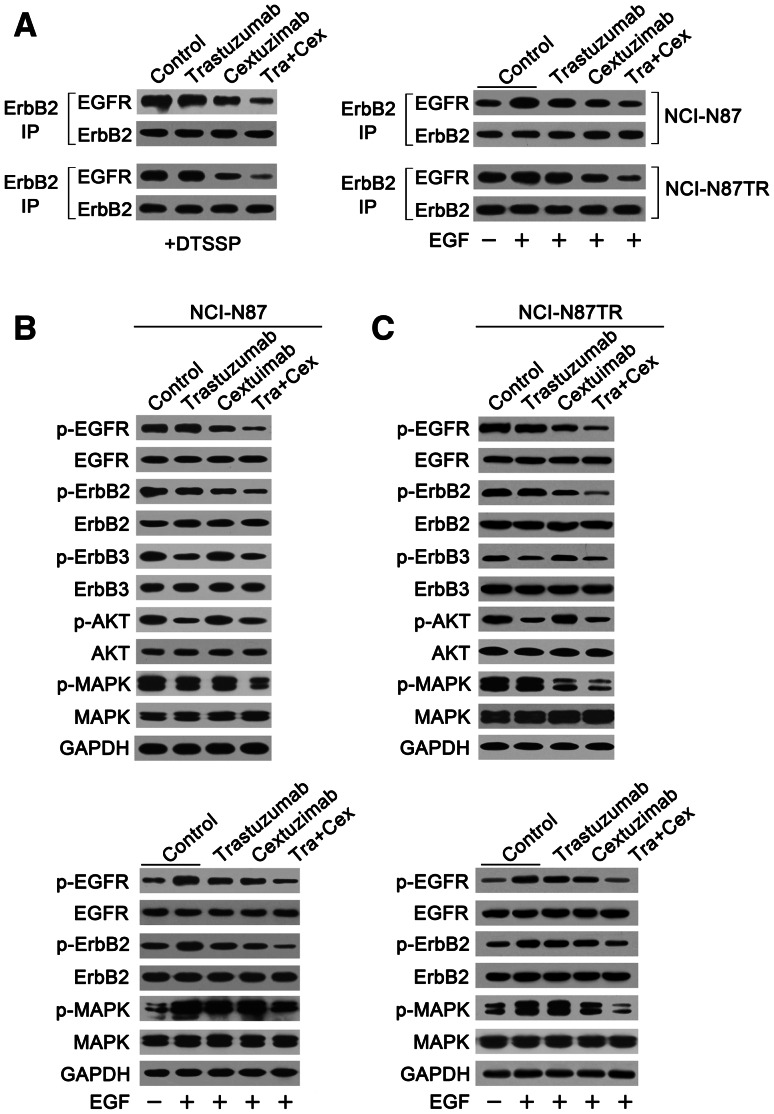
We investigated the capability of trastuzumab, cetuximab, or trastuzumab plus cetuximab to disrupt EGFR/ErbB2 heterodimerization in the NCI-N87 and NCI-N87TR cell lines. In both cell lines, trastuzumab was unable to disrupt ligand-independent EGFR/ErbB2 association (Fig. 2a), whereas cetuximab showed an evident decrease in EGFR/ErbB2 heterodimers (Fig. 2a). For EGF-induced EGFR/ErbB2 dimerization, trastuzumab or cetuximab alone weakly inhibited EGFR/ErbB2 dimerization, although cetuximab exhibited a stronger effect (Fig. 2a). However, the amount of EGFR/ErbB2 heterodimers in the NCI-N87TR cell line was still higher than the parental cells. Notably, the combination of trastuzumab and cetuximab was more effective at blocking ligand-independent or EGF-induced EGFR/ErbB2 heterodimerization compared with either mAb alone, and the combination caused a marked decrease in the amount of the EGFR/ErbB2 complex in both cell lines (Fig. 2a).
Fig. 2.
Combining trastuzumab and cetuximab effectively inhibits the formation of EGFR/ErbB2 heterodimers and activation in both NCI-N87 and NCI-N87TR gastric cancer cell lines. a Co-immunoprecipitation assay evaluating the ability of 10 μg/mL of control IgG, trastuzumab, cetuximab, or trastuzumab plus cetuximab to disrupt the ligand-independent and EGF-induced ErbB2/EGFR heterodimerization in NCI-N87 and NCI-N87TR cell lines. The data are representative of three independent experiments. b Immunoblots assessing ErbB signaling in the NCI-N87 and NCI-N87TR cell lines upon treatment with 10 μg/mL of control IgG, trastuzumab, cetuximab, or trastuzumab plus cetuximab in the absence of EGF. The data are representative of three independent experiments. c Immunoblots evaluating the effects of 10 μg/mL of control IgG, trastuzumab, cetuximab, or trastuzumab plus cetuximab pretreatment on EGF-activated ErbB signaling in NCI-N87 and NCI-N87TR cell lines. The data are representative of three independent experiments
Next, we investigated the inhibitory effects of trastuzumab or cetuximab alone, or the two mAbs in combination, on ErbB signaling pathways in the NCI-N87 and NCI-N87TR cell lines. Our results indicated that the ability of the mAbs alone or in combination to inhibit EGFR/ErbB2 signaling pathways corresponded with their capacity to inhibit EGFR/ErbB2 heterodimerization, under both basal and EGF-stimulated conditions (Fig. 2b, c.). Additionally, in the case of the ligand-independent heterodimerization, both of the trastuzumab-treated cell lines showed a slightly decreased phosphorylation of MAPK, indicating that trastuzumab could affect the ErbB2/ErbB2 or ErbB2/ErbB3 complexes, regardless of sensitivity or resistance to trastuzumab (Fig. 2b, c). Accordingly, adding cetuximab to trastuzumab led to a significant decrease in the phosphorylation of EGFR and MAPK in both the trastuzumab-sensitive and trastuzumab-resistant gastric cancer cell lines (Fig. 2b, c).
Combining trastuzumab and cetuximab potently inhibits the in vitro and in vivo growth of trastuzumab-resistant gastric cancer xenografts
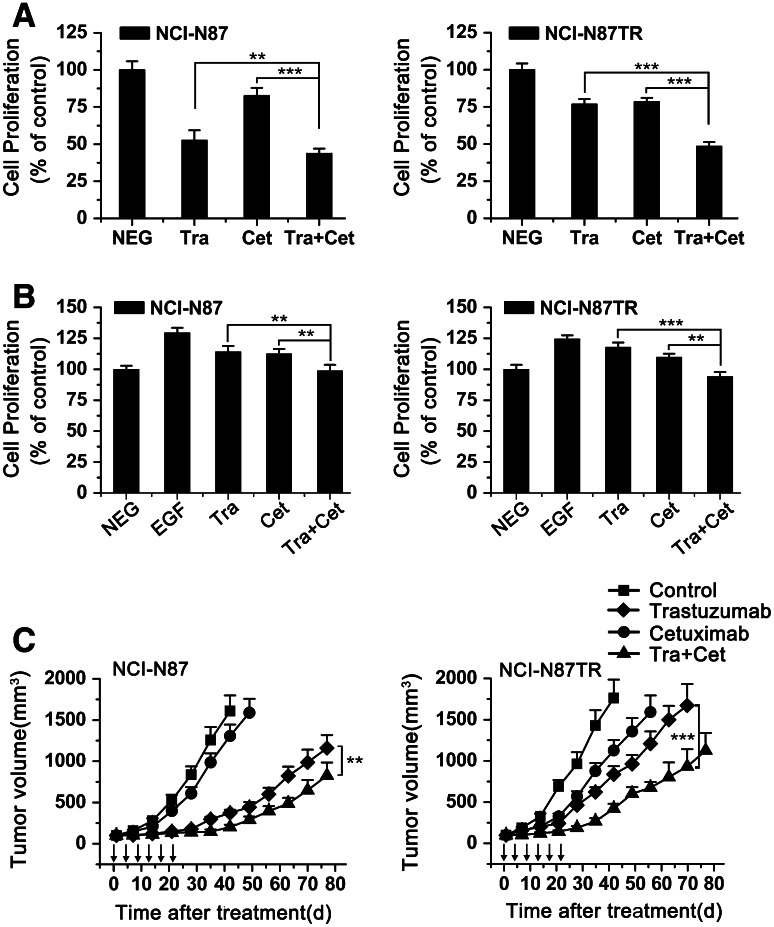
To examine whether over-enhanced EGFR/ErbB2 heterodimerization and activation is involved in the in vitro proliferation of NCI-N87TR, we evaluated the antitumor ability of trastuzumab or cetuximab alone or in combination for the NCI-N87 and NCI-N87TR cell lines. Under both the basal and EGF-stimulated conditions, trastuzumab was less efficient at inhibiting the NCI-N87TR cells, compared with parental cells, whereas cetuximab had only a minor effect (Fig. 3a, b). However, our data indicated that the antiproliferative activity of the combinatorial therapy was directly related to its ability to inhibit EGFR/ErbB2 heterodimerization and activation. In general, combining trastuzumab and cetuximab showed better antiproliferative activity than either mAb alone (Fig. 3a, b).
Fig. 3.
Combining trastuzumab and cetuximab potently suppresses the in vitro and in vivo growth of both trastuzumab-sensitive and trastuzumab-resistant gastric cancer cell lines. a MTS assay examining the effects of 10 μg/mL of control IgG, trastuzumab, cetuximab, or trastuzumab plus cetuximab on gastric cancer cell proliferation in the absence of EGF. The results are shown as percentage of control cell proliferation. Error bars, SD. **P < 0.001; ***P < 0.0001. The data are representative of three independent experiments. b MTS assay examining the effects of 10 μg/mL of control IgG, trastuzumab, cetuximab, or trastuzumab plus cetuximab on gastric cancer cell proliferation in the presence of EGF. The results are shown as percentage of control cell proliferation. Error bars, SD. **P < 0.001; ***P < 0.0001. The data are representative of three independent experiments. c Tumor volume of NCI-N87 or NCI-N87TR xenografts after treatment with control IgG (10 mg/kg), trastuzumab (10 mg/kg), cetuximab (10 mg/kg), or trastuzumab (10 mg/kg) plus cetuximab (10 mg/kg). The data are shown as the mean ± SEM. **P < 0.001; ***P < 0.0001, Mann–Whitney test
Next, to assess whether combining trastuzumab and cetuximab could be an effective strategy to overcome trastuzumab resistance in vivo, we examined the therapeutic efficacy of trastuzumab and cetuximab alone or in combination for nude mice bearing established NCI-N87 or NCI-N87TR tumor xenografts. As shown in Fig. 3c, in the NCI-N87 xenograft model trastuzumab led to a significant suppression of tumor growth and that suppression was better than cetuximab. In the NCI-N87TR tumor xenograft, both trastuzumab and cetuximab alone showed poor ability to inhibit the tumor growth. It is particularly noteworthy that combinatorial treatment with trastuzumab plus cetuximab was significantly more efficient in eliminating tumors, and most tumors were barely detectable 32 days after treatment (Fig. 3c). Collectively, these data indicate that the addition of cetuximab to trastuzumab sensitizes trastuzumab-resistant cell lines to trastuzumab, in vitro and in vivo.
Discussion
The molecular mechanisms underlying trastuzumab resistance in gastric cancer are not clear. Recent studies have highlighted that prolonged treatment with trastuzumab may induce tumor cells to reprogram themselves by various RTKs to develop alternative compensatory pathways to sustain cell proliferation, ultimately leading to trastuzumab resistance. In this study, we obtained a trastuzumab-resistant sub-line of the NCI-N87 gastric cancer cell line by modeling the development of acquired resistance in gastric cancer patients. Compared with the parental cell line, NCI-N87TR showed a notable increase in EGFR/ErbB2 heterodimers and signaling. Thus, the identification of the ‘EGFR/ErbB2 heterodimers and activation’ involved in the trastuzumab resistance mechanisms may help researchers to rationally develop new combinatorial therapies.
Previous studies have suggested that EGFR plays little or no role in driving the biology of ErbB2-overexpressing tumor cells. Consistent with this, our data indicated that trastuzumab effectively suppressed the growth of NCI-N87 cells, whereas cetuximab had only a minor effect. However, EGFR seems to play an important role in the trastuzumab-resistant NCI-N87 subline. In agreement with Narayan et al.’s study, our results indicated that the amounts of the EGFR and the EGFR/ErbB2 signaling pathways were remarkably up-regulated [7]. These results indicated a role for EGFR in compensating for resistance to trastuzumab, and suggested the basis for a novel combinatorial approach in clinical trials in gastric cancer. Furthermore, Narayan et al.’s results indicated that acquired trastuzumab resistance might be not synonymous with lack of responsiveness to trastuzumab and, importantly, suggested that trastuzumab priming might sensitize trastuzumab-resistant tumors to other EGFR family receptors-directed therapeutics [7]. Thus, the use of a combination of antibodies inhibiting EGFR/ErbB2 signaling pathways that confer trastuzumab resistance may have a potential clinical benefit.
Recently, trastuzumab in combination with cetuximab has proven useful in the treatment of carcinomas co-expressing EGFR and ErbB2, which our data support. Previously, Larbouret et al.’s study demonstrated that combing cetuximab and trastuzumab synergistically inhibited the survival of ErbB2low human pancreatic carcinoma xenografts, and thus showed superior EGFR/ErbB2 heterodimers-blocking activity over either mAb alone [14]. In agreement with this, we observed that the combination of these two antibodies has shown encouraging results in the trastuzumab-resistant NCI-N87 sub-line, both in vitro and in vivo. In addition, the NCI-N87TR cell line expressed higher levels of EGF than the parental cell line. The ineffectiveness of trastuzumab to block EGF-induced EGFR/ErbB2 heterodimerization and activation suggests that enhanced ligand-activated EGFR/ErbB2 signaling may be involved in trastuzumab resistance mechanisms. Due to the unique epitope of cetuximab, which overlaps substantially with the EGF binding site on domain III of EGFR, combinatorial therapy provides a more comprehensive blockade of EGFR/ErbB2 heterodimers and signaling, and results in greater antitumor activity in the NCI-N87TR tumor models. Thus, we conclude that combining trastuzumab and cetuximab may lead to a better therapeutic outcome for acquired trastuzumab-resistant ErbB2-overexpressing gastric cancer patients than either mAb alone.
In conclusion, this study identified enhanced EGFR/ErbB2 heterodimers and activation as an important step in the mechanisms of acquired trastuzumab resistance. Combining trastuzumab and cetuximab is effective in overcoming acquired trastuzumab resistance, suggesting that combinatorial therapy has the great potential to be translated to the clinic.
Acknowledgments
We thank Bohua Li for critical comments on the manuscript. This work was supported by the Hebei Medical science research program (20130345) from the Health Department of Hebei Province, and the technology support program (201202) from Harrison International Peace Hospital. The authors thank Ms. Yanchun Meng and Mr. Shi Hu for their technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Lei Zheng, Wenlong Tan and Jinrong Zhang have contributed equally to this work.
Contributor Information
Lei Zheng, Phone: +86-18-618370875, FAX: +86-18-903183657, Email: leinick@163.com.
Hongmei Liu, Phone: +86-18-618370875, FAX: +86-18-903183657, Email: hshylyz@163.com.
References
- 1.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 3.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, Elenius K, Isola J. Amplification of HER-2 in gastric carcinoma: association with topoisomerase II alpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 4.Matsubara J, Yamada Y, Nakajima TE, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74:76–83. doi: 10.1159/000139127. [DOI] [PubMed] [Google Scholar]
- 5.Hudis CA. Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 6.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009;69:2191–2194. doi: 10.1158/0008-5472.CAN-08-1056. [DOI] [PubMed] [Google Scholar]
- 8.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, Chin L, DePinho RA. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, Sahin AA, Esteva FJ, Hortobagyi GN, Yu D. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S–13S. [PubMed] [Google Scholar]
- 11.Schmitz KR, Ferguson KM. Interaction of antibodies with ErbB receptor extracellular regions. Exp Cell Res. 2009;315:659–670. doi: 10.1016/j.yexcr.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel D, Bassi R, Hooper A, Prewett M, Hicklin DJ, Kang X. Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int J Oncol. 2009;34:25–32. [PubMed] [Google Scholar]
- 13.Kondo N, Tsukuda M, Sakakibara A, Takahashi H, Hyakusoku H, Komatsu M, Niho T, Nakazaki K, Toth G. Combined molecular targeted drug therapy for EGFR and HER-2 in head and neck squamous cell carcinoma cell lines. Int J Oncol. 2012;40:1805–1812. doi: 10.3892/ijo.2012.1376. [DOI] [PubMed] [Google Scholar]
- 14.Larbouret C, Gaborit N, Chardès T, Coelho M, Campigna E, Bascoul-Mollevi C, Mach JP, Azria D, Robert B, Pèlegrin A. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatmentwith trastuzumab/erlotinib or lapatinib alone implication of receptors’ down-regulation and dimers’ disruption. Neoplasia. 2012;14:121–130. doi: 10.1593/neo.111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3 K complex is disrupted by trastuzumab and is effectively inhibited by thePI3 K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]