Abstract
Lung cancer is the second most common form of cancer and the leading cause of cancer death worldwide. Pleural effusions, containing high numbers of mononuclear and tumor cells, are frequent in patients with advanced stages of lung cancer. We reported that in pleural effusions from primary lung cancer, the CD8+ T cell subpopulation, and particularly the terminally differentiated subset, is reduced compared to that of non-malignant effusions. We analyzed the participation of activation-induced cell death (AICD) and extrinsic pathways (type I or II) as mechanisms for the decrease in pleural effusion CD8+ T cell subpopulation. Pleural effusion or peripheral blood CD4+ and CD8+ T cells, from lung cancer patients, were stimulated with anti-CD3 antibody and analyzed for (a) apoptosis by annexin-V-binding and TUNEL assay, (b) transcript levels of Fas ligand (FasL) and TRAIL by real-time RT–PCR, (c) expression of FasL and TRAIL, measured as integrated mean fluorescence intensities (iMFI) by flow cytometry, (d) expression of Bcl-2 and BIM molecules, measured as MFI, and (e) apoptosis inhibition using caspase-8 and -9 inhibitors. Pleural effusion CD8+ T cells, but not CD4+ T cells, from cancer patients underwent AICD. Blocking FasL/Fas pathway protected from AICD. Upregulation of FasL and TRAIL expressions was found in pleural effusion CD8+ T cells, which also showed a subset of Bcl-2 low cells. In memory CD8+ T cells, AICD depended on both extrinsic and intrinsic apoptotic pathways. Hence, in the pleural space of lung cancer patients, AICD might compromise the antitumor function of CD8+ T cells.
Keywords: Activation-induced cell death, CD8+ T cells, Lung cancer, Pleural effusion, Bcl-2 molecule, Fas ligand (FasL), Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
Introduction
Lung cancer is the second most common form of cancer and the leading cause of cancer death worldwide [1, 2]. Pleural effusion is a frequent clinical manifestation of metastatic lung tumors, and high numbers of mononuclear cells and tumor cells are frequently found within this fluid [3–5]. Because CD8+ T cells play a key role in the antitumor immune response, tumors have developed mechanisms to circumvent the immune system by suppressing antitumor CD8+ T cell activity [5–7].
T cell apoptosis has been proposed to severely compromise antitumor function of the host immune system and contributes to tumor progression [8–12]. Several mechanisms have been proposed for explaining T cell apoptosis. Spontaneous apoptosis of CD3+ T cells in peripheral blood from cancer patients (e.g., breast carcinoma, head and neck cancer, and oral carcinoma) is associated with high levels of Fas expression [10, 11]. The binding of Fas ligand (FasL) on the tumor cell to the Fas receptor on the T cell, a hypothesis known as tumor counterattack, has been suggested to be responsible for T cell death [8, 9]. However, T cell apoptosis still occurs in some types of tumors, including lung carcinomas, which do not express functional FasL [8, 12, 13].
Alternatively, chronic immune cell activation can lead to exhaustion of T cells in some chronic infections (e.g., HIV and HBV infections) [12]. One mechanism of T cell exhaustion is mediated by activation-induced cell death (AICD) [12, 14]. AICD is mediated by Fas/FasL interactions, which provoke T cells to kill each other and themselves [14–16]. Two types of Fas-mediated apoptotic signaling pathways have been described according to the type of cell involved. Type I cells are characterized by a high level of formation of the Fas-death-induced signaling complex (DISC) and activated caspase-8, which activates the downstream effector caspases. In type II cells, lower amounts of Fas-DISC are formed, leading to lower levels of activated caspase-8. This reduction is compensated by an amplification loop through the activation of the intrinsic pathway, in which caspase-8 cleaves Bid to generate truncated Bid (tBid). tBid then migrates to the mitochondria and induces the release of cytochrome c into the cytosol. This is followed by the formation of the apoptosome and caspase-9 activation and subsequent activation of effector caspases, leading to cell death [14, 16]. Type II apoptotic signaling, compared to type I signaling, can be blocked by high expression of anti-apoptotic Bcl-2 family members, such as Bcl-2 or Bcl-XL. In contrast, in type I cells, Fas-induced apoptosis cannot be blocked by the expression of Bcl-2 or Bcl-XL [14, 16].
In cancer patients, AICD has been hypothesized to mediate apoptosis of tumor-infiltrating lymphocytes (TIL) [12, 17, 18]. In some murine cancer models [19, 20] and cancer patients [18, 21, 22], CD8+ T cells undergo AICD after tumor antigen-specific or polyclonal stimulation. We previously reported that, in lung cancer, the CD8+ T cell subpopulation is reduced in pleural effusions compared to non-malignant pleural effusions. Furthermore, within this CD8+ T cell subpopulation, cells with a memory phenotype predominate over terminally differentiated cells [5]. To analyze the possible mechanisms involved in the decrease in CD8+ T cell subpopulation in pleural effusions, we evaluated whether polyclonal stimuli (anti-CD3 antibody) could induce AICD in vitro. Our data show that CD8+ T cells, but not CD4+ T cells, underwent AICD following anti-CD3 antibody stimulation, and this phenomenon is associated with the upregulation of FasL and TRAIL molecules. Moreover, two subsets of CD8+ T cells expressing Bcl-2 were found, and the percentage of Bcl-2hi CD8+ T cells decreased, with a concomitant increase in the Bcl-2lo CD8+ T cell subset. Furthermore, caspase-8 and -9 inhibitors blocked AICD. Taken together, our results suggest that tumor microenvironment sensitizes pleural effusion CD8+ T cells to AICD, which is amplified by the intrinsic pathway of apoptosis. Thus, in malignant pleural effusions, AICD compromises the antitumor functions of CD8+ T cells.
Materials and methods
Population studied
A total of 44 patients with lung cancer-associated pleural effusion were studied. Pleural fluid was obtained by thoracocentesis used for routine diagnostic procedures. Diagnosis was established by histological examination of pleural biopsy or cytological observation of malignant cells in pleural effusion. No patients received any type of anticancer therapy before the study. According to diagnosis, cancer group was composed of 39 pleural effusions of non-small cell lung carcinoma origin (32 adenocarcinomas and 7 squamous carcinomas) and 5 from mesotheliomas. Malignant pleural effusions included in this study presented high numbers of tumor and inflammatory cells. The median age of the cancer group was 62 years (range: 41–83 years). A second group of 15 patients with non-malignant effusions were included. This group consisted of pneumonias (nine), congestive heart failures (five), and hemothoraces (two), and the median age of this group was 66 (range: 25–78 years).
Peripheral blood was obtained from the cancer group and from 11 healthy non-smoking volunteers as a control group. The median age for the control group was 50 years (range: 41–72 years). Written informed consent was obtained from all participants included in this study. From pleural effusions, mononuclear cells (PEMCs) and tumor cells were separated as previously reported [5]. Peripheral blood mononuclear cells (PBMCs) from heparinized venous blood were separated on Lymphoprep (Axis-Shield, Oslo, Norway). The Committee of Science and Bioethics of the Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas approved the protocol for collection of biological samples.
Evaluation of spontaneous and anti-CD3-induced apoptosis in CD4+ and CD8+ T cells from PEMCs or PBMCs
Lung tumors may induce and amplify non-HLA restricted, inflammatory responses in the pleural compartment, through chemokines, cytokines, or other soluble factors, leading to increased susceptibility to AICD. Thus, we evaluated activation-induced cell death using anti-CD3 monoclonal antibody [22, 23] in CD4+ and CD8+ T cell subpopulation from PBMCs or PEMCs. Spontaneous apoptosis was also determined, and to evaluate this phenomenon, PBMCs or PEMCs were seeded at 1 × 106 cells/ml in RPMI-1640 supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate in 96-well culture plates. For anti-CD3-induced apoptosis, PBMCs or PEMCs were cultured in plates coated with 10 μg/ml anti-CD3 mAb (IgG, UCHT-1 clone; Pharmingen, CA). For all samples, plates were incubated with 5% CO2 at 37°C for 24 h.
After treatment, the percentages of apoptotic CD4+ and CD8+ T cells were determined by the detection of phosphatidylserine on the outer leaflet of the plasma membrane with annexin-V-FITC [23]. Briefly, mononuclear cells were collected and resuspended in PBS containing 1% BSA and 0.1% NaN3 and incubated with a cocktail of anti-CD4-PECy5 (BioLegend, San Diego) or anti-CD8-PECy5 (BioLegend) mAb on ice for 30 min. After washing, the cells were incubated with 1 μg/ml annexin-V-FITC (Santa Cruz Biotechnology, CA) to stain phosphatidylserine and 10 μg/ml propidium iodide to exclude necrotic cells. The cutoffs for PI cells were established, as previously reported [24], from a FSC versus PI dot-plot graph; then, from a FSC versus SSC dot-plot graph, 25,000 events from a lymphocyte-gated population were acquired; the gate was stringently set to eliminate cellular debris and necrotic cells. This allowed for a clear-cut discrimination between viable non-apoptotic and apoptotic cells in the CD4+ or CD8+ gate. Flow cytometric analyses were performed using a FACSCalibur (Becton–Dickinson) and FlowJo software (Tri Star Inc., v 8.8.6).
Apoptosis induced by anti-CD3-mAb treatment was calculated according to the following equation [23]:
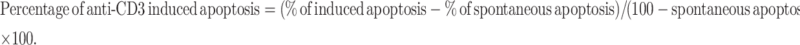 |
Evaluation of anti-CD3-induced apoptosis in CD4+ and CD8+ T cells from PEMCs by TUNEL assay
As annexin-V is an early marker of apoptosis, late apoptotic cells were detected using FlowTACS in situ terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-based Apoptosis Detection Kit (Trevigen, Gaithersburg, MD) according to manufacturer’s protocol. PEMCs were stimulated with 10 μg/ml anti-CD3 mAb as described above for 30 h, in presence of 400 nM non-peptide caspase-3 selective inhibitor (NSCI, Sigma-Aldrich, St. Louis, MO), antagonist 10 μg/ml anti-FasL mAb (clone 5G51, Enzo Life Sciences, San Diego, CA), or isotype control. Subsequently, 2 × 105 cells were harvested and incubated at room temperature (RT) for 30 min with anti-CD3 (PE-Texas Red, Invitrogen, Camarillo, CA), anti-CD4 (Alexa 700, BioLegend), and anti-CD8 mAbs (APC-Cy7, BioLegend), and then, cells were fixed with 1% paraformaldehyde at RT for 15 min. Cells were washed with PBS and permeabilized with 100 μl of Cytonin at RT for 30 min. The fragmented DNA was revealed by incubating the samples with the labeling reaction mix at 37°C for 1 h, and then cells were stained with strep-fluorescein solution for 10 min. Flow cytometric analysis was performed using a FACSCanto II (Becton–Dickinson). From a FSC versus SSC dot-plot graph, 30,000 events from a lymphocyte-gated population were acquired; the gate was stringently set to eliminate cellular debris and necrotic cells. Then, CD3+ cells were gated and CD4+ versus CD8+ cells dot-plot was set. TUNEL-positive cells were determined by TUNEL versus FSC 5% contour outlier plot of CD3+ CD4+- or CD3+ CD8+-gated cells.
Determination of FasL, TRAIL, Bcl-2, and BIM in anti-CD3-stimulated CD4+ and CD8+ T cells
After anti-CD3 mAb stimulation (10 μg/ml) of PBMCs or PEMCs (as described above), the percentages of FasL-, TRAIL-, or Bcl-2-positive cells and Bcl-2 and BIM protein levels were analyzed in CD4+ and CD8+ T cells by flow cytometry. For FasL and TRAIL membrane staining, PBMCs or PEMCs was harvested and incubated at RT for 30 min with anti-CD4 PECy5 or anti-CD8-PECy5 mAbs, in addition to either PE-conjugated anti-FasL, anti-TRAIL, or an isotype control mAb (all purchased from eBioscience, San Diego, CA). Cells were washed and fixed with 1% paraformaldehyde.
For intracellular detection of Bcl-2 and BIM, PBMCs or PEMCs, incubated previously with anti-CD4 PECy5 or anti-CD8-PECy5 mAbs, were washed and permeabilized with FACS Permeabilizing Solution (BD Pharmingen, San Diego, CA) at RT for 10 min. After washing, permeabilized cells were incubated with unlabeled rabbit anti-BIM polyclonal antibody (Cell Signaling, Danvers, MA), PE-conjugated mouse anti-Bcl-2 mAb (Bcl-2 100 clone, Santa Cruz Biotechnology, CA) or corresponding isotype control antibodies at RT for 30 min. For the detection of BIM, cells previously incubated with anti-BIM antibody were washed and incubated with Alexa 488 mouse anti-rabbit (Molecular Probes, Eugene OR) mAb at RT for 1 h. After washing, cells were fixed with 1% paraformaldehyde and analyzed using flow cytometry.
FSC versus SSC dot-plot graphs were done for cellular debris and necrotic cells exclusion. From a lymphocyte gate containing 50,000 lymphocytes, CD4+ or CD8+ cells were gated from a CD4+ or CD8+ versus SSC dot-plot graph. For the analysis of FasL and TRAIL expressions and to rule out non-specific antibody binding and autofluorescence, quadrants were set according to isotype controls obtained from stimulated CD4+ or CD8+ cells. The integrated mean fluorescence intensity (iMFI), which reflects the total functional response in terms of quality (mean fluorescence intensity, MFI) and magnitude (percentage), was calculated by multiplying the percentage of FasL+ or TRAIL+ CD8+-positive cells by their corresponding MFI, as described previously [25].
The percentage of Bcl-2-positive cells was determined by histograms, and for analysis of Bcl-2 and BIM expressions, the median fluorescence intensities (MFI) for each molecule were obtained by histograms.
CD8+ T cells purification and subsequent anti-CD3 stimulation
For some experiments, CD8+ T cells from PBMCs or PEMCs were purified by negative selection using a CD8+ T cell isolation kit (Miltenyi Biotec, Auburn, CA) following the manufacturer’s protocol. The purity of this subpopulation was always greater than 94%.
CD8+ T cells (1 × 106 cells/ml) were cultured in 96-well plates coated with 10 μg/ml anti-CD3 mAb. After incubation, the cells were harvested and analyzed for FasL and TRAIL transcript levels by real-time RT–PCR or the determination of apoptosis in CD8+ T cell subsets (naïve, memory, and terminally differentiated cells) by flow cytometry (see below).
RNA isolation and determination of FasL and TRAIL transcript levels in purified CD8+ T cells
Total RNA was isolated from non-stimulated or anti-CD3-stimulated CD8+ T cells for 4 h using the RNeasy micro Kit according to the manufacturer’s instructions (Qiagen). Contaminant DNA was removed from RNA samples using DNase provided by the manufacturer. RNA was reverse transcribed into cDNA and amplified. The transcript levels of FasL and TRAIL were determined using TaqMan probes (Applied Biosystems, Foster City, CA) with cDNA as template. Validation curves were obtained using 18S rRNA, GAPDH, and β-actin to determine the suitable endogenous control for TRAIL and FasL; β-actin was selected as the endogenous control for both transcripts. Each PCR reaction was performed in triplicate, and no template controls were included (value differences were less than 0.3 SD). Fluorescent emission from the released reporter dye was monitored using an ABI Prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA) in 96-well reaction plates using the recommended parameters (10 min at 95°C, 40 cycles of 95°C for 15 s and 60°C for 1 min). Data were analyzed with Sequence Detection software (v 1.3.1) to establish the PCR cycle at which the fluorescence exceeded a set threshold (Ct) for each sample. Data were analyzed according to the comparative Ct method, using β-actin transcript levels to normalize for differences in sample loading and preparation. The results are semiquantitative and represent the n-fold difference in the transcript levels from a particular sample compared to its calibrator cDNA (cDNA from non-stimulated CD8+ T cells from each subject).
Detection of apoptosis in CD8+ T cell subsets after anti-CD3 stimulation
After anti-CD3 stimulation of purified CD8+ T cells for 24 h (described above), the cells were stained using a combination of anti-CD28 PE-Cy5 and anti-CD45RO PE mAbs for the identification of CD45RO+ CD28+ (memory), CD45RO-CD28+ (naïve), CD45RO+ CD28-, and CD45RO-CD28- (terminally differentiated) CD8+ T cell subsets. Cells were further stained with annexin-V-FITC, as described above. In all cases, 20,000 events from the CD8+ lymphocyte-gated population were analyzed by flow cytometry by histograms, to evaluate the percentage of annexin-V-positive cells in each subset.
Effect of caspase-8 or caspase-9 inhibition on CD8+ T cell death after anti-CD3 stimulation
To evaluate the participation of the extrinsic or intrinsic apoptotic pathways in AICD, PEMCs from lung cancer patients were incubated with specific caspase inhibitors. Caspase-8 inhibitor II (Z-IE(OMe)TD(OMe)-FMK) and caspase-9 inhibitor III (Ac-LEHD-CMK) were purchased from Calbiochem (La Jolla, CA). These inhibitors are cell permeable and irreversible inhibitors of their respective caspases; caspase-8 inhibitor (5 mM) and caspase-9 inhibitor (10 mM) stock solutions were prepared in DMSO. Anti-CD3-stimulated cells were incubated with caspase-8 inhibitor (5 μM), caspase-9 inhibitor (20 μM), or a combination of the two for 24 h. After incubation, the cells were harvested and incubated with anti-CD45RA PE-Cy7, anti-CD28 PECy5, and anti-CD8 APC-Cy7 and then stained for the detection of phosphatidylserine with annexin-V-FITC and propidium iodide. Multiparametric analyses were performed using a FACSCanto II. To discriminate cellular debris and necrotic cells, cutoffs for PI cells were done as described above; then, the percentage of annexin-V-positive cells were determined by means of histograms after gating on CD8+ cells in total, or naïve, memory, and terminally differentiated CD8+ T cell subsets.
Statistical analysis
All values are expressed as the mean ± SE. Unpaired Student’s t tests were used for comparisons between peripheral blood from healthy donors and the cancer group or between malignant and non-malignant effusions. Paired Student’s t tests were used to compare data obtained from pleural effusion versus peripheral blood from the cancer group. Significant differences between groups were considered at P < 0.05.
Results
Pleural effusion but not peripheral blood CD8+ T cells from lung cancer patients are susceptible to AICD
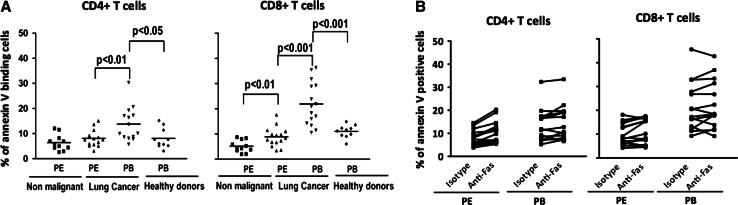
Spontaneous apoptosis was evaluated in CD4+ and CD8+ T cells from pleural effusions and peripheral blood of lung cancer patients (Fig. 1a). In malignant effusions, CD8+ T cells, but not CD4+ T cells, showed a significantly higher percentage of spontaneous apoptosis compared to those from non-malignant effusions (Fig. 1a). In cancer patients, the percentages of spontaneous apoptosis in CD4+ and CD8+ T cells were significantly higher in peripheral blood compared to pleural effusion samples. In peripheral blood, a significant increase in the percentage of spontaneous apoptosis was observed in both CD4+ and CD8+ T cells from lung cancer patients compared to healthy subjects (Fig. 1a). Thus, CD4+ and CD8+ T cells from the peripheral blood of lung cancer patients are more sensitive to apoptosis. Similar results have been found in other types of cancer [10, 11, 24]. Increase of spontaneous apoptosis in CD8+ T cells has been attributed to direct the activation of the extrinsic pathway [9, 10]; we tested this apoptosis by incubating PBMCs or PEMCs from lung cancer patients with an agonistic anti-Fas antibody (clone CH11) for 24 h. Contrary to these previous reports, we did not observe increases in the mean percentage of annexin-V-positive cells with respect to the isotype control in pleural effusion and peripheral blood CD4+ and CD8+ T cells (Fig. 1b).
Fig. 1.
CD4+ and CD8+ T cells from lung cancer patients do not undergo apoptosis after treatment with agonistic anti-Fas mAb. a Percentages of spontaneous apoptosis in CD4+ and CD8+ T cells from lung cancer and control groups after 24-h incubation of PEMCs or PBMCs in RPMI-1640 medium, determined by annexin-V binding. Bars depict the means. b PEMCs or PBMCs were incubated with anti-Fas mAb (1 μg/ml, clone IgM, clone CH11) or isotype control for 24 h; then, percentages of apoptotic cells were determined by annexin-V binding in CD4+ and CD8+ T cells from pleural effusion (PE) and peripheral blood (PB) of lung cancer patients (n = 12)
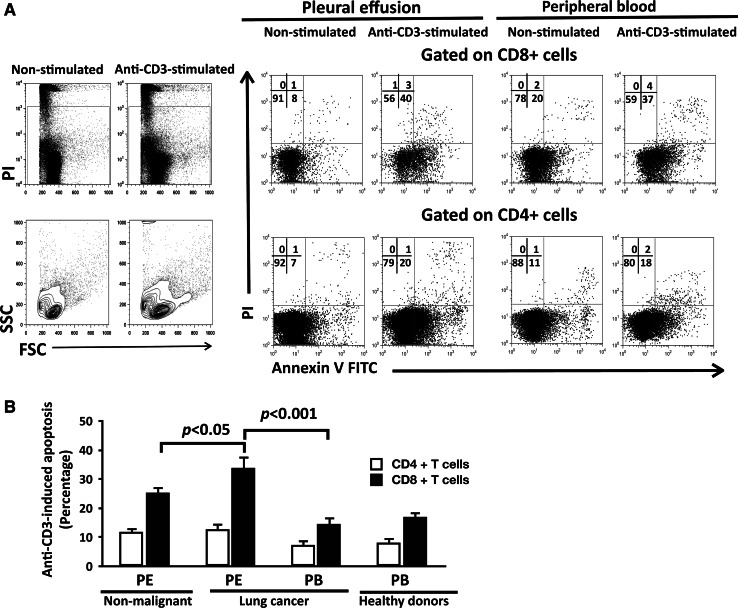
It has been hypothesized that AICD mediates apoptosis of TIL in cancer patients [12, 17]. Thus, we evaluated the susceptibility of CD4+ and CD8+ T cell subpopulations to AICD-mediated apoptosis. CD4+ and CD8+ T cells from lung cancer and control (healthy subjects and non-malignant diseases) groups that were stimulated with 10 μg/ml of anti-CD3 mAbs showed significantly higher percentages of annexin-V-positive cells compared to the corresponding non-stimulated subpopulations (Fig. 2a, data from a representative cancer patient).
Fig. 2.
Pleural effusion CD8+ T cells from lung cancer patients stimulated with anti-CD3 mAb presented high levels of apoptosis. a Plots showing the gating strategies for lymphocytes, cells were gated on the corresponding CD4+ or CD8+ T cell subpopulation, and analysis of annexin-V versus propidium iodide (PI) was performed, data from CD3-stimulated and non-stimulated pleural effusion (PE) and peripheral blood (PB) CD4+ and CD8+ T cells from a cancer patient. b Comparison of apoptosis induced by anti-CD3 mAb stimulation (10 μg/ml) in CD4+ and CD8+ T cells from all groups. PE from non-malignant patients (n = 11), PE and peripheral blood (PB) from lung cancer patients (n = 15) and PB from healthy donors (n = 13). Bars depict the mean ± SE
To evaluate only the apoptosis generated by anti-CD3 mAb treatment and for comparing this effect among the different groups studied, the percentage of anti-CD3-induced apoptosis was calculated. In CD4+ T cells, anti-CD3-induced apoptosis showed no statistical differences among the distinct groups studied (see Fig. 2b).
However, pleural effusion CD8+ T cells from cancer patients showed higher percentages of anti-CD3-induced apoptosis compared to those from the non-malignant group. When a comparison was done within the cancer patient group, anti-CD3-induced apoptosis in effusion CD8+ T cells was approximately twofold higher than in peripheral blood CD8+ T cells from the same patients (Fig. 2a, b). In peripheral blood CD8+ T cells, similar percentages of anti-CD3-induced apoptosis between lung cancer patients and healthy donors were found (Fig. 2b).
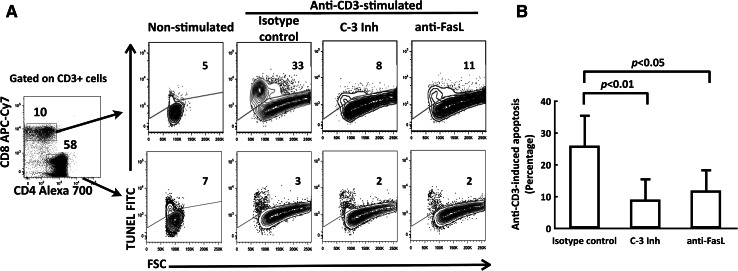
As annexin-V is an early marker of apoptosis, we corroborated that pleural effusion CD8+ T cells went through AICD by using TUNEL assay and FSC analysis. Pleural effusion CD8+ T cells, but not CD4+ T cells, from lung cancer patient and stimulated with 10 μg/ml anti-CD3 mAbs, showed higher percentages of TUNEL-stained cells compared to the corresponding non-stimulated subpopulations. Most of the TUNEL-stained apoptotic cells were smaller than unstained live cells (Fig. 3a). In addition, non-peptide caspase-3 inhibitor (400 nM) significantly reduced the percentage of anti-CD3-induced apoptosis in CD8+ T cells (Fig. 3a, b).
Fig. 3.
AICD of pleural effusion CD8+ T cells from lung cancer patients is blocked by caspase-3 inhibitor or FasL mAb. a Data from pleural effusion CD4+ and CD8+ T cells from a cancer patient are shown. PEMCs were stimulated with anti-CD3 mAbs for 30 h in the presence of a caspase-3 inhibitor, blocking anti-FasL mAb or isotype control. TUNEL-positive cells were determined by TUNEL versus FSC 5% contour outlier plot of CD4+ (lower row) or CD8+ cells (upper row), gated from CD3+ cell population, as described in “Materials and methods”. Percentage of TUNEL-positive cells is shown. b Comparison of apoptosis induced by anti-CD3 mAb stimulation (10 μg/ml) in pleural effusion CD8+ T cells. Percentage of anti-CD3-induced apoptosis is shown for isotype control, caspase-3 inhibitor (C-3 inh), and anti-FasL mAb (n = 5). Bars depict the mean ± SE
FasL and TRAIL molecules have been shown to mediate AICD [12, 15]. To assess the involvement of the Fas/FasL pathway in AICD, PEMCs stimulated with anti-CD3 mAb were incubated in the presence or in the absence of blocking anti-FasL antibody. Pleural effusion CD8+ T cells stimulated with anti-CD3 mAb, and in the presence of blocking anti-FasL mAb, showed a significantly lower percentage of anti-CD3-induced apoptosis compared to isotype control (Fig. 3a, b).
Taken together, these results indicate that, in cancer patients, CD4+ T cells from both peripheral blood and pleural effusion were insensitive to AICD. However, CD8+ T cells from malignant effusions, but not peripheral blood, were highly prone to AICD.
FasL and TRAIL expressions are increased in CD8+ T cells from lung cancer patients
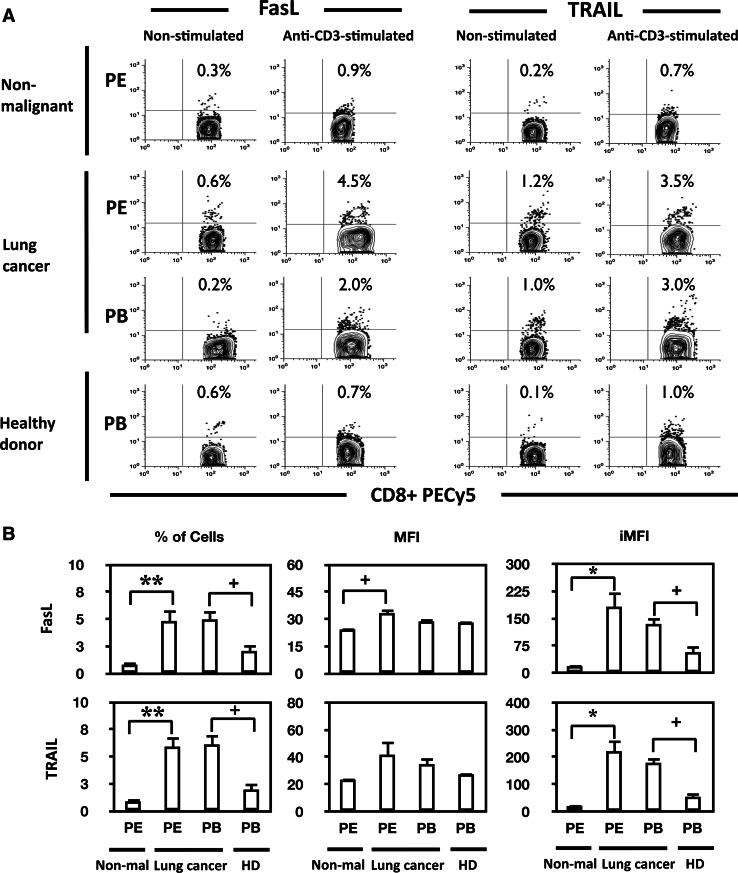
To further assess the involvement of FasL and TRAIL molecules in AICD, the expression of these molecules was analyzed by flow cytometry. The percentages of FasL- or TRAIL-positive CD4+ and CD8+ T cells were evaluated after anti-CD3 mAb stimulation (Fig. 4a for representative data). When stimulated and non-stimulated CD4+ and CD8+ T cells were compared, no significant differences were found in non-malignant effusion group and in peripheral blood samples from healthy donors (data not shown). In cancer patients, peripheral blood and pleural effusion samples of both stimulated and non-stimulated CD4+ T cells showed similar percentages of FasL-positive and TRAIL-positive cells (data not shown).
Fig. 4.
The expression of FasL and TRAIL is increased in anti-CD3-stimulated CD8+ T cells from lung cancer patients compared to non-malignant controls. a Representative 5% contour outlier plots showing the percentages of FasL- or TRAIL-positive CD8+ T cells from PEMC or PBMC of a cancer patient and controls under non-stimulated and anti-CD3-stimulated conditions. b Comparison of FasL (upper row) and TRAIL (lower row) production, the percentage of cells (left), MFI (middle), and iMFI (right) of FasL or TRAIL expressing CD8+ T cells induced by anti-CD3 mAb stimulation (10 μg/ml) from all groups is shown. PE from non-malignant patients (Non-mal, n = 11), PE and PB from lung cancer patients (n = 15) and PB from healthy donors (HD, n = 13). Bars depict the mean ± SE, *P < 0.0001, **P < 0.001, + P < 0.01
In peripheral blood and pleural effusion CD8+ T cells from cancer patients, the percentages of FasL- and TRAIL-positive cells were significantly higher in stimulated compared to non-stimulated cells (Fig. 4b). Furthermore, significantly higher percentages of FasL-positive cells were found in malignant effusion CD8+ T cells compared to the corresponding subpopulations from non-malignant effusions under non-stimulated (2.2 ± 0.9% vs. 0.1 ± 0.1%, P = 0.023) and anti-CD3-stimulated conditions (5.0 ± 1.1% vs. 1.2 ± 0.4%, P = 0.042). Respect to TRAIL-positive cells, similar results were found when comparing malignant effusion CD8+ T cells with the corresponding subpopulation from non-malignant effusions, under non-stimulated (2.9 ± 0.9% vs. 0.2 ± 0.1%, P = 0.026) and anti-CD3-stimulated conditions (6.5 ± 1.0% vs. 1.2 ± 0.4%, P = 0.042).
A recent report has described a new metric parameter conceived to increase the quantitative informational data obtained from flow cytometric analysis, the integrated mean fluorescence intensity (iMFI), which reflects the total functional response of activated T cells [25]. iMFI is calculated by multiplying the percentage of positive cells, which represents the magnitude of the response, by the MFI, which represents the quality of the response. iMFI for FasL and TRAIL from anti-CD3-stimulated CD8+ T cells, along with the percentage of cells and MFIs, is shown in Fig. 4b. Pleural effusion CD8+ T cells from cancer patients showed the highest iMFI for FasL and TRAIL compared with all other groups, and significant differences were found with respect to non-malignant group. Also, iMFI for FasL and TRAIL were significantly higher in peripheral blood CD8+ T cells from cancer patients compared to the corresponding subpopulation from healthy donors (Fig. 4b). Thus, CD8+ T cells from cancer patients showed an increase in FasL and TRAIL expressions.
FasL and TRAIL transcript levels are increased in CD8+ T cells from lung cancer patients
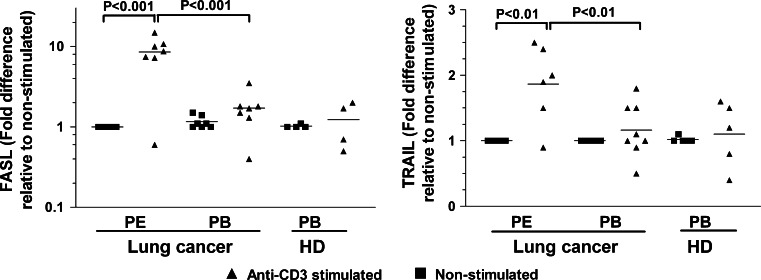
We next determined whether the increase in the iMFI values of FasL- and TRAIL-positive CD8+ T cells is associated with the upregulation of the corresponding transcripts. In healthy subjects, FasL mRNA levels did not change after anti-CD3 stimulation. As shown in Fig. 5, FasL mRNA levels were significantly increased following anti-CD3 stimulation in CD8+ T cells from malignant pleural effusion (about tenfold) compared to peripheral blood (onefold). A similar trend was observed for TRAIL mRNA; the upregulation of TRAIL transcripts in CD8+ T cells from malignant pleural effusions stimulated with anti-CD3 was higher than in other groups (Fig. 5).
Fig. 5.
Pleural effusion CD8+ T cells from lung cancer patients stimulated with anti-CD3 mAbs upregulate FasL and TRAIL mRNA levels. PE and PB CD8+ T cells from lung cancer patients and PB CD8+ T cells from healthy subjects (HD) were purified by negative selection using magnetic beads. Cell stimulation was performed by incubating with anti-CD3 mAb for 4 h. Total RNA was extracted, and qRT–PCR performed. The results were analyzed by the comparative Ct method, using β-actin as an internal control (see “Materials and methods”). The results are semiquantitative and represent the n-fold difference of the transcript levels in a particular sample compared to calibrator cDNA (cDNA samples from non-stimulated CD8+ T cells from each subject). Bars depict the mean
Anti-CD3 stimulation of pleural effusion CD8+ T cells from lung cancer patients induces two subsets of Bcl-2-positive cells
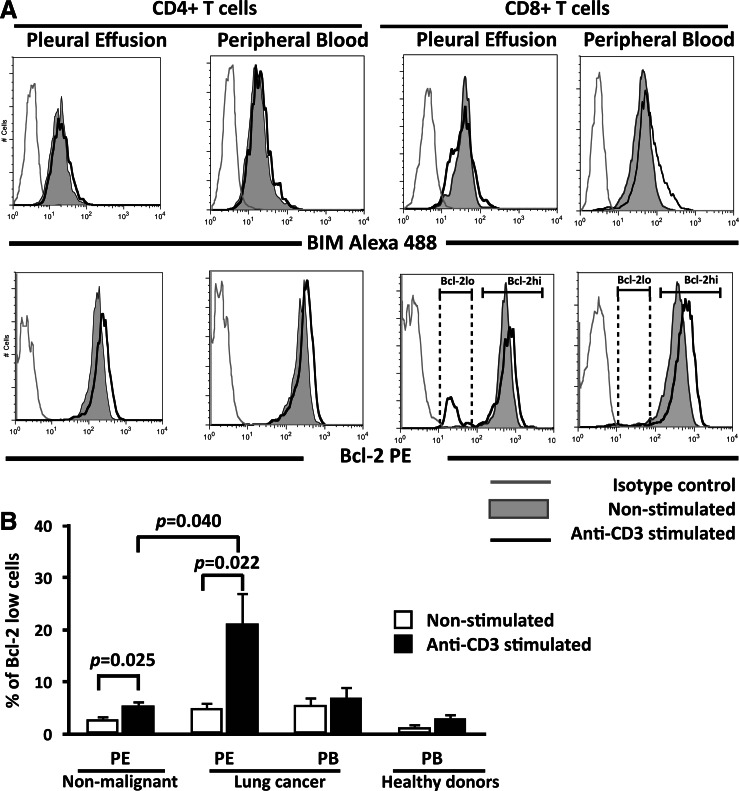
Spontaneous and Fas-induced apoptosis have been shown to be minimal in T cells from the peripheral blood of healthy donors, and this correlates with a high level of expression of Bcl-2 [26, 27]. Bcl-2 is upregulated upon T cell activation [28], suggesting that Bcl-2 family members may regulate T cell apoptosis. Indeed, the pro-apoptotic Bcl-2 family member, BIM, causes cell death of activated T cells in vivo [16]. Thus, we evaluated the expression of Bcl-2 and BIM in anti-CD3-stimulated CD8+ T cells. With respect to BIM expression, pleural effusion CD4+ and CD8+ T cells from non-malignant and malignant origins, and peripheral blood CD4+ and CD8+ T cells from cancer patients and healthy donors, did not modify the MFI values after stimulation with anti-CD3 mAb compared to corresponding non-stimulated groups (Fig. 6 and data not shown).
Fig. 6.
A Bcl-2lo subset is induced in pleural effusion CD8+, but not CD4+, T cells from lung cancer patients upon anti-CD3-stimulation. a BIM and Bcl-2 expression levels were analyzed in cells stimulated with anti-CD3 mAb (black line) and non-stimulated (gray area) for 24 h. Histograms obtained from CD4+ and CD8+ T cells from a lung cancer patient are shown. Isotype control is indicated by a gray line. b Percentages of Bcl-2lo cells in CD8+ T cells from lung cancer and control groups determined by analysis of Bcl-2 low region (n = 7). Bars depict the mean ± SE
Anti-CD3-stimulated CD4+ T cells from all groups significantly increased the MFI values of Bcl-2 (Table 1 and Fig. 6a). Anti-CD3-stimulated pleural effusion CD8+ T cells from the non-malignant group increased the MFI values approximately 1.7-fold compared to non-stimulated cells. Pleural effusion CD8+ T cells from the cancer group contained two subsets of Bcl-2-expressing cells (Bcl-2hi and Bcl-2lo) that were detected after anti-CD3 stimulation. The MFI values found in the Bcl-2hiCD8+ T cell subset were similar between anti-CD3-stimulated and non-stimulated conditions.
Table 1.
Bcl-2 expression in CD4+ and CD8+ cells from lung cancer and control groups after anti-CD3 stimulation
| Groups | Compartment | Mean fluorescence intensity (MFI) ± SE | |||||
|---|---|---|---|---|---|---|---|
| Bcl-2+ CD4+ cells | Bcl-2hi CD8+ cells | ||||||
| Non-stimulated | Anti-CD3 stimulated | P value | Non-stimulated | Anti-CD3 stimulated | P value | ||
| Non-malignant (n = 6) | PE | 323 ± 28 | 474 ± 36 | 0.040 | 260 ± 17 | 463 ± 20 | 0.004 |
| Lung cancer (n = 10) | PE | 350 ± 61 | 409 ± 59 | 0.017 | 305 ± 46 | 364 ± 60 | 0.030 |
| PB | 396 ± 66 | 516 ± 83 | 0.035 | 323 ± 50 | 353 ± 48 | 0.048 | |
| Healthy donors (n = 7) | PB | 120 ± 5 | 187 ± 16 | 0.049 | 120 ± 11 | 320 ± 23 | 0.007 |
P values obtained from comparison between non-stimulated versus anti-CD3-stimulated conditions
PB peripheral blood, PE pleural effusion
In peripheral blood CD8+ T cells from healthy donors, the stimulated cells increased the MFI values for Bcl-2 approximately 2.5-fold compared to non-stimulated cells. Stimulated peripheral blood CD8+ T cells from the lung cancer group exhibited similar MFI values compared to non-stimulated cells (Fig. 6a and Table 1).
When the percentages of Bcl-2-positive cells were analyzed in the groups mentioned above, pleural effusion CD8+ T cells from cancer patients significantly reduced the subset of Bcl-2hi cells after stimulation, this phenomenon was observed in 80% of the cancer patients. Reduction in Bcl-2hi cells was associated with a concomitant presence of a Bcl-2lo subset, in approximately 25% of the CD8+ T cell subpopulation (Fig. 6b). Pleural effusion CD8+ T cells from non-malignant controls also showed a slight but significant increase in the percentage of Bcl-2lo cells after stimulation; nevertheless, percentage of Bcl-2lo was significantly higher in pleural effusion CD8+ T cells from cancer patients (Fig. 6b). Thus, anti-CD3 stimulation of pleural effusion CD8+ T cells from lung cancer patients decreased the percentage of Bcl-2hi-positive cells, which may favor apoptosis.
Memory CD8+ T cell subsets from malignant effusions are susceptible to AICD
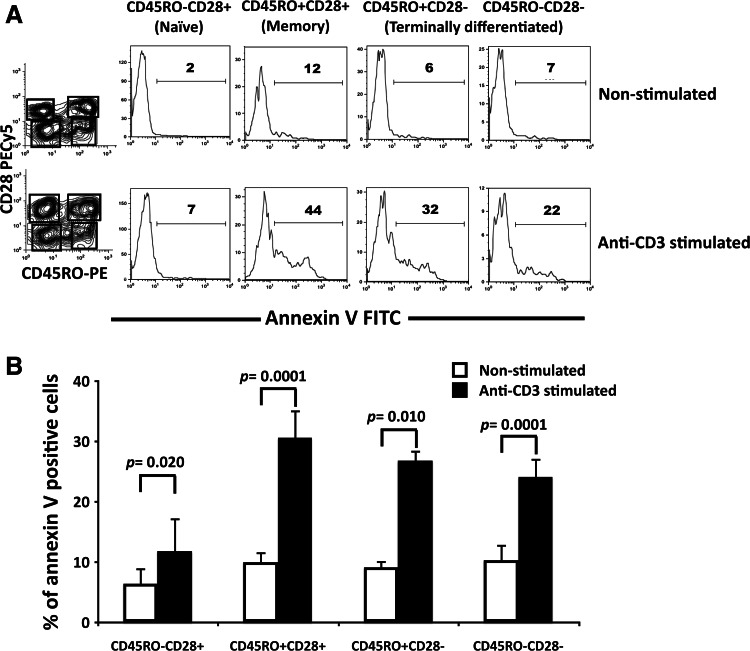
To study whether susceptibility to AICD is associated with a specific CD8+ T cell subset from malignant effusions, we identified memory, terminally differentiated, and naïve subsets using the markers CD45RO and CD28, as previously reported [5, 13, 29]. As shown in Fig. 7a and b, anti-CD3 stimulation significantly increased the percentages of annexin-V-positive cells in each analyzed subset. For comparison, anti-CD3-induced apoptosis was calculated in each CD8+ T cell subset. Memory (CD45RO+ CD28+) cells showed the highest percentages of anti-CD3-induced apoptosis (37 ± 3.5%). Terminally differentiated cells showed moderately lower percentages of apoptosis (for CD45RO+ CD28- cells: 22 ± 2.9%, and for CD45RO-CD28- cells: 17 ± 4.2%). Percentages of apoptosis in naïve (CD45RO-CD28+) CD8+ T cells were significantly lower respect to the other subsets (10 ± 3.1%, P < 0.001 with respect to CD45RO+ CD28+ cells and P < 0.05 with respect to CD45RO+ CD28- cells). Thus, our data suggest that memory and, to a lesser extent, terminally differentiated CD8+ T cell subsets were more susceptible to AICD.
Fig. 7.
CD8+ T cell subsets from malignant effusions are susceptible to apoptosis after CD3 stimulation. a Distribution of memory, terminally differentiated and naïve CD8+ T cell subsets and corresponding annexin-V histograms under non-stimulated and stimulated conditions. Percentages of annexin-V-positive cells are shown. b Percentages of annexin-V-positive cells in pleural effusion CD8+ T cell subsets from lung cancer patients (n = 7). Bars depict the mean ± SE
Caspase-8 and -9 inhibitors block AICD of pleural effusion CD8+ T cells
AICD stimulation is mediated through the extrinsic pathway [15, 16]. To corroborate this phenomenon, we determined the participation of caspase-8. As it has also been reported that the intrinsic pathway may be involved in some cell types [27], caspase-9 was also evaluated in this study.
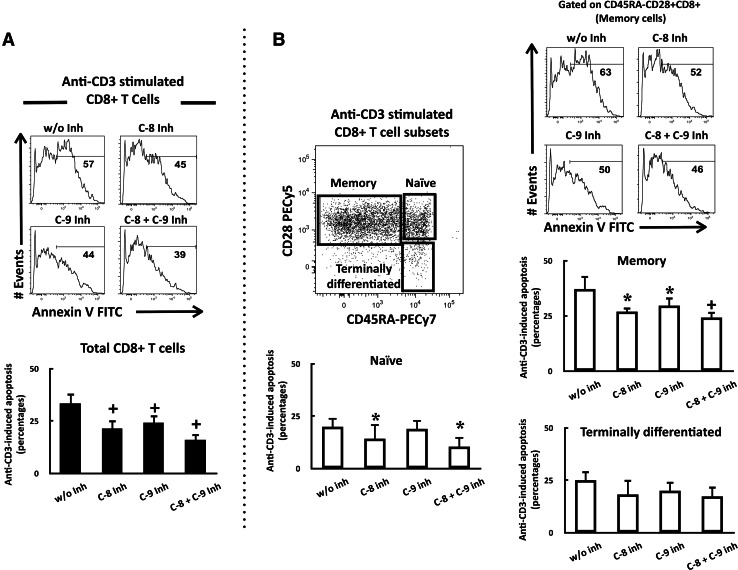
In CD8+ T cells from pleural effusion, caspase-8 and -9 inhibitors significantly reduced anti-CD3-mediated apoptosis (approximately by 35%). The percentages of apoptosis using both inhibitors together were slightly lower compared to those measured after using either caspase-8 or -9 inhibitors alone (Fig. 8a).
Fig. 8.
Caspase-8 and -9 inhibitors rescued memory CD8+ T cells from AICD. a Data from pleural effusion CD8+ T cells from a cancer patient are shown. PEMCs were stimulated with anti-CD3 mAbs in the presence of a caspase-8 inhibitor, a caspase-9 inhibitor, or both. Annexin-V-positive cells were analyzed by histograms within the PI CD8+ T cell population as described in “Materials and methods”. Percentages of anti-CD3-induced apoptosis are shown for each condition (n = 9). b Representative analysis of memory CD8+ T cells. PEMCs were stimulated with anti-CD3 mAbs in the presence of a caspase-8 inhibitor, a caspase-9 inhibitor, or both. Percentages of annexin-V-positive cells were then determined in memory, naïve, and terminally differentiated subsets. Percentages of anti-CD3-induced apoptosis are shown for each condition (n = 5). *P < 0.05 with respect to cells without inhibitor (w/o inh), + P < 0.001 with respect to cells without inhibitor (w/o inh)
In the naïve CD8+ T cell subset, caspase-8 inhibitor alone, or combined with caspase-9 inhibitor, significantly reduced anti-CD3-induced apoptosis in similar percentages. Caspase-9 inhibitor did not modify the percentages of anti-CD3-induced apoptosis in this subset. In terminally differentiated CD8+ T cells, caspase-8 and -9 inhibitors alone or combined tended to reduce anti-CD3-induced apoptosis in similar percentages. Remarkably, these inhibitors protected from apoptosis to the memory CD8+ T cell subset; specifically, each inhibitor reduced anti-CD3-induced apoptosis by approximately 30%, and addition of both inhibitors together caused a reduction in approximately 43% (Fig. 8b).
Discussion
Many studies have demonstrated that a high frequency of T cell apoptosis, particularly in the CD8+ T cell subpopulation, occurs in patients with cancer [10, 11, 24]. Remarkably, apoptosis of T cells is not limited to the tumor site, as an increased percentage of apoptosis has been found in T cells from the peripheral blood of patients with breast carcinoma, head and neck carcinoma, or melanoma [10, 11, 24]. Data from our study in lung cancer patients agree with these reports; peripheral blood CD4+ and CD8+ T cells show high susceptibility to spontaneous apoptosis compared to T cell subpopulations from healthy donors. Of the T cell subpopulations studied, spontaneous apoptosis was primarily observed in CD8+ T cells. Nevertheless, susceptibility to spontaneous apoptosis does not lead to a reduction in the CD8+ T cell subpopulation in peripheral blood. Indeed, no differences in the percentages of peripheral blood CD8+ T cells between lung cancer patients and healthy donors have been found [5].
The Fas/FasL pathway has been proposed to be responsible of spontaneous apoptosis observed in T cells [8, 9]. In particular, it has been reported that, in tumors of distinct origins, but not in lung cancer, peripheral blood CD8+ T cells increase cell death after treatment with agonistic anti-Fas antibodies, suggesting that high expression of Fas in CD8+ T cells may be responsible for T cell death [9, 10]. We have reported an increase in the percentage of Fas-positive CD8+ T cells in peripheral blood from lung carcinoma patients [5]. However, in the present study, using the same experimental conditions previously reported for agonistic anti-Fas antibody treatment [9], no apoptosis in CD4+ and CD8+ T cells was observed. These data indicate that the spontaneous apoptosis observed in this study is not mediated by the Fas receptor; however, other death receptors (such as TNFR1, DR4, DR5, or others) may be inducing this phenomenon. Furthermore, spontaneous apoptosis may be the consequence of other factors (described below) systemically released by tumor or stromal cells.
In malignant pleural effusions, both neoplastic and inflammatory cells are present [5], making them suitable models for studying the interactions between tumor cells and the host immune system [3–5, 30]. The ex vivo model allows for studying the effects of tumor cell-mediated alterations in pleural effusion lymphocytes. In pleural effusions of non-small cell carcinoma origin, some authors [5, 30] have described an increase in the number of CD4+ T cells and a concomitant decrease in CD8+ T cell subpopulation. Pace et al. [4] studied pleural effusions from tuberculosis and cancer patients and observed an increase in CD3+ T cell spontaneous apoptosis. Nevertheless, the susceptibility to spontaneous apoptosis for any particular T cell subpopulation was not determined. Our results show that pleural effusion CD8+ T cells from lung cancer patients were more susceptible to spontaneous apoptosis than CD8+ T cells from non-malignant pleural effusions, which suggests that the underlying pathology, rather than the anatomical compartment, mediates CD8+ T cell death. Of note, the percentages of spontaneous apoptosis in pleural effusion CD8+ T cells were lower than in peripheral blood CD8+ T cells. These observations may be explained by previous apoptotic elimination of a proportion of CD8+ T cells in the pleural compartment.
Tumor cells may be releasing tumor antigens that chronically stimulate CD8+ T cells, inducing a local activation of tumor-reactive T cells in the pleural compartment [31]. This chronic stimulation could sensitize CD8+ T cells to AICD. It has been shown that TILs from some human [18, 21, 22] and mouse tumors die by AICD [19, 20]. Based on these reports, AICD may participate in the reduction in the CD8+ T cell subpopulation observed in pleural effusions. Alternatively, through secreted chemokines, cytokines or other soluble factors, lung tumors may induce and amplify non-HLA restricted, inflammatory responses in the pleural compartment, leading to increased susceptibility to AICD. Accordingly, data obtained from our study show that CD8+, but not CD4+, T cells from pleural effusions underwent AICD, and this phenomenon was not observed in peripheral blood CD4+ and CD8+ T cells.
Inflammation caused by pleural effusion might be an important source of various mediators and proteins, such as damage-associated molecular patterns (DAMPs) molecules [32]. Given that DAMPs are not exclusively related to cancer, these molecules might sensitize CD8+ T cells to AICD in non-malignant effusions. Accordingly, our data show that non-malignant effusion CD8+ T cells were susceptible to AICD, which was associated with the presence of a low percentage of Bcl-2lo CD8+ T cells, suggesting that CD3-mediated apoptosis might be part of an inflammatory process. Nevertheless, AICD was significantly higher in pleural effusion CD8+ T cells from cancer patients with respect to non-malignant effusions; this may be attributed to the presence of tumor antigens in the pleural compartment.
Increased susceptibility to AICD observed in pleural effusion CD8+ T cells from cancer patients was associated with increased expression of FasL and TRAIL mRNAs. Percentages of FasL and TRAIL-positive cells in pleural effusion CD8+ T cells, although significantly higher than the corresponding stimulated CD8+ T cells from control groups, were nevertheless relatively low. This phenomenon might be the consequence of the release of TRAIL and FasL to the medium in microvesicles or soluble form [33]. On the other hand, a recent report has described a new metric parameter conceived to increase the quantitative informational data obtained from flow cytometric analysis, the integrated mean fluorescence intensity (iMFI), which reflects the total functional response of activated T cells [25]. Using this parameter, we found that expression of FasL and TRAIL is increased in anti-CD3-stimulated CD8+ T cells from cancer patients with respect to non-malignant CD8+ T cells and peripheral blood CD8+ T cells from healthy donors. In addition, blocking with antagonist anti-FasL mAb partially reduced the percentage of apoptotic cells in pleural effusion CD8+ T cells. Taken together, our results show that FasL pathway is involved in apoptosis induced by CD3 stimulation.
Pleural effusion CD8+ T cells showed a decrease in the percentage of Bcl-2hi cells with a concomitant presence of a Bcl-2lo population. A similar phenomenon has been observed ex vivo in antigen- and non-antigen-specific CD8+ T cells from HIV patients [34, 35]. Factors that could mediate Bcl-2 downregulation are cytokine deprivation (IL-2, IL-7c or IL-15); in particular, lung carcinoma cells express the alpha receptor for IL-2 ([36] and our unpublished observations), or increase in IL-21 in the microenvironment; it has been recently reported that CD8+ T cells express lower levels of Bcl-2 when stimulated in presence of IL-21 [37]. In the tumor microenvironment, reduced expression of anti-apoptotic Bcl-2 in pleural effusion CD8+ T cells, associated with increase in FasL and TRAIL expressions, might sensitize these cells to AICD and consequently impair their ability to eliminate tumor cells.
In cancer, there are few reports that study AICD in CD8+ T cell subsets [22, 38]. To our knowledge, this is the first study analyzing AICD susceptibility in CD8+ T cell subsets from lung cancer patients. As was previously reported, pleural effusions from lung cancer patients, which contain high proportions of tumor cells and lymphocytes, show high proportions of memory and low proportions of terminally differentiated CD8+ T cell subsets [5]. Our data show that the naïve CD8+ T cell subset underwent apoptosis after CD3 stimulation. Though surprising, the naïve subset has been demonstrated to be susceptible to AICD upon TCR stimulation [39]. Nevertheless, terminally differentiated CD8+ T cells were more susceptible to AICD compared to the naïve subset. Moreover, the highest susceptibility to AICD was observed in the memory subset. Interestingly, memory CD8+ T cells from healthy donors have been shown to be resistant to AICD [16, 27]. Given that polyclonal stimulation of pleural CD8+ T cells leaded to AICD, our results suggest that cell death is a general phenomenon of both tumor- and non-tumor-specific memory CD8+ T cells. This phenomenon is similar to that reported by Kilinc et al. [38], who, in a murine lung tumor model, delivered IL-12 to tumors in situ, resulting in activation and subsequent death of total effector/memory CD8+ T cells. Thus, susceptibility to AICD in malignant effusion-derived memory CD8+ T cells might hamper these cells from becoming terminally differentiated.
Previous studies have reported two types of Fas-mediated extrinsic apoptotic pathways according to the type of cell involved [14, 16]. Our data show that pleural effusion CD8+ T cells were rescued from AICD by caspase-8 and -9 inhibitors. Although caspase-8 inhibitor, but not caspase-9 inhibitor, protected naïve CD8+ T cells, memory CD8+ T cells were protected from AICD by both inhibitors. Thus, in memory CD8+ T cells, both caspase-8 and caspase-9 participate in AICD. These results are in agreement with the presence of a Bcl-2lo CD8+ T cell population detected after CD3 stimulation. Nevertheless, in memory CD8+ T cells incubated with both caspase inhibitors, the protective effect was not additive, as would be expected if type I and type II pathways were participating independently. Moreover, type II apoptotic signaling can be blocked by high expression of anti-apoptotic Bcl-2 molecules. In contrast, in type I cells, receptor-induced apoptosis cannot be blocked by the expression of Bcl-2 or caspase-9 inhibitor. Type I cells, therefore, can die despite the lack of mitochondrial involvement [14, 16]. Hence, memory, but not naïve, CD8+ T cells from malignant pleural effusion behave like type II cells after stimulation and require the amplification loop of the intrinsic apoptotic pathway.
The increased susceptibility of pleural effusion CD8+ T cells to AICD might be attributed to the following: (a) gangliosides released by tumor or stromal cells could sensitize activated T cells to apoptosis, which involves both the extrinsic and intrinsic apoptotic pathways, as demonstrated in vitro [40, 41]; (b) diminished levels of CD3ε in CD8+ T cells, as we previously reported in lung carcinoma patients; the tumor microenvironment could alter the complex CD3 stoichiometry necessary to induce efficient T cell signaling, leading to dysfunction of CD8+ T cell responses and enhanced T cell apoptosis [13]; and (c) production of reactive oxygen and nitrogen species, induced by chronic presence of tumor antigens, DAMPs or other factors secreted by tumor or stromal cells in the pleural compartment [42]. In this regard, nitric oxide has been shown to induce AICD dependent on Fas/FasL pathway in vitro [43]; also, memory CD8+ T cells have been reported to die by AICD, under both antigen-specific recognition and oxidative stress [44, 45].
Given the high proportion of CD8+ T cells undergoing AICD after polyclonal stimulation, we suggest that this phenomenon is both tumor antigen-specific and non-tumor antigen-specific. A similar phenomenon has been described in viral infections, where non-antigen-specific CD8+ T cells become prone, in a bystander fashion, to AICD after TCR stimulation [46]. Bystander sensitization to AICD has been proposed as a mechanism for immune deficiencies associated with persistent viral infections involving chronic T cell responses [46]. In lung cancer patients, a similar but deregulated phenomenon might explain the immunosuppression detected in peripheral blood [7] as well as the reduced numbers of CD8+ T cells in the pleural compartment [5].
Several clinical trials using intrapleural administration of diverse combinations of biological response modifiers have been conducted to increase the host immune response against tumor cells [47–49]; however, these treatments have failed to show significant clinical benefits in terms of patient survival or quality of life. Blocking the apoptotic loop may be essential for the success of T cell-based immunotherapeutic regimens for pleural effusions in patients with primary thoracic malignancy and possibly for other metastatic malignancies to the thorax.
Acknowledgments
This work was supported by Conacyt grant 102106.
References
- 1.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Atanackovic D, Block A, de Weerth A, Faltz C, Hossfeld DK, Hegewisch-Becker S. Characterization of effusion-infiltrating T cells: benign versus malignant effusions. Clin Cancer Res. 2004;10:2600–2608. doi: 10.1158/1078-0432.CCR-03-0239. [DOI] [PubMed] [Google Scholar]
- 4.Pace E, Bruno TF, Berenger B, Mody CH, Melis M, Ferraro M, Tipa A, Bruno A, Profita M, Bonsignore G, Gjomarkaj M. Elevated expression of prostaglandin receptor and increased release of prostaglandin E2 maintain the survival of CD45RO+ T cells in the inflamed human pleural space. Immunology. 2007;121:427–436. doi: 10.1111/j.1365-2567.2007.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prado-Garcia H, Aguilar-Cazares D, Flores-Vergara H, Mandoki JJ, Lopez-Gonzalez JS. Effector, memory and naïve CD8+ T cells in peripheral blood and pleural effusion from lung adenocarcinoma patients. Lung Cancer. 2005;47:361–371. doi: 10.1016/j.lungcan.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Broderick L, Brooks SP, Takita H, Baer AN, Bernstein JM, Bankert RB. IL-12 reverses anergy to T cell receptor triggering in human lung tumor-associated memory T cells. Clin Immunol. 2006;118:159–169. doi: 10.1016/j.clim.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Caras I, Grigorescu A, Stavaru C, Radu DL, Mogos I, Szegli G, Salageanu A. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol Immunother. 2004;53:1146–1152. doi: 10.1007/s00262-004-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilige and tumour counterattack. Immunol Cell Biol. 2002;80:131–137. doi: 10.1046/j.1440-1711.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 9.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 11.Kuss I, Donnenberg AD, Gooding W, Whiteside TL. Effector CD8+ CD45RO-CD27-T cells have signalling defects in patients with squamous cell carcinoma of the head and neck. Br J Cancer. 2003;88:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15:70–79. doi: 10.1038/sj.cdd.4402274. [DOI] [PubMed] [Google Scholar]
- 13.Prado-Garcia H, Aguilar-Cazares D, Meneses-Flores M, Morales-Fuentes J, Lopez-Gonzalez JS. Lung carcinomas do not induce T-cell apoptosis via the Fas/Fas ligand pathway but down-regulate CD3 epsilon expression. Cancer Immunol Immunother. 2008;57:325–336. doi: 10.1007/s00262-007-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 15.Gorak-Stolinska P, Truman JP, Kemeny DM, Noble A. Activation-induced cell death of human T-cell subsets is mediated by Fas and granzyme B but is independent of TNF-alpha. J Leuk Biol. 2001;70:756–766. [PubMed] [Google Scholar]
- 16.Bouillet P, O’Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–519. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 17.Restifo NP. Not so Fas: re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med. 2000;6:493–495. doi: 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–3279. [PMC free article] [PubMed] [Google Scholar]
- 19.Li JH, Rosen D, Sondel P, Berke G. Immune privilege and FasL: two ways to inactivate effector cytotoxic T lymphocytes by FasL-expressing cells. Immunology. 2002;105:267–277. doi: 10.1046/j.1365-2567.2002.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radoja S, Saio M, Frey AB. CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ. J Immunol. 2001;166:6074–6083. doi: 10.4049/jimmunol.166.10.6074. [DOI] [PubMed] [Google Scholar]
- 21.Friedlein G, El Hage F, Vergnon I, Richon C, Saulnier P, Lécluse Y, Caignard A, Boumsell L, Bismuth G, Chouaib S, Mami-Chouaib F. Human CD5 protects circulating tumor antigen-specific CTL from tumor-mediated activation-induced cell death. J Immunol. 2007;178:6821–6827. doi: 10.4049/jimmunol.178.11.6821. [DOI] [PubMed] [Google Scholar]
- 22.Gati A, Guerra N, Gaudin C, Da Rocha S, Escudier B, Lécluse Y, Bettaieb A, Chouaib S, Caignard A. CD158 receptor controls cytotoxic T-lymphocyte susceptibility to tumor-mediated activation-induced cell death by interfering with Fas signaling. Cancer Res. 2003;63:7475–7482. [PubMed] [Google Scholar]
- 23.Mueller YM, Makar V, Bojczuk PM, Witek J, Katsikis PD. IL-15 enhances the function and inhibits CD95/Fas-induced apoptosis of human CD4+ and CD8+ effector-memory T cells. Int Immunol. 2003;15:49–58. doi: 10.1093/intimm/dxg013. [DOI] [PubMed] [Google Scholar]
- 24.Albers AE, Schaefer C, Visus C, Gooding W, DeLeo AB, Whiteside TL. Spontaneous apoptosis of tumor-specific tetramer+ CD8+ T lymphocytes in the peripheral circulation of patients with head and neck cancer. Head Neck. 2009;31:773–781. doi: 10.1002/hed.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 26.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–1208. [PubMed] [Google Scholar]
- 27.Fas SC, Baumann S, Krueger A, Frey CR, Schulze-Bergkamen H, Brenner D, Stumpf C, Kappes K, Krammer PH. In vitro generated human memory-like T cells are CD95 type II cells and resistant towards CD95-mediated apoptosis. Eur J Immunol. 2006;36:2894–2903. doi: 10.1002/eji.200635925. [DOI] [PubMed] [Google Scholar]
- 28.Bosque A, Pardo J, Martínez-Lorenzo MJ, Iturralde M, Marzo I, Piñeiro A, Alava MA, Naval J, Anel A. Down-regulation of normal human T cell blast activation: roles of APO2L/TRAIL, FasL, and c-FLIP, Bim, or Bcl-x isoform expression. J Leuk Biol. 2005;77:568–578. doi: 10.1189/jlb.0904514. [DOI] [PubMed] [Google Scholar]
- 29.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, Douek DC, Mueller YM, Jacobson JM, Kulkarni V, Felber BK, Pavlakis GN, Katsikis PD, Koup RA. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol. 2009;183:1120–1132. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto M, Hasegawa Y, Hara T, Hashimoto N, Imaizumi K, Shimokata K, Kawabe T. T-helper type 1/T-helper type 2 balance in malignant pleural effusions compared to tuberculous pleural effusions. Chest. 2005;128:4030–4035. doi: 10.1378/chest.128.6.4030. [DOI] [PubMed] [Google Scholar]
- 31.Duncan SR, Elias DJ, Roglic M, Pekny KW, Theofilopoulos AN. T-cell receptor biases and clonal proliferations in blood and pleural effusions of patients with lung cancer. Hum Immunol. 1997;53:39–48. doi: 10.1016/S0198-8859(96)00296-0. [DOI] [PubMed] [Google Scholar]
- 32.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Lorenzo MJ, Anel A, Gamen S, Monleon I, Lasierra P, Larrad L, Pineiro A, Alava MA, Naval J. Activated human T cell release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163:1274–1281. [PubMed] [Google Scholar]
- 34.Boudet F, Lecoeur H, Gougeon ML. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J Immunol. 1996;156:2282–2293. [PubMed] [Google Scholar]
- 35.Lecoeur H, Ledru E, Gougeon ML. A cytofluorometric method for the simultaneous detection of both intracellular and surface antigens of apoptotic peripheral lymphocytes. J Immunol Methods. 1998;217:11–26. doi: 10.1016/S0022-1759(98)00060-X. [DOI] [PubMed] [Google Scholar]
- 36.Yano T, Fukuyama Y, Yokoyama H, Takai E, Tanaka Y, Asoh H, Ichinose Y. Interleukin-2 receptors in pulmonary adenocarcinoma tissue. Lung Cancer. 1996;16:13–19. doi: 10.1016/S0169-5002(96)00608-3. [DOI] [PubMed] [Google Scholar]
- 37.Barker BR, Parvani JG, Meyer D, Hey AS, Skak K, Letvin NL. IL-21 induces apoptosis of antigen-specific CD8+ T lymphocytes. J Immunol. 2007;179:3596–3603. doi: 10.4049/jimmunol.179.6.3596. [DOI] [PubMed] [Google Scholar]
- 38.Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, Egilmez NK. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–6973. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- 39.Inaba M, Kurasawa K, Mamura M, Kumano K, Saito Y, Iwamoto I. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J Immunol. 1999;163:1315–1320. [PubMed] [Google Scholar]
- 40.Batra RK, Lin Y, Sharma S, Dohadwala M, Luo J, Pold M, Dubinett SM. Non-small cell lung cancer-derived soluble mediators enhance apoptosis in activated T lymphocytes through an I kappa B kinase-dependent mechanism. Cancer Res. 2003;63:642–646. [PubMed] [Google Scholar]
- 41.Das T, Sa G, Paszkiewicz-Kozik E, Hilston C, Molto L, Rayman P, Kudo D, Biswas K, Bukowski RM, Finke JH, Tannenbaum CS. Renal cell carcinoma tumors induce T cell apoptosis through receptor-dependent and receptor-independent pathways. J Immunol. 2008;180:4687–4696. doi: 10.4049/jimmunol.180.7.4687. [DOI] [PubMed] [Google Scholar]
- 42.Spooner R, Yilmaz O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int J Mol Sci. 2011;12:334–352. doi: 10.3390/ijms12010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams MS, Noguchi S, Henkart PA, Osawa Y. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J Immunol. 1998;161:6526–6531. [PubMed] [Google Scholar]
- 44.Norell H, Martins da Palma T, Lesher A, Kaur N, Mehrotra M, Naga OS, Spivey N, Olafimihan S, Chakraborty NG, Voelkel-Johnson C, Nishimura MI, Mukherji B, Mehrotra S. Inhibition of superoxide generation upon T-cell receptor engagement rescues Mart-1(27–35)-reactive T cells from activation-induced cell death. Cancer Res. 2009;169:6282–6289. doi: 10.1158/0008-5472.CAN-09-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi A, Hanson MG, Norell HR, Havelka AM, Kono K, Malmberg KJ, Kiessling RV. Preferential cell death of CD8+ effector memory (CCR7-CD45RA-) T cells by hydrogen peroxide-induced oxidative stress. J Immunol. 2005;174:6080–6087. doi: 10.4049/jimmunol.174.10.6080. [DOI] [PubMed] [Google Scholar]
- 46.Zarozinski CC, McNally JM, Lohman BL, Daniels KA, Welsh RM. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J Virol. 2000;74:3650–3658. doi: 10.1128/JVI.74.8.3650-3658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoniou KM, Ferdoutsis E, Bouros D. Interferons and their application in the diseases of the lung. Chest. 2003;123:209–216. doi: 10.1378/chest.123.1.209. [DOI] [PubMed] [Google Scholar]
- 48.Castagneto B, Zai S, Mutti L, Lazzaro A, Ridolfi R, Piccolini E, Ardizzoni A, Fumagalli L, Valsuani G, Botta M. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: results of a phase II study on 31 consecutive patients. Lung Cancer. 2001;31:303–310. doi: 10.1016/S0169-5002(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 49.Timoshenko AV, Lan Y, Gabius HJ, Lala PK. Immunotherapy of C3H/HeJ mammary adenocarcinoma with interleukin-2, mistletoe lectin or their combination. Effects on tumour growth, capillary leakage and nitric oxide (NO) production. Eur J Cancer. 2001;37:1910–1920. doi: 10.1016/S0959-8049(01)00156-3. [DOI] [PubMed] [Google Scholar]