Abstract
The exact function of MAGE-C1/CT7 and MAGE-C2/CT10 is not yet understood in multiple myeloma (MM). However, the homologs MAGE-C1/CT7 and MAGE-C2/CT10 genes encode highly immunogeneic cancer/testis antigens (CTAs) and can be potential targets for T cell-based immunotherapy. MAGE-C1/CT7 and MAGE-C2/CT10 mRNA expression were investigated in MM patients, solitary plasmacytomas, monoclonal gammopathies of undetermined significance (MGUS) and bone marrow (BM) aspirates from healthy donors by RT-PCR. MAGE-C1/CT7 and MAGE-C1/CT10 were expressed in 67 and 59 % of the 46 MM analyzed patients. At least one of the genes was expressed in 76 % of MM cases. Solitary plasmacytoma also showed MAGE-C1/CT7 and MAGE-C2/CT10 expression. MAGE-C1/CT7 and MAGE-C2/CT10 were not expressed in normal BM samples, showing restricted expression of these CTA genes in MM, solitary plasmacytoma and MGUS. In the present study, we found high expression of the homologs MAGE-C1/CT7 and MAGE-C2/CT10 in monoclonal gammopathies and speculate whether these genes might represent a valuable therapeutic option for myeloma, in particular for combined immunotherapy.
Keywords: MAGE-C1/CT7, MAGE-C2/CT10, Cancer/testis antigen, Conventional RT-PCR, Monoclonal gammopathies
Introduction
Multiple myeloma (MM) is a malignant proliferation of B-cells with plasma cell differentiation [1]. MM accounts for 10 % of all blood malignancies and 1 % of all cancers. The number of new cases diagnosed each year with MM in the United States is approximately 20,000 cases [2, 3]. MM is considered incurable, with median overall survival of 7–8 years, after the recent advances in chemotherapy and autologous stem cells transplant [4]. In MM, distinct clinical phases can be recognized, including monoclonal gammopathy of undetermined significance (MGUS) and solitary plasmacytoma. Although these other monoclonal gammopathies do not present the same clinical features of MM, they share some genetic features of myeloma [5].
There are evidences that small fractions of myeloma cells survive after treatment, remaining undetected by conventional methods and being responsible for recurrence of disease. These cell fractions are considered to be attractive targets for immunotherapy, which can be recognized and destroyed by cytotoxic T lymphocytes (CTL) [6]. Vaccines formulated with antigens associated with myeloma cells can instruct the immune system to eliminate malignant cells, but identification of the best antigens expressed by MM is required [7].
Tumor-associated antigens were initially described in patients with melanoma and subsequently identified in several human malignant neoplasms. In normal somatic tissues, these antigens were identified in germ cells of testis, fetal ovary and placental cells (trophoblast) and are therefore being called cancer/testis antigens (CTAs) [6].
Many CTAs have been described in human tumors (CTA database—http://www.cta.lncc.br). Approximately 10 % of these antigens are located on chromosome X and often co-expressed in tumor cells. In normal human testis, CTAs are expressed in spermatocytes and act in meiosis, which justifies that aberrant expression of these antigens in tumor cells can lead to abnormal chromosomal segregation and aneuploidy [6, 8]. On the chromosome X, we can find the big MAGE family, and all their members have a 200 amino acids common domain, called to MHD (MAGE homology domain), and involved in protein–protein interactions. In tumor cells, they appear to contribute directly to the malignant phenotype and poor response to therapy. MAGE CTA genes are recognized by CTL and are strictly tumor-specific [6, 8, 9].
In MM, among the members of MAGE family, we can highlight the MAGE-C1/CT7 gene. MAGE-C1/CT7 gene represents the first member of a new subfamily (MAGE-C), and its expression seems to be restricted to malignant plasma cells in MM [7, 10]. In our previous study, MAGE-C1/CT7 gene was the most frequently expressed in MM patients with advanced stage and seems to have prognostic impact in overall survival [11].
MAGE-C2/CT10 gene, another member of the MAGE-C subfamily, was identified in melanoma cell lines and shows significant homology (>70 % nucleotide identity) with MAGE-C1/CT7 gene, and both CTA genes are located in the q26–27 region of chromosome X [10, 12–14].
Immunogenicity of MAGE-C1/CT7 and MAGE-C2/CT10 proteins has been previously shown in various human tumors, suggesting its potential role to evoke both humoral and cellular immune responses. Therefore, both CTA genes are considered highly immunogenic and have been described as a potential target for T cell-based immunotherapy [11, 15, 16].
In this study, we evaluated MAGE-C1/CT7 and MAGE-C2/CT10 genes expression in bone marrow (BM) aspirates collected from patients with MM, solitary plasmacytoma and MGUS and normal controls to evaluate potential combination of the homolog genes for immunotherapy in this disease by RT-PCR.
Materials and methods
Patients
BM aspirates from 46 MM patients, five solitary plasmacytomas and four MGUS were collected between 2009 and 2011 from Hospital São Paulo, UNIFESP/EPM, São Paulo, Brazil (Table 1). We used five normal bone marrow (BM) aspirates obtained from allogeneic stem cell transplant normal donors at the GRAACC, São Paulo, Brazil, for control experiments. This study was approved by the Ethics Committee of Hospital São Paulo, Universidade Federal de São Paulo, UNIFESP/EPM (#1230/09), and informed consent was obtained from all patients and normal BM donors.
Table 1.
Baseline clinical characteristics of patients with MM
| Number of patients | 46 |
|---|---|
| Sex (n) | |
| Male | 25 |
| Female | 21 |
| Median age, years (range) | 65 (27–95) |
| Paraprotein isotype (n) | |
| IgA | 9 |
| IgG | 25 |
| IgM | 1 |
| LC | 4 |
| NA | 7 |
| Light chain | |
| λ | 15 |
| k | 31 |
| Mean tumor cells | |
| Percentage (range) | 65 % (10–100) |
| Durie-Salmon stage | |
| IA | 2 |
| IIA | 1 |
| IIIA | 25 |
| IIIB | 18 |
| ISS | |
| 1 | 6 |
| 2 | 13 |
| 3 | 23 |
| NA | 4 |
MM multiple myeloma, LC light chain, ISS international staging system, NA not available
RT-PCR
RNA was prepared from total BM aspirates using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Two micrograms of total RNA was reverse-transcribed with SuperScript III Reverse Transcriptase (Invitrogen). MAGE-C1/CT7 and MAGE-C2/CT10 were analyzed by RT-PCR (reverse transcription-polymerase chain reaction) and 2 % agarose gel electrophoresis and visualized by SYBR® Safe DNA gel stain (Invitrogen). Normal testis was used as template for positive control in all RT-PCRs. PCRs were performed using Platinum Taq DNA Polymerase (Invitrogen). The PCR steps performed in an Applied Biosystems GeneAmp PCR 9700 thermocycler, and cycle conditions are initial denaturation at 94 °C for 2 min, 35 cycles of denaturation 45 s at 94 °C, annealing 63 °C, extending 1 min at 72 °C and final extending at 72 °C for 7 min [11]. The sequences of MAGE-C1/CT7 primers were: forward 5′-GACGAGGATCGTCTCAGGTCAGG-3′ and reverse 5′-ACATCCTCACCCTCAGGAGGG-3′ [11], and the sequences of MAGE-C2/CT10 primers were: forward 5′-CGGATCGAAGGCATTTGTGAG 3′ and reverse 5′-CATGAACTCACGGGCTCTCTTG-3′ [17]. The RT-PCRs were performed in duplicate, and at least three independent experiments were performed for each sample.
Results
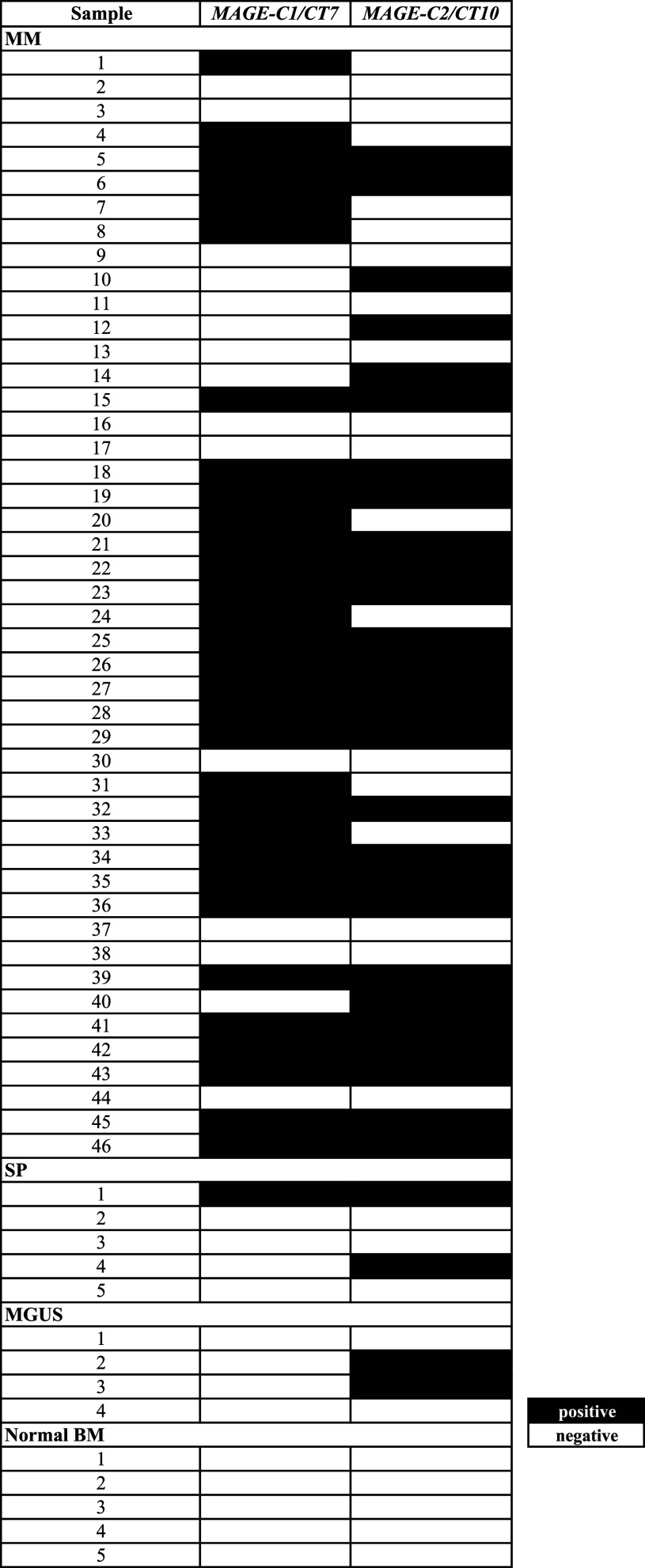
For the 46 MM analyzed samples, MAGE-C1/CT7 was positive in 67 % (31/46) and MAGE-C2/CT-10 in 59 % (27/46) of the cases. In 23 out of 46 MM cases (50 %), both CTAs genes were positive, and in 35 out of 46 samples (76 %), the expression of at least one of the genes could be detected by RT-PCR (Table 1). Solitary plasmacytoma, MAGE-C1/CT7 and MAGE-C2/CT10 genes were expressed in at least one of the analyzed samples. MAGE-C2/CT10 was expressed in 50 % (2/4) of the small sample size MGUS analyzed. All BM aspirates collected from healthy donors were negative for both CTA genes evaluated (Table 2).
Table 2.
MAGE-C1/CT7 and MAGE-C1/CT10 expression in MM, SP, MGUS and normal BM samples by RT-PCR
MM multiple myeloma, SP solitary plasmacytoma, MGUS monoclonal gammopathy of undetermined significance, BM bone marrow
We did not find an association between the clinical characteristics of the 46 MM patients studied and the percentage of MAGE-C1/CT7 and MAGE-C2/CT10 CTAs expressed by RT-PCR (Table 1). Considering the International Staging System (ISS), the data show that expression of both CTA genes was widely expressed in MM samples studied, independently on the stage of disease (Table 1).
Discussion
Using conventional RT-PCR, we found MAGE-C1/CT7 and MAGE-C1/CT10 mRNA expression in 67 and 59 % of the 46 MM analyzed patients. At least one of the genes was expressed in 76 % of myeloma cases. Although the number of patients analyzed for other monoclonal gammopathies (solitary plasmacytoma and MGUS) was small, we also showed MAGE-C1/CT7 and MAGE-C2/CT10 expression in some of these patients. These findings suggest that both CTA genes can have a biological role in MM distinct clinical phases and maybe contribute to malignant plasma cell development. MAGE-C1/CT7 and MAGE-C2/CT10 were not expressed in normal BM samples, showing restricted expression of these CTA genes in monoclonal gammopathies (MM, solitary plasmacytoma and MGUS).
MAGE-C1/CT7 expression is testis-restricted CTA, and MAGE-C2/CT10 expression is testis/brain-restricted (Purkinje cells of the normal cerebellum) by the current classification [18, 19], and several studies have shown that these CTA genes are expressed in a wide variety of human tumors [6, 11, 17]. In the pathophysiology of MM, the exact function of MAGE-C1/CT7 and MAGE-C2/CT10 is not yet understood [20].
Functional studies for the MAGE-C1/CT7 gene, using gene silencing (transient and stable) by RNAi, have suggested that this CTA gene is involved in the survival of myeloma cells, protecting malignant plasma cells against spontaneous as well as decreasing apoptosis induced by chemotherapy drugs [20, 21]. Studies have demonstrated that MAGE-C1/CT7 gene expression may be related to myeloma progression because of its high expression in the MM samples in advanced stage [7, 11].
The high expression of MAGE-C1/CT7 gene has also been observed in MM patients post therapy and which had relapsed. Quantitative analysis of MAGE-C1/CT7 gene expression by qPCR (quantitative PCR) has also been demonstrated a relationship between the presence of this CTA gene and disease burden following therapy [17]. MAGE-C1/CT7 is unique compared to MAGE family proteins because this CTA gene has, in the amino-terminal region, a segment composed of tandem repeats presenting several SNPs (single nucleotide polymorphisms) and to leading a distinct conformation [6]. Heterogeneity in the expression of MAGE-C1/CT7 protein has been observed in myeloma patients by immunohistochemistry, and this variation seems to be correlated with survival and proliferation [17]. Several studies have identified and characterized naturally occurring MAGE-C1/CT7-specific CD8+ T lymphocytes in patients with myeloma expressing this CTA gene, suggesting a strong immunogenicity and confirming its role as a potential target for immunotherapy in MM [6, 22].
The MAGE-C2/CT10 gene is homologous to MAGE-C1/CT7 gene (73–77 % nucleotide identity). The mainly difference between the two CTA genes is that MAGE-C2/CT10 does not have the large repeated region which is found in the coding region (exon 4) of MAGE-C1/CT7. The coding region of MAGE-C2/CT10 is located in exon 3, and the translated protein is 56 % identical to MAGE-C1/CT7 protein [13, 14]. MAGE-C2/CT10 encodes for an alternative splicing isoform named MAGE-C2/CT10M but does not affect translation of protein, and it seems that all tumors expressing MAGE-C1/CT10 have also MAGE-C2/CT10M isoform expressed, although at a lower level [23]. It was observed that multiple MAGE proteins, including human MAGE-C2/CT10 protein, form complexes with Kap-1, co-repressor of p53, and the silencing these MAGE genes by siRNA induced apoptosis and increased expression of p53 in vitro [24].
In a melanoma patient, it was demonstrated the MAGE-C2/CT10 antigenic peptides were recognized by patient’s cytotoxic T cells. In the presence of INF-γ, they lead to tumor regression, confirming this CTA gene as a potential target for vaccine in cancer [15].
In myeloma, it was demonstrated that the level of MAGE-C1/CT7 and MAGE-C1/CT10 expression was considerably low in bone marrow (BM) of MM patients that expressed these CTA genes after autologous stem cell transplantation (autoSCT) and allogeneic stem cell transplantation (alloSCT) [17]. MAGE-C1/CT7 and MAGE-C2/CT10 proteins were also found frequently expressed in paraffin-embedded tissue samples of bone lesions derived from MM patients who had submitted surgical interventions and bone marrow of MM patients, suggesting that these CTAs proteins could be used for diagnostic, prognostic and immunotherapy in myeloma [25].
In summary, the present study confirmed MAGE-C1/CT7 and MAGE-C2/CT7 expression in monoclonal gammopathies. The high-frequency found in MM patients suggests the subfamily of MAGE-C on the development of myeloma. Although the function of these genes is still poorly understood, several studies have shown that MAGE-C1/CT7 and MAGE-C2/CT10 genes are able to elicit spontaneous immune responses and therefore can be considered potential targets for cell therapy in this incurable disease. Myeloma cells have the potential of presenting antigens directly to T lymphocytes. However, this ability is limited, and thus, the antigen presentation of CTA proteins can be cross-priming potentialized by dendritic cells [6]. Dendritic cells (DCs) are known to be the only cells able to induce the activation of naïve tumor-specific T lymphocytes, and currently, the number of clinical trials studies using tumor antigen-loaded DCs as cellular immunotherapy in cancer patients has been increasing [26]. Finally according to this and other studies previously published, we could speculate that targeting MAGE-C1/CT7 and MAGE-C2/CT10 genes might represent a valuable therapeutic option for myeloma, in particular when applied in cell immunotherapy and specific-tumor vaccines.
Acknowledgments
We are grateful to Dr. Adriana Seber (Instituto de Oncologia Pediatrica—GRAACC, UNIFESP, São Paulo, Brazil) for normal bone marrow aspirates collected from healthy donors.
Conflict of interest
The authors have declared no conflict of interest.
References
- 1.Pingali SR, Haddad RY, Saad A. Current concepts of clinical management of multiple myeloma. Dis Mon. 2012;58(4):195–207. doi: 10.1016/j.disamonth.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87(1):78–88. doi: 10.1002/ajh.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philips S, Menias C, Vikram R, et al. Abdominal manifestations of extraosseous myeloma: cross-sectional imaging spectrum. J Comput Assist Tomogr. 2012;36(2):207–212. doi: 10.1097/RCT.0b013e318245c261. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KC. New insights into therapeutic targets in myeloma. Hematol Am Soc Hematol Educ Program. 2011;2011:184–190. doi: 10.1182/asheducation-2011.1.184. [DOI] [PubMed] [Google Scholar]
- 5.Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 6.de Carvalho F, Vettore AL, Colleoni GW. Cancer/testis antigen MAGE-C1/CT7: new target for multiple myeloma therapy. Clin Dev Immunol. 2012;2012:257695. doi: 10.1155/2012/257695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jungbluth AA, Ely S, DiLiberto M, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106(1):167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 8.Simpson AJ, Caballero OL, Jungbluth A, et al. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5(8):615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 9.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. J Immunol. 1991;178(5):2617–2621. [PubMed] [Google Scholar]
- 10.Lucas S, De Smet C, Arden KC, et al. Identification of a new MAGE gene with tumor-specific expression by representational difference analysis. Cancer Res. 1998;58(4):743–752. [PubMed] [Google Scholar]
- 11.Andrade VC, Vettore AL, Felix RS, et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YT, Güre AO, Tsang S, et al. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc Natl Acad Sci USA. 1998;95(2):6919–6923. doi: 10.1073/pnas.95.12.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Güre AO, Stockert E, Arden KC, et al. CT10: a new cancer cancer-testis (CT) antigen homologous to CT7 and the MAGE family, identified by representational-difference analysis. Int J Cancer. 2000;85(5):726–732. doi: 10.1002/(SICI)1097-0215(20000301)85:5<726::AID-IJC21>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Lucas S, De Plaen E, Boon T. MAGE-B5, MAGE-B6, MAGE-C2, and MAGE-C3: four new members of the MAGE Family with tumor-specific expression. Int J Cancer. 2000;87(1):55–60. doi: 10.1002/1097-0215(20000701)87:1<55::AID-IJC8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Ma W, Vigneron N, Chapiro J, et al. A MAGE-C2 antigenic peptide processed by the immunoproteasome is recognized by cytolytic T cells isolated from a melanoma patient after successful immunotherapy. Int J Cancer. 2011;129(10):2427–2434. doi: 10.1002/ijc.25911. [DOI] [PubMed] [Google Scholar]
- 16.Curioni-Fontecedro A, Nuber N, Mihic-Probst D, et al. Expression of MAGE-C1/CT7 and MAGE-C2/CT10 predicts lymph node metastasis in melanoma patients. PLoS ONE. 2011;6(6):e21418. doi: 10.1371/journal.pone.0021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atanackovic A, Luetkens T, Hildebrandt Y, et al. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin Cancer Res. 2009;15(4):1343–1352. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann O, Caballero OL, Stevenson BJ, et al. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci USA. 2008;105(51):20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oba-Shinjo SM, Caballero OL, Jungbluth AA, et al. Cancer-testis (CT) antigen expression in medulloblastoma. Cancer Immun. 2008;8:7. [PMC free article] [PubMed] [Google Scholar]
- 20.de Carvalho F, Costa ET, Camargo AA, et al. Targeting MAGE-C1/CT7 expression increases cell sensitivity to the proteasome inhibitor bortezomib in multiple myeloma cell lines. PLoS ONE. 2011;6(11):e27707. doi: 10.1371/journal.pone.0027707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atanackovic D, Hildebrandt Y, Jadczak A, et al. Cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 promote the survival of multiple myeloma cells. Haematologica. 2010;95(5):785–793. doi: 10.3324/haematol.2009.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson LD, Jr, Cook DR, Yamamoto TN, et al. Identification of MAGE-C1 (CT-7) epitopes for T-cell therapy of multiple myeloma. Cancer Immunol Immunother. 2011;60(7):985–997. doi: 10.1007/s00262-011-1009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W, Germeau C, Vigneron N, et al. Two new tumor-specific antigenic peptides encoded by gene MAGE-C2 and presented to cytolytic T lymphocytes by HLA-A2. Int J Cancer. 2004;109(5):698–702. doi: 10.1002/ijc.20038. [DOI] [PubMed] [Google Scholar]
- 24.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100(11):2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pabst C, Zustin J, Jacobsen F, et al. Expression and prognostic relevance of MAGE-C1/CT7 and MAGE-C2/CT10 in osteolytic lesions of patients with multiple myeloma. Exp Mol Pathol. 2010;89(2):175–181. doi: 10.1016/j.yexmp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Van Nuffel AM, Benteyn D, Wilgenhof S, et al. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol Immunother. 2011;61(7):1033–1043. doi: 10.1007/s00262-011-1176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]