Abstract
Myeloid cells play a crucial role in tumor progression. The most common tumor-infiltrating myeloid cells are myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAMs). These cells promote tumor growth by their inherent immune suppressive activity which is enhanced by their cross-talk. The root extract of the plant Withania somnifera (Ashwagandha) (WRE) has been reported to reduce tumor growth. HPLC analysis identified Withaferin A (WA) as the most abundant constituent of WRE and led us to determine whether the anti-tumor effects of WRE and WA involve modulating MDSC and TAM activity. A prominent effect of MDSC is their production of IL-10 which increases upon cross-talk with macrophages, thus polarizing immunity to a pro-tumor type 2 phenotype. In vitro treatment with WA decreased MDSC production of IL-10 and prevented additional MDSC production of IL-10 generated by MDSC–macrophage cross-talk. Macrophage secretion of IL-6 and TNFα, cytokines that increase MDSC accumulation and function, was also reduced by in vitro treatment with WA. Much of the T-cell suppressive activity of MDSC is due to MDSC production of reactive oxygen species (ROS), and WA significantly reduced MDSC production of ROS through a STAT3-dependent mechanism. In vivo treatment of tumor-bearing mice with WA decreased tumor weight, reduced the quantity of granulocytic MDSC, and reduced the ability of MDSC to suppress antigen-driven activation of CD4+ and CD8+ T cells. Thus, adjunctive treatment with WA reduced myeloid cell-mediated immune suppression, polarized immunity toward a tumor-rejecting type 1 phenotype, and may facilitate the development of anti-tumor immunity.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1470-2) contains supplementary material, which is available to authorized users.
Keywords: Tumor immunology, Tumor-induced immune suppression, T-cell activation, Immunotherapy, Withaferin A, Withania somnifera
Introduction
Immunotherapy to stimulate a patient’s anti-tumor immune response is a promising therapeutic approach; however, most patients contain immune suppressive cells, which prevent the activation of tumor-specific T lymphocytes. Myeloid-derived suppressor cells (MDSC) play a major role in inhibiting T-cell responses and are a dominant cause of immune suppression because they are present in most cancer patients [1]. Clinical studies have confirmed that immunotherapy will be maximally effective if MDSC activity is blocked [2, 3]. In addition to inhibiting T-cell activation, MDSC also perturb innate anti-tumor immunity through their interaction with macrophages. Classically activated M1 macrophages promote tumor regression; however, in the presence of MDSC, macrophages are converted to an alternatively activated phenotype, which enhances tumor progression [4, 5]. This re-polarization is the result of cross-talk between MDSC and macrophages, which increases MDSC production of IL-10 and decreases macrophage production of IL-12 [6].
Natural products are increasingly being recognized as having anti-tumor activity. In particular, the root extract of the plant Withania somnifera Dunal, commonly known as Ashwagandha (WRE), inhibits primary tumor growth and metastatic disease [7–11] and enhances the activation of tumor-reactive CTL [12]. In vitro studies have identified Withaferin A (WA), a component of WRE, as having the following direct effects on tumor cells: (a) inducing apoptosis [13–16]; (b) increasing intracellular levels of antioxidants [17]; (c) inhibiting Notch signaling [18, 19]; (d) inhibiting NF-kB activation [20, 21]; and (e) altering cytoskeletal architecture [22]. Additional in vitro studies have shown that WA inhibits angiogenesis [23]. Because MDSC are a major contributor to tumor progression, the present study was undertaken to determine whether the anti-tumor effects of WA also involve reducing MDSC-mediated immune suppression. Our studies confirm earlier reports that WA delays tumor progression and demonstrate that it reduces MDSC suppression through several mechanisms. It inhibits macrophage production of two of the pro-inflammatory cytokines that drive MDSC accumulation and potency. WA also decreases MDSC production of reactive oxygen species (ROS), which mediate many of the suppressive functions of MDSC. In addition, WA decreases MDSC production of IL-10, a cytokine that polarizes immunity toward a tumor-promoting type 2 phenotype. Therefore, in addition to its nonimmunological effects, WA impairs tumor progression by reducing MDSC-mediated immune suppression.
Materials and methods
Reagents
Withania somnifera root extract, WA, Withanolide A, and 12-deoxywithastramonolide (12-deoxy) were purchased from Chromadex (Irvine, CA, USA). Ethanol and methanol were from Pharmco-Aaper (Shelbyville, KY, USA) and VWR International (West Chester, PA, USA), respectively. Phorbol 12-myristate 13-acetate (PMA) was from Sigma (St. Louis, MO, USA). Dulbecco’s PBS with glucose and sodium pyruvate (DPBS) was from Invitrogen, and BBL™ Brewer’s modified thioglycolate medium was from BD Diagnostics (Sparks, MD, USA).
WRE and its components
To prepare 250 mg/ml WRE in ethanol, 10 g WRE was added to 40 ml of 60 °C 200 proof ethanol and the mixture was stirred for 30 min, followed by addition of 4 ml of distilled water and stirring for another 30 min at 60 °C. Solution was spun at 3,000g for 15 min, supernatant was collected and passed through a 0.22-μm filter, and 0.5 ml aliquots of WRE were stored at −80 °C. WA (2 mg/ml) was prepared in 90 % ethanol. Withanolide A and 12-deoxy were prepared in methanol as 2 mg/ml stock solutions.
HPLC analysis
Withania somnifera root extract, WA, Withanolide A, and 12-deoxy were analyzed using a PerkinElmer FX 10 HPLC equipped with an autosampler, peltier column oven, and photodiode array detector set at 227 nm. A 100-μl sample loop was used for loading the 20-μl sample at 4 °C. The analytical column was a Phenomenex Luna C18 150 mm × 4.6 mm, 5-μm particle size set to 25 °C at the column oven. Chromatographic separation was achieved using 40-min-long isocratic elution. The mobile phase consisted of 70 % 0.1 % phosphoric acid in water and 30 % acetonitrile. The solvent flow rate was 1.5 ml/min.
Mice
BALB/c mice, BALB/c DO11.10 TCR-transgenic mice expressing an αβ-TCR restricted to chicken OVA peptide 323–339 restricted by I-Ad, and BALB/c clone 4 TCR-transgenic mice expressing an αβ-TCR specific for amino acids 518–526 of influenza hemagglutinin (HA) restricted to H-2Kd [24] were bred and maintained in the University of Maryland Baltimore County (UMBC) animal facility according to the NIH guidelines for the humane treatment of laboratory animals. All animal procedures have been approved by the UMBC Institutional Animal Care and Use Committee.
Tumor cells, tumor inoculations, tumor growth, WA treatment, and MDSC measurements
The 4T1 mammary carcinoma was maintained as described [25]. Mice were inoculated s.c. in the abdominal mammary gland with 7,000 4T1 mammary carcinoma cells on day 0 and monitored for tumor progression as described [25]. Mice were fed orally with WA (1, 2, 4, 8 mg/kg body weight WA, or vehicle control) every other day (three times a week) beginning on the day tumors were palpable and continued until mice were moribund. To determine total MDSC and MDSC subpopulation percentages, mice were bleed into heparinized tubes, RBC were depleted, and WBC were stained with fluorescently coupled antibodies as described [5].
Antibodies and flow cytometry
Fluorescently coupled Gr1 (clone RB68C5), CD11b, Ly6C (clone AL-21), Ly6G (clone 1A8), CD11c, F4/80, B220, CD3, CD4, CD8, DO11.10 TCR (clone KJ1-26), Vβ8.1&8.2 mAbs, isotype control mAbs, purified mouse anti-arginase I mAb, and rat anti-mouse IgG1-FITC were from BD Pharmingen (San Diego, CA, USA). Cells were labeled for direct immunofluorescence as described [25]. Samples were analyzed on a Beckman/Coulter CyAn ADP flow cytometer using Summit software.
ROS (H2O2) detection
Reactive oxygen species was measured by H2O2 detection using an Amplex Red Hydrogen Peroxide Assay Kit (Invitrogen) as described by the manufacturer. Briefly, 5 × 104 MDSC in DPBS (50 μl/well) were activated with 30 ng/ml PMA and co-cultured with 50 μl/well Amplex Red Reagent in the presence or absence of WRE, WA, Withanolide A, or 12-deoxy. Resorufin fluorescence was measured at 37 °C at 5-min intervals for 1 h using a microplate plate reader (BioTek Synergy 2, Winooski, VT, USA) with excitation at 530 nm and emission at 590 nm. Fluorescent readings were transformed using Gen5 software to μM H2O2 values using a standard curve generated by serial dilutions of 20 μM H2O2.
Phospho-STAT3 (pSTAT3) detection
Blood leukocytes were either untreated or incubated for 15 min at 37 °C with tumor cell-conditioned media (TCCM) [26] in the presence of WA (1 μg/ml) and subsequently stained according to the manufacturer’s protocol (BD Biosciences) with mAbs to phospho-STAT3, CD11b and Gr1.
Macrophage and MDSC stimulation and cytokine production
BALB/c mice were inoculated i.p. on day 0 with 2.5 ml of 3 % thioglycolate, peritoneal exudate cells (PEC) were harvested, and RBC were lysed on day 4–5. PEC were >90 % macrophages as measured by F4/80 staining. Macrophages were plated in 24-well plates at 7.5 × 105 cells/well/500 μl DMEM containing 10 % FBS and incubated at 37 °C in 5 % CO2 for 3 h. Nonadherent cells were then removed, the attached cells washed once with macrophage medium (DMEM with 5 % FBS), and the remaining macrophages activated with 2 ng/ml IFN-γ and 100 ng/ml LPS for 16 h as described [6], in presence or absence of WRE, WA, Withanolide A, or 12-deoxy. Culture supernatants were analyzed for NO using the Greiss reagent and for TNF-α, IL-12, IL-6, and IL-10 using ELISA kits (R&D Systems) as recommended by the manufacturer. MDSC (>90 % CD11b+Gr1+ cells) were plated in 24-well plates at 1.5 × 106 cells/well/500 μl macrophage medium and stimulated with 2 ng/ml IFN-γ and 100 ng/ml LPS for 16 h in the presence or absence of WRE, WA, Withanolide A, or 12-deoxy. Culture supernatants were analyzed as for macrophages.
Macrophage and MDSC co-cultures
Thioglycolate-induced peritoneal macrophages were co-cultured with 4T1-induced blood-derived MDSC as described [6]. Briefly, MDSC (>90 % CD11b+Gr1+ cells) and macrophages were co-cultured in 24-well plates (1.5 × 106 MDSC and 7.5 × 105 macrophages/well/500 μl macrophage medium) with IFNγ and LPS in the presence or absence of WRE, WA, Withanolide A, or 12-deoxy. Culture supernatants were collected after 16 h and assayed for NO and cytokines.
T-cell suppression
Transgenic DO11.10 or Clone4 splenocytes were co-cultured with cognate peptide and irradiated MDSC (>90 % Gr1+CD11b+ cells) from tumor-bearing mice as described [5].
Cell viability
Blood MDSC from 4T1 tumor-bearing mice or peritoneal macrophages from tumor-free mice were cultured with WA or vehicle control, and viability was assessed after 4 h by trypan blue dye exclusion. 4T1 tumor cells were cultured with WA or vehicle control with or without 1 μCi [3H]thymidine/105 cells/200 μl/well (96-well plates) (ICN Biochemicals) for 24 h. Cells were harvested onto glass fiber filter mats and counted in a liquid scintillation counter or stained with trypan blue and unstained cells counted using a hemocytometer, respectively.
Statistics
Student’s two-tailed t test was performed with Microsoft Excel 2007. Mann–Whitney and log-rank tests were performed using the Web links http://faculty.vassar.edu/lowry/VassarStats.html and http://bioinf.wehi.edu.au/software/russell/logrank/. p values <0.05 were considered significant.
Results
Withaferin A reduces MDSC production of reactive oxygen species
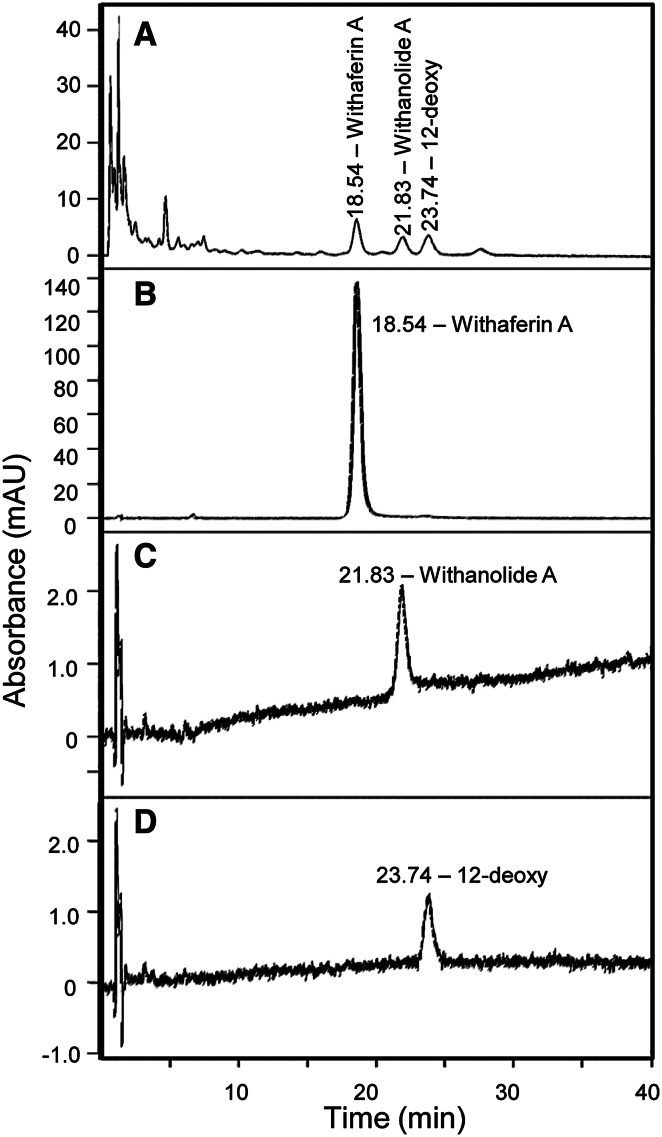
To identify the components of WRE and to determine which, if any, of the components modulate the function of MDSC and TAMs, we analyzed the root extract using HPLC. HPLC analysis yielded three major peaks with retention times of 18.54, 21.83, and 23.74 min. They were identified using reference standards as Withaferin A (WA), Withanolide A, and 12-deoxywithastramonolide (12-deoxy) (Fig. 1).
Fig. 1.
Withania somnifera root extract (WRE) contains three components: Withaferin A (WA), Withanolide A, and 12-deoxywithastramonolide, as assessed by HPLC analysis. HPLC chromatogram of a WRE, b Withaferin A, c Withanolide A, and d 12-deoxywithastramonolide
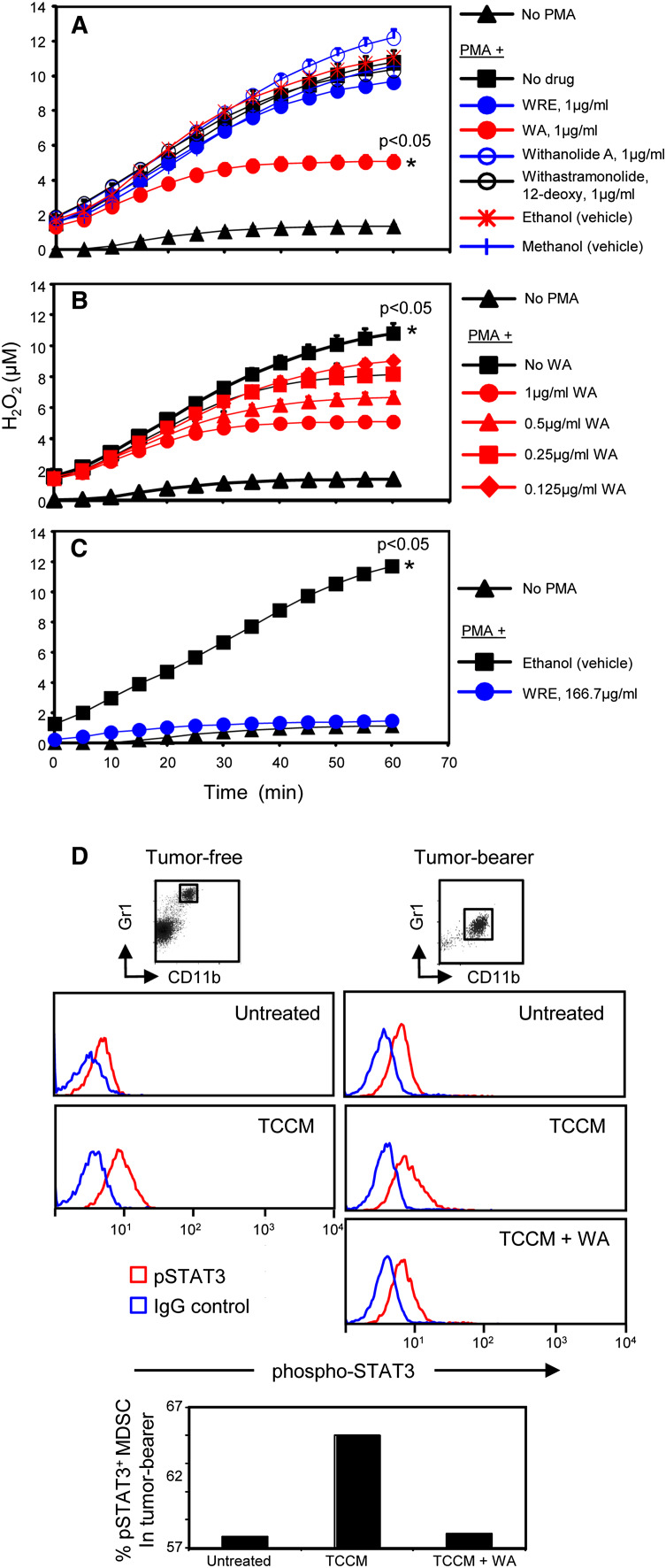
To determine whether WRE, WA, Withanolide A, or 12-deoxy affects the ability of MDSC to suppress T cells, we assessed the effects of these compounds on MDSC production of reactive oxygen species (ROS) since ROS are a major mechanism MDSC use to impair T-cell activation [27]. MDSC were harvested from the blood of 4T1 tumor-bearing BALB/c mice and activated with PMA in the presence or absence of 1 μg/ml WRE, WA, Withanolide A, or 12-deoxy, and H2O2 production was recorded (Fig. 2a). WA reduces MDSC production of H2O2 by >50 %. In contrast, equivalent quantities of the intact root extract, Withanolide A, or 12-deoxy do not reduce H2O2 production. WA-induced reduction of H2O2 is dose dependent since decreasing the concentration of WA decreased H2O2 release (Fig. 2b). To confirm that WA is the active component in WRE, the dose of WRE was increased to 166.7 μg/ml so that the concentration of WA in WRE was the same as the concentration of purified WA used in Fig. 2a (1 μg/ml). This concentration of WRE completely inhibited the production of H2O2 (Fig. 2c) and was not toxic as assessed by cell viability (not shown).
Fig. 2.
WA, but not Withanolide A, or 12-deoxy, reduces MDSC production of hydrogen peroxide. BALB/c mice were inoculated on day 0 with 4T1 mammary carcinoma cells. Mice were bled 38 days later and blood MDSC (>90 % CD11b+Gr1+ cells) were stimulated with PMA. H2O2 production was measured in the presence of a 1 μg/ml WRE, WA, Withanolide A, or 12-deoxy; b decreasing doses of WA; and c 166.7 μg/ml WRE to match the concentration of WA (1 μg/ml) in WRE (0.60 % WA in WRE samples). Each point is the average + SD of triplicate measurements. Data are representative of three independent experiments. For a–c, * Indicates curves that are significantly different from all other curves at p < 0.05. d WA inhibits STAT3 activation in MDSC. Gr1+CD11b+ cells from the blood of tumor-free or 4T1 tumor-bearing BALB/c mice (pooled from 3 mice) were untreated or treated in vitro for 15 min with TCCM ± WA and then stained for Gr1, CD11b, and pSTAT3. Gated Gr1+CD11b+ cells were analyzed for pSTAT3. Data are from one of three experiments
To further determine whether WA impacts MDSC activation, we assessed phosphorylated STAT3 levels since H2O2 production by MDSC is regulated by STAT3 [28] (Fig. 2d). Gr1+CD11b+ cells from tumor-free mice contain minimal activated STAT3. In contrast, MDSC from tumor-bearing mice contain modest levels of phosphorylated STAT3, and addition of TCCM significantly increased activated STAT3. Inclusion of WA inhibited the TCCM activation and reduced the levels of phosphorylated STAT3, indicating that WA-mediated reduction in ROS production in MDSC is regulated by STAT3. These results demonstrate that WA significantly reduces MDSC production of H2O2 and is the key bioactive component of the root extract and acts by reducing the phosphorylation of STAT3.
WA minimizes MDSC production of the pro-tumor cytokine IL-10
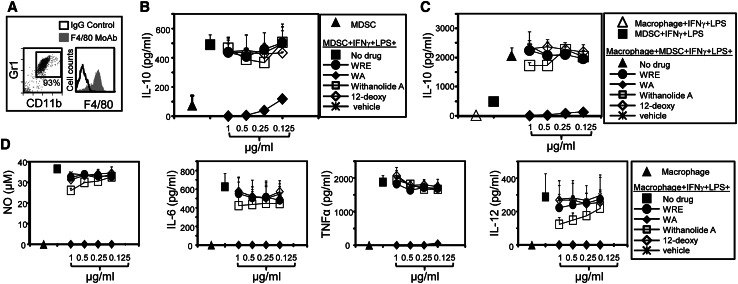
In addition to their inhibiting T-cell activation, MDSC also inhibit tumor immunity by producing IL-10, which polarizes immunity toward a tumor-promoting type 2 response [6]. To determine whether MDSC production of IL-10 is altered by WRE, MDSC isolated from the blood of 4T1 tumor-bearing mice (Fig. 3a left histogram) were stimulated in vitro with IFNγ plus LPS in the presence or absence of WRE, WA, Withanolide A, or 12-deoxy, and the supernatants evaluated by ELISA for IL-10 (Fig. 3b). WA decreased IL-10 in a dose-dependent manner without affecting cell viability (supplementary figure 1), whereas WRE, Withanolide A, and 12-deoxy did not affect IL-10 production.
Fig. 3.
WA reduces IL-10 and macrophage-amplified IL-10 production by MDSC, and macrophage production of pro-inflammatory cytokines. a Leukocytes from 4T1-tumor-bearing mice and PEC from thioglycolate-treated healthy mice were stained for Gr1+CD11b+ (MDSC) or F4/80 (macrophages), and analyzed by flow cytometry. b MDSC or c MDSC plus macrophages were co-cultured with IFNγ and LPS ± escalating doses of WRE or its components. Supernatants were collected after 16 h and assayed for IL-10 by ELISA. d LPS plus IFNγ-activated macrophages were incubated for 16 h ± WRE or its components and supernatants assayed for NO, IL-6, TNFα, or IL-12. Data are the average + SD from three mice/group and are representative of three experiments
Cross-talk between MDSC and macrophages increases MDSC production of IL-10 and further skews immunity toward a tumor-promoting type 2 phenotype [6]. To determine whether WRE or its components regulate MDSC–macrophage cross-talk, macrophages and MDSC were stimulated with IFNγ plus LPS and co-cultured with or without WRE or its components. Supernatants were analyzed for IL-10 (Fig. 3c). As previously observed, macrophages increased MDSC production of IL-10. WA reduced IL-10 in a dose-dependent manner without affecting cell viability (supplementary figure 1), while the other components of WRE had no effect. These data together with the results of Fig. 3b demonstrate that WA inhibits MDSC production of IL-10 and prevents additional IL-10 production generated by MDSC–macrophage cross-talk.
WA reduces macrophage production of pro-inflammatory cytokines
Inflammation is functionally associated with tumor progression, and many of the pro-inflammatory mediators in the tumor microenvironment are produced by resident macrophages. We and others have previously demonstrated that multiple pro-inflammatory mediators drive the accumulation and suppressive potency of MDSC, thus explaining how inflammatory effects impair anti-tumor immunity [29]. To determine whether the anti-tumor effects of WRE and its components include downregulating inflammation, we assessed the production of inflammatory cytokines from activated macrophages. PEC were harvested from thioglycolate-treated BALB/c mice and were >93 % macrophages as assessed by F4/80 expression (Fig. 3a right histogram). These cells were stimulated in vitro with IFNγ plus LPS with or without WRE, WA, Withanolide A, or 12-deoxy, and the supernatants assayed for nitric oxide (NO) and cytokines. WA eliminated macrophage release of NO and the pro-inflammatory cytokines IL-6, TNFα, and IL-12 (Fig. 3d) without affecting cell viability (supplementary figure 1). WRE and 12-deoxy had no effect on NO, IL-6, TNFα, or IL-12, while Withanolide A modestly reduced IL-12 and had no effect on NO, IL-6, or TNFα. These results demonstrate that WA is a potent inhibitor of pro-inflammatory mediators produced by macrophages.
Myeloid-derived suppressor cells and macrophages also generate arginase which suppresses T-cell activation [30]. To assess whether WA alters this enzyme, MDSC and macrophages were treated with vehicle or WA and then stained for surface CD11b, Gr1, and intracellular arginase (supplementary figure 2). WA did not alter production of arginase from either MDSC or macrophages.
Withaferin A reduces MDSC accumulation in tumor-bearing mice
The results of Figs. 2 and 3 demonstrate that in vitro treatment with WA inhibits multiple suppressive functions of MDSC, suggesting that WA may impair MDSC function in vivo. To test this possibility, BALB/c mice were inoculated on day 0 with 4T1 tumor. When tumor masses were palpable, the mice were randomized into five groups and oral WA feeding every other day was started. A dose range of 0, 1, 2, 4, and 8 mg/kg body weight was used [31]. In agreement with previous publications [15, 31], tumor growth was modestly, but significantly reduced in all the treatment groups as compared to control (Fig. 4a). To further confirm the effect of WA, mice were killed when they became moribund, and the primary tumors excised and weighed. All doses of WA significantly decreased tumor weight compared to the control group (Fig. 4b). Since in vitro cultured tumor cells were not killed by WA (supplementary figure 3), the ability of WA to delay tumor growth was most likely an indirect effect mediated by host cells. We therefore determined whether WA reduces MDSC accumulation in vivo. Tumor-bearing WA-treated mice were bled on days 0, 11, and 24, and the percent of MDSC in the blood was determined by flow cytometry. All doses of WA reduced granulocytic MDSC (PMN-MDSC, CD11b+Ly6G+Ly6Clow/−) (Fig. 4c), but did not affect monocytic MDSC (MO-MDSC, CD11b+Ly6G−Ly6C+) (data not shown). Because MDSC levels are driven by tumor mass, WA-treated mice may have fewer MDSC because their primary tumors are smaller and not because WA is directly reducing MDSC quantity. To address this issue, we compared the percentage of MDSC in the blood of WA-treated and vehicle-treated BALB/c mice with the same size primary tumors (Fig. 4c). WA-treated mice with 10–11-mm-diameter primary tumors had 42 % fewer PMN-MDSC as compared to vehicle-treated tumor-bearing mice with equal-sized primary tumors. To investigate whether WA reduces MDSC because it is toxic to blood cells in general, tumor-free BALB/c mice were fed WA (4 mg/kg body weight) or vehicle control, and the percentages of different populations of blood cells were determined by flow cytometry (supplementary figure 4a). No significant differences in the levels of CD4+ or CD8+ T cells, B cells, dendritic cells, or monocytes were observed between vehicle-treated and WA-treated mice before and after WA treatment. There was also no effect on the ability of T cells from WA-treated mice to be activated by peptide (supplementary figure 4b), indicating that at the doses used to inhibit MDSC in vivo, T-cell proliferation was not impaired. These data demonstrate that WA delays tumor progression and reduces the accumulation of PMN-MDSC in tumor-bearing mice without impacting other cells in the blood.
Fig. 4.
WA treatment delays primary tumor growth and decreases the quantity and suppressive activity of PMN-MDSC. a BALB/c mice were inoculated in the mammary gland on day 0 with 4T1 cells. Starting on day 9 when tumors were 2–2.5 mm in diameter and continuing through day 31, WA was fed orally every other day and tumor diameters were measured. N = 5 mice/group. b Mice from panel a were killed on day 31 and their tumors excised and weighed. * Indicates the curve (a) or value (b) that is significantly different from other measurements at p < 0.05. c Left panels Leukocytes from the tumor-bearing mice of panel A were labeled for CD11b, Ly6C, and Ly6G, and CD11b+ cells were gated and analyzed for expression of Ly6C and Ly6G. MO-MDSC = CD11b+Ly6G−Ly6Chi; PMN-MDSC = CD11b+Ly6G+Ly6C−/low. Middle panel WA reduces the quantity of MDSC in tumor-bearing mice. Mice of panel a were bled on days 0, 11, and 24, and the leukocytes gated as shown in the left panels and assayed for the percentages of PMN-MDSC. Values are the average + SD of 4–5 mice per group. * Indicates the value that is significantly different from other values in the same group at p < 0.05. Right panel Percentage PMN-MDSC in WA-treated mice with 10–11-mm-diameter 4T1 tumors. d MDSC from WA-treated mice are less suppressive than MDSC from control-treated mice. CD8+ (Clone 4) or CD4+ (DO11.10) transgenic splenocytes were stimulated with cognate peptide and co-cultured with ± MDSC (>85 % CD11b+Gr1+ cells) from the blood of WA or control-treated 4T1 tumor-bearing mice. T-cell proliferation was measured by incorporation of [3H]thymidine. Data are representative of three independent experiments
To determine whether in vivo treatment with WA reduces MDSC function, MDSC were harvested from control or WA-treated mice and tested for their ability to suppress the activation of antigen-specific CD8+ or CD4+ T cells (Fig. 4d). MDSC from WA-treated mice were significantly less suppressive on per cell basis as compared to MDSC from control-treated mice, demonstrating that WA reduces the suppressive capability of MDSC.
Discussion
Natural products that possess anti-carcinogenic properties have been used for centuries in Ayurvedic medicine. Withaferin A, the most abundant component of WRE, is a natural product with demonstrated anti-tumor efficacy against cultured and xenografted tumor cells. Previous studies have attributed the in vivo efficacy of WRE/WA to its antioxidant properties of increasing glutathione, glutathione S transferase, superoxide dismutase, and catalase. WRE/WA also inhibits angiogenesis and enhances NK and CTL function [12, 23, 32]. MDSC contribute to many of the mechanisms that are downregulated by WRE/WA. For example, MDSC promote oxidative stress in the tumor microenvironment [33], drive angiogenesis [34], and suppress T- and NK-cell function [35]. We have therefore investigated whether the anti-tumor effects of WRE/WA involve neutralization of MDSC. The studies reported here demonstrate that WA is the most potent bioactive component of WRE and that WA regulates MDSC quantity and function, and inhibits multiple pro-inflammatory mediators that drive MDSC accumulation and suppressive potency.
Myeloid-derived suppressor cell levels are driven by tumor burden, so WA effects on MDSC could be explained by decreased tumor burden due to tumor cell apoptosis. However, the doses of WA used in this study are less than the minimum dose required for tumor cell apoptosis (≥2 μM [13]; ≥0.5 μM [15, 31]) and are not toxic to tumor cells in vitro (supplementary figure 3). Therefore, WA affects MDSC independent of its effects on tumor cells. However, a concomitant in vivo effect on tumor cells cannot be ruled out and may also contribute to decreased tumor growth. The WA doses used in vitro in this report to reduce MDSC function (0.265–2.12 μM) are likely to be effective in vivo because pharmacological studies demonstrated that effective in vivo doses of WA (4 mg/kg) give a mean peak plasma concentration of 1.8 μM [31].
Reactive oxygen species are major immune suppressive molecules produced by murine and human MDSC [27, 28, 36]. Hydrogen peroxide, which is reduced by WA in MDSC (Fig. 2), generates peroxynitrite which is the most potent oxidant. Peroxynitrite nitrates the TcR of T cells and MHC class I molecules. These structural alterations sterically prevent the recognition of tumor cell MHC I–peptide complexes by tumor-reactive T cells [37] and thereby prevent T-cell activation. Although WA has been reported to increase constitutive ROS production in tumor cells [38–40], the data reported here (Fig. 2) demonstrate that WA decreases PMA-induced ROS production in MDSC. Our finding that WA reduces ROS production by MDSC is supported by earlier studies, demonstrating that MDSC production of ROS is regulated by STAT3 [28] and that WA inhibits STAT3 signaling in other cells [41, 42]. Interestingly, IL-6, COX2, and PGE2 also activate MDSC, and WA also inhibits these pro-inflammatory mediators by preventing STAT3 activation [41–45]. Therefore, WA reduces MDSC potency directly by reducing ROS and indirectly by inhibiting pro-inflammatory mediators that drive MDSC.
The transcription factor NF-κB is also activated by many pro-inflammatory mediators and is considered a central feature of how inflammation promotes tumor progression [46–48]. Previous studies have established that inflammation activates MDSC via NF-κB [49]. Our finding that WA, which is also an NF-κB inhibitor [21, 50, 51], impairs MDSC suggests that the efficacy of WA may be due to its combined effect on STAT3 and NF-κB. NF-κB signaling is involved in multiple inflammatory processes in a diversity of cells, so it is likely that MDSC are only one of the tumor-promoting cell populations inhibited by WA. Therefore, these results not only confirm WA as an inhibitor of inflammatory networks that drive MDSC, but also identify immune suppression as another tumor-promoting mechanism regulated by NF-κB.
In mice, IL-10 has been reported to have both pro- and anti-tumor activities depending on the mouse strain and tumor system [52, 53]. In the BALB/c 4T1 tumor system used in this report, IL-10−/− mice have delayed tumor progression indicating that IL-10 contributes to tumor growth (D. Beury and S. Ostrand-Rosenberg, unpublished). For tumor systems in which IL-10 has pro-tumor activity, IL-10 facilitates the development of T regulatory cells (Tregs) [54], drives type 2 immune responses including tumor-promoting M2-like macrophages and type 2 CD4+ T cells, promotes tumor cell invasion, metastasis [55], and angiogenesis [56], and protects tumor cells from chemotherapy-induced apoptosis [57]. IL-10 also counteracts the development of anti-tumor type 1 responses including type 1 CD4+ T cells, cytotoxic CD8+ T cells and NK cells, and tumoricidal M1-like macrophages [58]. Since MDSC produce relatively high levels of IL-10 and are known inducers of Tregs [59, 60], they are likely to contribute to these processes. Therefore, by downregulating IL-10, WA may not only polarize immunity toward an anti-tumor type 1 response, but may also reduce angiogenesis and increase chemotherapy-induced tumor cell apoptosis.
Withaferin A is appreciated as a natural product that inhibits key transcription factors that regulate cell proliferation, and at prescribed in vivo levels directly reduces tumor cell growth. The studies reported here demonstrate that WA also reduces MDSC-mediated immune suppression. Therefore, WA is a natural product with the potential to regulate tumor progression by directly acting on tumor cells and by simultaneously reducing immune suppression and generating a favorable environment for active immunotherapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Drs. James B. McMahon, Kirk R. Gustafson, and Thomas J. Sayers (NIH) for helpful discussions, and Dr. Adam I. Marcus (Emory) for sharing his protocol for the preparation of the WRE extract. This study was supported by NIH RO1CA84232, NIH RO1CA115880, NIHRO1GM021248, and American Cancer Society IRG-97-153-07.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 2.Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol. 2012;189:5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 5.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 6.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 7.Christina AJ, Joseph DG, Packialakshmi M, Kothai R, Robert SJ, Chidambaranathan N, Ramasamy M. Anticarcinogenic activity of Withania somnifera Dunal against Dalton’s ascitic lymphoma. J Ethnopharmacol. 2004;93:359–361. doi: 10.1016/j.jep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Muralikrishnan G, Dinda AK, Shakeel F. Immunomodulatory effects of Withania somnifera on azoxymethane induced experimental colon cancer in mice. Immunol Invest. 2010;39:688–698. doi: 10.3109/08820139.2010.487083. [DOI] [PubMed] [Google Scholar]
- 9.Padmavathi B, Rath PC, Rao AR, Singh RP. Roots of Withania somnifera inhibit forestomach and skin carcinogenesis in mice. Evid Based Complement Alternat Med. 2005;2:99–105. doi: 10.1093/ecam/neh064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senthilnathan P, Padmavathi R, Magesh V, Sakthisekaran D. Chemotherapeutic efficacy of paclitaxel in combination with Withania somnifera on benzo(a)pyrene-induced experimental lung cancer. Cancer Sci. 2006;97:658–664. doi: 10.1111/j.1349-7006.2006.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leyon PV, Kuttan G. Effect of Withania somnifera on B16F-10 melanoma induced metastasis in mice. Phytother Res. 2004;18:118–122. doi: 10.1002/ptr.1378. [DOI] [PubMed] [Google Scholar]
- 12.Davis L, Kuttan G. Effect of Withania somnifera on CTL activity. J Exp Clin Cancer Res. 2002;21:115–118. [PubMed] [Google Scholar]
- 13.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound Withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 14.Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH, Lee JM, Kim YH, Park JW, Kwon TK. Induction of apoptosis by Withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis. 2008;13:1494–1504. doi: 10.1007/s10495-008-0273-y. [DOI] [PubMed] [Google Scholar]
- 15.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal C, Dutta A, Mallick A, Chandra S, Misra L, Sangwan RS. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis. 2008;13:1450–1464. doi: 10.1007/s10495-008-0271-0. [DOI] [PubMed] [Google Scholar]
- 17.Davis L, Kuttan G. Effect of Withania somnifera on DMBA induced carcinogenesis. J Ethnopharmacol. 2001;75:165–168. doi: 10.1016/S0378-8741(00)00404-9. [DOI] [PubMed] [Google Scholar]
- 18.Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther. 2010;9:202–210. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Sehrawat A, Singh SV. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat. 2012;136:45–56. doi: 10.1007/s10549-012-2239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006;5:1434–1445. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 21.Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, Libert C, De Keukeleire D, Essawi T, Haegeman G. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 22.Falsey RR, Marron MT, Gunaherath GM, Shirahatti N, Mahadevan D, Gunatilaka AA, Whitesell L. Actin microfilament aggregation induced by Withaferin A is mediated by annexin II. Nat Chem Biol. 2006;2:33–38. doi: 10.1038/nchembio755. [DOI] [PubMed] [Google Scholar]
- 23.Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 24.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 25.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]
- 26.Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–5390. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 28.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 31.Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, Tighiouart M, Vertino PM, Harvey RD, Garcia A, Marcus AI. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–2755. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 32.Davis L, Kuttan G. Effect of Withania somnifera on cell mediated immune responses in mice. J Exp Clin Cancer Res. 2002;21:585–590. [PubMed] [Google Scholar]
- 33.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+ CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343–353. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 40.Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, Suri KA, Qazi GN, Singh J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 41.Um HJ, Min KJ, Kim DE, Kwon TK. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem Biophys Res Commun. 2012;427:24–29. doi: 10.1016/j.bbrc.2012.08.133. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991–1998. doi: 10.1093/carcin/bgq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malara N, Foca D, Casadonte F, Sesto MF, Macrina L, Santoro L, Scaramuzzino M, Terracciano R, Savino R. Simultaneous inhibition of the constitutively activated nuclear factor kappaB and of the interleukin-6 pathways is necessary and sufficient to completely overcome apoptosis resistance of human U266 myeloma cells. Cell Cycle. 2008;7:3235–3245. doi: 10.4161/cc.7.20.6832. [DOI] [PubMed] [Google Scholar]
- 44.Min KJ, Choi K, Kwon TK. Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglia cells. Int Immunopharmacol. 2011;11:1137–1142. doi: 10.1016/j.intimp.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 46.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 47.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 48.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 49.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ndlovu MN, Van Lint C, Van Wesemael K, Callebert P, Chalbos D, Haegeman G, Vanden Berghe W. Hyperactivated NF-{kappa}B and AP-1 transcription factors promote highly accessible chromatin and constitutive transcription across the interleukin-6 gene promoter in metastatic breast cancer cells. Mol Cell Biol. 2009;29:5488–5504. doi: 10.1128/MCB.01657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol. 2009;9:614–619. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 53.Tanikawa T, Wilke CM, Kryczek I, Chen GY, Kao J, Nunez G, Zou W. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72:420–429. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+ CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 60.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.