Abstract
Osteosarcoma and Ewing’s sarcoma tumor cells are susceptible to IL15-induced or antibody-mediated cytolytic activity of NK cells in short-term cytotoxicity assays. When encountering the tumor environment in vivo, NK cells may be in contact with tumor cells for a prolonged time period. We explored whether a prolonged interaction with sarcoma cells can modulate the activation and cytotoxic activity of NK cells. The 40 h coculture of NK cells with sarcoma cells reversibly interfered with the IL15-induced expression of NKG2D, DNAM-1 and NKp30 and inhibited the cytolytic activity of NK cells. The inhibitory effects on receptor expression required physical contact between NK cells and sarcoma cells and were independent of TGF-β. Five days pre-incubation of NK cells with IL15 prevented the down-regulation of NKG2D and cytolytic activity in subsequent cocultures with sarcoma cells. NK cell FcγRIIIa/CD16 receptor expression and antibody-mediated cytotoxicity were not affected after the coculture. Inhibition of NK cell cytotoxicity was directly linked to the down-regulation of the respective NK cell-activating receptors. Our data demonstrate that the inhibitory effects of sarcoma cells on the cytolytic activity of NK cells do not affect the antibody-dependent cytotoxicity and can be prevented by pre-activation of NK cells with IL15. Thus, the combination of cytokine-activated NK cells and monoclonal antibody therapy may be required to improve tumor targeting and NK cell functionality in the tumor environment.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1406-x) contains supplementary material, which is available to authorized users.
Keywords: NK cell, NKG2D, ADCC, IL15, Sarcoma
Introduction
Osteosarcoma and Ewing’s sarcoma represent the most frequent osseous, malignant tumors in adolescents and young adults. The current treatment consists of a combination of systemic multi-drug chemotherapy and surgical resection [1–3]. Up to 70 % of patients with localized disease achieve persistent remission. In contrast, the prognosis of patients with metastasized and recurrent disease has remained dismal, despite advancements in surgical techniques and intensification of chemotherapy regimens during the last decades. In the quest for novel cytolytic therapies, we have previously reported the sensitivity of sarcoma cells to NK cell-based cellular immunotherapy [4–7].
Human NK cells express a broad repertoire of germline-encoded inhibitory and activating receptors [8–10]. The proportion of inhibiting and activating signals perceived from target cells tunes the cytotoxic activity of NK cells. The inhibitory ‘killer cell immunoglobulin-like receptors’ (KIR) recognize HLA-A, B, C alleles of the MHC class I complex [11], whereas the NKG2A/CD94 complex binds HLA-E [12]. The activating receptor NKG2D recognizes the stress ligands MHC class I chain-related protein (MIC) A, MICB and UL-binding proteins (ULBP) 1 to ULBP6 [13–17], and DNAM-1 binds to CD112 and CD155 [9, 18]. B7-H6 has recently been identified as a ligand for the ‘natural cytotoxicity receptors’ (NCR) NKp30 [19], whereas ligands for NKp44 and NKp46 are insufficiently characterized on tumor cells. Loss of MHC class I expression and ligation of NK cell-activating receptors increase the sensitivity of tumor cells to NK cell-mediated lysis [8–10]. In addition to the properties of target cells, NK cell cytotoxicity can be ‘primed’ by cytokines, such as IL2 and IL15 [20–22].
Apart from killing tumor cell lines in vitro, NK cells have been shown to be involved in the rejection of transplanted hematopoietic tumors or chemically induced tumors in mice, preventing tumor outgrowth and supporting tumor-specific T cell responses [23–26]. A role of NK cells in anticancer responses in humans has been emphasized by the observation that patients with leukemia have a better outcome when receiving KIR ligand-mismatched allogeneic stem cell transplantations [27]. However, tumor cells may acquire diverse mechanisms to evade NK cell responses. Shedding of NKG2D ligands [28, 29], down-regulation of NKG2D and DNAM-1 surface expression [30–33] by sustained ligand–receptor interactions, and secretion of TGF-β [34, 35] have been associated with defective NK cell functions, hampering tumor cell recognition and killing.
We and others have established that ligands for the NK cell-activating receptors NKG2D and DNAM-1 are differentially expressed in sarcoma tissue and on sarcoma cell lines. Sarcoma cell lysis by NK cells involves NKG2D and DNAM-1 as measured in short-term cytotoxicity assays [4, 5, 7]. In vivo, however, NK cells may be in contact with tumor cells over a long-time period which may alter the cytotoxic function of NK cells. Indeed, we have previously observed that peripheral blood NK cells from Ewing’s sarcoma patients exerted low cytolytic activity [7]. In the present study, we demonstrate that prolonged interactions with sarcoma cells can inhibit the cytotoxic activity of NK cells as well as NK cells receptor expression. Remarkably, IL15-activated NK cells and antibody-dependent cytotoxicity by NK cells were resistant to this inhibition. Therefore, the combination of IL15-activated NK cells and monoclonal antibodies may sustain anti-tumor functions of NK cell-based immunotherapy in sarcoma patients.
Materials and methods
Cell lines
The following previously characterized sarcoma cells were included in this study: Ewing’s sarcoma cell lines A673, CADO-ES, SK-ES-1, SK-N-MC, STA-ET2.1, TC71 and L1062; osteosarcoma cell lines HOS, OHS, OSA, SAOS-2 and U2OS. All sarcoma cell lines were obtained from the EuroBoNeT cell line repository (by 2007) and were confirmed for their identity by short tandem repeat DNA fingerprinting in 2011. The TC71 cell line was cultured in IMDM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal calf serum (FCS, Invitrogen) and 100 U/ml penicillin and 100 ug/ml streptomycin (Invitrogen); all other sarcoma cell lines as well as the erythroleukemic cell line K562, the Burkitt’s lymphoma cell line Daudi and the murine mastocytoma cell line P815 (ATCC, Manassas, VA, USA) were maintained in RPMI 1640 supplemented with 10 % FCS and penicillin/streptomycin. All Ewing’s sarcoma cell lines were grown in 0.1 % gelatin-coated culture flasks. All cell lines were negative for mycoplasma infection as regularly tested by RT-PCR.
T cell depletion and NK cell isolation
PBMC were separated from buffy coats of healthy adult donors (Sanquin Blood bank, Region Southwest, Rotterdam, the Netherlands) by Ficoll–Hypaque density gradient centrifugation. T cells were depleted from PBMC (TCD PBMC) by positive selection using anti-CD3 MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany) to exclude NK cell activation via T cell-derived IL2 due to potential alloreactivity toward sarcoma cells. TCD PBMC contained <0.5 % of contaminating CD3+ T cells as analyzed by flow cytometry. NK cells were purified from PBMC by negative selection, depleting non-NK cells through a combination of biotin-conjugated monoclonal anti-human antibodies and MicroBeads using the ‘Human NK cell Isolation Kit’ (Miltenyi Biotech); NK cell purity was >95 % as determined by flow cytometry, analyzing NK cells as CD56+, CD3−, CD14− and CD19− cells. IL15-activated NK cells were obtained by incubating purified NK cells for 5 days with 10 ng/ml of IL15 (Peprotech, Rocky Hill, NJ, USA) in RPMI medium (with 10 % FCS and penicillin/streptomycin) in 24-well tissue culture plates.
Cocultures
Sarcoma cell lines were seeded in 24-well plates in 1 ml of RPMI medium and incubated for 18 h. According to cell size and growth rate, the cell lines were seeded at the following cell numbers per well, allowing sub-confluent cell densities at the start of cocultures: TC71 and HOS at 50,000; OHS, OSA, SAOS-2 and U2OS at 75,000; A673, CADO-ES and L1062 at 80,000; SK-ES-1 and STA-ET2.1 at 150,000; SK-N-MC at 200,000. Cocultures were assembled by carefully adding 1 × 106 TCD PBMC, 1.5 × 105 purified NK cells or 5 days IL15-activated NK cells to the attached sarcoma cells, achieving NK cell-tumor cell ratios of 1:1–3:1 according to the tumor cell numbers seeded. In trans-well experiments, TCD PBMC (upper compartment) were separated from STA-ET2.1 or TC71 cells (lower compartment) by a porous membrane (0.4-μm pore size). Cocultures were incubated for approximately 40 h with or without IL15. The following TGF-β inhibitors were applied: TGF-β-neutralizing antibody (10 μg/ml; anti-TGFβ1/TGFβ2/TGFβ3; clone 1D11; R&D Systems, Minneapolis, MN, USA), ALK (TGF-βRI kinase) small molecule inhibitor SB-431542 (10 μM, kindly provided by Dr. Luuk J.A.C. Hawinkels, Department of Molecular Cell Biology, Leiden University Medical Center, Leiden, Netherlands) or recombinant human latency-associated peptide (LAP; 250 ng/ml; R&D Systems). As controls, TCD PBMC or purified NK cells were incubated in the absence of sarcoma cells with or without IL15, recombinant TGF-β (1 ng/ml; R&D Systems) or TGF-β inhibitors. Subsequently, non-attached cell populations were harvested to analyze the expression of NK cell activation markers by flow cytometry and the cytolytic activity of NK cells in a 4 h 51chromium (51Cr) release assay.
Flow cytometry
Determination of NK cell percentages in TCD PBMC, validation of NK cell purity and T cell depletion, and the expression of NK cell activation markers were analyzed phenotypically with fluorescently labeled antibodies and flow cytometry. The following mouse anti-human monoclonal antibodies were applied according to the manufacturer’s instructions: HLA-GFITC (MEM-G/9) (Abcam, Cambridge, UK); CD3FITC (SK7), CD3PerCP-Cy5.5 (SK7), CD14PerCP-Cy5.5 (M5E2), CD69PE (L78), CD69FITC (L78), DNAM-1FITC (DX11), MICA/BPE (6D4), PD-1 (CD279, MIH4), goat anti-mouse IgAPC (550826) (BD Biosciences, Franklin Lakes, NJ, USA); HLA-EPE (3D12), PD-1L (CD274, MIH1) (eBiosciences, San Diego, CA, USA); CD16FITC (3G8), CD56APC (N901 NKH1), CD112PE (R2.477.1), CD155 (PV404.10), NKG2APE (Z199), NKG2DPE (ON72), NKp30PE (Z25), NKp44PE (Z231), NKp46PE (BAB281) (IOTEST Immunotech, Marseille, France); MICA (159227), MICB (236511), ULBP1 (170818), ULBP2 (165903), ULBP3 (166510) (R&D Systems); perforinFITC (deltaG9, Ancell, Bayport, MN, USA). To analyze ligands for NKG2D, NKp30 and NKp44 on sarcoma cells, freshly harvested tumor cells were incubated with the respective Fc fusion protein constructs for 2 h (2.5 μg/ml; R&D Systems, Minneapolis, MN, USA) followed by the Alexa Fluor 647 goat anti-human IgG secondary antibody (A21445; Invitrogen). The anti-CD20 mAb rituximab (MabThera; Roche, Basel, Switzerland) was used as an IgG1 isotype-matched negative control for fusion proteins. FACS measurements were performed with the FACSCalibur (BD Biosciences) and analyzed with the ‘BD Cell Quest ProTM’ software (version 5.2.1). The indicated fold expression data were calculated by dividing the geometric mean fluorescence intensity after the 40 h cocultures by the geometric mean fluorescence intensity after 40 h incubation in medium or IL15 only (as set to 1.00).
51Chromium release assay
The cytolytic activity of NK cells in TCD PBMC or purified NK cells against K562 (sensitive to unstimulated and activated NK cells), Daudi (sensitive to activated NK cells only) and sarcoma cell lines was measured in 4 h 51Cr release assays. Effector cells were unstimulated non-cocultured TCD PBMC or purified NK cells, or the non-adherent cell fraction from the 40 h cocultures. For blocking experiments, effector cells were incubated with 10 μg/ml of mouse anti-human NKG2D (149810, R&D Systems), DNAM-1 (DX11, BD Biosciences) and NKp30 (P30-15, Biolegend, San Diego, CA, USA) for 30 min. Target cells were labeled with 100 μl Na-chromate (51Cr, 3.7 MBq) for 1 h. To measure antibody-dependent cellular cytotoxicity (ADCC), sarcoma cells were incubated with the chimeric monoclonal antibody cetuximab (anti-epidermal growth factor receptor, 10 μg/ml; Erbitux®, Merck KGaA, Darmstadt, Germany) for 30 min. To measure redirected lysis, murine FcγR+ P815 cells were incubated with 1 μg/ml of mouse anti-human CD16FITC (3G8, 1/100, IOTEST Immunotech), DNAM-1 (DX11, BD Biosciences), NKG2D (149810, R&D Systems), NKp30 (P30-15, Biolegend) and NKp46 (9E2, Biolegend) for 30 min. 2.5 × 103 target cells ± antibodies were added to effector cells in triplicate or duplicate at the indicated effector / target (E:T) ratios and incubated for 4 h at 37 °C. Supernatants were collected, and the release of 51Cr was measured with a β-counter (Wallac/PerkinElmer, Waltham, MA, USA). Spontaneous and total release were obtained by incubation in medium and Triton X-100 (2.5 %; Merck Chemicals, Darmstadt, Germany), respectively. The specific lysis was calculated by the following formula: percentage of specific lysis = 100 × (experimental release-spontaneous release/total release-spontaneous release).
Statistical analysis
Statistical analyses were performed with Graphpad Prism version 5.04 (La Jolla, CA, USA) or SPSS version 16.0 (IBM, Armonk, NJ, USA) using paired Student’s t-test, comparing means between groups of samples. Error bars represent the standard error of the mean. A P value of <0.05 was considered statistically significant. ns, not statistically significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Sarcoma cells can interfere with NK cell receptor expression and cytolysis
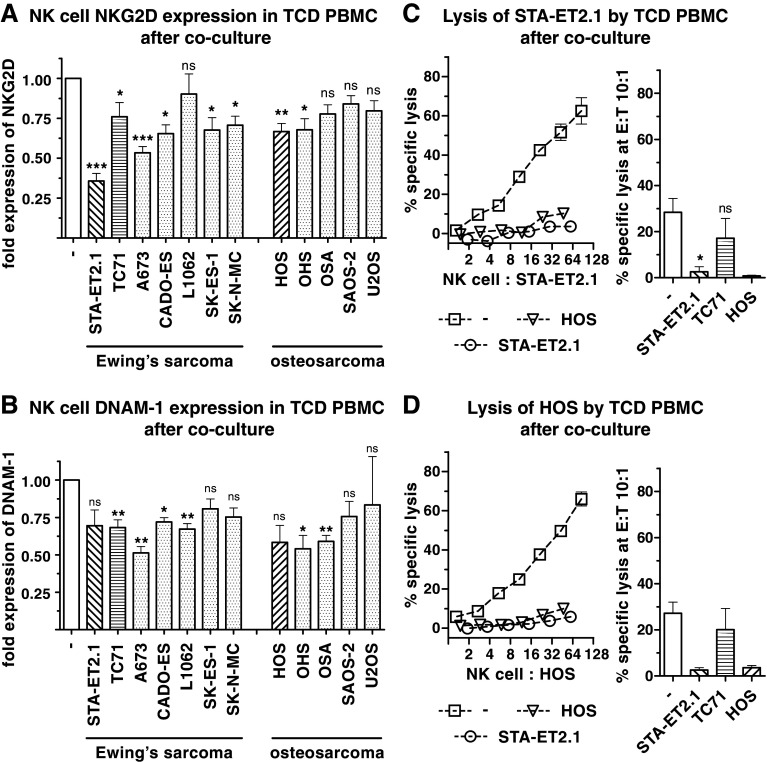
To explore whether sarcoma cell lines are able to influence NK cell function, T cell-depleted PBMC (TCD PBMC) were cocultured with osteosarcoma and Ewing’s sarcoma cell lines for 40 h. After the coculture, NKG2D and DNAM-1 were down-regulated on resting NK cells by most of the cell lines (8/12 and 6/12, respectively) (Fig. 1, panel a, b). Four cell lines significantly down-regulated both NKG2D and DNAM-1 expression.
Fig. 1.
Down-regulation of NK cell NKG2D, DNAM-1 and cytolytic activity by sarcoma cells. Surface expression of NKG2D (a) and DNAM-1 (b) on NK cells was analyzed after 40 h cocultures of TCD PBMC with the indicated Ewing’s sarcoma and osteosarcoma cell lines by flow cytometry. The indicated fold expression was calculated by dividing the geometric mean fluorescence intensity after 40 h cocultures by the geometric mean fluorescence intensity after 40 h incubation in medium only (as set to 1.00). Within the lymphocyte population of life-gated TCD PBMC, NK cells were identified as CD56+ cells devoid of CD3 and CD14 expression. Diagonal- and horizontal-lined patterns in bars indicate the cocultures mainly focused on in the following experiments (relatively strong inhibitors of NKG2D, STA-ET2.1 and HOS; relatively weak inhibitor, TC71). Combined data of the fold expression of multiple experiments are depicted. Lysis of (non-cocultured) STA-ET2.1 (c) and HOS cells (d) by NK cells after the 40 h coculture of TCD PBMC with STA-ET2.1, TC71 and HOS cells or 40 h incubation in medium only was measured in 4 h 51Cr release assays. The specific lysis (%) is depicted as representative data of one experiment and as combined data of two or multiple experiments analyzed at a 10:1 E:T ratio. ns, not statistically significant; *P < 0.05; **P < 0.01; ***P < 0.001
In addition to the phenotypical changes, it was investigated whether sarcoma cells can affect the cytolytic potential of resting NK cells. TCD PBMC were removed from cocultures with STA-ET2.1, HOS (relatively strong inhibitors of NKG2D expression) and TC71 cells (relatively weak inhibitor of NKG2D). Afterward, NK cell-mediated cytolytic activity was tested against non-cocultured sarcoma and non-sarcoma target cells in 4 h 51Cr release assays. The 40 h coculture of TCD PBMC with STA-ET2.1 and HOS cells substantially reduced the cytolytic activity against (non-cocultured) STA-ET2.1 and HOS target cells (Fig. 1, panel c, d). Likewise, lysis of the NK cell target cell line K562 was decreased after these cocultures (Supplementary Fig. 1, panel a, available online), indicating that there was no sarcoma cell-specific inhibitory effect. In contrast, coculture with TC71 cells had little effect on NK cell cytolysis.
Hence, sarcoma cell lines differed in their efficacy to down-regulate the cytolytic activity of NK cells as well as the expression of the NK cell-activating receptors NKG2D and DNAM-1.
IL15-induced NK cell activation is impaired by sarcoma cells
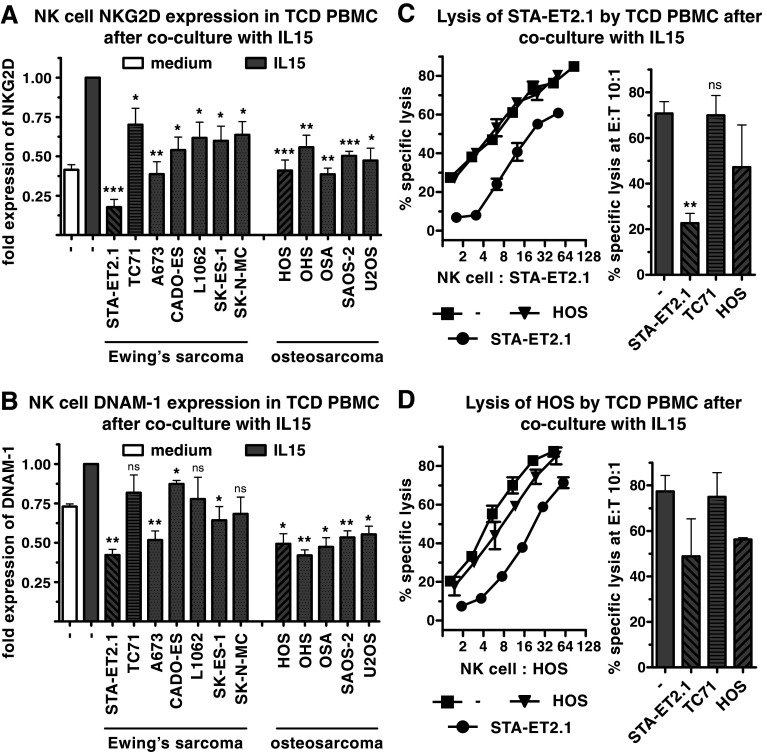
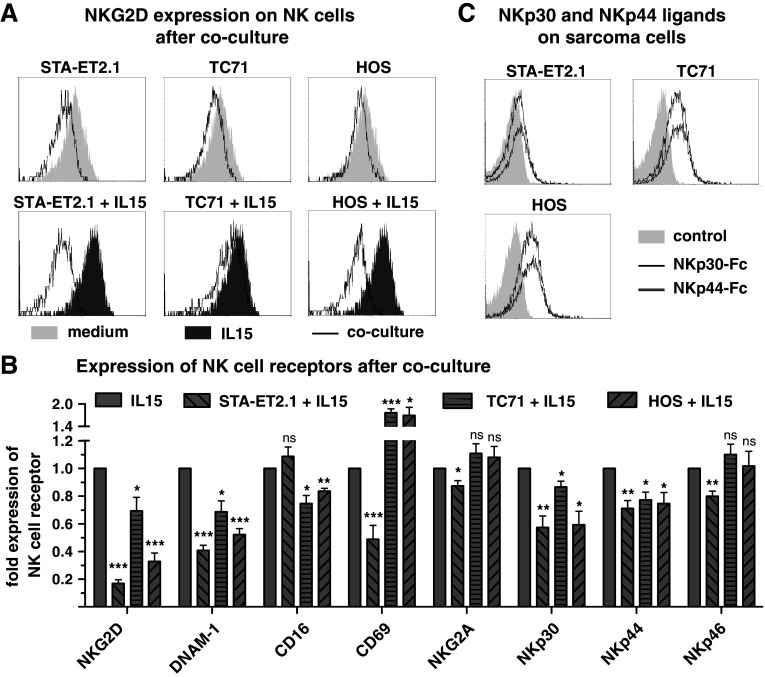
IL15 is a potent NK cell-activating cytokine which enhances the expression of NKG2D and DNAM-1 on NK cells and boosts their cytolytic activity against sarcoma cells [4, 7]. It was investigated whether IL15-induced activation of NK cells can also be inhibited by sarcoma cells. When cocultured with TCD PBMC in the presence of IL15, sarcoma cell lines significantly interfered with the IL15-induced up-regulation of NKG2D (12/12) and DNAM-1 (9/12) on NK cells (Figs. 2, panel a, b and 3, panel a). In addition, STA-ET2.1 cells inhibited the IL15-induced expression of CD69, NKp30, NKp44 and NKp46, whereas HOS and TC71 cells inhibited only NKp30 and NKp44 (Fig. 3, panel b).
Fig. 2.
Down-regulation of IL15-induced NK cell cytolytic activity and NKG2D and DNAM-1 expression by sarcoma cells. Surface expression of NKG2D (a) and DNAM-1 (b) on NK cells was analyzed after IL15-stimulated cocultures of TCD PBMC with Ewing’s sarcoma and osteosarcoma cell lines by flow cytometry. The indicated fold expression is combined data of multiple experiments, comparing expression levels of IL15-stimulated 40 h cocultures with expression levels after incubation with IL15 only. Gray bars depict TCD PBMC incubated with IL15 only (no pattern) or IL15-stimulated cocultures (diagonal- and horizontal- lined patterns with STA-ET2.1, HOS or TC71 cells), and white bars depict TCD PBMC incubated in medium only. The expression of NKG2D after IL15 stimulation only was set to 1.00. Lysis of STA-ET2.1 (c) and HOS cells (d) by NK cells after IL15-stimulated coculture of TCD PBMC with sarcoma cells or incubation in IL15 only. Representative data of one experiment and combined data of two or multiple experiments analyzed at a 10:1 E:T ratio are depicted
Fig. 3.
NKp30 and NKp44 down-regulation on NK cells is not associated with NKp30 and NKp44 ligands expression on sarcoma cells. a Representative FACS overlay plots of the geometric mean fluorescent intensity of NKG2D expression on NK cells after 40 h incubation of TCD PBMC with medium (light gray area) and with IL15 (dark gray area) and after 40 h coculture (black solid line) with the sarcoma cell lines STA-ET2.1 (left panel), TC71 (middle panel) and HOS (right panel) in the presence or absence of IL15. b Expression of the NK cell receptors NKG2D, DNAM–1, FcγRIIIa/CD16, NKG2A, NKp30, NKp44 and NKp46 and of the NK cell activation marker CD69 was investigated after the coculture of TCD PBMC with STA-ET2.1, TC71 or HOS cells in the presence of IL15. Fold expression data were calculated from geometric mean fluorescence intensities and combined from multiple experiments. c FACS overlay plots, detecting NKp30 ligands by NKp30-Fc fusion protein (black solid line), NKp44 ligand by NKp44-Fc fusion protein (gray solid line) and isotype-matched negative control by the anti-CD20 mAb rituximab (light gray area), all followed by secondary antibody, on STA-ET2.1, TC71 and HOS cells
Stimulation of TCD PBMC with IL15 for 40 h enhanced the lysis of non-cocultured STA-ET2.1 and HOS target cells (Fig. 2, panel c, d). The IL15-induced lysis of both cell lines was reduced after the coculture with STA-ET2.1 cells, while the cocultures with HOS or TC71 had little or no effect. Furthermore, the IL15-induced lysis of Daudi cells, which are only susceptible to activated NK cells, was greatly diminished after the coculture with STA-ET2.1 cells (Supplementary Fig. 1, panel b, available online).
These results indicate that sarcoma cells could also interfere with NK cell function in the presence of the activating cytokine IL15.
NKG2D down-regulation on NK cells requires direct contact with sarcoma cells and is independent of TGF-β
The mechanism responsible for NK cell receptor down-regulation by certain sarcoma cells could be based on sustained ligand–receptor interactions. Although the STA-ET2.1 cell line had the strongest inhibitory effect on NK cell NKG2D and DNAM-1 expression, the NKG2D ligands MICA, ULBP1 and ULPB3 were hardly detected on STA-ET2.1 cells, while MICB, ULBP2, the NKG2D-Fc fusion construct and the DNAM-1 ligands CD112 and CD155 were detected at lower intensities as compared to other sarcoma cell lines (Supplementary Fig. 2, available online). Notably, the STA-ET2.1 cell line down-regulated NKp30 and NKp44 expression on NK cells, but ligands for NKp30 and NKp44 were barely detected on STA-ET2.1 cells by Fc fusion constructs as compared to TC71 and HOS cells (Fig. 3, panel c). Ligands for NKp30 and NKp44 were only expressed on a few Ewing’s sarcoma and osteosarcoma cell lines (Supplementary Table 1, available online). Overall, when comparing surface densities of NKG2D, DNAM-1, NKp30 and NKp44 ligands on the various sarcoma cell lines, differences in surface densities and expression profiles did not correspond to the level of NKG2D, DNAM-1, NKp30 or NKp44 receptor down-regulation by the respective cell lines (Supplementary Table 1, available online). Thus, variability in ligand surface densities alone did not explain the differences among sarcoma cell lines in down-regulating NK cell receptors. In addition, despite the strong inhibitory effect of STA-ET2.1 cells, expression levels of the known NK cell-inhibitory surface ligands HLA-E, HLA-G and PD-1L were not particularly high on STA-ET2.1 cells (Supplementary Fig. 2, available online). PD-1 expression was not specifically increased on NK cells after coculture with STA-ET2.1 cells (Supplementary Fig. 3, panel a, available online).
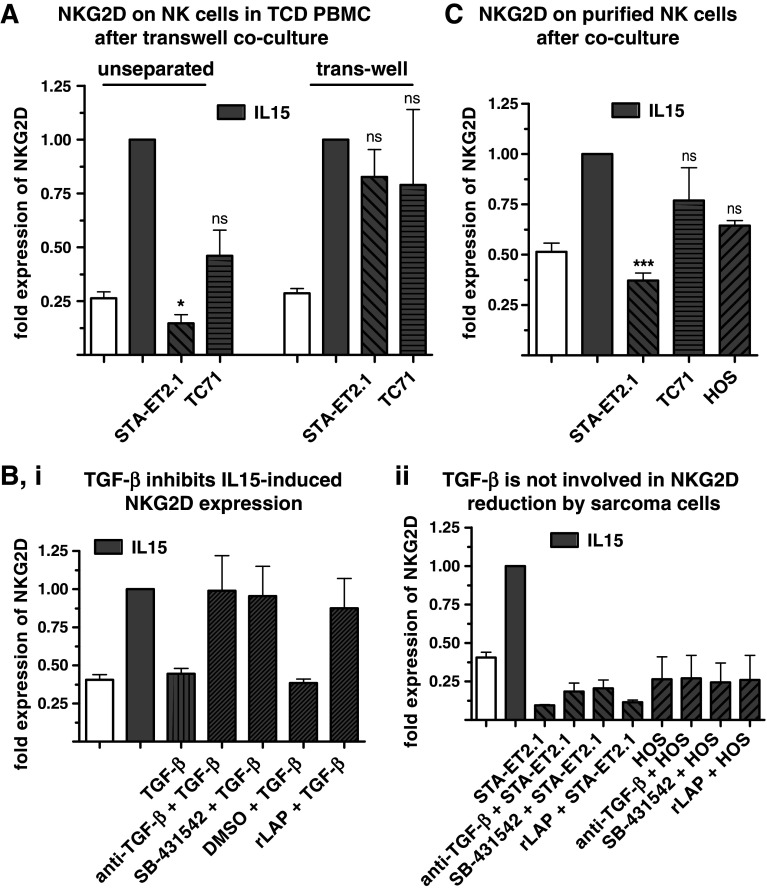
To investigate the role of soluble factors and/or cell–cell contact in the down-regulation of the NKG2D receptor, it was tested whether cell-free supernatants of long-term, high-density cultures of STA-ET2.1, TC71 and HOS cells influenced NK cell NKG2D and DNAM-1 expression. Incubation of NK cells with supernatant of sarcoma cell cultures did not alter NKG2D or DNAM-1 expression on NK cells (Supplementary Fig. 1, panel c, available online). When STA-ET2.1 or TC71 cells (lower compartment) were separated from TCD PBMC (upper compartment) in trans-well cultures, both cell lines failed to interfere with IL15-induced NKG2D expression on NK cells (Fig. 4, panel a). Thus, cell–cell contact was either required directly or because it resulted in the production of inhibitory soluble factors mediating NKG2D down-regulation. Incubation of TCD PBMC with supernatant of cocultures of TCD PBMC and STA-ET2.1 or TC71 cells did also not down-regulate NKG2D expression on NK cells (Supplementary Fig. 1, panel c, available online). Altogether, these data indicate that the down-regulation of NKG2D and DNAM-1 was mediated by close cell–cell contact between sarcoma cells and NK cells and not by soluble mediators derived from sarcoma cell lines or produced after cell–cell contact during the cocultures.
Fig. 4.
Down-regulation of NKG2D on NK cells requires cell–cell contact and is independent of TGF-β. a TCD PBMC (upper compartment) were separated from sarcoma cells (lower compartment) by a porous membrane (0.4 μm) in trans-well experiments. Surface expression of NKG2D on NK cells was investigated after the unseparated coculture and the trans-well coculture, both in the presence of IL15 and incubated for 40 h. Data are combined from multiple experiments. b (i) NKG2D expression on NK cells was investigated after incubating TCD PBMC with IL15 in the presence or absence of recombinant TGF-β and TGF-β inhibitors (TGF-β-neutralizing antibody, SB-431542 and recombinant LAP). Data are combined from two experiments. (ii) NKG2D expression on NK cells in TCD PBMC was analyzed after the IL15-stimulated coculture with sarcoma cells in the presence or absence of TGF-β inhibitors. Data are combined from two experiments. c Surface expression of NKG2D on NK cells was measured after coculture of purified NK cells with sarcoma cells in the presence of IL15. Data are combined from multiple experiments. In all panels, IL15-stimulated samples are indicated by gray bars, white bars depict control incubations in medium only. Expression of NKG2D after IL15 stimulation was set to 1.00
In previous studies, membrane-associated TGF-β has been described to interfere with the activation of NK cells in a cell–cell contact dependent-manner [36–38]. Therefore, it was investigated whether neutralization of TGF-β can abolish the inhibitory effect of sarcoma cells. The inhibition of IL15-induced up-regulation of NKG2D on NK cells by recombinant TGF-β could be prevented by the addition of a TGF-β-neutralizing antibody, the small molecule inhibitor SB-431542 (TGF-βRI kinase inhibitor) or recombinant LAP (inactivator of TGF-β). In contrast, addition of the TGF-β inhibitors to the cocultures of TCD PBMC with STA-ET2.1 or HOS cells did not prevent the interference with the IL15-induced NKG2D expression on NK cells, suggesting that TGF-β did not play a role in the inhibitory effects of the sarcoma cell lines (Fig. 4, panel b, ii).
Since until now all experiments have been performed using TCD PBMC, sarcoma cell-dependent inhibition of NK cells could have been indirect and mediated by non-NK, non-T cell populations in PBMC. Therefore, NK cells were purified and cocultured with sarcoma cells. Similar to NK cells in TCD PBMC, also on purified NK cells, the IL15-induced up-regulation of NKG2D was inhibited after the coculture with, in particular, STA-ET2.1 cells (Fig. 4, panel c). Consequently, sarcoma cells could influence NK cells by direct cell–cell interaction without requiring the presence of other PBMC populations.
Pre-activation of NK cells with IL15 prevents NK cell inhibition
To explore whether the inhibitory effect of sarcoma cells resulted in irreversible alterations in NK cells, TCD PBMC were removed from STA–ET2.1 cells after the 40 h coculture and subsequently incubated with IL15 alone. The down-regulated expression of NKG2D and DNAM-1 expression after the 40 h coculture was restored when the TCD PBMC were incubated for three additional days with IL15 in the absence of STA-ET2.1 cells (Fig. 5, panel a and data not shown). NK cell NKG2D and DNAM-1 expression remained inhibited when STA-ET2.1 cells were present during the additional 3 days of incubation with IL15 (Fig. 5, panel a).
Fig. 5.
NKG2D expression and cytotoxic activity of 5 days (d) IL15-stimulated NK cells are not inhibited by sarcoma cells. a After the IL15-stimulated 40 h coculture (second bar), TCD PBMC were removed from STA-ET2.1 cells, as indicated by the arrows, and subsequently incubated with IL15 for three additional days in the presence (fourth bar) or absence of fresh STA-ET2.1 cells (fifth bar). NKG2D expression on NK cells was analyzed after the 40 h coculture and after the 40 h plus 3 days (d) coculture. Data are combined from multiple experiments. b Purified NK cells were activated with IL15 for 5 days and afterward cocultured with sarcoma cells for 40 h (gray bars, IL15 stimulated samples). Data of NKG2D expression are combined from multiple experiments. c Lysis of non-cocultured STA-ET2.1 and HOS cells after the coculture of 5 days IL15-activated NK cells with sarcoma cells. Data are analyzed at a 10:1 E:T ratio and combined from three to two experiments, respectively
Next, it was investigated whether NK cells activated with IL15 prior to the coculture are still susceptible to sarcoma cell-induced inhibition. Remarkably, when using 5 days pre-activated NK cells, neither NKG2D expression nor the cytolytic activity of the NK cells was altered by subsequent 40 h cocultures with sarcoma cells (Fig. 5, panel b, c).
Overall, the sarcoma cell-induced inhibitory effect on NK cell activation was reversible and could be prevented by pre-activation of NK cells with IL15.
Antibody-dependent cytotoxicity by NK cells is not affected by sarcoma cells
In the previous experiments, the coculture with the STA-ET2.1 cell line significantly down-regulated NKG2D, DNAM-1 and NKp30 expression as well as the cytolytic activity against sarcoma cell lines. These receptors play an important role in sarcoma cell lysis by NK cells as illustrated in 51Cr release assays performed in the presence of specific neutralizing antibodies against the respective receptors. Lysis of STA-ET2.1, TC71 and HOS cells by resting NK cells was significantly reduced by antibodies against DNAM-1 and fully inhibited by combined blocking of DNAM-1 and NKG2D while lysis was only partly dependent on NKG2D alone (Supplementary Fig. 1, panel d, available online). Lysis by IL15-activated NK cells could only be significantly reduced by the combination of DNAM-1 and NKG2D antibodies and was fully inhibited by the addition of NKp30 antibodies.
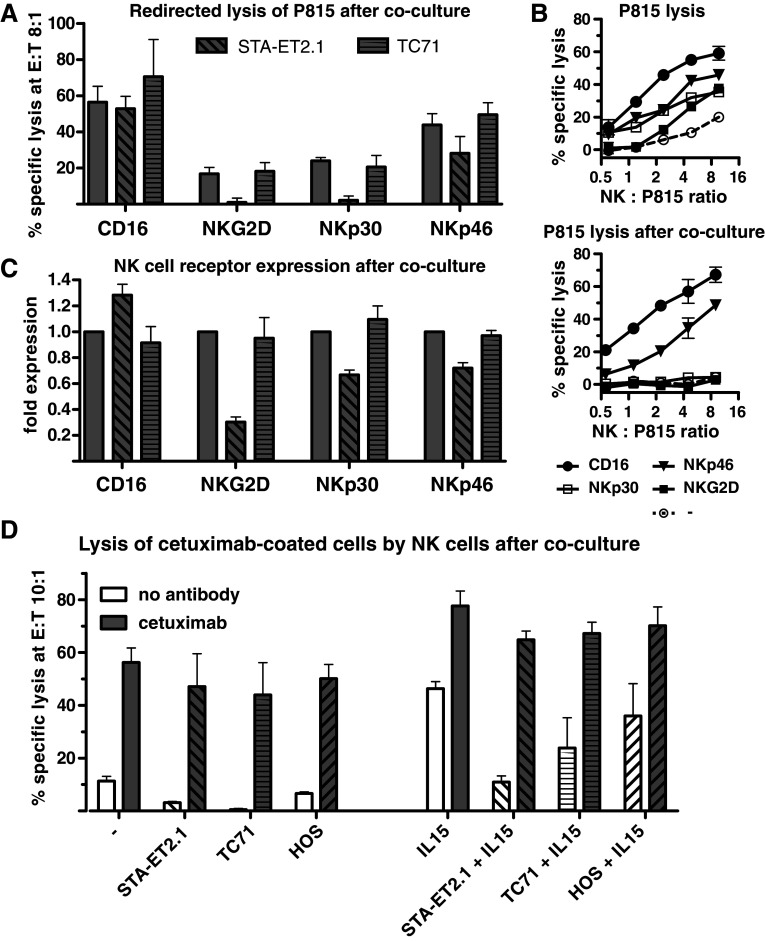
Therefore, down-regulation of these NK cell receptors may be an explanation for the reduced cytolytic activity. To investigate whether down-regulation of a single receptor resulted in reduced lysis mediated by this receptor, NK cell cytotoxicity after coculture was analyzed in redirected lysis assays against the murine FcγR+ P815 cell line. Lysis of P815 cells by 2 days IL15-stimulated NK cells was enhanced when P815 cells were coated with mouse anti-human FcγRIIIa/CD16, NKG2D, NKp30 or NKp46, but not DNAM-1 antibodies (Fig. 6, panel a, b and data not shown). After the IL15-stimulated coculture with STA-ET2.1 cells, however, redirected lysis through NKG2D and NKp30 was severely diminished (Fig. 6, panel a, b). Lysis through NKp46 was partly reduced, whereas FcγRIIIa/CD16-mediated lysis remained intact. These results were closely correlated with the down-regulation of NKG2D and NKp30 expression and the partly reduced levels of NKp46 on these NK cells, whereas FcγRIIIa/CD16 expression remained intact (Fig. 6, panel c). In contrast, after coculture with TC71 cells, redirected lysis via CD16, NKG2D, NKp30 and NKp46 as well as expression of the respective receptors was unaffected (Fig. 6, panel a–c). These data demonstrate that the inhibition of NKG2D, NKp30 and partly NKp46-dependent NK cell cytotoxicity were directly linked to the down-regulation of the respective NK cell receptors. As indicated by functional FcγRIIIa/CD16-mediated redirected lysis, the components of the cytolytic machinery appeared to be intact after the coculture. This was further corroborated by the fact that intracellular perforin expression in NK cells remained normal (Supplementary Fig. 3, panel B, available online).
Fig. 6.
Lysis of antibody-coated tumor cells by NK cells is not affected after the coculture. a Redirected lysis of P815 cells coated with FcγRIIIa/CD16, NKG2D, NKp30 and NKp46 antibodies by NK cells after for 2 days incubation of purified NK cells with IL15 only (bars without pattern) and in the presence or absence of STA-ET2.1 or TC71 cells (bars with pattern). The specific lysis (%) is depicted as representative data and as combined data of three experiments analyzed at a 8:1 E:T ratio (lysis of P815 in the absence of antibodies was subtracted from the total lysis in the presence of antibodies). b Representative data of redirected lysis of P815 cells by NK cells with or without antibody coating in the absence (upper panel) or presence of STA-ET2.1 cells (lower panel). c FcγRIIIa/CD16, NKG2D, NKp30 and NKp46 expression on NK cells after coculture used in redirected lysis experiments above. Data are combined from three experiments. d Lysis of non-cocultured, uncoated (white bar, no antibody addition) and cetuximab-coated (gray bar) STA-ET2.1 cells by NK cells after coculture of purified NK cells with STA-ET2.1, TC71 or HOS cells in the presence or absence of IL15. Data are combined from two experiments
Since FcγRIIIa/CD16-mediated redirected lysis as well as FcγRIIIa/CD16 receptor expression was intact after the coculture, this raised the question whether the FcγRIIIa/CD16-mediated antibody-dependent cytotoxic function of NK cells can be exploited to preserve sarcoma cell lysis after the coculture. Coating of STA-ET2.1 cells with the anti-epidermal growth factor receptor antibody cetuximab substantially enhanced their lysis by resting NK cells and, moderately, by IL15-stimulated NK cells (Fig. 6, panel d) [6]. Remarkably, lysis of antibody-coated STA-ET2.1 cells by NK cells was not inhibited after the coculture with STA-ET2.1, TC71 or HOS cells in the absence or presence of IL15.
Thus, despite the inhibitory effects of certain sarcoma cells, the FcγRIIIa/CD16-mediated, antibody-dependent cytotoxicity of NK cells was preserved after the coculture.
Discussion
Tumor escape from NK cell immunosurveillance may occur by selection of tumor cells with no or low expression of ligands for NK cell-activating receptors or increased expression of ligands for inhibitory receptors. Alternatively, tumor cells may produce factors which inhibit NK cell activity [39]. In our study, the prolonged, that is, 40 h, coculture of sarcoma cells with NK cells resulted in a sarcoma cell line-specific modulation of NK cell-activating receptors, in particular reducing (IL15-induced) expression of NKG2D and DNAM-1. Most remarkably, the STA-ET2.1 cell line reduced the IL15-induced expression of NKG2D even to lower levels than expressed on resting NK cells. In addition, the IL15-induced cytolytic activity of NK cells was reduced against sarcoma and non-sarcoma cell targets after the coculture. The down-regulation of NK cell-activating receptors, such as NKG2D, NKp30 and NKp46, was directly linked to the reduction in cytotoxicity mediated through these receptors as shown in redirected lysis experiments. This may offer an explanation for the inhibited sarcoma cell lysis which was dependent on NKG2D, DNAM-1 and NKp30.
It was notable that the different sarcoma cell lines were differentially able to down-regulate NK cell phenotype and function. We attempted to define whether one or more of the described mechanisms were responsible for this effect. Sustained interactions of NKG2D with its ligands expressed on tumor cells have been described to induce receptor internalization and degradation, resulting in NK cell hyporesponsiveness [33, 40–43]. However, in our study, the degree of down-regulation of NK cell receptors by different sarcoma cell lines did not correlate with the expression of the cognate ligands or known NK cell-inhibitory molecules, such as HLA-E, HLA-G and PD-1L, on these tumor cells. In particular, the sarcoma cell line with the strongest inhibitory potential (STA-ET2.1) exhibited only few NKG2D ligands (MICB and ULBP2), had relatively low intensities of NKG2D and DNAM-1 ligands and had undetectable expression of NKp30 and NKp44 ligands. Thus, the differences in ligand on the sarcoma cell lines do not sufficiently explain the differences in down-regulation of NK cell receptors. This suggests that other inhibiting factors than sustained ligand–receptor interactions are involved. Another potential mechanism of NK cell receptor down-regulation could be through soluble mediators as has been demonstrated for melanoma cells [44, 45]. However, NKG2D and DNAM-1 down-regulation after coculture with sarcoma cells was not mediated by soluble factors. Instead, direct cell–cell contact between sarcoma cells and NK cells was required as similarly shown for other tumor cell types [30, 38, 45, 46]. This requirement for cell–cell contact could be mediated by membrane-associated TGF-β as has been shown for various other tumor cells perturbing NK cell NKG2D expression [36–38]. However, in our experiments, TGF-β, either membrane-associated or soluble, was not involved since blockage of TGF-β or its signaling pathway during the coculture did not prevent NKG2D down-regulation on NK cells. Hence, we have determined that the inhibition of NK cell cytotoxicity was linked to the down-regulation of the respective NK cell-activating receptors. However, the mechanism of NKG2D and DNAM-1 down-regulation by some of the sarcoma cell lines remains to be further explored. It is tempting to speculate that certain sarcoma cells express a membrane-bound, inhibiting factor other than TGF-β that can globally impair NK cell receptor expression and thus NK cell cytolytic activity but leaving FcγRIIIa/CD16-mediated lysis intact.
Several studies have reported on defective cytotoxic functions of tumor-associated and peripheral blood NK cells from patients with cancer (prior to the start of anticancer therapies) in association with reduced expression of NK cell-activating receptors [30, 47–50]. As an exception to these studies, we have previously shown that peripheral blood NK cells from patients with Ewing’s sarcoma exerted low cytolytic activity despite higher levels of NKG2D, suggesting that another mechanism may be responsible for the in vivo down-regulation of NK cell reactivity [7]. Interestingly, when the NK cells were activated with IL15 for 5 days prior to the coculture, NKG2D expression and cytolytic activity were no longer affected by sarcoma cells. Conversely, the impaired expression of NKG2D and DNAM-1 could be reversed when the NK cells were removed from the coculture and subsequently stimulated with IL15 [32, 38, 40, 42]. Thus, the reduction in NK cell receptor expression requires the permanent presence of tumor cells. Ex vivo stimulation with IL15 of NK cells from patients with Ewing’s sarcoma restored the cytolytic activity [7], indicating that the inhibited cytolytic function of patient’s NK cells can also be reversed. Similarly, it has been shown that the decreased cytolytic activity of NK cells from patients with leukemia normalizes when patients have achieved complete remission [48].
In vivo, the excess of tumor cells, imperfect NK cell activation and incomplete tumor cell elimination may result in prolonged tumor-NK cell interactions, rendering NK cells non-functional over time. In this perspective, adoptively transferred NK cells have been shown to lose antibody-independent cytolytic activity in murine cancer models and cancer patients but retain antibody-dependent cytotoxic activity in patients [51, 52]. Importantly, in our experiments, antibody-dependent target cell lysis remained functional after the interaction with sarcoma cells, whereas FcγRIIIa/CD16-independent cytotoxicity of NK cells was inhibited. In several other studies, defective antibody-dependent cytotoxicity of NK cells from patients with cancer was associated with reduced expression of FcγRIIIa/CD16 on NK cells, whereas in our experiments, FcγRIIIa/CD16 expression was unaffected and mediated antibody-dependent target cell lysis. Different tumors apparently employ different mechanisms to interfere with cytolytic pathways of NK cells [30, 41, 53]. Hence, the coculture with sarcoma cells neither exhausted the cytolytic potential nor caused overall NK cell hyporesponsiveness. The inhibition of both ITAM-dependent (NKp30 and NKp46) and ITAM-independent (NKG2D) NK cell cytotoxicity appeared to be directly linked to the down-regulation of the respective NK cell receptors. Thus, target cell recognition was presumably impaired, while other prerequisites for target cell lysis, such as perforin expression, remained intact.
In our study, neither the cytotoxicity of 5 days IL15-activated NK cells nor the antibody-dependent cytolytic function of resting and IL15-stimulated NK cells was affected by the inhibitory effects of sarcoma cells. Thus, a combination of adoptively-transferred, IL15-activated NK cells with infusion of tumor-reactive antibodies may improve tumor targeting and conserve NK cell functionality in vivo in a potentially NK cell-inhibiting tumor environment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank F. Schaper, M. Verheul and L. Oudejans for technical contributions. We thank T. van Hall, G. de Groot-Swings, J. Suurmond and S. de Jong for kindly providing antibodies against HLA-E, HLA-G, PD-1 and PD-1L, respectively. This work was financially supported by a grant from the foundation ‘Quality of Life Gala 2007,’ the European Commission (EuroBoNeT, grant No 018814) and the Dutch Foundation Children Cancer Free (grant 2009-052).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing’s sarcoma family of tumors: current management. Oncologist. 2006;11:503–519. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 2.Hattinger CM, Pasello M, Ferrari S, Picci P, Serra M. Emerging drugs for high-grade osteosarcoma. Expert Opin Emerg Drugs. 2010;15:615–634. doi: 10.1517/14728214.2010.505603. [DOI] [PubMed] [Google Scholar]
- 3.van den Berg H, Kroon HM, Slaar A, Hogendoorn PCW. Incidence of biopsy-proven bone tumors in children: a report based on the Dutch pathology registration “PALGA”. J Pediatr Orthop. 2008;28:29–35. doi: 10.1097/BPO.0b013e3181558cb5. [DOI] [PubMed] [Google Scholar]
- 4.Berghuis D, Schilham M.W., Voss HI, Santos SJ, Kloess S, Buddingh EP, Egeler RM, Hogendoorn PCW, Lankester AC. Histone Deacetylase Inhibitors Enhance Expression of NKG2D Ligands in Ewing Sarcoma and Sensitize for Natural Killer Cell-Mediated Cytolysis. Clin Sarcoma Res. 2012;2:8. doi: 10.1186/2045-3329-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buddingh EP, Schilham MW, Ruslan SE, Berghuis D, Szuhai K, Suurmond J, Taminiau AH, Gelderblom H, Egeler RM, Serra M, Hogendoorn PCW, Lankester AC. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol Immunother. 2011;60:575–586. doi: 10.1007/s00262-010-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pahl JH, Ruslan SE, Buddingh EP, Santos SJ, Szuhai K, Serra M, Gelderblom H, Hogendoorn PC, Egeler RM, Schilham MW, Lankester AC. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin Cancer Res. 2012;18:432–441. doi: 10.1158/1078-0432.CCR-11-2277. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven DHJ, de Hooge AS, Mooiman EC, Santos SJ, ten Dam MM, Gelderblom H, Melief CJ, Hogendoorn PCW, Egeler RM, van Tol MJD, Schilham MW, Lankester AC. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol. 2008;45:3917–3925. doi: 10.1016/j.molimm.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–158. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 11.Thielens A, Vivier E, Romagne F. NK cell MHC class i specific receptors (KIR): from biology to clinical intervention. Curr Opin Immunol. 2012;24:239–245. doi: 10.1016/j.coi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813–818. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacon L, Eagle RA, Meyer M, Easom N, Young NT, Trowsdale J. Two human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D. J Immunol. 2004;173:1078–1084. doi: 10.4049/jimmunol.173.2.1078. [DOI] [PubMed] [Google Scholar]
- 14.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 15.Chalupny NJ, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophys Res Commun. 2003;305:129–135. doi: 10.1016/S0006-291X(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 16.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 17.Eagle RA, Traherne JA, Hair JR, Jafferji I, Trowsdale J. ULBP6/RAET1L is an additional human NKG2D ligand. Eur J Immunol. 2009;39:3207–3216. doi: 10.1002/eji.200939502. [DOI] [PubMed] [Google Scholar]
- 18.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7–H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. 2007;8:1345–1352. doi: 10.1038/ni1524. [DOI] [PubMed] [Google Scholar]
- 22.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 26.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 28.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 29.Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of nkg2d ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189:1360–1371. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 30.Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, Kiessling R, Malmberg KJ. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–4930. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 31.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 32.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/S1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 33.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 34.Castriconi R, Cantoni C, Della CM, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, Lane HC, Fauci AS. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136:3916–3920. [PubMed] [Google Scholar]
- 36.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 37.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 38.Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, Cook GP. Human tumour immune evasion via TGF-beta blocks nk cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS One. 2011;6:e22842. doi: 10.1371/journal.pone.0022842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 40.Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–1717. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 41.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–3578. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 42.Hanaoka N, Jabri B, Dai Z, Ciszewski C, Stevens AM, Yee C, Nakakuma H, Spies T, Groh V. NKG2D initiates caspase-mediated CD3{zeta} degradation and lymphocyte receptor impairments associated with human cancer and autoimmune disease. J Immunol. 2010;185:5732–5742. doi: 10.4049/jimmunol.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roda-Navarro P, Reyburn HT. The traffic of the NKG2D/Dap10 receptor complex during natural killer (NK) cell activation. J Biol Chem. 2009;284:16463–16472. doi: 10.1074/jbc.M808561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S, Solari N, Gualco M, Queirolo P, Moretta L, Mingari MC. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72:1407–1415. doi: 10.1158/0008-5472.CAN-11-2544. [DOI] [PubMed] [Google Scholar]
- 45.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, Moretta L, Mingari MC, Vitale M. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Lilienfeld-Toal M, Frank S, Leyendecker C, Feyler S, Jarmin S, Morgan R, Glasmacher A, Marten A, Schmidt-Wolf IG, Brossart P, Cook G. Reduced immune effector cell NKG2D expression and increased levels of soluble NKG2D ligands in multiple myeloma may not be causally linked. Cancer Immunol Immunother. 2009;59:829–839. doi: 10.1007/s00262-009-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 48.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109:323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 49.Le Maux CB, Moretta A, Vergnon I, Opolon P, Lecluse Y, Grunenwald D, Kubin M, Soria JC, Chouaib S, Mami-Chouaib F. NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. J Immunol. 2005;175:5790–5798. doi: 10.4049/jimmunol.175.9.5790. [DOI] [PubMed] [Google Scholar]
- 50.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 51.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62:5813–5817. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.