Abstract
It has been demonstrated that aggressive in situ tumor destruction (ablation) could lead to the release of tumor antigens, which can stimulate anti-tumor immune responses. We developed an innovative method of tumor ablation based on intratumoral alpha-irradiation, diffusing alpha-emitters radiation therapy (DaRT), which efficiently ablates local tumors and enhances anti-tumor immunity. In this study, we investigated the anti-tumor potency of a treatment strategy, which combines DaRT tumor ablation with two approaches for the enhancement of anti-tumor reactivity: (1) neutralization of immunosuppressive cells such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) and (2) boost the immune response by the immunoadjuvant CpG. Mice bearing DA3 mammary adenocarcinoma with metastases were treated with DaRT wires in combination with a MDSC inhibitor (sildenafil), Treg inhibitor (cyclophosphamide at low dose), and the immunostimulant, CpG. Combination of all four therapies led to a complete rejection of primary tumors (in 3 out of 20 tumor-bearing mice) and to the elimination of lung metastases. The treatment with DaRT and Treg or MDSC inhibitors (without CpG) also resulted in a significant reduction in tumor size, reduced the lung metastatic burden, and extended survival compared to the corresponding controls. We suggest that the therapy with DaRT combined with the inhibition of immunosuppressive cells and CpG reinforced both local and systemic anti-tumor immune responses and displayed a significant anti-tumor effect in tumor-bearing mice.
Keywords: Immunotherapy, Alpha-radiation, Brachytherapy, Tumor ablation, Myeloid-derived suppressor cells, Regulatory T cells
Introduction
A major effort is required to develop systemic therapeutic strategies, which enhance anti-tumor immune responses in order to reduce the high mortality of cancer patients from metastases. Anti-tumor immune-mediated mechanisms are operative during tumor development, and immune surveillance plays an important role in cancer control [1]. The difficulties to develop efficient cancer vaccines due to problems with the identification of tumor-specific antigens stimulated different efforts aiming to prime an anti-tumor immune response. After it has been reported that irradiation of a tumor site can cause the decrease in size of distant tumor tissue (the abscopal effect) [2], many publications demonstrated that aggressive in situ tumor destruction (ablation) and mainly gamma-irradiation external beam radiotherapy could stimulate anti-tumor immune reactivity [3, 4]. Tumor destruction releases tumor antigens, which trigger anti-tumor immune responses, resulting in the destruction of residual malignant cells in primary tumors and distant metastases. Such an immune response can be further reinforced [5].
We developed a potent tumor ablation brachytherapy based on alpha-irradiation. Although being highly cytotoxic, alpha-based radiotherapy has not been used so far for the treatment of solid tumors due to the short distance of alpha-particles in tissue (40–90 μm). Our approach termed diffusing alpha-emitters radiation therapy (DaRT) is based on the intratumoral insertion of radium-224-loaded wires (3.66-d half-life), which release by recoil short-lived alpha-emitting atoms into the tumor [6]. These atoms disperse in the tumor and spray it with highly destructive alpha particles. DaRT wires retarded tumor development, extended survival, and reduced lung metastases in mice bearing different mouse- and human-derived tumors [6–10]. DaRT is the only modality currently available, which provides an efficient method for prolonged treatment of the entire volume of solid tumors by alpha-radiation.
Applied as a monotherapy, DaRT enhanced the anti-tumor immune responses in both high and low immunogenic experimental tumor models [11, 12]. Moreover, DaRT in combination with CpG retarded the growth of DA3-derived tumors more effectively than each treatment alone [12].
A substantial amount of data suggests that host immune cells with a suppressive phenotype represent a significant hurdle to successful immunotherapy of metastatic cancer. The function of suppressor cells which facilitate tumor growth and confer immune tolerance against the tumor was already suggested in 1972 [for review see 13], and their elimination by radiation was postulated to boost anti-tumor immunity [14]. Among the suppressor cells, Tregs and MDSCs are significantly increased in hosts with advanced malignancies [15, 16]. Tumor-derived immunosuppression constantly diminishes anti-tumor immune responses; therefore, therapies neutralizing immunosuppression should be given before vaccination and continued throughout treatment.
Tregs are crucial in mediating immune homeostasis and promoting peripheral tolerance. However, in most cancers, they play a central role in contributing to the progression of the disease [17]. Suppression mechanisms mediated by Tregs are thought to contribute significantly to the failure of current therapies that rely on induction or potentiation of anti-tumor responses [18]. Tregs enrichment was also reported to correlate with breast cancer metastasis [19]. MDSCs are a heterogeneous population of immature myeloid cells that are increased in many cancer types. MDSCs play a central role in suppression of the host immune system through mechanisms such as arginase-1, release of immunosuppressive factors such as reactive oxygen species (ROS), nitric oxide (NO), and certain cytokines [15]. Moreover, the appearance of spontaneous distant metastasis is correlated with MDSCs recruitment in breast cancer [20].
In this study, we investigated the potency of a treatment strategy, which combines tumor ablation based on the intratumoral alpha-irradiation with two approaches for immune stimulation: (1) neutralization of Tregs and MDSCs (2) boost the immune response by immunoadjuvants. The study was performed with mice bearing the murine breast adenocarcinoma, DA3, which is a weakly immunogenic tumor model. DA3 tumors have an immunosuppressive microenvironment involving both Tregs [21] and MDSCs [22]. For Tregs inhibition, low-dose cyclophosphamide (CP) was used [23], and to suppress MDSCs activity, mice were treated with the phosphodiesterase-5 (PDE-5) inhibitor sildenafil. Sildenafil is used for the treatment of erectile dysfunction, pulmonary hypertension, and cardiac hypertrophy and also affects tumors by eliminating MDSCs immunosuppressive functions that results in accumulation and activation of tumor-infiltrating lymphocytes [24]. Immunostimulation was induced with the adjuvant CpG [25].
Materials and methods
Animals
Balb/c female mice (a body weight of 20–25 g, 10 weeks old) delivered by Tel Aviv University (Tel Aviv, Israel) and Harlan (Jerusalem, Israel) were kept in the animal facility of Tel Aviv University. Experiments were performed according to government and institute guidelines and regulations (ethical committees permit No. M-11-104). The survival and general performance of mice were monitored daily.
Tumor cell lines
The DA3 cell line is a dimethylbenzanthracene (DMBA)-induced, undifferentiated breast adenocarcinoma cell line. The cells were grown in DMEM (Biological industries, Israel), supplemented with 10 % fetal calf serum, l-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) (Biological Industries, Israel). The cell line was stored in a humid incubator at a temperature of 37 °C and 5 % CO2.
Anesthesia
All surgical and invasive procedures were held under anesthesia using ketamin (100 mg/kg, Fort Dodge, IA) and xylazine hydrochloride (10 mg/kg, VMD, Arendonk, Belgium) solution in 0.25 mL of PBS. Intraperitoneal inoculation was given 10 min before starting the treatment.
Tumor cell inoculation
DA3 cells were injected into either the low lateral side of the back or the low left mammary gland of female mice (5 × 105 DA3 cells in 0.1 or 0.05 mL HBSS) (Biological Industries, Israel), respectively.
Diffusing alpha-emitter radiation therapy wire (Ra-224-loaded) preparation
Ra-224 (3.66 d half-life)-loaded platinum wires were prepared using a Th-228 (1.91-y half-life) generator. About 50 % of Ra-224 atoms recoil out of the generator surface as positive ions and collected electrostatically on a thin wire. To prevent the Ra-224 desorption, the atoms are embedded few atomic layers into the wire surface through thermal diffusion as described [6].
DaRT treatment
Mice with tumors 6–8 mm long (6.5 ± 0.1) (~50–60 mm3 average volume) were treated by 7-mm Ra-224-loaded wires. As control group; tumor-bearing mice treated by an inert wire. A 23-gauge needle attached to a 2.5-mL syringe was used for wire insertion (Pic indolor, Italy). For the wires implantation, mice were sedated.
Low-dose CP administration
Tumor-bearing mice were injected with 125 mg/kg CP (Sigma, Israel) a day before and a week after insertion of Ra-224-loaded or inert wires. As control groups served inert wire + CP or DaRT/inert wire + PBS treated mice.
Sildenafil administration
Tumor-bearing mice were treated orally with sildenafil (Pfizer, NY) dissolved in the drinking water as published before [24]. Glass bottles were used to avoid absorption. Bottles were covered by aluminum foil to protect from light. Bottles were shaken five times a week, and water was exchanged twice a week. The treatment started as tumors reached 2–3 mm (8.2 ± 2 mm3) and lasted for 6 weeks. Control mice with tumors of similar size received only drinking water.
Adjuvant administration
On the day of wire insertion, mice were injected with 100 μg of CpG (Syntezza, Jerusalem, Israel) in 30 μL PBS (three peri-tumoral injections with 10 μL each). In addition, a day after the wire insertion, mice were inoculated i.v. with 100 μg CpG in 100 μL PBS.
Flow cytometry
Four days after a single i.p injection of 50 or 125 mg/kg CP or PBS, all spleens were harvested from DA3 tumor-bearing mice. A single cell suspension was prepared, and cells were triple stained using anti-CD25 (PE), anti-CD4 (FITC), anti-FoxP3 (APC) antibodies (Biolegend, San Diego, CA). The fluorescent intensity was measured using a FACSort (Becton Dickinson, Mountain View, CA), and results were analyzed using “Cyflogic” software.
Tumor volume calculation
Local tumor growth was determined by measuring 3 mutually orthogonal tumor dimensions 2–3 times per week, according to the following formula: Tumor volume = π/6 × Diameter 1 × Diameter 2 × Height. Local tumor responsiveness to DaRT and immunomodulator treatment was expressed by the following ratios:
Response = Tumor volume on day × after treatment/Tumor volume on the day of wire insertion. Ratio = 0: Complete response (CR); Ratio <1 was considered partial response (PR); Ratio = 1, stable disease; Ratio >1, No response (NR).
Challenge assay
Seventeen to 25 days post-wire insertion, mice treated by DaRT and the immunostimulators were re-inoculated with the same amount of cells that were used in order to induce the primary tumor (5 × 105 tumor cells/mouse). Naïve mice were inoculated with tumor cells for the first time as control for tumor growth.
Statistical analysis
Statistical significance (P < 0.05) between the experimental groups was determined by two-side Student’s T test or two-way ANOVA without replication, statistical tests.
Results
Inhibition of local DA3 tumor development by combined treatment with Ra-224-loaded wires and suppression of either MDSCs or Tregs
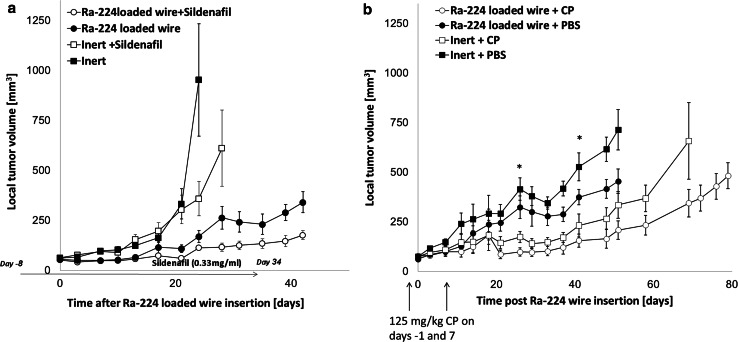
DA3 tumors were treated by DaRT wires and the MDSCs inhibitor, sildenafil. Sildenafil significantly enhanced the ability of the radioactive wires to lower tumor growth rate (P < 0.05; Fig. 1a). Sildenafil also exhibited a limited attenuation effect on tumor growth.
Fig. 1.
Ra-224-loaded wires and sildenafil or CP arrest local DA3 tumor growth. a Tumor growth curves of mice bearing DA3 tumors (57.7 ± 6 mm3) were treated with either Ra-224-loaded wire (27-39 kBq/wire) combined with the administration of sildenafil in drinking water (20 mg/kg/24 h) for 6 weeks (Ra-224-loaded wire + sildenafil) (n = 10); or with Ra-224-loaded wire only (Ra-224-loaded wire + regular drinking water) (n = 10); or with inert wire and sildenafil (inert + sildenafil; n = 10); or inert wire alone (inert + regular drinking water; n = 10). P (ANOVA two ways without replication) <0.05. b Tumor growth curves of mice bearing DA3 tumors (71.8 ± 5.8 mm3) treated with either Ra-224-loaded wire (35-42 kBq/wire) combined with the administration of low-dose CP (125 mg/kg) (Ra-224 wire + CP, n = 7); or Ra-224-loaded wire only (Ra-224 wire + PBS, n = 6); or inert wire combined with low-dose CP (inert wire + CP, n = 7); or inert wire (inert wire + PBS, n = 7). CP was injected i.p. 1 day before the wire insertion and at day 7 after the irradiation. Ra-224-loaded wires insertion is defined as day 0. P < 0.05. (ANOVA—two ways without replication)
Next, we investigated the effect of low-dose CP and DaRT on tumor-bearing mice. CP was injected as a monotherapy to DA3-bearing mice at two doses (50 or 125 mg/kg, n = 3/group) and 4 days later spleens were collected for Tregs FACS analysis. Our findings show that CP efficiently decreases Tregs in the DA3 model and 125 mg/kg were found to be more effective in inhibiting Tregs than 50 mg/kg CP. The percentage of Tregs in the spleen (out of total leukocytes) of DA3 tumor-bearing mice is more than 4 %. Fifty mg/kg CP decreased Tregs percentage to 3.5 %, and 125 mg/kg inhibited Tregs to <2 % (data not shown). Our results show that the combined treatment with DaRT and low-dose CP significantly inhibited DA3 tumor growth. The size of the tumor treated by DaRT + CP was twofold smaller than DaRT alone 42 days post-wire insertion (P < 0.05: Fig. 1b). DaRT + CP also extended the mean survival time of the animals to 116 day compared to the control groups (inert wires + CP, 99 days, DaRT alone, 71 days, or inert wires, 63 days).
We also investigated the effects of the treatment of local tumor with DaRT in combination with low-dose CP administration on the development of lung metastases. It was found that all mice treated with DaRT had metastases in the lungs 55 days after wire insertion, whereas only 80 % of mice treated with DaRT + CP carried lung metastases. Furthermore, the average volume of metastases in the DaRT + CP group was 6.85 times lower than in the DaRT-treated group (8.7 ± 1.1 and 60 ± 29 mm3, respectively; P < 0.05).
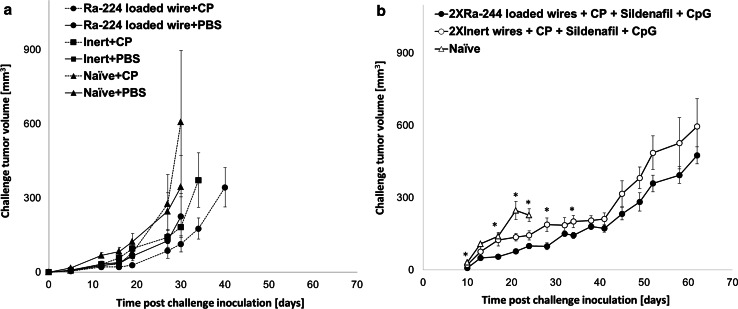
Next, we studied whether treated tumor-bearing mice could develop resistance to an additional tumor challenge. Tumors were treated by a radioactive or inert wire with or without CP (125 mg/kg) and excised 10 days after wire insertion. The mice were re-injected with a tumorigenic dose of DA3 cells and monitored for tumor growth. The experiment revealed that mice treated with both DaRT plus low-dose CP displayed a significantly delayed tumor growth as compared to any other treatment including DaRT or CP alone. Twenty days post-tumor re-inoculation tumors in DaRT and low-dose CP-treated mice were 2.3–4.3 smaller than in mice treated only by Ra-224-loaded or inert wires, mice treated by inert wires plus CP, or normal mice treated by CP, respectively (P < 0.05, Fig. 3a).
Fig. 3.
Combined treatment with Ra-224-loaded wire and CP or CP and sildenafil and CpG stimulated anti-tumor immunity in DA3 tumor-bearing mice. a Mice bearing DA3 tumors were treated with either three Ra-224-loaded wire (25–29 kBq/wire) combined with the administration of low-dose CP (125 mg/kg) (Ra-224 wire + CP, n = 15); or three Ra-224-loaded wire only (Ra-224 wire + PBS, n = 13); or 3 inert wires combined with low-dose CP (inert wire + CP, n = 12); or 3 inert wires (inert wire + PBS, n = 14). CP was injected i.p. 1 day before the wire insertion and at day 7 after the irradiation. Naïve + CP (n = 6) or naïve + PBS (n = 16) were injected with challenge tumor cells only. Residual tumors were resected 10 days after wire insertion, and 7 days later mice were re-inoculated with a tumor challenge. Presented are tumor growth curves of re-inoculated tumors. P < 0.05 Ra-224-loaded wire + CP versus any other group. (ANOVA—two ways without replication). b Mice bearing DA3 tumors (80 ± 41 mm3) were each treated with either two Ra-224-loaded (37-48 kBq/wire) (n=10), or 2 inert wires (n=7) in combination with low-dose CP, sildenafil, and CpG. CP was injected i.p. 1 day before the wire insertion and at day 7 after the irradiation. Mice received sildenafil in the drinking water (20 mg/kg/24 h) for 6 weeks. CpG (100 µg) was administrated 1 day before wire insertion i.t and 1 day after wire insertion. Residual tumors were resected 15 days after wire insertion, and 10 days later mice were re-inoculated with a challenge tumor. Presented are the tumor growth curves of re-inoculated mice. Naïve mice (n=8) that were inoculated for the first time with DA3 served as internal control for tumor development. P (Two-way ANOVA without replication) <0.05. *P (T test) <0.05 (b)
Application of DaRT ablation combined with low-dose CP, sildenafil, and CpG resulted in regression of local tumors and systemic anti-tumor response
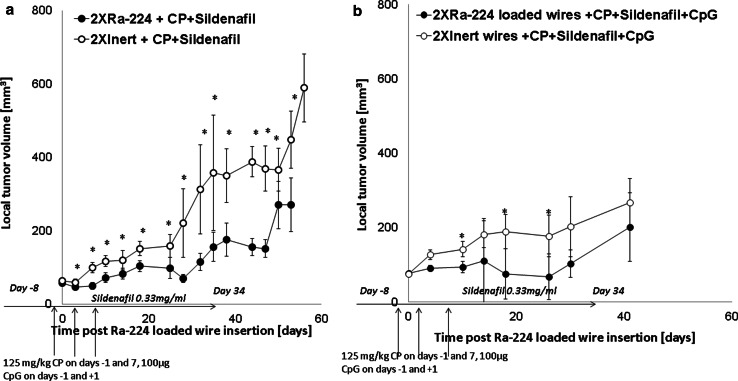
After showing that the combinations of DaRT with either CpG (12) or MDSC or Treg inhibitors significantly inhibited DA3 local tumors, we tested the effect of local alpha-irradiation combined with the inhibition of both Tregs and MDSCs. The results presented in Fig. 2a indicate that the combination of radioactive wire with Treg and MDSC inhibitors was more effective in retardation of tumor development than DaRT with each inhibitor alone (Fig. 1a, b).
Fig. 2.
DaRT in combination with both cyclophosphamide, sildenafil with or without CpG resulted in DA3 delayed tumor growth. Mice bearing DA3 tumors (76 ± 16 mm3) were each treated with either two Ra-224-loaded (28-45 kBq/wire) or inert wires in combination with low-dose CP, sildenafil, and CpG. CP was injected i.p. 1 day before the wire insertion and at day 7 after the irradiation. Mice received sildenafil in the drinking water (20 mg/kg/24 h) for 6 weeks. CpG (100 µg) was administrated one day before wire insertion i.t and one day after wire insertion. a The tumor growth curves of DA3 tumor-bearing mice treated by either 2XRa-224 wires + CP + sildenafil, (n = 20) or 2Xinert wires + CP + sildenafil, (n = 18), P (Two-way ANOVA without replication) <0.05. *P (T test) <0.05. b The tumor growth curve of DA3 tumor-bearing mice treated by two Ra-224 + CP + sildenafil + CpG, (n = 20) or two inert wires + CP + sildenafil + CpG, (n = 20), P (Two-way ANOVA without replication) <0.05. *P (T test) <0.05
In view of these results, we went a step further and treated tumors with DaRT combined with all three immunomodulators, MDSCs and Tregs inhibition and CpG, and compared it with treatment with the immunomodulators alone (Fig. 2b). Out of twenty mice in the DaRT plus immunomodulators group, in six the tumors completely regressed. In two of the six, the tumors recurred 44 days after treatment, and in one animal, metastasic lesions developed in the neck 120 days post-treatment and lung metastases were detected by histology. Three animals remained primary tumor-free; however, metastases were detected in the lungs of one animal by histology. In the other 16 animals in this treatment group, tumor growth inhibition was observed, and the average tumor volume 26 days post-treatment was 67.6 ± 61.3 versus 177.7 ± 56.4 mm3 in the control group. Complete tumor regression was observed in one mouse in the inert wires + 3 immunomodulators group, and inhibition of tumor growth was also observed, as eight out of twenty animals demonstrated a partial response (Table 1). In addition, in this group, two mice survived 150 days post-treatment and lung metastases were detected by histological examination, thereafter.
Table 1.
Effect of treatment with DaRT cyclophosphamide, sildenafil, and CpG on tumor response
| Treatmenta | Elimination of primary tumorb | PRc | NRd |
|---|---|---|---|
| 2XRa-224-loaded wire + CP + sildenafil + CpG | 3 | 16 | 1 |
| 2XInert + CP + sildenafil + CpG | 1 | 8 | 11 |
| 2XRa-224-loaded wire + CP + sildenafil | 0 | 2 | 8 |
| 2XInert + CP + sildenafil | 0 | 0 | 9 |
aMice bearing DA3 tumors (62.4 ± 5.4 mm3) were treated with either 2 Ra-224-loaded (40–50 kBq/wire) or inert wires in combination with low-dose CP, sildenafil, and CpG
bElimination of primary tumor—number of mice in which the primary tumor was eliminated and the mice survived for 39–150 days after treatment
cPR (partial response)—number of mice in which the tumor shrunk or did not grow after treatment
dNR (no response)—number of mice in which progressive tumor growth was scored
P (Chi-square) <0.05
The combination of DaRT and 3 immunomodulators was examined for the induction of a systemic immune response. Ra-224 loaded wires or inert wires were inserted to DA3 primary tumors (tumor volumes on treatment day: 91.7 ± 13.27 vs. 65.89 ± 11.9 mm3). All tumors were excised 15 days after wires insertion, and 10 days later, all mice were re-inoculated with DA3 cells (5 × 105 cells/mice). Naïve mice inoculated with DA3 cells served as an internal control for DA3 tumor development. The group that was treated by inert wires and the 3 immunomodulators resulted in tumor inhibition compared to the naïve group. The group that was initially treated by Ra-224-loaded wires and three immunomodulators had the smallest tumors after the challenge (Fig. 3b).
The combined DaRT treatment with the three immunomodulators reduced the metastatic burden in the treated mice (Table 2). About a third of such treated animals carried lung metastases compared with more than a half of the mice treated with an inert wire and the three drugs. Lung metastases were detected in about two-thirds of the animals treated with Ra-224 wires, low-dose CP and sildenafil, and in most of the animals treated only by inhibitors of Tregs and MDSCs. All of the DaRT-treated or non-treated animals carried lung metastases (Table 2).
Table 2.
Effect of treatment with DaRT cyclophosphamide, sildenafil with or without CpG on elimination of lung metastases
| Total volume of metastases (mm3)c | % Metastases bearing miceb | Treatmenta |
|---|---|---|
| 60 ± 29 | 100 | DaRT alone (n = 10) |
| 16.6 ± 5.6 | 77 | Inert + CP + sildenafil (n = 5) |
| 13.3 ± 6.5 | 60 | DaRT + CP + sildenafil (n = 8) |
| 19.8 ± 14.2 | 55 | Inert + CP + sildenafil + CpG (n = 9) |
| 10.6 ± 9.7 | 30 | DaRT + CP + sildenafil + CpG (n = 8) |
aMice bearing DA3 tumors (60 ± 5 mm3) were treated with either two Ra-224-loaded (40-50 kBq/wire) or inert wires in combination with low-dose CP, Sildenafil ± CpG (n = 5–9). CP was injected i.p. 1 day before the wire insertion and at day 7 after the irradiation. Mice received sildenafil in the drinking water (20 mg/kg/24 h) for 6 weeks. CpG (100 µg) was administrated 1 day before wires insertion i.t and 1 day after wires insertion
bMice were examined by CT, and the number of mice with lung metastases was scored
cMice were examined by CT, and the metastatic load was scored
In addition, the synergism between DaRT and the three immune manipulators resulted in prolongation in life expectancy. Fifty percent of the mice in this group survived 124 days post-Ra-224-loaded wires insertion compared to only 10 % survival in the group treated by inert wire and the 3 immunomodulators (P = 0.051 by Chi-square test).
Discussion
The destruction of tumors by external beam gamma-radiotherapy was reported to boost systemic anti-tumor immunity and serve as a strategy for in situ cancer vaccination [3, 4, 26, 27]. Thus, we investigated whether local destruction of solid tumors by our novel alpha-based radiation brachytherapy (DaRT ablation) boosted the development of effective anti-tumor immunity.
In previous studies, we were the first to report that following ablation of the primary tumor by alpha-irradiation-mediated brachytherapy, mice bearing the immunogenic colon cancer, CT26, exhibited an effective anti-tumor immune response, as manifested by resistance to a tumor challenge both in the skin and in the lungs [12]. Similar experiments using a low immunogenic breast cancer, DA3 cells, treatment with DaRT alone resulted in a weak anti-tumor immune response, which was accompanied by a reduced load of metastases in the lungs [11, 12]. Another report using radioimmunotherapy with alpha-emitters also demonstrated the induction of anti-tumor immunity [28].
A better control of the primary tumor and metastases can be achieved by neutralizing cells that suppress anti-tumor immunity such as Tregs and MDSCs or boost the immune response by immunoadjuvants or using the combination of both approaches.
Elimination of Tregs [29, 30] or MDSCs [31, 32] was found to be essential for an effective anti-tumor immunotherapy. MDSCs depletion was associated with restoration of immune dysfunction in hepatocellular carcinoma patients [33], suggesting that their inhibition might improve the control of cancer development.
The immune microenvironment of the weakly immunogenic DA3 tumor was shown to involve both Tregs [21] and MDSCs [22]. Thus, in the present study, we investigated whether the weak anti-tumor immunity induced by an alpha-irradiation-mediated destruction of DA3 tumors could be enhanced using Treg and MDSC inhibitors and cause to significant retardation of primary and metastatic tumor progression. It was also examined if integrating the radioactive-mediated ablation with suppressor cell inhibitors and the immunoadjuvant, CpG, can acheive a therapeutic effect.
The effect of the various treatment combinations was examined on two levels: (1) growth retardation of the local tumor and (2) inhibition of metastatic lesions in the lungs. First, DaRT ablation was applied simultaneously with either the PDE-5 inhibitor sildenafil that was shown previously to block MDSCs functions [24] or the Tregs inhibitor, cyclophosphamide at low dose [23]. It was shown that the inhibition of MDSCs by sildenafil concomitantly with the radioactive treatment retarded tumor progression more efficiently than DaRT alone (Fig. 1a). Death in DA3 bearing mice is caused by lung metastases, yet DaRT with inhibition of MDSCs alone did not extend the survival of the treated animals as compared to the group treated with DaRT alone. It might be that MDSCs may not affect lung metastases development in the DA3 model. In other studies, inhibition of MDSCs with a selective blocker of CSF1 receptor together with gamma-radiotherapy suppressed tumor growth more effectively than irradiation alone [34]. The possible role of MDSCs in the outcome of tumor ablation was pointed out by the study, which showed that increased MDSC-related functions are an early indicator for incomplete radiofrequency ablation of NSCLC [35].
Tregs inhibition using low-dose CP in synergism with DaRT ablation was significantly more effective than CP alone or DaRT alone in blocking tumor growth (Fig. 1b) and extending overall survival. Life prolongation was due to reduced lung metastases. It has been earlier reported that Tregs depletion also improved the effect of other ablation modalities. Targeting Tregs targeting together with tumor gamma-irradiation significantly reduced tumor burden and improved overall survival [36]. When combined with Tregs depletion, cryoablation was significantly more effective than either surgical excision or cautery at inducing systemic anti-tumor immunity, resulting in the cure of a fraction of animals with established metastatic disease and resistance to re-injection of tumor cells [37]. The combination of Tregs depletion and ablation by photodynamic therapy (PDT) potentiated PDT-mediated immunity, leading to a long-term survival and a development of immunological memory [23].
Next, we attempted to overcome the suppressive microenvironment of the DA3 tumor by combining inhibition of both Tregs and MDSCs with the alpha-irradiation-mediated ablation. We showed for the first time that the combined treatment was significantly better in retarding the tumor growth and reducing lung metastases than alpha-irradiation and inhibition of each suppressor cell population alone (Fig. 2a). Other studies reported that synergistic suppression of Tregs and MDSCs cells in mice with tumors promoted their anti-tumor immunity [38]. Another study using a combination of a fusion protein vaccine with both Tregs and MDSCs inhibition demonstrated a complete eradication of large tumors in mice [39]. Treatment of the murine 4T1 breast cancer with a combination of anti-TGF-β immunotherapy and Tregs inhibitor, low-dose CP, reduced MDSCs numbers and promoted their differentiation indicated by a higher expression of MHC class II and the immune co-stimulatory molecule CD80 [40]. This study also showed that such therapy led to a growth inhibition of lung metastasis and resistance to a tumor challenge.
Finally, since we previously demonstrated that CpG can augment anti-tumor immunity in combination with DaRT [12], we aimed to maximize the anti-tumor immunity following ablation of the low immunogenic DA3 primary tumor by adding CpG to the inhibitors of Tregs and MDSCs. The combined treatment was effective in eliminating both primary tumors and lung metastases and stimulating systemic immune response. This combination, cured three animals with an established tumor and resulted in the highest rate of partial response in several animals with established tumors and lung metastases. To our knowledge, this is the only report on a curative effect of such combined treatment. Gamma-irradiation in combination with an immunostimulant and inhibitor of Tregs was earlier reported to be the most effective treatment of a breast cancer [41]. Furthermore, it was claimed in another report that the key mechanisms of augmenting the functions of adoptively transferred T cells by total body irradiation in melanoma patients include the depletion of Tregs and MDSCs and the activation of the innate immune system via Toll-like receptor 4 signaling [42].
This alpha-based radiotherapy may also prove to be more effective against cancer stem cells. The targeting of CSCs is highly important due to their ability to acquire resistance to cytotoxic therapies and their propensity to form metastases [43]. Therapy based on alpha-irradiation has been recently shown to kill cancer stem cells (CSCs) [44].
Taken together, DaRT is a promising cancer treatment modality that is capable of both local tumor destruction and immune stimulation and can be used either instead or before surgery. However, the treatment with DaRT alone in some cases is not completely curative due to the tumor-induced immune cell dysfunction and immune suppression. Here it was shown that DaRT ablative treatment could be perfectly combined with other immunotherapies to maximize the anticancer immunity induced by DaRT. Our findings suggest that the combination strategy including DaRT, the neutralization of immunosuppressive cells and immunostimulation with immunoadjuvants could be applied for the treatment of cancer patients. Ra-224-loaded wires can be inserted a few days before a subsequent operation leading not only to primary tumor shrinking but also to the stimulation of an anti-tumor immune response that can react against circulating cells or distant metastases.
Acknowledgments
We thank Dr. Gideon Halpern for assistance with the statistical analysis and Kathrin Frank for technical assistance. This work was supported in part by Roberts–Guthman Chair in Immunopharmacology and The German-Israeli Foundation for Scientific Research and Development (GIF) (to Prof. Yona Keisari and Prof. Viktor Umansky). This work was performed in partial fulfillment of the requirements toward a Ph.D. degree of Hila Confino, Sackler Faculty of Medicine, Tel Aviv University.
Abbreviations
- Bq
Becquerel
- CP
Cyclophosphamide
- CSC
Cancer stem cell
- CT
Computed tomography
- DaRT
Diffusing alpha-emitters radiation therapy
- i.p
Intra-peritoneal
- i.v
Intra-venous
- MDSC
Myeloid-derived suppressor cell
- NR
No response
- NT
Non-treated
- PBS
Phosphate buffered saline
- PR
Partial response
- Ra
Radium
- s.c
Subcutaneous
- Treg
Regulatory T cell
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Note on previous publications:
1. European Association for Cancer Research (EACR 23), Munich, Germany, July 5–8, 2014. Eur. J. Cancer, 2014, 50, Suppl 5, S213.
2. International Cong. Anti Cancer Res. Sithonia, Greece, Oct. 5–10, 2014. Anti Cancer Res. 2014, 34 (10), 5994–5.
3. Seventh International Conference on Tumor Microenvironment: Progression, Therapy and Prevention, Tel Aviv, Israel, Oct. 11–16, 2015. Cancer Microenvironment, 2015, 8, suppl. 1, S111.
References
- 1.Corthay A. Does the immune system naturally protect against cancer? Front Immunol. 2014;5:197. doi: 10.3389/fimmu.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nobler MP. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology. 1969;93:410–412. doi: 10.1148/93.2.410. [DOI] [PubMed] [Google Scholar]
- 3.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Crittenden M, Kohrt H, Levy R, et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol. 2015;25:54–64. doi: 10.1016/j.semradonc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frey B, Rubner Y, Kulzer L, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arazi L, Cooks T, Schmidt M, et al. Treatment of solid tumours by interstitial release of recoiling short-lived alpha emitters. Phys Med Biol. 2007;52:5025–5042. doi: 10.1088/0031-9155/52/16/021. [DOI] [PubMed] [Google Scholar]
- 7.Cooks T, Schmidt M, Bittan H, et al. Local control of lung derived tumors by diffusing alpha-emitting atoms released from intratumoral wires loaded with radium-224. Int J Radiat Oncol Biol Phys. 2009;74:966–973. doi: 10.1016/j.ijrobp.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Horev-Drori G, Cooks T, Bittan H, et al. Local control of experimental malignant pancreatic tumors by treatment with a combination of chemotherapy and intratumoral 224 radium-loaded wires releasing alpha-emitting atoms. Transl Res. 2012;159:32–41. doi: 10.1016/j.trsl.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Cooks T, Tal M, Raab S, et al. Intratumoral 224Ra-loaded wires spread alpha-emitters inside solid human tumors in athymic mice achieving tumor control. Anticancer Res. 2012;32:5315–5321. [PubMed] [Google Scholar]
- 10.Reitkopf-Brodutch S, Confino H, Schmidt M, et al. Ablation of experimental colon cancer by intratumoral 224Radium loaded wires is mediated by alpha particles released from atoms which spread in the tumor and can be augmented by chemotherapy. Int J Radiat Biol. 2015;91(2):179–186. doi: 10.3109/09553002.2015.959666. [DOI] [PubMed] [Google Scholar]
- 11.Keisari Y, Hochman I, Confino H, et al. Activation of local and systemic anti-tumor immune responses by ablation of solid tumors with intra-tumoral electrochemical or alpha radiation treatments. Cancer Immunol Immunother. 2014;63:1–9. doi: 10.1007/s00262-013-1462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Confino H, Hochman I, Efrati M, et al. Induction of anti-tumor immunity against experimental metastatic tumors following tumor ablation by intratumoral Ra-224 loaded wires. Cancer Immunol Immunother. 2015;64:191–199. doi: 10.1007/s00262-014-1626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prehn RT, Prehn LM. The flip side of immune surveillance: immune dependency. Immunol Rev. 2008;222:341–356. doi: 10.1111/j.1600-065X.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- 14.North RJ. Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother. 1984;16(3):175–181. doi: 10.1007/BF00205425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanchot C, Terme M, Pere H, et al. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron. 2013;6:147–157. doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee A, Vasanthakumar A, Grigoriadis G. Modulating T regulatory cells in cancer: how close are we? Immunol Cell Biol. 2013;91(5):340–349. doi: 10.1038/icb.2013.12. [DOI] [PubMed] [Google Scholar]
- 18.Oleinika K, Nibbs RJ, Graham GJ, et al. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol. 2013;171:36–45. doi: 10.1111/j.1365-2249.2012.04657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe MA, Oda JM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 2010;29(4):569–579. doi: 10.1007/s10555-010-9247-y. [DOI] [PubMed] [Google Scholar]
- 20.Danilin S, Merkel AR, Johnson JR, et al. Myeloid—derived suppressor cells expand during breast cancer progression and promote tumor—induced bone destruction. Oncoimmunology. 2012;1(9):1484–1494. doi: 10.4161/onci.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrio R, Torroella-Kouri M, Libreros S, et al. Decreased accumulation of immune regulatory cells is correlated to the antitumor effect of IFN-γ overexpression in the tumor. Int J Oncol. 2011;39:1619–1627. doi: 10.3892/ijo.2011.1169. [DOI] [PubMed] [Google Scholar]
- 22.Youn JI, Nagaraj S, Gabrilovich MI. Subset of myeloid—derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reginato E, Mroz P, Chung H, et al. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen. Br J Cancer. 2013;109:2167–2174. doi: 10.1038/bjc.2013.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer C, Sevko A, Ramacher M, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci USA. 2011;108:17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bode C, Zhao G, Steinhagen F, et al. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 27.Ahmed MM, Hodge JW, Guha C, et al. Harnessing the potential of radiation-induced immune modulation for cancer therapy. Cancer Immunol Res. 2013;1:280–284. doi: 10.1158/2326-6066.CIR-13-0141. [DOI] [PubMed] [Google Scholar]
- 28.Gorin JB, Ménager J, Gouard S, et al. Antitumor immunity induced after α irradiation. Neoplasia. 2014;16:319–328. doi: 10.1016/j.neo.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casares N, Arribillaga L, Sarobe P, et al. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 30.Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 31.Kusmartsev S, Cheng F, Yu B, et al. All- trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 32.Iclozan C, Antonia S, Chiappori A, et al. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62(5):909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalathil S, Lugade AA, Miller A, et al. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider T, Sevko A, Heussel CP, et al. Serum inflammatory factors and circulating immunosuppressive cells are predictive markers for efficacy of radiofrequency ablation in non-small cell lung cancer. Clin Exp Immunol. 2015;180:467–474. doi: 10.1111/cei.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bos PD, Plitas G, Rudra D, et al. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210(11):2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy MY, Sidana A, Chowdhury WH, et al. Cyclophosphamide unmasks an antimetastatic effect of local tumor cryoablation. J Pharmacol Exp Ther. 2009;330(2):596–601. doi: 10.1124/jpet.109.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tongu M, Harashima N, Monma H, et al. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother. 2013;62:383–391. doi: 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song X, Guo W, Cui J, et al. A tritherapy combination of a fusion protein vaccine with immune-modulating doses of sequential chemotherapies in an optimized regimen completely eradicates large tumors in mice. Int J Cancer. 2011;128(5):1129–1138. doi: 10.1002/ijc.25451. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Yang Y, Zhou Q, et al. Effective chemoimmunotherapy with anti-TGFβ antibody and cyclophosphamide in a mouse model of breast cancer. PLoS one. 2014;9:e85398. doi: 10.1371/journal.pone.0085398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewan MZ, Vanpouille-Box C, Kawashima N, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res. 2012;18:6668–6678. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patabiraman DR, Weinberg RA. Tackling the cancer stem cells—what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sgouros G, Song H. Cancer stem cells targeting using the alpha-particle emitter, 213Bi: mathematical modeling and feasibility analysis. Cancer Biother Radioparm. 2008;23:74–81. doi: 10.1089/cbr.2007.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]