Abstract
The capacity of natural killer (NK) cells to kill tumor cells without specific antigen recognition provides an advantage over T cells and makes them potential effectors for tumor immunotherapy. However, the efficacy of NK cell adoptive therapy can be limited by the immunosuppressive tumor microenvironment. Transforming growth factor-β (TGF-β) is a potent immunosuppressive cytokine that can suppress NK cell function. To convert the suppressive signal induced by TGF-β to an activating signal, we genetically modified NK-92 cells to express a chimeric receptor with TGF-β type II receptor extracellular and transmembrane domains and the intracellular domain of NK cell-activating receptor NKG2D (TN chimeric receptor). NK-92 cells expressing TN receptors were resistant to TGF-β-induced suppressive signaling and did not down-regulate NKG2D. These modified NK-92 cells had higher killing capacity and interferon γ (IFN-γ) production against tumor cells compared with the control cells and their cytotoxicity could be further enhanced by TGF-β. More interestingly, the NK-92 cells expressing TN receptors were better chemo-attracted to the tumor cells expressing TGF-β. The presence of these modified NK-92 cells significantly inhibited the differentiation of human naïve CD4+ T cells to regulatory T cells. NK-92-TN cells could also inhibit tumor growth in vivo in a hepatocellular carcinoma xenograft tumor model. Therefore, TN chimeric receptors can be a novel strategy to augment anti-tumor efficacy in NK cell adoptive therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-1959-1) contains supplementary material, which is available to authorized users.
Keywords: NK-92 cell, TGF-βR II, NKG2D, Chimeric receptor, Immunotherapy
Introduction
Natural killer (NK) cells are innate immune cells that lyse tumor cells in a non-major histocompatibility complex (MHC) restricted manner. The capacity of NK cells to kill tumor cells is regulated by the inhibitory and stimulatory signals transduced by surface receptors. Inhibitory signals result from the interaction between NK inhibitory receptors and human leucocyte antigen (HLA) molecules on target cells. Engagement of activating receptors by ligands expressed predominantly by virus-infected cells or tumor cells leads to direct cytotoxicity. Although NK cell therapy is a promising strategy for tumor immunotherapy, the reported results of the clinical trials are less impressive [1, 2]. The in vivo function of the adoptively transferred NK cells could be inhibited by immunosuppressive tumor microenvironment and the insufficient infiltration of the NK cells to the tumor sites [3].
Transforming growth factor-β (TGF-β), a cytokine produced by tumors and almost all immunologic cell types, can regulate proliferation, differentiation, embryonic development, angiogenesis, wound healing, and other functions in many cell types [4]. TGF-β plays an important role in tumor initiation and progression and is one of the major immunosuppressive cytokines in the tumor microenvironment [5, 6]. TGF-β signals regulate a large number of biological processes via activating protein kinase receptors and SMAD mediators [7]. TGF-β induces differentiation of regulatory T (Treg) and Th17 cells and inhibits B-cell proliferation and immunoglobulin A (IgA) secretion. TGF-β can inhibit the function of NK cells and CD8+ T cells via blocking the cytotoxic key proteins, such as perforin and granzymes. Recent studies also demonstrate that TGF-β downregulates the activating receptor NK Group 2 member D (NKG2D) on NK cells and CD8+ T cells thereby inhibits NK cell activation [8, 9]. Neutralizing TGF-β enhances CD8+ T cell and NK-cell-mediated anti-tumor immune responses [10].
NK-92 is a highly activated NK cell line which was originally established from a non-Hodgkin’s lymphoma that had NK cell-like morphology and expressed CD56 but not CD3 or CD16 [11]. The NK-92 cells do not express the inhibitory killer cell Ig-like receptors (KIR) and thus exhibit strong cytotoxicity against tumor cells that express MHC class I molecules [12, 13]. They can be expanded substantially in the presence of interleukin-2 (IL-2) without the need for allogeneic feeder cells, which makes them suitable for adoptive immunotherapy.
NKG2D is a type II transmembrane-anchored C-type lectin-like protein expressed on all NK cells and some T cell subsets. It is a key activation receptor of NK cells. NKG2D is associated with DNAX-activating protein 10 (DAP10), which promotes and stabilizes its surface membrane expression. NKG2D signal can significantly promote the cytotoxicity of NK cells [14]. In the current study, we genetically modified NK-92 cells to express extracellular and transmembrane domains of TGF-β receptor II with intracellular domain of NKG2D (TN chimeric receptor). NK-92 cells expressing this chimeric receptor are resistant to suppressive TGF-β signaling. Most importantly, they can be activated through NKG2D pathway by TGF-β, and chemo-attracted to TGF-β-expressing tumor cells, as well as suppress the differentiation of regulatory T cells.
Materials and methods
Cells and culture conditions
HEK293T cell line, hepatocellular carcinoma cell lines SMMC7721, SK-HEP-1, and prostate cancer cell line PC-3 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in Dulbecco-modified Eagle medium (DMEM) (Gibco, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (FBS, Biological Industries, Israel), 2 mM L-glutamine, 100 units/ml penicillin, and 100 units/ml streptomycin (Gibco, Carlsbad, CA). Human NK-92 cell line (gift from Prof. Feili Gong at Tongji Medical College of Huazhong University of Science and Technology), NK-92-TN, and NK-92-Vector cell lines were maintained in minimum essential medium alpha (α-MEM) containing heat-inactivated 12.5% horse serum (Gibco, Carlsbad, CA), 12.5% heat-inactivated FBS (Biological Industries, Israel), 0.1 mM 2-mercaptoethanol (Sigma–Aldrich, St Louis), and 200 U/ml recombinant human IL-2 (Beijing Four Rings Bio-Pharmaceutical, China).
Generation of NK-92 cells expressing chimeric antigen receptors
Human cDNA was obtained from peripheral blood. TGF-β receptor II and NKG2D were amplified from cDNA by polymerase chain reaction (PCR) with the primers as follows: TGF-β receptor II, forward 5′-GGATCCATGGGTCGGGGGCTGCTCAG-3′; reverse 5′-TCCACCCCATGTAGCAGTAGAAGATGATG-3′; NKG2D, forward 5′-CTACTGCTACATGGGGTGGATTCGTGGTC-3′; reverse 5′-GGATCCTTAAGATGCATTTTCTCTACATTTG-3′. The productions of PCR were fused, digested with BamHI, and ligated into the lentiviral vector pRRL-Venus at the same restriction enzyme sites. HEK293T cells were cultured in 10 cm cell culture dishes and achieved 50–80% confluence 18 h to 24 h later. The recombinant plasmid pRRL-Venus-TN and three packaging plasmids [ΔR, Rev, vesicular stomatitis virus G (VSV-G)] were co-transfected into the cells by 2*HBS and CaCl2. HEK293T cells producing lenti-virus were grown overnight in DMEM, and then, the supernatant was taken out and fresh media were added. After 20 h of culture, the cultured supernatant was directly added to NK-92 cells, and incubated with NK-92 cells for 20 h at 37 °C. Then, the cells were grown for 48 h in fresh NK-92 medium. The infected NK-92 cells were analyzed for yellow fluorescent protein (YFP) expressions and sorted by FACS Aria™ III flow cytometer (BD Biosciences, San Jose, CA).
Flow cytometry
Cell-surface expressions of TGF-βR II and NKG2D were determined by fluorescence-activated cell sorter (FACS) analysis. Single-cell suspensions of NK-92, NK-92-TN, or control NK-92-Vector cells were incubated for 30 min at 4 °C with monoclonal antibody (mAb) against TGF-βR II (Abcam, Cambridge, MA). Cells were washed twice with PBS and then incubated for another 1 h at 4 °C with a fluorescein phycoerythrin (PE)—labeled goat anti-mouse IgG (Multisciences, Hangzhou, China) secondary antibody. Cells were washed twice with PBS and analyzed with FACS Calibur (BD Bioscience, San Jose, CA). For NKG2D expression, NK-92-TN or control NK-92-Vector cells were collected, washed with PBS then counted, and seeded in 96-well-U-bottom plates, in the presence of 200 U/ml IL-2 and various concentration of TGF-β1. Cells were then incubated with APC-conjugated anti-NKG2D mAbs (Biolegend, San Diego, CA). Cells were analyzed by flow cytometry on a FACS Calibur (BD Bioscience, San Jose, CA). For intracellular staining of IFN-γ, NK-92-Vector and NK-92-TN cells were first treated with 5 ng/ml phorbol myristate acetate (PMA) and 50 ng/ml ionomycin (Beyotime, Shanghai, China) and with or without TGF-β (Peprotech, Rocky Hill, NJ) in the presence of 4 mM Brefeldin A (BFA) (eBioscience, San Diego, CA) for 4 h. They were fixed in 4% formaldehyde (Sinopharm, Shanghai, China) for 30 min and permeabilized with saponin (Sigma–Aldrich, St Louis, MO) for 15 min at room temperature. Cells were then incubated with 0.2 ug APC-labeled anti-human IFN-γ (Biolegend, San Diego, CA) in the dark for 30 min at 4 °C. Cells were then washed and analyzed with a FACS Calibur (BD Biosciences, San Jose, CA).
For apoptosis analysis, NK-92-Vector or NK-92-TN cells were seeded into six-well plates at 2 × 106 cells/2 ml in each well. Human recombinant TGF-β1 (Peprotech, Rocky Hill, NJ) was used in treating cells at the concentration of 10, 50, and 100 ng/ml. Lethally irradiated SMMC-7721 cells were also co-cultured with NK-92-Vector or NK-92-TN cells. 24 h later, cells were collected and centrifuged at 1000 r/min for 5 min and the supernatant was discarded. Cells were suspended gently by PBS and counted. 1 × Annexin V bind buffer was prepared. Then, 200 μL of the buffer was added into every tube to suspend cells. The mixed solution of 5 μL 7-AAD and 5 μl Annexin V-PE (Biolegend, San Diego, CA) was added into every tube. After incubated in dark place at room temperature for 15 min, 200 μl buffer was added into every tube and flow cytometry was performed with a FACS Calibur (BD Biosciences, San Jose, CA).
Western blotting
NK-92-TN or control NK-92-Vector cells (1 × 106/ml) were stimulated with TGF-β1 (50 ng/ml, Peprotech, Rocky Hill, NJ, USA) for 16–20 h, and the cells were collected and extracted for cytoplasm and membrane proteins (Beyotime, Shanghai, China). The extracted proteins were separated by SDS–PAGE on 12.5% resolving gel and 5% stacking gel. Then, the proteins on the gel were transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Darmstadt, Germany). The transferred membrane was blocked with 5% non-fat milk in PBS at 4 °C overnight, which was then incubated with primary antibody against Smad2 & Phospho-Smad2 rabbit mAb (Cell Signaling Technology, Danvers, MA, USA) diluted in PBS with 0.1% Tween-20 for 2.5 h at room temperature. Then, it was washed four times for 10 min each in PBS with 0.1% Tween-20 and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Multisciences, Hangzhou, China) for 1.5 h at room temperature. The membrane was then washed and developed with the enhanced chemiluminescent system (Mutisciences, Hangzhou, China).
Quantitative real-time PCR analysis
mRNA of NK-92-Vector and NK-92-TN cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA was reversely transcribed into cDNA. Quantitative real-time PCR (RT-PCR) for CCR1, CCR3, CCR6, CXCR3, CXCR4, CX3CR1, NKG2A, NKG2C, NKp30, NKp44, and NKp80 was performed using a Mastercycler ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) and Absolute qPCR SYBR Green mix (Thermo Fisher, Waltham, MA). Primers used were listed in supplemental Table 1.
CCK8 proliferation assay
NK92-Vector or NK92-TN cells were seeded into 96-well plates at 1 × 105cells/100 ul in each well. Human recombinant TGF-β1 (Peprotech, Rocky Hill, NJ) was used in treating NK92-Vector or NK92-TN cells at the concentration of 10, 50 and 100 ng/ml. Lethally irradiated SMMC-7721 cells were also co-cultured with NK92-Vector or NK92-TN cells. A total of 10 μl CCK8 solution was added into every well of 96-well plate after culturing for 24 h. The culture plate was incubated in an incubator for 2 h. A microplate reader was employed to determine the absorbance at 450 nm.
Cytotoxicity assays
Cytotoxicity of NK-92-TN cells against tumor cells was determined by the lactic dehydrogenase (LDH)-release assay using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Fitchburg, WI). The assay is based on the measurement of lactate dehydrogenase that is released upon cell lysis. Briefly, the NK-92-TN and NK-92-Vector cells were resuspended in α-MEM complete medium and mixed with tumor cells in U-bottom 96-micro well plates at various E:T ratios in triplicate. Microplates were incubated at 37 °C with 5% CO2 for 5 h and spun for 4 min at 250 g to pellet cells. Supernatant (50 μl) was collected from each well and added to 50 μl reconstituted substrate mix for 30 min in the dark at room temperature. Enzymatic reaction was stopped by adding stop solution. Absorbance was recorded at 490 nm. Spontaneous release was determined from wells with targets only and total release from wells with targets plus 1% Triton X-100 (Sinopharm, Shanghai, China). Results are expressed as percentage of cytotoxicity, using the formula: percentage of cytotoxicity = (experimental-effector spontaneous − target spontaneous)/(target maximum − target spontaneous) × 100%.
Regulatory T cell differentiation
Human lymphocytes were isolated from umbilical cord blood using Ficoll density gradient centrifugation (Haoyang Biotech, Tianjin, China). CD4+ T cells were separated by MACS sorting (Miltenyi, Bergisch Gladbach, Germany). Cells were cultured at 1 × 106 cell/ml in anti-human CD3, anti-CD28-coated (1 ug/ml BD Biosciences, San Diego, CA) 96-well-U-bottom plates, with recombinant human TGF-β (5 ng/ml Peprotech, Rocky Hill, NJ), r-human IL-2 (100 U/ml, Four Rings Bio-Pharmaceutical, Beijing, China). NK-92-Vector and NK-92-TN cells were harvested, washed with PBS, and seeded with the CD4+ T cells in 96-well-U-bottom plates with an NK/T ratio at 5:1. RPMI1640 media were added every 2 days with the same concentrations of cytokines. After 1 week, the percentages of regulatory T cells were analyzed by flow cytometry. Briefly, cells were stained with FITC-labeled anti-human CD4 and PE-labeled anti-human CD25 (BD Biosciences, San Diego, CA) for 30 min at 4 °C. After fixation and permeabilization, APC-labeled anti-human Foxp3 (eBioscience, San Diego, CA) was added and incubated for 30 min at 4 °C. Cells were then washed and analyzed by FACS Calibur (BD Biosciences, San Jose, CA, USA). The apoptosis of induced regulatory T cells was measured using the method described in “Flow cytometry” section. In the apoptosis assay, regulatory T cells were also stained with PE-labeled anti-human CD127 (Biolegend, San Diego, CA) and APC-Cy7-labeled anti-human CD25 (Biolegend, San Diego, CA).
Transwell assay for cell migration
1 × 105 SMMC7721 cells were cultured overnight in 600 ul DMEM per well in the lower chambers of the transwell plates. Anti-human TGF-β1 (Biolegend, San Diego, CA) or isotype control (Biolegend, San Diego, CA) was added into the lower chambers. Then, 2 × 105 (100 ul) NK-92-TN or NK-92-Vector cells were added to the upper chambers. 24 h later, we counted the number of NK-92-TN and NK-92-Vector cells in the low chambers by flow cytometry.
Animal model
4–5-week-old athymic nude mice were obtained from Shanghai Laboratory Animal Center (Shanghai, China). They were kept in a specific pathogen-free facility. All animal protocols were approved by Institutional Laboratory Animal Care and Use Committee of Soochow University. 2 × 106 hepatocellular carcinoma SMMC7721 cells were injected into nude mice subcutaneously. On day 10 after tumor cell inoculation, tumor-bearing mice were treated with 3 Gy total body irradiation (0.16 Gy/min), then 5 × 106 NK-92-TN or NK-92-Vector cells suspended in 0.1 ml PBS or PBS alone were injected intravenously. Tumors were measured, and tumor volumes were calculated using the formula: (length × (width)2)/2. On day 28, mice were sacrificed and tumors were separated and weighed. The tumor infiltrating NK-92 cells were analyzed 5 days after adoptive transfer by flow cytometry (7AAD negative and YFP positive).
Results
Generation of NK-92 cells expressing chimeric TN antigen receptors
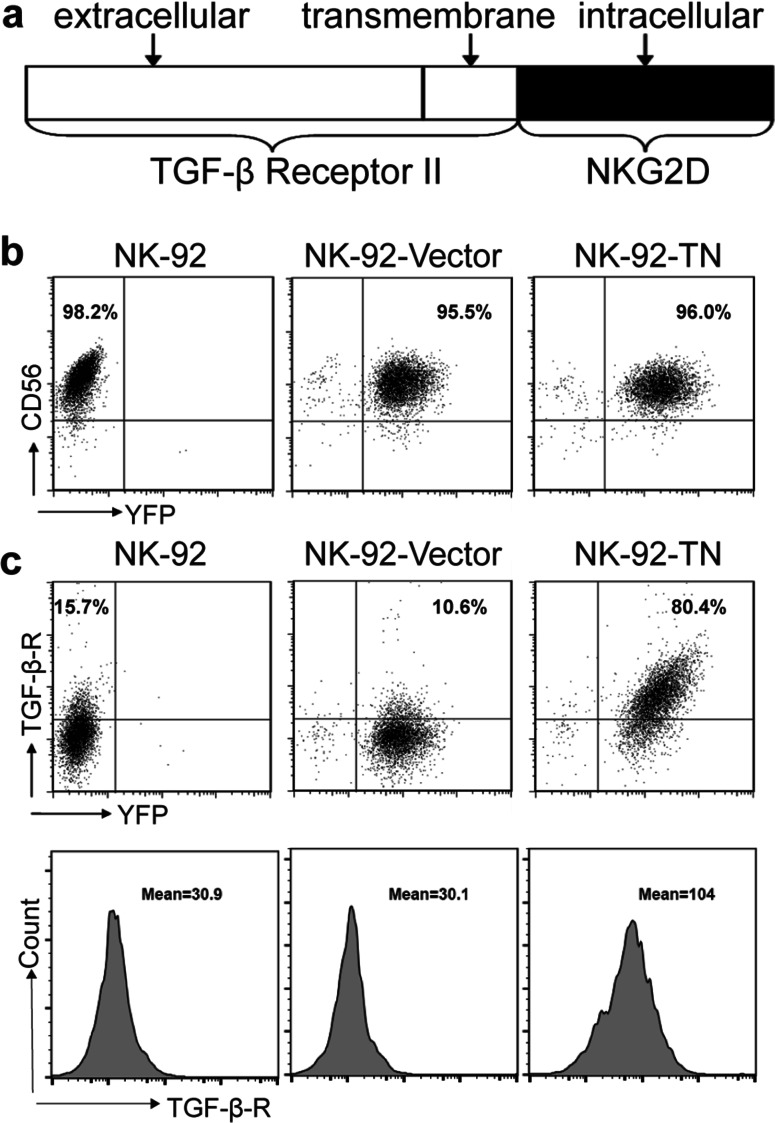
To generate human NK-92 cells expressing chimeric TN antigen receptor, NK-92 cells were infected with lenti-virus expressing pRRL-TN-Venus construct (Fig. 1a) or the control vector. The pRRL-TN-Venus construct contained the extracellular and transmembrane domains of TGF-βR II and the intracellular domain of NKG2D. The infected cells, denoted as NK-92-TN or NK-92-Vector cells, were sorted by FACS, and surface expressions of CD56 (Fig. 1b) and TGF-βR II (Fig. 1c) were analyzed by FACS analysis. The parental and the infected NK-92 cells expressed high level of CD56 (Fig. 1b). As shown in Fig. 1c, the parental and the NK-92 cell infected with the vector had only moderate levels of TGF-βR II expression (15.7 and 10.6%), while NK-92-TN cells expressed high level of TGF-βR II on the cell surface (80.4%). The mean fluorescent intensity of TGF-βR II was also significantly higher on NK-92-TN cells than that on the parental and vector control cells (mean fluorescent intensity (MFI): 104, 30.9, and 30.1, respectively). These results suggested that the chimeric TN receptors were successfully expressed on the NK-92-TN cells.
Fig. 1.
Generation of NK-92 cells expressing chimeric TN antigen receptors. a Schematic representation of the TN chimeric receptor. TN chimeric receptor includes extracellular and transmembrane domains of TGF-βR II and intracellular domain of NKG2D. b Expression of YFP and CD56 in NK92, NK-92-TN, and NK-92-Vector cells analyzed by flow cytometry using PE-labeled anti-CD56 antibody. c Expression of YFP and surface TGF-βR in NK92, NK-92-TN, and NK-92-Vector cells analyzed by flow cytometry using PE-labeled anti-TGF-βR II antibody. The mean fluorescence of TGF- βR II expression was also shown. Data shown are the representatives of at least three independent experiments
NK-92-TN cells are resistant to TGF-β-induced suppression
To examine whether the TGF-β signaling pathway could still be activated in NK-92-TN cells, we analyzed the level of smad-2 phosphorylation in NK-92-TN and control cells. NK-92-TN and NK-92-Vector cells were treated with TGF-β overnight. Western blot results indicated that smad-2 was equally expressed in both cell lines (Fig. 2a). However, the phosphorylation level of smad-2 in NK-92-TN cells was significantly lower than that in the control cells (Fig. 2a). These results suggested that TGF-β could not activate the suppressive signaling pathway in NK-92-TN cells.
Fig. 2.
NK-92-TN cells are resistant to TGF-β induced suppression. a Western blot for total Smad-2 and phosphorylated Smad-2 in NK-92-TN and NK-92-Vector cells after TGF-β (50 ng/ml) treatment. b NK-92-TN and NK-92-Vector cells were treated with TGF-β (10 or 100 ng/ml), and the expression of YFP and NKG2D was analyzed with flow cytometry using APC-labeled anti-NKG2D antibody. c Quantitative results of the percentages and mean fluorescent intensity (MFI) of NKG2D+NK-92 cells. d Proliferation of NK-92-TN and NK-92-Vector cells in the absence or presence of TGF-β or tumor supernatants was analyzed by CCK8 staining. e Apoptosis of NK-92-TN and NK-92-Vector cells in the absence or presence of TGF-β or tumor supernatants was measured by Annexin V and 7-AAD staining. Data shown are the representatives of at least three independent experiments. Results are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
TGF-β has been shown to down-regulate NKG2D expression in NK cells [8, 9]. To investigate whether TGF-β could affect NKG2D expression in NK-92-TN cells, flow cytometry was performed to determine the expression of NKG2D in NK-92-TN and control cells (Fig. 2b). TGF-β1 significantly down-regulated NKG2D expression in NK-92-Vector cells in a dose-dependent manner (from 33.5 to 22.5%), similar to the parental NK-92 cells (Supplemental Fig. 1; from 31.6 to 19.3%), while the NKG2D expression remained unchanged in both percent of positive cells (from 41.8 to 38.9%) and mean fluorescent intensity in NK-92-TN cells with TGF-β treatment (Fig. 2b, c). Therefore, these results demonstrated that NK-92-TN cells were resistant to TGF-β-induced suppression. Despite the changes in signaling pathway and NKG2D expression, the proliferation (Fig. 2d) and apoptosis (Fig. 2e) of both NK-92-TN and control cells were not significantly affected by the TGF-β treatment.
Cytotoxicity against tumor cells is enhanced in NK-92-TN cells
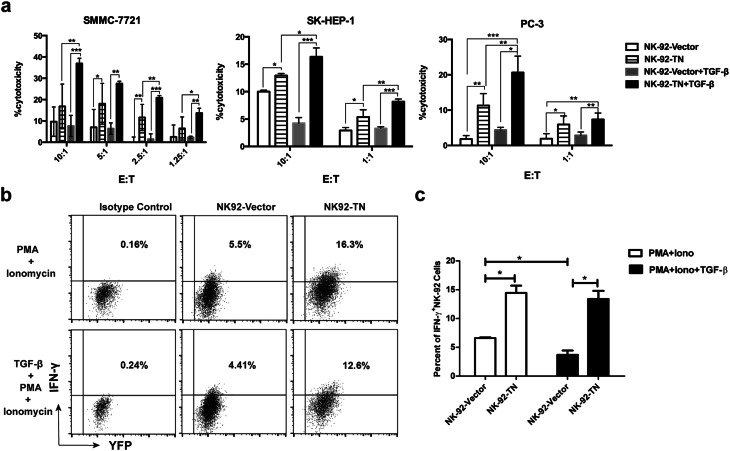
To investigate the NK-92-TN cells’ cytotoxicity against tumor cells, human hepatocellular carcinoma cell lines SMMC-7721, SK-HEP-1, and human prostate cancer cell line PC-3 were used as tumor targets in the cytotoxicity assay. NK-92-TN cells exhibited higher levels of cytotoxicity against all three cell lines than the control cells (Fig. 3a). When the cells were treated with TGF-β, the cytotoxicity of the control cells was either unchanged when it was already very low (SMMC-7721 and PC-3), or slightly suppressed when it was at a relatively higher level (SK-HEP-1). On the other hand, the killing capacities of NK-92-TN cells against all three tumor cell lines were significantly enhanced by TGF-β treatment.
Fig. 3.
Cytotoxicity against tumor cells is enhanced in NK-92-TN cells. a Cytotoxicity of NK-92-TN and NK-92-Vector cells with or without TGF-β treatment against SMMC7721, SK-HEP-1, and PC-3 tumor cells was analyzed by CytoTox 96 non-radioactive cytotoxicity assay. b IFN-γ production was analyzed by intracellular staining in NK-92-TN and NK-92-vector cells stimulated with 5 ng/ml PMA and 50 ng/ml ionomycin and with or without TGF-β in the presence of 4 mM BFA. c Quantitative analysis of percent of IFN-γ+NK-92 cells. Data shown are the representatives of at least three independent experiments. Results are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
IFN-γ is an important effector molecule produced by NK cells that has anti-tumor activity [15–17]. We analyzed IFN-γ production by NK-92-TN and NK-92-Vector cells by intracellular staining (Fig. 3b, c). NK-92-TN cells produced more IFN-γ than the control cells after PMA and ionomycin stimulation. TGF-β1 treatment significantly down-regulated IFN-γ production by NK-92-Vector cells, while the IFN-γ production by NK-92-TN cells was not affected. Therefore, the cytotoxicity and IFN-γ production of NK-92-TN cells were greatly enhanced compared with the control NK-92 cells.
NK-92-TN cells are chemo-attracted and migrate to the TGF-β1-producing tumor cells
TGF-β is one if the major suppressive cytokines in the tumor microenvironment. To investigate whether the TGF-βR-expressing NK-92-TN cells could be attracted by TGF-β and migrate to the tumor microenvironment, we established an in vitro experimental model. We seeded SMMC 7721 cells, which expressed high level of TGF-β (data not shown) in the lower chamber of the transwell overnight, then added NK-92-TN or NK-92-Vector cells in the upper chamber (Fig. 4a). The cells were cultured for 48 h to allow for migration and the cells that migrated to the lower chamber were counted (using YFP and morphology to distinguish between NK cells and tumor cells; gating strategy, as shown in Supplemental Fig. 2). The results showed that significantly more NK-92-TN than NK-92-Vector cells migrated to the lower chamber, and this enhanced migration was diminished by adding neutralizing anti-TGF-β antibodies (Fig. 4b). These results suggest that NK-92-TN cells were better attracted by the TGF-β-producing tumor cells than the control cells in a TGF-β-dependent manner.
Fig. 4.
NK-92-TN cells are chemo-attracted and migrate to the TGF-β1-producing tumor cells. a Schematic illustration of the experiments. SMMC7721 cells were seeded in the lower chambers and cultured overnight. NK-92-TN or NK-92-Vector cells were then seeded in the upper chamber. After 24h, NK-92-TN or NK-92-Vector cells that migrated to the lower chambers were counted by flow cytometry. b Quantitative analysis of the number of NK-92-TN or NK-92-Vector cells that migrated to the lower chambers in the presence or absence of anti-TGF-β antibodies (1 and 5 μg/ml). c Expressions of chemokine receptors on NK-92-TN or NK-92-Vector cells, including CCR1, CCR3, CCR6, CXCR3, CXCR4, and CX3CR1, were analyzed by real-time PCR. d Expression of NKG2A, NKG2C, NKp30, NKp44, and NKp80 on NK-92-TN or NK-92-Vector cells analyzed by real-time PCR. Data shown are the representatives of at least three independent experiments. Results are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
The expression of surface chemokine (Fig. 4c) and activating/inhibitory (Fig. 4d) receptors was also examined on both NK-92-TN and NK-92-Vector cells by real-time PCR. The expressions of CCR3, CCR6, CXCR4, CX3CR1, and NKG2C were increased, while NKp80 expression was slightly decreased on NK-92-TN cells. The overall profile of the receptor expressions suggested increased tissue migration and activation of NK-92-TN cells.
Presence of NK-92-TN cells inhibits Treg differentiation
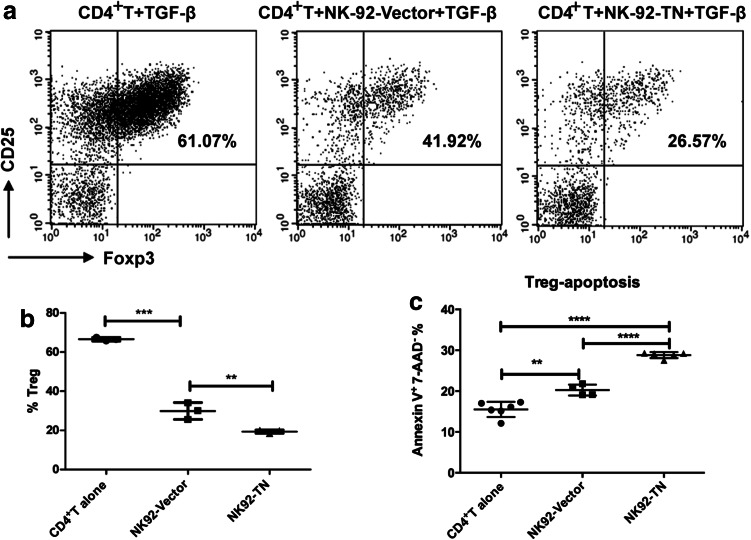
Regulatory T cells can be induced by TGF-β and suppress anti-tumor immune response [18]. To examine whether NK-92-TN cells could neutralize some of TGF-β and inhibit Treg differentiation, we co-cultured the NK-92-TN or NK-92-Vector cells with human naïve CD4+ T cells separated from cord blood in the presence of TGF-β. After 1 week, flow cytometry was performed to analyze CD25 and Foxp3 expressions to determine the percent of Tregs. After TGF-β induction, about 60% of the CD4+T cells became Tregs (Fig. 5a, b). Coculture with NK-92-Vector cells decreased the percent of Tregs, while coculture with NK-92-TN cells further decreased the percent of Tregs to less than 20%. This result indicated that the presence of NK-92-TN cells could inhibit Treg differentiation.
Fig. 5.
Presence of NK-92-TN cells inhibits regulatory T cell differentiation. a NK-92-TN or NK-92-Vector cells were co-cultured with human CD4+ T cells at 5:1 ratio in the presence of TGF-β for 1 week. The percent of regulatory T cells was analyzed by flow cytometry using PE-labeled anti-CD25 and APC-labeled anti-Foxp3 antibodies. b Quantitative analysis of the percent of regulatory T cells. c Quantitative analysis of the percent of apoptotic regulatory T cells examine by Annexin V and 7-AAD staining. Data shown are the representatives of at least three independent experiments. Results are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
We further examined whether the apoptosis of the Tregs could be affected by coculture with NK-92-TN or NK-92-Vector cells (Fig. 5c). The apoptotic Treg cells increased from 15% to about 20% in the coculture with NK-92-Vector cells, while it further increased to about 30% in the coculture with NK-92-TN cells. This result suggested that coculture with NK-92 cells could promote the apoptosis of the Tregs and the expression of chimeric receptors on NK-92 cells further enhanced this activity. Therefore, the reduced Treg differentiation by coculture with NK-92-TN cells could be due to the combined effects of neutralizing TGF-β and apoptosis induction of the Tregs.
Adoptive transfer of NK-92-TN cells slightly augmented the anti-tumor effect of NK-92 cells in xenograft tumor model
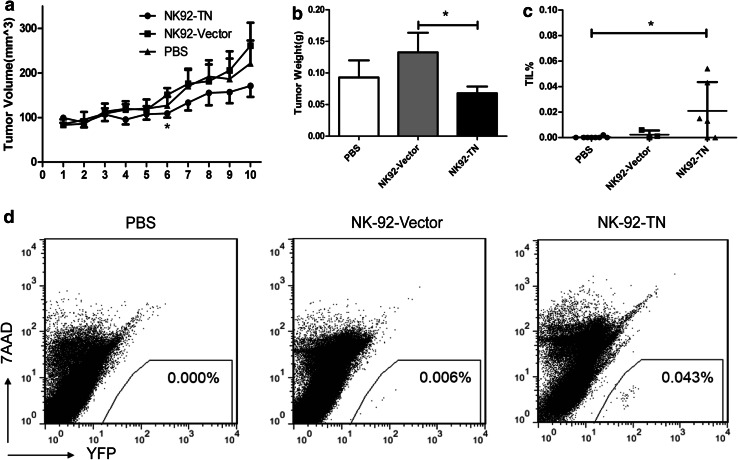
To compare the anti-tumor effect of NK-92-TN and NK-92-Vector cells in vivo, we established a xenograft model of hepatocellular Carcinoma by subcutaneously injecting SMMC7721 cells (2 × 106) in BALB/c athymic nude mice. Mice were treated with a single intravenous injection of PBS, NK-92-TN, or NK-92-Vector (Fig. 6). Tumor volumes were monitored (Fig. 6a), and tumor weights (Fig. 6b) were determined on day 28. Although the immune regulatory role of the NK-92-TN cells could not be achieved in this model, the results showed that both the tumor volumes and tumor weights could be moderately reduced by NK-92-TN cell transfer.
Fig. 6.
Adoptive transfer of NK-92-TN cells augmented the anti-tumor effect of NK-92 cells in vivo. The BALB/c athymic nude mice were inoculated subcutaneously with 2 × 106 hepatocellular carcinoma SMMC7721 cells. Ten days later, 5 × 106 NK-92-TN or NK-92-Vector cells were adoptively transferred, and a tumor volumes and b tumor weights were measured on day 28. c Percent of tumor infiltrating YFP+NK cells was analyzed by flow cytometry. d Representative flow cytometry plots detecting YFP+NK cells were shown. Data shown are the average of five independent experiments (including 30 mice per group). Results are expressed as mean ± SE. *p < 0.05
The tumor infiltrating NK-92 cells were analyzed by flow cytometry (Fig. 6c, d). The percentages of tumor infiltrating NK-92-TN cells were significantly higher than the percentages of NK-92-Vector cells, suggesting that the NK-92-TN cells could better infiltrate into the tumors and perform their anti-tumor activities.
Discussion
NK cells are vital effector cells of innate immunity and play important roles in immune surveillance [19]. Numerous NK cell-based anticancer therapies are currently under investigation [1]. However, the clinical trials of NK adoptive transfer, either autologous or allogeneic, were largely unsuccessful to treat patients with solid tumors [1, 2, 20]. This is largely due to the inability of NK cells to traffic to the tumor site and the suppressed anti-tumor activity of the transferred NK cells in the tumor microenvironment [9, 21–23]. There were previous studies expressing dominant negative TGF-βR II on NK cells to render resistance to TGF-β [10, 24]. Here, for the first time, we reported a chimeric NK cell receptor with the extracellular domain of TGF-βR II and the intracellular domain of NKG2D, which may help NK cells infiltrate to the TGF-β-high tumor microenvironment and convert the suppressive TGF-β signal to an activating NKG2D signal to facilitate the effector function of the transferred NK cells.
TGF-β is a pleiotropic cytokine mediating immune suppression in the tumor microenvironment [25]. TGF-β can induce Tregs which can suppress effector T cell function [26]. It has also been shown recently that ALL blast can induced NK cell dysfunction through the TGF-β/SMAD pathway [27]. TGF-β-induced microRNA-183 suppresses DAP12 transcription, which is critical for surface NK receptor stabilization and downstream signal transduction [28]. TGF-β in the tumor microenvironment can cause the immaturation and suppression of NK cells and neutralizing TGF-β promotes NK function and tumor regression [29, 30]. The chimeric receptor with extracellular TGF-βR II expression can still bind to TGF-β and be attracted to the tumor microenvironment. However, since it does not transduce TGF-β signal, the NK cells expressing this chimeric receptor are resistant to the suppressive TGF-β signal, and instead activated by the TGF-β in the tumor microenvironment. Because of the high-level expression of TGF-βR II on these NK cells carrying the chimeric receptors, they can also absorb and reduce the level of TGF-β within the tumor, which could affect the induction of Tregs and the function of the effector T cells, or even endogenous NK cells. CD4+ CD25+ Tregs are reported to inhibit NKG2D-mediated NK cell cytotoxicity in vitro, and depletion of these cells in vivo significantly promoted NK cell-mediated tumor rejection [31]. Our in vitro experiments showed that the presence of the NK-92 cells expressing the chimeric receptor (TN) indeed could reduce the generation of human Tregs (Fig. 5). Therefore, these TN-expressing NK cells may not only possess potent anti-tumor capacity, but also promote endogenous T and NK cell function after adoptive transfer.
Decreased level of NKG2D expression impairs NK cell cytotoxicity and IFN-γ production [32]. IFN-γ is predominantly produced by activated NK cells, and is one of the markers for NK cell activation [33]. Both NKG2D expression and IFN-γ production were down-regulated by TGF-β in control NK cells (Figs. 2, 3). However, the NK-92-TN cells expressing the chimeric receptors were resistant to TGF-β suppression, with NKG2D expression and IFN-γ production unchanged during TGF-β treatment. Although the cytotoxicity of the NK-92-TN cells can be enhanced by TGF-β (Fig. 3), TGF-β treatment did not up-regulate the NKG2D expression or IFN-γ production, suggesting that the increased killing capacity could be due to the activation of NKG2D signaling and other effector molecules. The overall profile of the receptor expressions (Fig. 4c, d) suggested increased tissue migration and activation of NK-92-TN cells [34, 35]. However, the changes were not drastic (within twofolds). Therefore, the expression of the chimeric receptor on NK-92-TN cells may not greatly influence their receptor expression patterns other than increasing their resistance to TGF-β-induced suppressions and chemotaxis towards TGF-β-high tumor areas.
Despite the substantial enhancement of the killing capacity of the NK-92-TN cells by expressing the chimeric receptors, the in vivo reduction of tumor growth with NK-92-TN cell adoptive transfer was quite moderate (Fig. 6). We only administered one dosage of 5 × 106 cells to match the practical cell numbers per kg in adoptive therapy for humans. The therapeutic efficacy could be enhanced by transferring more cells of multiple dosages. Moreover, the immune regulatory function of this chimeric receptor cannot be examined with the current animal model because of the lack of endogenous human NK and T cells. Humanized mouse tumor model could be used in the future to fully examine the anti-tumor effect of the NK cells expressing chimeric TN receptor in vivo.
The current study is based on the NK-92 cell line adoptive therapy. Future study of this TN chimeric receptor can also be expanded to autologous or allogeneic primary NK cells. Our results provided the basis for expressing the chimeric TN receptor as a novel strategy for enhancing the therapeutic efficacy of NK cell adoptive therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Feili Gong for providing NK-92 cells and Dr. Yun Zhao for providing lenti-virus vectors. This work has been supported by grants from the National Natural Science Foundation of China (81273268, 81471586), the project funding from Suzhou city (SWG0904), Priority Academic Program Development of Jiangsu Higher Education Institutions, and the start-up grant from the National University of Singapore.
Abbreviations
- α-MEM
Minimum essential medium alpha
- BFA
Brefeldin A
- DAP
DNAX-activating protein
- DMEM
Dulbecco-modified eagle medium
- FACS
Fluorescence-activated cell sorter
- FBS
Fetal bovine serum
- HLA
Human leucocyte antigen
- HRP
Horseradish Peroxidase
- IFN-γ
Interferonγ
- IgA
Immunoglobulin A
- IL-2
Interleukin 2
- KIR
Killer cell Ig-like Receptors
- LDH
Lactic dehydrogenase
- mAb
Monoclonal antibody
- MFI
Mean fluorescent intensity
- MHC
Major histocompatibility complex
- NK
Natural killer
- NK-92-TN
NK-92 expressing extracellular and transmembrane domains of TGF-β receptor II with intracellular domain of NKG2D
- NK-92-Vector
NK-92 expressing vector
- NKG2C
NK Group 2 member C
- NKG2D
NK Group 2 member D
- PCR
Polymerase chain reaction
- PE
Phycoerythrin
- PMA
Phorbol myristate acetate
- PVDF
Polyvinylidene fluoride
- RPMI
Roswell Park Memorial Institute
- RT-PCR
Real-time PCR
- SDS–PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TGF-β
Transforming growth factor-β
- TGF-βR
Transforming growth factor-β receptor
- Treg
Regulatory T
- VSVG
Vesicular stomatitis virus G
- YFP
Yellow fluorescent protein
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interest.
Footnotes
Z. Wang, L. Guo, and Y. Song contributed equally.
References
- 1.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 2.Barkholt L, Alici E, Conrad R, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1:753–764. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 3.Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res. 2014;20:3390–3400. doi: 10.1158/1078-0432.CCR-13-1766. [DOI] [PubMed] [Google Scholar]
- 4.Jung B, Staudacher JJ, Beauchamp D. Transforming growth factor β superfamily signaling in development of colorectal cancer. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 6.Massagué J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drabsch Y, ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31:553–568. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 8.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010;12:7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Liu H, Shi W, Wang Z, Sun S, Zhang G, Hu Y, Liu T, Jiao S. Blocking transforming growth factor-beta signaling pathway augments antitumor effect of adoptive NK-92 cell therapy. Int Immunopharmacol. 2013;17:198–204. doi: 10.1016/j.intimp.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 12.Wu L, Zhang C, Tian Z, Zhang J. NK cell-based approach for screening novel functional immune genes. Int Immunopharmacol. 2011;11:274–279. doi: 10.1016/j.intimp.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother. Stem Cell Res. 2001;10:369–383. doi: 10.1089/152581601750288975. [DOI] [PubMed] [Google Scholar]
- 14.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 15.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.V97.1.192. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/S1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev. 2005;203:38–47. doi: 10.1111/j.0105-2896.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+ CD25+ regulatory T†„cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 19.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Souza AP, Bonorino C. Tumor immunosuppressive environment: effects on tumor-specific and nontumor antigen immune responses. Expert Rev Anticancer Ther. 2009;9:1317–1332. doi: 10.1586/era.09.88. [DOI] [PubMed] [Google Scholar]
- 22.Jones E, Pu H, Kyprianou N. Targeting TGF-beta in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets. 2009;13:227–234. doi: 10.1517/14728220802705696. [DOI] [PubMed] [Google Scholar]
- 23.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Hu J, Li R, Song J, Kang Y, Liu S, Zhang D. Enhanced NK cell adoptive antitumor effects against breast cancer in vitro via blockade of the transforming growth factor-beta signaling pathway. Onco Targets Ther. 2015;8:1553–1559. doi: 10.2147/OTT.S82616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Zhang LJ, Zhang HR, et al. Tumor-derived transforming growth factor-beta is critical for tumor progression and evasion from immune surveillance. Asian Pac J Cancer Prev. 2014;15:5181–5186. doi: 10.7314/APJCP.2014.15.13.5181. [DOI] [PubMed] [Google Scholar]
- 26.Carambia A, Freund B, Schwinge D, et al. TGF-beta-dependent induction of CD4(+)CD25(+)Foxp3(+) Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594–599. doi: 10.1016/j.jhep.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Rouce RH, Shaim H, Sekine T, et al. The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B acute lymphoblastic leukemia. Leukemia. 2015;30(4):800–811. doi: 10.1038/leu.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donatelli SS, Zhou JM, Gilvary DL, et al. TGF- -inducible microRNA-183 silences tumor-associated natural killer cells. Proc Natl Acad Sci. 2014;111:4203–4208. doi: 10.1073/pnas.1319269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krneta T, Gillgrass A, Chew M, Ashkar AA. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell Mol Immunol. 2015;13(5):628–639. doi: 10.1038/cmi.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez M, Bouchlaka MN, Sckisel GD, Sungur CM, Chen M, Murphy WJ. Increased antitumor effects using IL-2 with anti-TGF-beta reveals competition between mouse NK and CD8 T cells. J Immunol. 2014;193:1709–1716. doi: 10.4049/jimmunol.1400034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4 + CD25 + T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 32.Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 34.Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11(10):658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.