Abstract
Immune-based therapies that induce remarkable and durable responses against melanoma and lung cancer have unfortunately demonstrated limited success in ovarian cancer patients. This is likely due to the exceptional immunoregulatory nature of ovarian tumors, which employ numerous strategies to effectively suppress anti-tumor immunity. Here, we summarize a decade of research indicating that ovarian cancers possess an exquisite capacity to subvert the activity of host dendritic cells (DCs) as a key mechanism to impede the development and maintenance of protective T cell-based immune responses. Identifying, understanding, and disabling the precise mechanisms promoting DC dysfunction in ovarian cancer are, therefore, fundamental requirements for devising the next generation of successful immunotherapies against this devastating malignancy.
Keywords: Dendritic cells, Immunotherapy, Ovarian cancer, Immunosuppression, Tumor microenvironment, Regulatory myeloid suppressor cells
Introduction
Ovarian carcinoma claims the lives of more than 14,000 American women every year [1]. The vast majority of ovarian tumors are diagnosed at advanced stages, when the disease has already disseminated throughout the peritoneal cavity [2]. While ovarian cancer patients initially respond to cytoreductive surgery and platinum-based chemotherapy, about 70% of them relapse with more aggressive and drug-resistant disease [3]. Classical treatments based on surgery and chemotherapy have met with very limited success during the past four decades and the 5-year survival rate for metastatic ovarian cancer patients remains at less than 30% [1]. Hence, new and more effective therapies are urgently needed in the clinic to improve the dismal prognosis of ~22,000 American women diagnosed with ovarian cancer each year [1].
Harnessing the intrinsic ability of our immune system to recognize and eliminate malignant cells is the most attractive anti-cancer intervention since the development of chemotherapy. As tumor-reactive T cells can exert some immune pressure against ovarian cancer progression [4–6], immunotherapy has recently emerged as an appealing approach to complement the standard ovarian cancer treatments. Nonetheless, multiple studies have demonstrated that the generation of protective and durable T-cell responses against ovarian cancer is persistently controlled by several immunosuppressive factors and conditions present in the hostile ovarian cancer microenvironment [7–11].
Immune checkpoints comprise inhibitory pathways needed to maintain self-tolerance and modulate the duration and amplitude of physiological immune responses. Tumors, however, co-opt immune-checkpoint pathways as a strategy to abrogate protective T-cell-mediated immune responses [12]. Antibodies targeting Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) and programmed cell death protein 1 (PD-1), two critical immune checkpoints, elicit impressive clinical responses in a subset of melanoma and lung cancer patients [13]. Adoptively transferred tumor-reactive T cells have also been demonstrated to induce robust anti-tumor responses that lead to clinical control of otherwise incurable tumors [14, 15]. Unfortunately, the current overall response rate of platinum-resistant ovarian cancer patients to checkpoint blockade is less than 15% [16] and adoptive T-cell immunotherapies have also shown minimal success in this aggressive malignancy [17]. Furthermore, ovarian cancer patients seem to be refractory to therapeutic vaccines based on autologous transfer of tumor antigen-pulsed DCs [18]. New interventions that reverse tumor-induced immunosuppression and that enhance the protective activity of innate immune cells in the tumor microenvironment are necessary to potentiate the efficacy of standard treatments and emerging immunotherapies in ovarian cancer patients.
Optimal DC function is necessary for the initiation and maintenance of protective anti-tumor immunity. Yet, aggressive cancers can proficiently evade immune control by crippling normal DC functions. This review summarizes the body of evidence, demonstrating that ovarian cancers potently suppress anti-tumor immunity by provoking severe DC dysfunction in the tumor microenvironment. We discuss diverse DC molecular pathways subverted by ovarian tumors, and we explore various therapeutic approaches to restore normal DC function in ovarian cancer, which arises as a critical requirement for developing successful and more definite interventions against this disease.
Ovarian tumors actively recruit and manipulate DCs
Conventional DCs (cDCs) are specialized antigen-presenting cells that exhibit robust phagocytic ability in their immature state, and high cytokine-producing and immunostimulatory capacity when functionally mature [19]. cDCs are generally divided into two main subsets based on the surface expression of CD8α and CD11b. CD8α+ cDCs are functionally specialized in antigen cross-presentation to CD8+ T cells via MHC class I molecules. In contrast, CD11b+ cDCs preferentially induce of CD4+ T-cell immunity due to their prominent expression of MHC class II and its associated antigen presentation machinery [20]. Plasmacytoid dendritic cells (pDCs) constitute another group of DCs that are long-lived and critical for the induction of potent anti-viral immunity due to their unique capacity to produce copious amounts of type I interferons (IFN) [19]. DCs with poor antigen presentation capacity or reduced expression of co-stimulatory molecules have generally been considered to be “tolerogenic”, whereas mature DCs expressing elevated levels of cytokines and co-stimulatory molecules demonstrate potent T-cell-activating and stimulatory attributes. Aggressive cancers induce local and systemic inflammation by triggering “emergency myelopoiesis”, which is a process that mediates the rapid expansion and mobilization of myeloid leukocytes [21]. This mechanism is critical to confront viral and bacterial infections in response to inflammatory cytokines, but tumors exploit this process to promote the homing of immature myeloid progenitors to lymphatic tissues and tumor locations [22, 23]. Pioneering work by Conejo-Garcia and colleagues first demonstrated that DCs massively infiltrate advanced human and mouse ovarian tumors [7]. Strikingly, β-defensins secreted by ovarian cancer epithelial cells recruited DC precursors into the tumor microenvironment via the chemokine receptor 6 (CCR6) [7]. Overexpression of Vascular Endothelial Growth Factor-A (VEGF-A) by ovarian tumors transformed the arriving DC populations into pro-angiogenic cells that facilitated tumor vascularization and growth [7]. The ontology of ovarian cancer-associated DCs has recently been reviewed [24]. Importantly, while these DCs cells engulf tumor-derived materials and express relatively high levels of co-stimulatory molecules, they consistently demonstrate defective antigen-presenting capacity and potent immunosuppressive activity, which ultimately blocks the local activation and expansion of intratumoral T cells [25, 26]. Due to these particular functional features, ovarian cancer-associated DCs have been interchangeably classified as regulatory/tolerogenic/dysfunctional. Accordingly, sophisticated in vivo depletion approaches demonstrated that elimination of CD11c+ DCs delayed ovarian cancer progression by reducing tumor angiogenesis while concomitantly boosting endogenous anti-tumor T-cell-based responses [10, 26]. As expected, therapeutic DC ablation potentiated the effects of chemotherapy [10] and enhanced adoptive T-cell immunotherapy [5, 6], thereby extending host survival in pre-clinical models of metastatic ovarian cancer. Altogether, these key initial studies revealed that ovarian cancers dynamically promote intratumoral DC accumulation to support malignant progression via pro-angiogenic and immunoregulatory mechanisms.
Immunosuppressive factors expressed by ovarian cancer-associated DCs
Seminal studies by Curiel and colleagues demonstrated that myeloid DC populations isolated from human ovarian cancer specimens express high levels of the immunosuppressive ligand PD-L1, also known as B7-H1 [9]. Extensive ex vivo approaches indicated that blockade of DC-intrinsic PD-L1 enhanced T-cell activation in these specimens, a process that was accompanied by down-regulation of T-cell-derived IL-10 and up-regulation of IL-2 and IFN-γ [9]. Accordingly, tumor-reactive T cells expanded by PD-L1-neutralized DCs exhibited an enhanced ability to inhibit the growth of autologous human ovarian cancer in immunodeficient mice [9].
DCs isolated from ovarian cancer lesions, but not DCs from tumor-free locations of the same host, also exhibited strong Arginase 1 activity [25], which is an enzyme that metabolizes L-arginine to L-ornithine and urea, and hence depletes the arginine T cells that require for optimal activation and expansion [27]. Interestingly, the immunosuppressive capacity of ovarian cancer-associated DCs was functionally confirmed via coculture assays in which the expansion of transgenic T cells in response to cognate antigen was abrogated upon introduction of freshly isolated DCs from ovarian tumors [25].
Human ovarian cancer-associated DCs have also been shown to express CD277 (BTN3A1), [28], which is a type I transmembrane member of the butyrophilin family that shares striking similarities with immunosuppressive B7-H4. Abundant expression of CD277 was evidenced on myeloid and malignant cells in the human ovarian carcinoma microenvironment [28]. Interestingly, expression of CD277 in monocyte-derived human DCs was up-regulated by tumor microenvironmental cytokines and hypoxia associated mediators, such as IL-6, IL-10, VEGF, PIGF-1, and CCL3 [28] (Fig. 1). Enforced expression of CD277 on artificial antigen-presenting cells inhibited TCR-mediated proliferation of human T cells as well as Th1-related cytokine secretion [28]. These results uncovered a new immunomodulatory role for CD277 in ovarian cancer and suggested that CD277 neutralization could represent a new therapeutic approach to control DC-mediated immunosuppression in ovarian cancer patients. DCs recruited to ovarian tumors, therefore, overexpress PD-L1, BTN family members and functional Arginase 1, which operate in conjunction to restrain anti-cancer T-cell function. Since ovarian cancer-associated DCs home to perivascular locations within the tumor [7], they represent a critical immunosuppressive gateway for transmigrating T cells that attempt to infiltrate and eliminate malignant cells (Fig. 1).
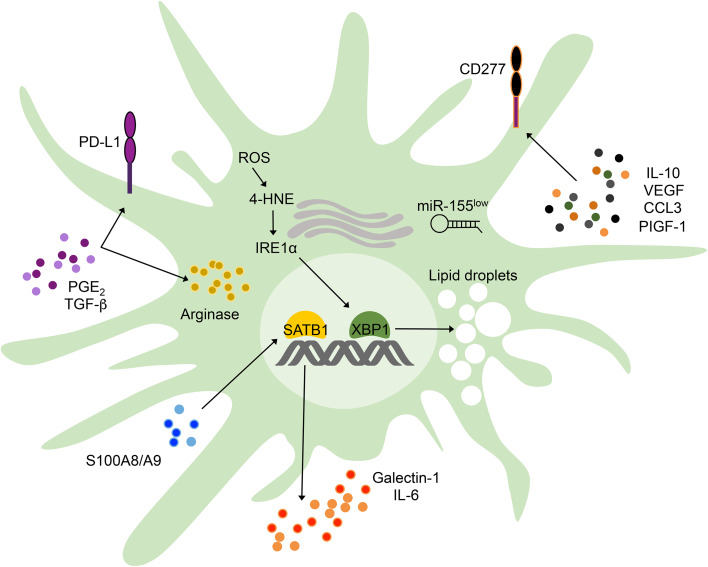
Fig. 1.
Factors promoting DC malfunction in ovarian tumors. Ovarian cancer cells secrete PGE2 and TGF-β, which induce PD-L1 expression and increase Arginase activity in DCs to restrain anti-cancer T-cell activity. IL-10, VEGF, CCL3, and PIGF-1 are commonly present in the ovarian cancer microenvironment and induce overexpression of immunoregulatory CD277 in DCs. In addition, microenvironmental S100A8/A9 proteins provoke relentless SATB1 activation in tumor-associated DCs, which endows them with the capacity to secrete pro-inflammatory IL-6 and immunosuppressive Galectin-1. Furthermore, the local antigen-presenting capacity of ovarian cancer-associated DCs is severely deteriorated due to reduced expression of functional miR-155 and to the accumulation of ROS-driven lipid peroxidation byproducts, such as 4-HNE, which cause ER stress, IRE1α-XBP1 overactivation, and abnormal lipid droplet accumulation
Drivers of DC dysfunction in ovarian cancer
The tumor microenvironmental factors and cell-intrinsic mechanisms dictating DC dysfunction in ovarian cancer have just begun to be characterized. Ovarian cancer cells were found to secrete copious amounts of PGE2 and TGF-β, which operated in conjunction to transform conventional splenic DCs from immunocompetent to immunosuppressive cells via induction of PD-L1 and Arginase activity [26] (Fig. 1). Interestingly, microRNAs (miRNAs) were also demonstrated to play a substantial role in controlling the tolerogenic phenotype of ovarian cancer-associated DCs. These small non-coding RNAs constitute a major regulatory mechanism controlling global gene expression profiles, and thus, dysregulated miRNA expression has been shown to mediate tumorigenesis, metastasis, and malignant progression in several cancer types (Reviewed in Ref. [29]). Ovarian cancer-associated DCs demonstrated severe down-regulation of miR-155, a miRNA that is required for the optimal antigen-presenting and immunostimulatory activity of DCs [30]. Notably, in vivo miR-155 replacement therapy altered nearly 50% of the transcriptome of tumor-associated DCs, and potently repressed critical genes encoding immunosuppressive and tolerogenic mediators, such as Cd200, C/epbβ, Tgfβ1, Smad1, Smad6, Smad7, and Ccl22 [31]. Consequently, restoring functional miR-155 expression in ovarian cancer-associated DCs enhanced their antigen-presenting capacity at tumor locations, induced in vivo production of Th1 cytokines with anti-tumor potential, such as TNFα, IL-12, IFNγ, and CCL5, and elicited protective T-cell-based immune responses against ovarian cancer [31]. Additional groups subsequently confirmed the critical immunostimulatory role of miR-155 in cancer-associated myeloid cells, including Naldini and colleagues, who elegantly demonstrated that myeloid-specific knockdown of miR-155 accelerated tumor growth in a spontaneous model of breast cancer [32]. Besides controlling common tolerogenic and immunosuppressive mediators, miR-155 was unexpectedly found to regulate the master genomic organizer SATB1 in ovarian cancer-associated DCs [31]. Interestingly, subsequent studies showed that S100A8/A9 proteins commonly found in the tumor microenvironment perpetuated SATB1 expression in DCs and this process enhanced their ability to release IL-6 and immunosuppressive Galectin-1, which potently inhibited the function of ovarian cancer-reactive T cells [33] (Fig. 1).
Most recently, the endoplasmic reticulum (ER) stress response was demonstrated to play a crucial role as an additional driver of DC dysfunction in ovarian cancer [34]. Aggressive tumors thrive under adverse conditions, such as hypoxia, nutrient starvation, and oxidative stress by adjusting their protein folding capacity through the ER stress response pathway [35]. The most conserved arm of the ER stress response is the dual enzyme, IRE1α. Activated during periods of ER stress incited by the accumulation of misfolded proteins in this organelle, the IRE1α endoribonuclease domain excises a 26-nucleotide fragment from the Xbp1 mRNA to generate a spliced version that codes for the functionally active transcription factor, XBP1 [36]. By inducing expression of critical genes involved in protein folding and quality control, XBP1 facilitates adaptation to ER stress and promotes cell survival [37]. Indeed, sustained XBP1 activation in malignant cells renders tumors with greater angiogenic, metastatic, and drug-resistant capacity in various cancer types [38–40]. Accordingly, overexpression of ER stress response markers in multiple malignancies correlates with unfavorable prognosis and poor clinical outcome [39, 41–43]. Most of the tumorigenic effects of aberrant IRE1α-XBP1 signaling have been attributed to its direct function on the cancer cell, but whether this arm of the ER stress responses also contributes to malignant progression by inhibiting host immune responses had not been considered. Interestingly, our group found that ovarian cancer-associated DCs exhibited robust IRE1α-XBP1 activation and overexpression of various ER stress response markers, compared with DCs isolated from non-tumor locations [34]. DCs in the ovarian cancer microenvironment possessed high levels of intracellular reactive oxygen species (ROS) that induced lipid peroxidation and generation of reactive byproducts that diffused into the ER, thereby modifying critical chaperones in this compartment and triggering ER stress [34] (Fig. 1). This process provoked persistent IRE1α-XBP1 activation in tumor-infiltrating DCs and such effects could be prevented using agents that sequestered ROS or lipid peroxidation byproducts [34]. Strikingly, primary and metastatic ovarian cancer progression was compromised in immunocompetent hosts selectively deleting XBP1 in DCs, and these effects were accompanied by the infiltration and accumulation of activated IFNγ-secreting T cells inside the tumor. These observations suggested that ovarian cancer-associated DCs devoid of XBP1 had stimulatory rather than tolerogenic capacity. Indeed, extensive in vitro and in vivo assays confirmed that XBP1-deficient DCs isolated from ovarian tumors demonstrated enhanced antigen-presenting capacity. Abnormal XBP1 activation disrupted lipid metabolism in DCs and re-programmed them towards aberrant triglyceride synthesis and accumulation (Fig. 1), a process that was associated with their inability to efficiently present antigens. Of note, abnormal lipid accumulation by DCs in cancer had been demonstrated to be a critical process inhibiting their optimal antigen-presenting capacity [44]. In addition, and supporting the key immunoregulatory role of IRE1α-XBP1 signaling in cancer-associated myeloid cells, Gabrilovich and colleagues recently demonstrated that human peripheral neutrophils could be rapidly converted into potent immunosuppressive cells via ER stress-driven XBP1 overactivation [45]. The novel task of the IRE1α-XBP1 branch of the ER stress response as a major modulator of myeloid cell function in tumors emerges as a potential “Achilles heel” of the disease, and, therefore, constitutes a new attractive target for cancer immunotherapy.
Therapeutic approaches to restore DC function in ovarian cancer
Re-educating tumor-associated DCs via CD40 and TLR stimulation
Engagement of CD40 on the surface of antigen-presenting cells, such as dendritic cells, macrophages, and B cells, greatly increases their capacity to elicit potent T-cell activation and expansion [46, 47]. As such, CD40 agonists have demonstrated efficacy against aggressive and highly immunosuppressive cancers, such as pancreatic ductal adenocarcinoma [48, 49] and effective T-cell-based anti-cancer therapies in lymphoma models require CD40-CD40L activation of tumor necrosis factor (TNF) and DCs producing inducible nitric oxide synthase [50]. In mice bearing metastatic ovarian cancer, intraperitoneal treatment with Poly I:C synergized with concomitant CD40 stimulation to induce potent activation and maturation of tumor-infiltrating DCs [25]. These effects reversed the tolerogenic and immunosuppressive phenotype of DCs at tumor locations, and induced remarkable T-cell responses leading to prolonged host survival and even tumor rejection [25]. CD40 and TLR3 co-activation dramatically enhanced the capacity of tumor-infiltrating DCs to process and present the antigens that they spontaneous phagocytize at tumor locations, and induced up-regulation of co-stimulatory molecules [25]. Furthermore, in vivo co-stimulation of CD40 and TLR3 promoted the migration of activated DCs carrying antigens from the tumor to lymph nodes [25]. These findings indicate that restoring DC function in tumors via CD40 and TLR3 co-activation represents a promising immunotherapeutic strategy against ovarian cancer (Fig. 2). Since CD40 and TLR3 agonistic agents have been independently tested in multiple clinical trials [48, 51, 52], this combination approach could have rapid clinical applicability in the setting of metastatic ovarian cancer.
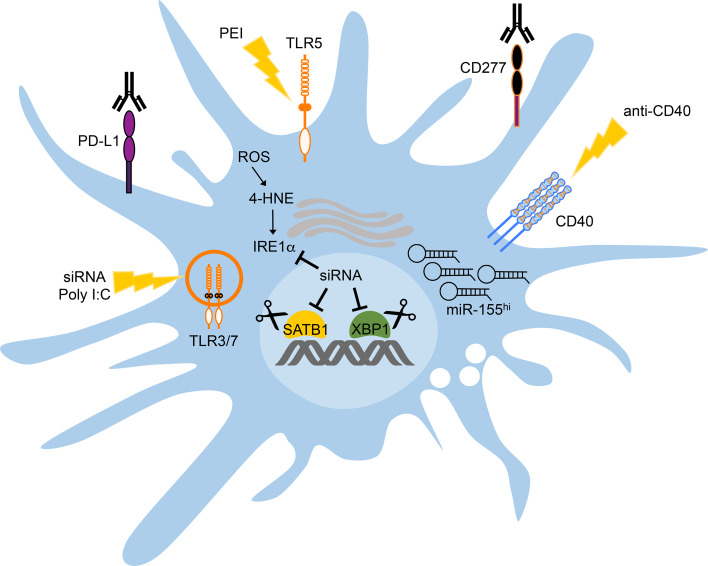
Fig. 2.
Therapeutic strategies to improve DC function in ovarian cancer. CD40 and TLR3 co-activation enhances the capacity of tumor-infiltrating DCs to process and present tumor antigens, and up-regulates expression of co-stimulatory molecules. Selective targeting of ovarian cancer-associated DCs using siRNA-PEI nanoparticles stimulates TLR3/7 (siRNA) and TLR5 (PEI), thus promoting DC activation in situ. PEI-based nanoparticles loaded with siRNA targeting SATB1 or IRE1α/XBP1 further reduces the suppressive capacity of tumor-associated DCs while enhancing their antigen-presenting capacity. miR-155 replacement therapy via PEI-based nanoparticles encapsulating miRNA mimetics can be used to globally re-program ovarian cancer-associated DCs from immunosuppressive to immunostimulatory cells. Furthermore, neutralizing PD-L1 and CD277 could also decrease the T-cell-suppressive capacity of tumor-associated DCs. These experimental interventions have proven effective at restoring DC function in pre-clinical models of ovarian cancer, and lead to the generation of therapeutic anti-tumor immune responses. Gene-editing technologies based on CRISPR/Cas9 could also be exploited to simultaneously ablate SATB1 and XBP1 in therapeutic DC-based vaccines prior to transfer into the patient
Selective targeting of ovarian cancer-associated DCs using nanoparticles
Since phagocytes demonstrate unparalleled capacity to “ingest” particles in the nanometer range, they represent a major obstacle for the optimal performance and selectivity of nanotherapies aiming at targeting non-myeloid cell types. This is considered a limitation in some diseases, but it also represents a competitive advantage in the setting of metastatic ovarian cancer. Specialized leukocytes optimally sequester charged particles of ~200 nm, and in most experimental models, stealth nanomaterials smaller than 100 nm are significantly sequestered by macrophages and DCs [53]. As immature DCs with strong phagocytic capacity infiltrate ovarian carcinomas, delivery of nanoparticles containing nucleic acids has been demonstrated to be a feasible strategy to re-program their function in situ to turn them against the tumor—the “Trojan horse” concept [54]. Intraperitoneal administration of polyethylenimine (PEI)-based nanocomplexes encapsulating small-interfering RNA (siRNA) into hosts bearing metastatic ovarian cancer resulted in the rapid and preferential targeting of cancer-associated DCs, both in the solid tumor and in malignant ascites [11]. This first-in-class system has proven effective for therapeutically silencing expression of PD-L1 [11], SATB1 [33] and XBP1 [34] in ovarian cancer-associated DCs (Fig. 2), and consequently extended host survival by evoking robust T-cell-driven anti-tumor immune responses. Of note, siRNA oligonucleotides within the nanoparticle contributed to reversing the regulatory phenotype of ovarian cancer-associated DCs via activation of TLR3 and TLR7 upon intracellular delivery [11] (Fig. 2). Unexpectedly, PEI was found to act as a novel TLR5 agonist that further promoted the activation of tumor-associated DCs engulfing nanoparticles in vivo [11] (Fig. 2). Ovarian cancer-bearing hosts treated with PEI-based nanocomplexes containing non-targeting siRNA, therefore, showed a significant MyD88-dependent increase in survival compared with untreated mice, uncovering the inherent immunoadjuvant activity of this nanoparticle class [11]. Thus, treatment with siRNA-PEI nanoparticles can elicit relevant T-cell-mediated responses against ovarian cancer independently of the siRNA sequence, but such immunotherapeutic effects are substantially enhanced when expression of an immunosuppressive mediator, such as PD-L1, SATB1, or XBP1, is specifically targeted in DCs [11, 33, 34]. PEI-based nanoparticles were also effective at delivering miRNA mimetic compounds to ovarian cancer-associated DCs as a strategy to restore the expression of functionally mature miR-155 and revamp the natural T-cell stimulatory capacity of these myeloid cells at tumor locations [31] (Fig. 2). Indeed, in vivo miR-155 replacement therapy endowed ovarian cancer-associated DCs with potent antigen-presenting capacity while simultaneously silencing multiple immunosuppressive mediators, and this process elicited potent anti-tumor immune responses that extended host survival and that induced tumor rejection in some hosts, especially when combined with concomitant CD40 stimulation [31].
Gene-editing technologies to empower therapeutic DC-based vaccines
Despite enormous translational efforts, treatment of metastatic ovarian cancer patients with autologous DCs pulsed ex vivo with tumor lysates induced limited clinical benefit [18]. This is possibly due to the multiple ovarian cancer-driven mechanisms discussed above, which cause severe DC dysfunction in the host. In proof-of-principle experiments, our group recently demonstrated that intraperitoneal treatment of ovarian cancer-bearing mice with XBP1-deficient bone marrow-derived DCs (BMDCs) induced substantial anti-tumor effects compared with treatment using common wild-type BMDCs [34]. These observations suggested that adoptively transferred DCs lacking XBP1 were likely refractory to the detrimental effects of ER stress in the tumor microenvironment, and, therefore, demonstrated superior immunostimulatory activity capable of triggering protective T-cell-based immune responses. We propose that cutting-edge genome editing technologies, such as CRISPR/Cas9 [55], could be exploited to enable precise, efficient, and simultaneous ablation of detrimental DC-intrinsic factors, such as XBP1 and SATB1, prior to adoptive transfer (Fig. 2), a process that should improve DC function in ovarian cancer hosts and that could elicit superior therapeutic immunity against this malignancy. Interestingly, unlike other programmable nuclease systems used for genome editing, the Cas9 system allows the use of various single-guide RNAs (sgRNAs) to achieve efficient multiplexed genome editing even in primary mammalian cells [56]. Molecular tailoring of DCs prior to adoptive transfer, therefore, represents an attractive approach to enhance the efficacy of therapeutic vaccines against ovarian cancer in the clinic.
Concluding remarks
Ovarian cancer is a lethal malignancy with a striking ability to cripple anti-tumor immune responses. By causing severe DC dysfunction in the host, ovarian tumors efficiently escape immune control. Over the last 10 years, multiple mechanisms responsible of driving DC dysfunction in ovarian cancer have been identified, characterized, and targeted in pre-clinical models of disease, which has unraveled novel interventions to elicit therapeutic immunity and control ovarian cancer progression. So far, these detrimental mechanisms include the induction of immunosuppressive mediators, deregulation of crucial miRNAs, and abnormal activation of key genomic organizers and ER stress response factors (Fig. 1). Current immunotherapies that induce remarkable responses in melanoma and lung cancer have unfortunately shown limited success in ovarian cancer patients, likely because normal DC function is dramatically compromised in these women. While checkpoint blockade “releases the brakes” on tumor-reactive T cells, this strategy has minimal chance of success if DC function, which provides the “gas” for T cells, is suboptimal. Disruptive approaches capable of restoring the T-cell-stimulatory capacity of DCs in ovarian cancer are urgently needed to create the next generation of potent and hopefully definite immunotherapies against this devastating disease.
Acknowledgements
Our research has been supported by the John H. Copenhaver, Jr. and William H. Thomas, MD 1952 Fellowship of the Geisel School of Medicine at Dartmouth (Juan R. Cubillos-Ruiz), the Irvington Institute Fellowship Program of the Cancer Research Institute (Juan R. Cubillos-Ruiz), the Ann Schreiber Mentored Investigator Award of the Ovarian Cancer Research Fund Alliance (Juan R. Cubillos-Ruiz), and the Ovarian Cancer Academy—Early Career Investigator Award of the Department of Defense (Juan R. Cubillos-Ruiz). We apologize to colleagues whose work was not cited in this review due to space limitations.
Abbreviations
- 4-HNE
4-Hydroxynonenal
- BMDC
Bone marrow-derived DC
- CCL3
Chemokine (C-C motif) ligand 3
- CCL5
Chemokine (C-C motif) ligand 5
- CCR6
Chemokine receptor 6
- cDC
Conventional DCs
- CRISPR/Cas9
Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9
- CTLA4
Cytotoxic T-lymphocyte-associated antigen 4
- DC
Dendritic cell
- ER
Endoplasmic reticulum
- IFN
Interferon
- IRE1α
Inositol-requiring enzyme 1
- miRNA
MicroRNA
- MyD88
Myeloid differentiation primary response gene 88
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death protein ligand 1
- pDC
Plasmacytoid dendritic cells
- PEI
Polyethylenimine
- PGE2
Prostaglandin E2
- PIGF-1
Placenta growth factor
- ROS
Reactive oxygen species
- SATB1
Special AT-rich sequence-binding protein-1
- sgRNA
Single-guide RNA
- siRNA
Small-interfering RNA
- tDC
Tumor-associated DC
- TGF-β
Tumor growth factor -β
- Th1
T helper 1
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- VEGF-A
Vascular Endothelial Growth Factor-A
- XBP1
X-box-binding protein 1
Compliance with ethical standards
Conflict of interest
Juan R. Cubillos-Ruiz is co-founder and scientific advisor for Quentis Therapeutics, Inc. The other authors declare no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the conference Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application which was hosted by the Wistar Institute in Philadelphia, PA, USA, 16th–19th June, 2016. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Matulonis UA, et al. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giornelli GH. Management of relapsed ovarian cancer: a review. SpringerPlus. 2016;5(1):1197. doi: 10.1186/s40064-016-2660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Nesbeth Y, et al. CCL5-mediated endogenous antitumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res. 2009;69(15):6331–6338. doi: 10.1158/0008-5472.CAN-08-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nesbeth YC, et al. CD4 + T cells elicit host immune responses to MHC class II-negative ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol. 2010;184(10):5654–5662. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conejo-Garcia JR, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10(9):950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 8.Barnett B, et al. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54(6):369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 9.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 10.Huarte E, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68(18):7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cubillos-Ruiz JR, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119(8):2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamanishi J, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 17.Kershaw MH, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandalaft LE, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2(1):e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40(5):642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119(13):2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conejo-Garcia JR, Rutkowski MR, Cubillos-Ruiz JR. State-of-the-art of regulatory dendritic cells in cancer. Pharmacol Ther. 2016;164:97–104. doi: 10.1016/j.pharmthera.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarlett UK, et al. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69(18):7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarlett UK, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209(3):495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munder M, Arginase an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158(3):638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cubillos-Ruiz JR, et al. CD277 is a negative co-stimulatory molecule universally expressed by ovarian cancer microenvironmental cells. Oncotarget. 2010;1(5):329–338. doi: 10.18632/oncotarget.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu HT, Gao P. The roles of microRNAs related with progression and metastasis in human cancers. Tumor Biol. 2016;37(12):15385–15397. doi: 10.1007/s13277-016-5436-9. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez A, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cubillos-Ruiz JR, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72(7):1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zonari E, et al. A role for miR-155 in enabling tumor-infiltrating innate immune cells to mount effective antitumor responses in mice. Blood. 2013;122(2):243–252. doi: 10.1182/blood-2012-08-449306. [DOI] [PubMed] [Google Scholar]
- 33.Tesone AJ, et al. Satb1 overexpression drives tumor-promoting activities in cancer-associated dendritic cells. Cell Rep. 2016;14(7):1774–1786. doi: 10.1016/j.celrep.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cubillos-Ruiz JR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12(9):703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida H, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 37.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang CH, et al. Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduces leukemic cell survival. J Clin Invest. 2014;124(6):2585–2598. doi: 10.1172/JCI73448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508(7494):103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auf G, et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci USA. 2010;107(35):15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalton LE, et al. The endoplasmic reticulum stress marker CHOP predicts survival in malignant mesothelioma. Br J Cancer. 2013;108(6):1340–1347. doi: 10.1038/bjc.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies MP, et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer. 2008;123(1):85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 43.Matsuo K, et al. The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecol Oncol. 2013;128(3):552–559. doi: 10.1016/j.ygyno.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herber DL, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16(8):880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Condamine T, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2):aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoenberger SP, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 47.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 48.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vonderheide RH, et al. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62(5):949–954. doi: 10.1007/s00262-013-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marigo I, et al. T cell cancer therapy requires CD40-CD40L activation of tumor necrosis factor and inducible nitric-oxide-synthase-producing dendritic cells. Cancer Cell. 2016;30(3):377–390. doi: 10.1016/j.ccell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beatty GL, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19(22):6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenfeld MR, et al. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12(10):1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. CMLS Cell Mol Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cubillos-Ruiz JR, Fiering S, Conejo-Garcia JR. Nanomolecular targeting of dendritic cells for ovarian cancer therapy. Future Oncol. 2009;5(8):1189–1192. doi: 10.2217/fon.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaj T, Gersbach CA, Barbas CF 3rd (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31(7):397–405 [DOI] [PMC free article] [PubMed]
- 56.Platt RJ, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159(2):440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]