Abstract
The treatment of human melanoma has progressed markedly in recent years. Building on the observation that immune recognition is a frequent event in melanoma, a series of immunotherapeutic approaches have been evaluated in clinical trials, culminating in the first phase III study improving overall survival of melanoma patients since 20 years. However, the response rates seen upon immunotherapeutic interventions such as anti-CTLA4 treatment are often low. Furthermore, clinical responses can take several weeks to develop, during which time stage IV melanoma patients often deteriorate. Recent advances in our understanding of the genetic lesions in human melanoma now also allow the specific targeting of the signaling pathway alterations in this disease. Such targeted therapies can lead to high response rates, although the duration of these responses is thus far relatively short. We suggest that the combination of immuno and targeted therapy offers potential for synergy for both conceptual and practical reasons. In this review, we will discuss the potential and possible limitations for such combination therapy, and we describe the most promising combinations of targeted therapy and immunotherapy that can be tested in the clinic in the coming years. The concept of induction therapy by small molecule administration and consolidation by immunotherapeutics also has potential for the treatment of other human cancers.
Keywords: Targeted therapy, Immunotherapy, Melanoma, T cell, PLX4032, Ipilimumab
Introduction
The incidence of metastatic melanoma has been increasing in the United States as well as in Europe over the past two decades. Death rates have been rising faster than those of other cancers, making melanoma one of the human malignancies with the worst prognosis [1, 2]. Despite considerable efforts, the mean overall survival of melanoma patients with unresectable distant metastases remains less than 1 year [3–5].
The clinical treatment of melanoma patients faces two major problems. First, none of the “standard” treatments such as DTIC (Europe) or DTIC and IL-2 (US) has shown a significant survival benefit in randomized trials, both because of low response rates and short response duration. Furthermore, no biomarkers have been identified that can be utilized to select patients who could benefit from these treatments. Second, patients that progress after first line therapy often deteriorate rapidly. However, experimental (immunotherapeutic) approaches, such as vaccination or adoptive T cell therapies, require a certain time to be prepared or to exert their effect in vivo. As an example, responses upon anti-CTLA4 treatment (ipilimumab) are generally not observed before the third infusion (6 weeks after start of the therapy). Thus, many patients drop out from promising therapies only on the basis of fast progression before clinical response can be expected.
For a significant improvement of the overall survival rate of the whole melanoma patient population, an initial fast response at high response rates will be crucial (induction phase). Long-term survival could then be achieved by an increased rate of complete responses or long-term stabilization of partial responses (consolidation phase).
Achieving fast responses at high response rates—small molecule inhibitors
Genetic alterations in melanoma
The analysis of the genetic alterations in human melanoma over the past years has revealed that the mitogen-activated protein kinase (MAPK) pathway is activated in more than 80% of melanomas. This dysregulation of the MAPK pathway is often caused by an activating mutation in the gene encoding the serine–threonine protein kinase B-RAF (BRAF) or, more upstream, by expression of the neuroblastoma RAS viral oncogene homolog (NRas). In addition, mutations in the oncogenes C-KIT, GNAQ, and GNA11, as well as mutations in the tumor suppressor genes PTEN or p53, and loss of the CDKN2A gene products p16 and p15, have been described [6–9]. The most common mutation, the BRAFV600E mutation, is found in 40–60% of all melanomas. Alterations of PTEN are found in up to 55% of melanoma metastases, and combined MAPK pathway/PTEN alterations have been found in 25–50% of melanoma cell lines [10–12] (D. Peeper, NKI-AVL Amsterdam, personal communication).
Blocking the MAPK pathway
Based on the finding that MAPK pathway hyperactivation is a common denominator for the majority of melanomas, a large effort has been made over the past few years to assess the potential of inhibitors of this pathway in clinical trials. First-generation inhibitors targeting the MAPK pathway (Raf/MEK/ERK), such as the Raf inhibitor sorafenib or MEK inhibitor AZD6244, failed to display measurable responses in clinical trials, likely due to the fact that the level of MAPK pathway inhibition that was achieved at maximum-tolerated doses was insufficient [13–15]. These results led to the development of next-generation, more specific small molecules that preferentially inhibit signaling by mutant BRAF (e.g., BRAFV600E) over wild-type BRAF. For the most prominent of these compounds, PLX4032 (RG7204/Vemurafenib) [16], the first phase III trial has just been completed. Data from the prior phase II study that enrolled 132 patients showed an impressive response rate of more than 50% (68% yet unconfirmed), consistent with the response rates seen at MTD in the BRAFV600E subgroup in the phase I trial of this drug [17] (J. Sosman, oral presentation, 7th International Melanoma Research Meeting, Sydney 2010). In patients in whom tumor control occurs, this can be clinically observed already after 1–2 weeks ([17] and authors’ own observations). However, complete responses are rare upon PLX4032 (about 2% of the patients in the phase II trial), and response duration is often short, with a mean progression-free survival of 6.2 months. Other BRAF inhibitors currently tested in clinical trials are RAF265, XL281, and GSK2118436, of which the latter also appears highly specific for V600E mutant BRAF.
Resistance against MAPK pathway blockade
A significant clinical challenge in the use of these BRAF-targeting therapeutics is the drug resistance that can already be observed after only few weeks of treatment (authors’ own observations), and a considerable research effort is currently ongoing to understand the underlying mechanisms. In vitro experiments indicate that PLX4032/PLX4720-resistant melanoma cell lines exhibit cross-resistance to other specific BRAF inhibitors [18]. Several groups have found that BRAF inhibitor-resistant melanoma lines can recover phospho-ERK expression independent of the presence of the BRAF inhibitor [18–22]. Different mechanisms have been postulated that can explain this escape from BRAF inhibition and thereby causing melanoma cell survival: a) reactivation of the MAPK pathway and thus the RAF/MEK/ERK signaling cascade via bypass signaling from ARAF and CRAF [18, 23, 24], b) MAPK-kinase pathway activation via the agonist COT [20], and/or c) via upstream signal cascade activation of oncogenic RAS [21, 23]. Small molecules that inhibit downstream of MEK could counteract such “upstream resistance mechanisms”. Indeed, dual BRAF/MEK inhibition prevented onset of resistance observed upon single BRAF inhibition [19]. Furthermore, after onset of resistance, MEK inhibitors could mediate cell cycle arrest [18]. Thus, the combination of a BRAF inhibitor and a MEK inhibitor (such as AZD6244, GSK1120212, or MEK 162) might overcome this mode of BRAF inhibitor resistance. However, inhibition of ERK phosphorylation and reduction of cell viability was only achieved at very high concentrations of MEK inhibitors [18]. Considering the fact that clinical responses are observed only under conditions in which significant pERK inhibition is achieved [25], it may be challenging to achieve the required high serum levels of MEK inhibitors without intolerable toxicities. A new pathway leading to BRAF inhibitor resistance has been identified by Levi Garraway and his colleagues. By means of massive parallel sequencing of 138 cancer genes, these authors identified an activating mutation of MEK in melanoma patients that became resistant after an initial near-complete response upon PLX4032 [26]. Cells expressing this mutation were also resistant to the MEK inhibitor AZD6244, implying that the above combinations of BRAF and MEK inhibitor might not overcome resistance in such patients. Overcoming mutations of MEK would require inhibition downstream of MEK (e.g. ERK inhibition) or alternative mechanisms of inhibiting mutated MEK.
The role of the PI3K/AKT pathway in MAPK-pathway blockade resistance
The observation that inhibition of MEK only reduced the viability of BRAF inhibitor–resistant cells, to some extent, is consistent with the possibility that additional pathways promote cell survival in such cells [18]. Indeed, BRAF inhibitor–resistant melanoma cell lines have been shown to display increased IGF-1R and PDGFR-beta expression, and IGF-1R blockade was shown to improve cell growth inhibition by PLX4032 [18, 21]. Whereas the downstream mechanism that leads to PDGFR-mediated cell survival is yet to be determined, IGF-1R can activate both the MAPK and the PI3K/AKT pathway [27]. MEK-independent survival of BRAF inhibitor–resistant cells has also been shown to be mediated via the PI3K pathway in a second study [22]. In line with this model is the observation that PTEN-deficient BRAFV600E mutation–positive melanoma lines are often substantially less sensitive to BRAF inhibitors than PTEN-expressing cells (unpublished data, mentioned in [18] and [28]). Furthermore, the targeting of the PI3K/AKT pathway by the pan-PI3K inhibitor GSK2126458 led to the synergistic induction of apoptosis in BRAF inhibitor–resistant cells, when administered concomitant with MEK inhibitor [18]. Thus, the combination of BRAF inhibitors with inhibitors of the PI3K pathway (e.g., GSK2126458 or BEZ235) that are in early clinical testing may also be of value in selected melanoma patients.
Small molecule inhibitor treatment challenges
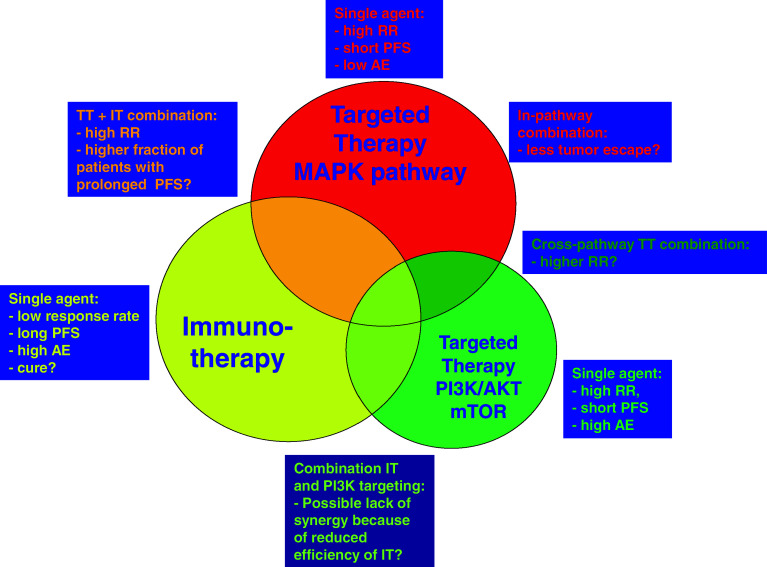
These recent and certainly still incomplete data on resistance mechanisms upon BRAF inhibition offer clear suggestions for combination therapy. As a first possibility, the targeting of two checkpoints within one pathway (“in-pathway combination therapy”), for instance, by combined BRAF inhibition and MEK or ERK inhibition, might delay tumor escape from single-agent therapy and thereby improve clinical outcome. Alternatively, as discussed above, the combined targeting of two signaling pathways (“cross-pathway combination therapy”), for instance, by the joint targeting of the MAPK and the PI3K/AKT pathway, could be attempted (see also Fig. 1). However, two important issues remain. First, as is also noted above, treatment-related toxicity may limit the feasibility of some of such combination therapies. Second, and as a more fundamental problem, the pathway alterations that have thus far been proposed to mediate resistance are observed in only a minority of patients with tumor recurrence upon treatment with PLX4032. For example, PDGFR-beta upregulation was observed only in 4 out of 11 PLX4032-resistant patients, and the combined increase of IGF-1R and pAKT expression was observed in only 1 out of 5 [18, 21]. Thus, it seems that melanoma cells can be extremely versatile in the way they use different signaling pathways to become resistant to BRAF-targeted therapy. This flexibility in the way drug resistance is achieved could limit our ability to obtain long-term melanoma growth inhibition by targeted therapies only.
Fig. 1.
Schematic overview of single agent, in pathway or cross pathway combinations of targeted and/or immunotherapy
Achieving long-term responses—immunotherapeutic approaches
Melanoma is an immunogenic malignancy, as demonstrated by its ability to undergo spontaneous regression and by the presence of melanoma-antigen specific T cells in the peripheral blood of many patients [29, 30]. Furthermore, melanoma displays a number of cellular properties that can be explained by immunoselection, such as downregulation of MHC class I expression or release of immunosuppressive cytokines like TGF-β. Most likely, immunosuppressive entities produced by melanoma are also responsible for the lymphopenia that is observed in treatment-naïve, progressive stage melanoma patients [31].
A large effort has been made to stimulate the tumor-specific immune response in melanoma patients, and three conceptually different approaches can be distinguished. First, it has been attempted to activate the endogenously present T cell repertoire against shared melanoma antigens by vaccination. Thus far, the clinical data obtained with this approach have been disappointing [4]. Second, supply of exogenously expanded or genetically engineered T cells has been used with the aim to create a large tumor-reactive T cell compartment by adoptive therapy. While data obtained over the past few years indicate that adoptive T cell therapy can lead to clinically meaningful effects [32], it is still in a relatively early phase of clinical development. Thus, even though the combination of adoptive cell therapy with targeted therapy appears attractive, it will likely take some time before this concept will be tested in the clinic. Third, the supply of “pro-inflammatory molecules”—mostly in the form of recombinant antibodies—has been used to enhance the T cell response against non-defined melanoma antigens. This class of immunotherapeutic interventions includes blockade of T cell checkpoint molecules such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) and programmed death receptor 1 (PD-1), but also activation of T cell or DC stimulatory molecules such as CD137 and CD40. Clinical development of these immune-modulating molecules has progressed to a stage where combination with targeted therapies forms a logical next step, and in this review, we will therefore focus specifically on this class of immunotherapeutics.
Anti-CTLA-4 antibodies
CTLA-4 is an inhibitory receptor that is expressed on the surface of activated T cells. There it competes with the stimulatory receptor CD28 for binding to CD80/86 on the surface of antigen-presenting cells (APCs). Signaling through CTLA-4 leads to diminished T cell function by shutdown of T cell receptor (TCR) signaling pathways, alterations in cytokine and chemokine production and induction of indolamine 2,3 deoxygenase (IDO) production by the APC [33–35]. Blockade of CTLA-4 signaling in preclinical studies was shown to lead to the regression of established tumors in prostate and melanoma tumor models [36, 37]. These findings led to the development of two fully human monoclonal anti-CTLA4 antibodies for clinical testing, ipilimumab and tremelimumab. Although both antibodies have been extensively studied in humans, only ipilimumab has been shown to induce a statistically significant improvement in overall survival in a randomized phase III trial [38–40].
In this recently published phase III study, 676 patients with unresectable stage III or IV melanoma were either treated with ipilimumab, with a glycoprotein 100 (gp100) peptide vaccine, or with the combination of the two [38]. Importantly, median overall survival was significantly improved by ipilimumab treatment relative to gp100 vaccination (10.1 months vs. 6.4 months). Within the ipilimumab-alone treatment group, 28.5% of the patients showed a clinical benefit (complete response, partial response, or stable disease), compared to 11% in the gp100 vaccine group. While the number of patients that respond to ipilimumab treatment is limited, in patients that do show a clinical response, these responses are often long lasting. Indeed, in 60% of the patients that experienced a clinical response in the ipilimumab-alone treatment group, this response was still ongoing after more than 2 years.
Treatment with ipilimumab is associated with the occurrence of immune-related adverse events (irAE), most commonly involving the skin and gastrointestinal tract. Although serious adverse events have been registered, most irAE are mild and (medically) manageable, while not affecting the anti-tumor effect of the treatment. Interestingly, the occurrence of auto-immunity during anti-CTLA-4 treatment appears predictive of an objective response, although this effect is not absolute [41, 42]. In addition, an increase in the number of lymphocytes during treatment has been shown to correlate with response to treatment [42, 43]. Furthermore, it has been reported that patients who have high levels of FoxP3+ cells and IDO present in the tumor microenviroment at baseline are more likely to respond to ipilimumab treatment [44].
Strikingly, the kinetics of the response to ipilimumab differs from those seen with most anti-cancer drugs. Patients can initially experience a period of stable disease or even disease progression before showing an objective response to the treatment. Indeed, for some patients, it took 5–6 months after start of treatment before tumor regression could be observed [45, 46]. This slow onset of clinical responses may have added to the fact that only a little over 60% of the patients treated within the phase III study all received planned ipilimumab doses, with the most frequent reason for discontinuation of therapy being disease progression.
Although the combination of ipilimumab with targeted therapy has not yet been tested, combinations of ipilimumab plus IL-2 and ipilimumab plus DTIC have already been analyzed in clinical trials. Whereas no synergy between IL-2 and ipilimumab was observed [47], the combination of ipilimumab with the standard chemotherapeutic dacarbazine (DTIC) led to a three-fold increase in response rate relative to ipilimumab-alone in a phase II clinical trial by Weber et al. [46]. While unconfirmed, this study suggests that agents that directly affect the viability of tumor cells can act synergistically with immunotherapy, possibly through the release of antigen from dying tumor cells, thereby providing the T cell receptor trigger that is the condition sine qua non in T cell activation.
Anti-PD-1 antibodies
A second inhibitory receptor that has been demonstrated to play a role in tumor immune evasion in preclinical studies is PD-1. This inhibitory molecule is expressed by activated and by exhausted T and B cells and is involved in peripheral tolerance. PD-1 signaling leads to a negative regulation of T cell activity, as demonstrated by a decreased TCR triggering–induced proliferation, cytokine production, and cytolytic activity [48]. One of the PD-1 ligands, PD-L1, is frequently expressed on tumor cells, and preclinical studies have shown that the disruption of PD-1/PD-L1 interactions can result in enhanced tumor control [49–52]. These findings have led to the development of two fully human monoclonal anti-PD-1 antibodies, MDX-1106 and CT-011, that have been evaluated in phase I trials [53, 54]. In these trials, patients with different types of malignancies were included, but only the MDX-1106 phase I trial included metastatic melanoma patients. In this trial, 39 patients with various types of solid tumors were treated with different doses of MDX-1106. The study showed that anti-PD-1 antibody administration was well tolerated (unfortunately the maximum-tolerated dose was not reached), and only few (immune-related) adverse events occurred. The anti-tumor activity of MDX-1106 treatment was demonstrated by one complete response, two partial responses, and two significant lesional regressions. Clinical responses seemed to be associated with the extent of PD-L1 expression by the tumor cells. Further testing of this antibody in the phase II study part has confirmed its clinical efficacy in renal cell carcinoma and melanoma [55].
When comparing anti-CTLA-4 treatment to anti-PD-1 treatment, it becomes apparent that immune-related adverse events occur with both treatments, albeit that those occurring in anti-PD-1 treated patients are far less severe and less frequent. Compared with anti-CTLA-4, the clinical experience with anti-PD-1 treatment is still rather limited, and the MTD has not yet been reached, so this issue of irAEs may need to be revisited in the coming years. Although both CTLA-4 and PD-1 are inhibitors of T cell activity, their function is not completely redundant. Because of this, it is very well possible that the combination of anti-CTLA-4 treatment and anti-PD-1 may result in synergy.
Blockade of BTLA
The inhibitory receptor B and T lymphocyte attenuator (BTLA, CD272), another member of the CD28:B7 immunoglobulin superfamily, is structurally and most likely functionally related to CTLA-4 and PD-1. BTLA is transiently expressed during T cell activation and seems to be constitutively expressed on tumor-specific T cells, inhibiting T cells functions [56]. Many aspects of the exact function of BTLA in humans are still unknown. Interestingly, restoration of the function of tumor-specific T cells by CpG vaccination was shown to be associated with a reduction in BTLA expression [57]. Even though the preclinical data on BTLA are still scant, the fact that positive clinical results have been obtained by the targeting of its family members CTLA-4 and PD-1 makes it very likely that the effects of BTLA blockade will also be analyzed in clinical trials soon.
Blockade of CD200
Recently, it was discovered that cell surface expression of CD200 can adversely affect melanoma-specific T cell responses. CD200 expression by melanoma cells appears to be driven by MAPK-pathway activity and can result in immuno-suppressive effects [58, 59]. The exact mechanism of CD200-mediated immune modulation is not yet fully understood, but may be related to diminished dendritic cell function [60]. Preclinical studies have shown that anti-CD200 administration can restore anti-tumor responses both in vitro and in vivo [58, 59]. A human monoclonal antibody targeting CD200 has been generated and is in early clinical development for the treatment of human cancer.
Agonistic antibodies directed against stimulatory receptors
While the alleviation of inhibitory signals that are received by T cells forms one way to improve melanoma-specific T cell responses, the activity of tumor-specific T cells can also be enhanced by artificial triggering of stimulatory receptors on their surface or on the surface of APCs. Following this rationale, an agonistic antibody against CD137 (4-1BB) has been tested in a phase I dose-escalating trial [61]. The binding of this antibody to CD137 on the surface of activated lymphocytes leads to CD28-independent co-stimulation of T cells, enhanced T cell proliferation, and protection against activation-induced T cell death [62–64]. In the phase I clinical trial that has been carried out with this antibody, 9 out of 47 melanoma patients showed stable disease or a partial regression, demonstrating the potential of CD137 stimulation, and phase II clinical trials are currently ongoing.
A second stimulatory receptor on T cells, for which an agonistic murine antibody has been developed, is OX40. Upon their activation, T cells transiently express this molecule on their surface, and signaling through OX40 promotes both T cell function and survival [65]. Furthermore, OX40 stimulation on T-regulatory cells leads to an inhibition of their suppressive function. A phase I clinical trial has shown some clinical effects, but further studies are required to evaluate the value of agonistic OX40 antibody treatments [66].
As a third possibility, the tumor-specific T cell response may be stimulated through the use of agonistic anti-CD40 antibodies. CD40 is expressed by many immune cells and binding of its ligand, CD40L, promotes B cell, APC, and T cell activation. Administration of agonistic anti-CD40 has been shown to lead to clinical benefit in various phase I trials in melanoma patients [67, 68]. Of particular interest in the context of this review is the observation that the combination of anti-CD40 treatment with carboplatinum and paclitaxel chemotherapy resulted in a marked improvement in efficacy, with 6/36 patients showing a partial response and 14/36 experiencing stable disease.
Achieving long-term responses at high response rates by the combination of targeted therapy and immunotherapy?
Potential for synergy in combination treatments
The above-described trials, in which ipilimumab and anti-CD40 treatment, were combined with cytotoxic agents (DTIC and carboplantinum/paclitaxel, resp.) [46, 68] form a clear example of the increased interest in (and potential value of) the combination of classical genotoxic drugs and immuno-active compounds. The idea of enhancing tumor immunogenicity by chemotherapy induced antigen expression has been established in the eighties by Bonmassar and others and underwent a renaissance with the work of Zitvogel and Kroemer that suggested that metronomic chemotherapy induced cell death could result in a calreticulin-mediated DC activation and polarization of T cells [69–71]. In addition to the proposed immunogenic effects of chemotherapeutics, preclinical experiments suggest that other strategies that induce tumor cell death, such as cryotherapy, radiofrequency ablation (RFA), or radiotherapy (RT), can also synergize with CTLA-4 blockade [72, 73].
Strong support for the notion that immune components may also potentially form an important element in the effect of targeted therapies comes from recent work by Rakhra and colleagues. Specifically, in in vivo murine tumor models, an intact immune system was shown to be required to obtain sustained tumor regression upon driver oncogene inactivation [74]. In this study, oncogene inactivation was shown to result in the recruitment of immune cells, in particular, CD4+ T cells, to the tumor site. This recruitment resulted in an altered cytokine production in the tumor microenvironment, and subsequent induction of cellular senescence and shutdown of angiogenesis. In another work, Lisa Coussens and colleagues showed in a murine breast tumor model that tumor control by paclitaxel (PTX) was abrogated when CD8+ T cells were depleted [75]. Vice versa, depletion of tissue-associated macrophages (TAM) or interference with their recruitment by αCSF-1 mAb or CSF1/cKIT inhibitor PLX3397 improved PTX-induced tumor control, arguing that the modulation of the tumor microenvironment towards a favorable immune signature (low TAM, high CD8+ T cells) might improve chemotherapy effects. Indeed, they further showed that the combination of high CD8+ T cell infiltration and low CD68+ macrophage infiltration was associated with increased overall survival of breast cancer patients treated with adjuvant chemotherapy. Building on these observations, it may be postulated that the combination of a targeted therapy with immune modulating compounds can work synergistically, by stimulating the activity of newly recruited immune cells.
A potential synergy between immuno-active compounds and targeted therapies (either single agent or “in-pathway”- or “cross-pathway”-combined; Fig. 1) in the treatment of melanoma may also occur for two other reasons. First, administration of small molecule inhibitors will induce tumor cell death, thereby leading to the release of tumor antigens that can be cross-presented by antigen-presenting cells. A possibility that needs to be considered is that certain types of cell death may be more immunogenic than others [76, 77]. However, at present, there is only limited data available with regard to potential differences in this respect between the different cytotoxic agents (let alone targeted therapies) that are used in the clinic, an area of research that deserves further attention.
Second, a striking feature of immunotherapeutic agents such as ipilimumab is the slow kinetics with which clinical responses develop. As discussed above, clinical responses are often delayed or even preceded by progressive disease [45, 46], and modified response evaluation criteria have in fact been developed (on the basis of the clinical data with ipilimumab) to allow a better assessment of the clinical benefit of immuno-active compounds. Targeted therapies that extend the time in which a metastatic melanoma patient remains free of disease progression, will give more patients the opportunity to receive all doses of immunotherapy, thereby possibly increasing immunotherapy response rates considerably just for that reason (see also Fig. 1).
Potential issues in combination therapy
Although synergy between immunotherapy and targeted therapy can be expected for the above-described reasons, the extent of this synergy is difficult to estimate. Specifically, it is presently unclear which biological factors determine whether a clinical response after immunotherapy does or does not take place in individual patients, and it is possible that the issues that are “fixed” by targeted therapy are not the relevant ones. As an example, the addition of a targeted therapy to an immunotherapy regimen will likely lead to an antigen boost that can stimulate the immune response. However, it is possible that the lack of clinical response that is still seen in most patients after immunotherapy is not caused (or even partly caused) by a suboptimal level of tumor antigen presentation. For instance, tumor antigens may be omnipresent, but loss of MHC expression could have made the tumor cells insensitive to T cell attack. Alternatively, a given tumor may simply lack antigenic determinants that can be recognized by the endogenous T cell repertoire, thereby making an increase in cell death without value. However, the prior studies in which CTLA-4 blockade or CD40 activation was combined with classical chemotherapy have already provided some evidence for synergy, forming some cause for optimism.
Would a potential synergy with immuno-active compounds be higher or lower for targeted therapies as compared to other treatment modalities such as RT, RFA, or classical chemotherapeutics? On the one hand, it is possible that the amount of inflammation could be lower in the case of targeted therapies, as cell death should largely be restricted to tumor cells. At the same time, this more selective cell death can also be expected to lead to a higher representation of tumor antigens in the set of antigens presented by antigen-presenting cells, and this could form an advantage. As far as potential toxicity is concerned, the cell death that is induced by targeted therapies will not only lead to the release of tumor antigens but also to (other) self-antigens that are expressed within the tumor cells. As a consequence, an auto-reactive T cell response could ensue which is then further boosted by immunotherapy. Most of the self-antigens expressed in melanoma and to which a self-reactive T cell repertoire is known to exist consist of the melanocyte differentiation antigens. Activation of melanocyte lineage-specific T cells may result in autoimmune vitiligo or uveitis, but these form conditions that are either considered acceptable or medically manageable.
Practical aspects and (pre) clinical development of combination therapy
While clinical trials that test the value of combination therapy will undoubtedly be initiated in the coming years, it is plausible that the preclinical testing of combination therapy in murine tumor models can help to optimize their design. Important aspects that could be addressed in such preclinical studies are the combinations that demonstrate the highest synergy, but also the timing of the different treatments. In addition, more experimental aspects, such as the effect of pulsed instead of continuous dosing of the small molecule inhibitor, may also be addressed in such preclinical models.
In the clinical trials that will evaluate the effects of combination therapy, it will be important to determine to what extent combination therapy influences tumor-specific T cell responses in melanoma patients relative to single-agent immunotherapy. Technology for the parallel analysis of peripheral blood T cell responses against very large collections of melanoma antigens should be very informative in this respect [30, 78]. In addition, it will be useful to not restrict biomarker analysis in these trials to the peripheral blood compartment, but to also include an analysis of tumor biopsies obtained prior to and during treatment. Immunohistochemical analyses of immune cell infiltration, senescence markers, such as β-gal, and apoptosis markers, such as caspase-3, should give insights into the effects of combination treatment at the tumor site itself.
Proposed combinations of small molecule inhibitors and immunotherapy
Although a large number of small molecule inhibitors and immuno-active compounds are currently undergoing (pre) clinical evaluation, only a few of these have completed phase II or III studies. This puts an upper limit to the number of combination therapies that can be tested in the near future, but this situation will change in the years to come, when monotherapies are approved. As a more fundamental issue with regard to the possibility of designing combination therapy trials, the recent data about possible mechanisms of resistance or impairment of immune cell functions should certainly be considered when designing such studies.
BRAFV600E inhibition and CTLA-4 blockade
PLX4720, a BRAF V600E inhibitor that is structuraly related to PLX4032/RG7204 (Vemurafenib) has been shown not to affect T cell functions in in vitro assays [79, 80], and this makes the combination of PLX4032 with ipilimumab highly attractive. Joint administration of these two drugs is very likely to increase the percentage of patients that can receive all four ipilimumab courses and by this sole fact may already improve overall response rates induced by ipilimumab. The clinical efficacy of the combination of PLX4032/Vemurafenib and ipilimumab will likely be tested soon, and as a subsequent step, it may also be attractive to explore the value of alternating the two drugs: all current vaccination approaches supply antigen in discrete waves (i.e., in the form of prime-boost combinations). By analogy, the repetitive release of antigen—perhaps best in the days just prior to ipilimumab administration—may potentially be superior to a continuous antigen exposure. In addition, pulsed application of the BRAF inhibitor will possibly delay the time to treatment resistance, thereby ensuring that antigen liberation also occurs during the later cycles of ipilimumab treatment. While the immunological rationale for pulsed BRAF inhibition is clearly there, it may be difficult to initiate a clinical trial that tests this concept in the absence of supporting preclinical data. For this reason, and also to evaluate the timing between PLX4032/RG7204 and ipilimumab administration, it will be worthwhile to test this concept in animal model systems.
BRAFV600E and MEK inhibition, possible inclusion of immune-activating compounds
The targeting of V600E-mutant BRAF, either with PLX4032/RG7204 or with GSK2118436, has been shown to lead to high response rates. These drugs have just completed or are currently tested in phase III studies. However, clinical responses are often only short-lived, and tumors evade the upstream MAPK pathway inhibition by reactivation of pERK. The combination of BRAF inhibition plus MEK inhibition has been shown to prevent drug resistance in in vitro assays [19], and a phase I study that will evaluate the combination of PLX4032 with the MEK inhibitor GDC-0973 is currently in preparation. The combination of BRAF inhibition and ERK inhibition is likely to improve response rates and in particular response duration. However, inclusion of an immune-activating compound in such a combination therapy appears less attractive because of the strong T cell inhibition induced by MEK inhibitors [79]. Potentially, the pulsed administration of the MEK inhibitor could be used to bypass this issue, a possibility that should be explored by preclinical testing in animal models.
BRAFV600E inhibition, PI3K pathway targeting, and CTLA-4 blockade
The targeting of the PI3K/AKT pathway in melanoma can be considered highly attractive not only because of the fact that many melanoma metastases carry genetic alterations in this pathway [10, 11] but also because recent work on resistance mechanisms upon MAPK-pathway inhibition suggests an involvement of the PI3K pathway [18, 81]. Consequently, the combination of MAPK pathway inhibition (either by BRAF inhibitors or by the combination of BRAF and MEK inhibitors) and PI3K pathway inhibition may result in enhanced tumor regression. However, the combination of PI3K inhibitors with immuno-active compounds such as anti-CTLA4 might be less attractive, as downstream TCR signaling has been shown to utilize the p85 subunit of phosphoinositide 3-kinase (PI3K) (reviewed in [82]). In addition, the fact that both pan-PI3K inhibitors that are currently tested in the clinic (BEZ235 and GSK2126458) do display mTOR inhibitory capacity also makes the combination of these drugs with immune modulating compounds less attractive.
Blockade of CTLA-4 and PD-1/PD-L1
In addition to the well-documented clinical effect of blockade of CTLA-4 in melanoma, there is evidence for clinical activity of PD-1 blocking antibodies from phase I/II studies [54, 55]. The effect of blockade of one of the PD-1 ligands, PD-L1, is currently evaluated in a phase I trial. Preclinical experiments using a transplantable melanoma model have revealed that the triple blockade of CTLA-4, PD-1, and PD-L1 results in superior tumor control [83]. Thus, in addition to the potential value of the combination of immune-activating antibodies with targeted therapies, the effect of the combination of different immuno-modulating antibodies in melanoma could also be explored. Other molecules, such as BTLA, CD200, CD40, OX40, CD27, and CD137, form further targets for combination therapies in melanoma. However, the combined blockade of CTLA-4 and PD-1 would be a logical first step, due to the more advanced clinical development of antibodies that target these receptors.
BRAFV600E inhibition and CTLA-4 + PD-1 blockade
Finally, in case the combination of PLX4032 and ipilimumab, and the combination of ipilimumab and MDX-1106 yield encouraging response data without a strong increase in (immune related) adverse events, the triple combination of BRAF V600E inhibition plus dual immune activation by anti-CTLA-4/anti-PD-1 would be a logical next step. We consider this combination particularly promising as any impairment of T cell functions would not be expected. Furthermore, based on the fact that PD-1 blockade was extremely well tolerated in the phase II extension part [55], the toxicity of the combination of PD-1 blockade and CTLA-4 blockade may also be acceptable.
Thus far, none of these proposed combinations have been tested in preclinical melanoma mouse models, perhaps due to the fact that an immune-proficient murine melanoma model that carries the relevant genetic alterations has been lacking. With the recent development of the BRAF V600E—PTEN-deficient murine melanoma model [84], the preclinical evaluation of the above-described targeted therapy/immunotherapy combinations should now be within reach.
Future perspectives
Clinical trials
Until very recently, the treatment options for patients with metastatic melanoma were highly limited, and mean overall survival of this patient population has been short. However, a number of recent clinical trials have shown that both targeted therapy and immunotherapy can induce clinical benefits, improving progression-free and overall survival of these patients. These encouraging results are expected to lead to the registration of these drugs in the near future, and more compounds belonging to these drug classes will be developed and tested in the coming years. In addition, we foresee the initiation of clinical trials in the near future that will explore the potential of the combination of targeted therapy plus immunotherapy.
Clinical trials in which the BRAFV600E inhibitor (PLX4032/RG7204) will be combined with MEK inhibition (GDC-0973) or with ipilimumab treatment are subject of discussion among melanoma medical oncologists and industry, and the results of these trials may well change the standard treatment of melanoma patients in the coming years. In addition, the number of immunotherapeutic strategies that can be combined with each other or with targeted therapies will increase significantly, as phase III clinical trials for a number of these compounds will be concluded soon. Finally, the clinical development of adoptive T cell therapy will possibly progress to a stage in which it can be combined with targeted therapy and/or other immunotherapies. For those—still few—driver mutations that do result in the generation of a MHC-presented neo-epitope (e.g., the CDKR24C mutation), such adoptive immunotherapy could conceivably target the very same mutations that are targeted by small molecules.
Preclinical research
Preclinical research in this area can be expected to yield new data on the following important issues in the coming years. First, the further analysis of the prospects of targeting other receptor molecules (either inhibitory or stimulatory) will yield new candidates for clinical testing. Second, an improved understanding of the pathways that limit a clinical response to immunotherapy will be extremely useful for the design of clinical protocols that aim to specifically address these issues. Third (and likewise), the analysis of escape mechanisms in response to targeted treatment will be crucial to improve response durations.
Finally, the development of additional small molecule inhibitors that (like PLX4032/Vemurafenib) specifically target mutated signaling proteins in melanoma can be expected. Most of the targeted therapies developed to date lead to dose-limiting toxicity due to their effect on cells from healthy tissues that also express the targeted proteins. In contrast, the use of small molecule inhibitors that are (at least to some extent) specific for mutant proteins will generally be associated with lower toxicity. A further advantage of these mutant protein-specific small molecule inhibitors will be that their activity will not affect immune cells, and they thereby form promising candidates for combination therapy.
Application in other malignancies
It is expected that the value of the combination of targeted therapy and immunotherapy will also be tested in other human malignancies. Renal cell carcinoma (RCC) forms a second human malignancy that is considered sensitive to immune attack. Many targeted therapies for the treatment of RCC have already been developed (e.g., bevacizumab, sunitinib, pazopanib, temsirolimus, everolimus), and also immunotherapeutic strategies such as the blockade of inhibitory receptors and adoptive T cell transfer are being evaluated in patients with RCC [61, 85]. Furthermore, the combination of anti-CTLA-4 treatment and irradiation (which, like targeted therapy, will induce antigen release as a consequence of tumor cell death) is currently tested in a phase III study in RCC.
It is likely that within a few years, malignancies will no longer (or at the least not solely) be classified on the basis of the originating tissue, but on the pathway alterations that are driving the development and progression of that tumor. Potential synergy between targeted therapies and immuno-active compounds seen in melanoma may therefore form the basis for the testing of the same treatment modalities in other patient groups. As an example, BRAF mutations are also present in 40–70% of papillary thyroid cancer, 5–20% of colorectal cancer, 10–20% of cholangiosarcomas, and 1–5% of lung cancers [86], and if a PLX4032—ipilimumab combination trial would be positive in melanoma, it would certainly also be attractive to test this combination for patients with these tumors.
Irrespective of the exact direction that preclinical and clinical research will take us in the coming years, it has become clear that the treatment of metastatic melanoma, after many years of stasis, will change dramatically in the years ahead of us.
Footnotes
We would like to thank Daniel Peeper, NKI-AVL, Amsterdam, for valuable suggestions and discussion.
Contributor Information
Christian U. Blank, Phone: +31-205-122570, FAX: +31-205-122572, Email: c.blank@nki.nl
Ton N. Schumacher, Phone: +31-205-122072, FAX: +31-205-122057, Email: t.schumacher@nki.nl
References
- 1.Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 2.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150(2):179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 3.Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9(5):587–595. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AM. Immunotherapy: vaccine trials in melanoma—time for reflection. Nature Rev. 2009;6(5):256–258. doi: 10.1038/nrclinonc.2009.42. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40(12):1825–1836. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 7.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 8.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharpless E, Chin L. The INK4a/ARF locus and melanoma. Oncogene. 2003;22(20):3092–3098. doi: 10.1038/sj.onc.1206461. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim N, Haluska FG. Molecular pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol. 2009;4:551–579. doi: 10.1146/annurev.pathol.3.121806.151541. [DOI] [PubMed] [Google Scholar]
- 11.Smalley KS. Understanding melanoma signaling networks as the basis for molecular targeted therapy. J Invest Dermatol. 2009;130(1):28–37. doi: 10.1038/jid.2009.177. [DOI] [PubMed] [Google Scholar]
- 12.Gast A, Scherer D, Chen B, Bloethner S, Melchert S, Sucker A, Hemminki K, Schadendorf D, Kumar R. Somatic alterations in the melanoma genome: a high-resolution array-based comparative genomic hybridization study. Genes Chromosomes Cancer. 2010;49(8):733–745. doi: 10.1002/gcc.20785. [DOI] [PubMed] [Google Scholar]
- 13.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, Gibbens I, Hackett S, James M, Schuchter LM, Nathanson KL, Xia C, Simantov R, Schwartz B, Poulin-Costello M, O’Dwyer PJ, Ratain MJ. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95(5):581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, Eggermont A, Grabbe S, Gonzalez R, Gille J, Peschel C, Schadendorf D, Garbe C, O’Day S, Daud A, White JM, Xia C, Patel K, Kirkwood JM, Keilholz U. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27(17):2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 15.Dummer R (2008) AZD6244 (Arry-a428896) versus temozolomide (TMZ) in patients with advanced melanoma: an open-label, randomized, multicenter, phase II study. J Clin Oncol 26(abstract 9033)
- 16.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, Smalley KS, Fong D, Zhu YL, Marimuthu A, Nguyen H, Lam B, Liu J, Cheung I, Rice J, Suzuki Y, Luu C, Settachatgul C, Shellooe R, Cantwell J, Kim SH, Schlessinger J, Zhang KY, West BL, Powell B, Habets G, Zhang C, Ibrahim PN, Hirth P, Artis DR, Herlyn M, Bollag G. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D’Andrea K, Pushparajan A, Hayden JE, Brown KD, Laquerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18(6):683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, Messina JL, Flaherty KT, Smalley KS. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102(12):1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang CC, Lai F, Thorne RF, Yang F, Liu H, Hersey P, Zhang XD (2010) MEK-independent survival of B-RAFV600E melanoma cells selected for resistance to apoptosis induced by the RAF inhibitor PLX4720. Clin Cancer Res [DOI] [PubMed]
- 23.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, Dias-Santagata D, Stubbs H, Lee DY, Singh A, Drew L, Haber DA, Settleman J. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68(12):4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KY, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D’Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu X, Nathanson KL, Nolop K. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA (2011) Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. doi:10.1200/JCO.2010.33.2312 [DOI] [PMC free article] [PubMed]
- 27.Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B, Baserga R. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol. 1999;19(10):7203–7215. doi: 10.1128/mcb.19.10.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smalley KS, Sondak VK. Melanoma—an unlikely poster child for personalized cancer therapy. N Engl J Med. 2010;363(9):876–878. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- 29.Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19(5):275–282. doi: 10.1097/CMR.0b013e32832eabd5. [DOI] [PubMed] [Google Scholar]
- 30.Hadrup SR, Bakker AH, Shu CJ, Andersen RS, van Veluw J, Hombrink P, Castermans E, Thor Straten P, Blank C, Haanen JB, Heemskerk MH, Schumacher TN. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat Methods. 2009;6(7):520–526. doi: 10.1038/nmeth.1345. [DOI] [PubMed] [Google Scholar]
- 31.Lui VK, Karpuchas J, Dent PB, McCulloch PB, Blajchman MA. Cellular immunocompetence in melanoma: effect of extent of disease and immunotherapy. Br J Cancer. 1975;32(3):323–330. doi: 10.1038/bjc.1975.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alegre ML, Shiels H, Thompson CB, Gajewski TF. Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol. 1998;161(7):3347–3356. [PubMed] [Google Scholar]
- 34.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172(7):4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 35.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94(15):8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 38.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA, Gomez-Navarro J, Ribas A. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27(7):1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 40.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12(7):864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 41.Lutzky JWJ, Hamid O, et al. Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol. 2009;27:9034. [Google Scholar]
- 42.Di Giacomo AM, Danielli R, Calabro L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C, Biagioli M, Altomonte M, Maio M (2010) Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother. doi:10.1007/s00262-010-0958-2 [DOI] [PMC free article] [PubMed]
- 43.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamid O, Chasalow SD, Tsuchihashi Z, Alaparthy S, Galbraith S, Berman D. Association of baseline and on-study tumor biopsy markers with clinical activity in patients (pts) with advanced melanoma treated with ipilimumab. J Clin Oncol. 2009;27:15s. [Google Scholar]
- 45.Di Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli M, Altomonte M, Maio M. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hersh EM, O’Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, Samlowski WE, Nichol GM, Yellin MJ, Weber JS (2010) A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. doi:10.1007/s10637-009-9376-8 [DOI] [PubMed]
- 47.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, Kleiner D, Mavroukakis SA, Yellin M, Rosenberg SA. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12(12):1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–1145. doi: 10.1158/0008-5472.CAN-03-3259. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwai Y, Terawaki S, Honjo T (2004) PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol [DOI] [PubMed]
- 52.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 53.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 54.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.M. Sznol JDP, Smith DC, Brahmer JR, Drake CG, McDermott DF, Lawrence DP, Wolchok JD, Topalian SL, Lowy I (2010) Safety and antitumor activity of biweekly MDX-1106 (Anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies. J Clin Oncol 28:15s (suppl; abstr 2506)
- 56.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 57.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, Olive D, Speiser DE. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120(1):157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petermann KB, Rozenberg GI, Zedek D, Groben P, McKinnon K, Buehler C, Kim WY, Shields JM, Penland S, Bear JE, Thomas NE, Serody JS, Sharpless NE. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Invest. 2007;117(12):3922–3929. doi: 10.1172/JCI32163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57(7):987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 61.Sznol MFSH, Margolin K, McDermott DF, Ernstoff MS, Kirkwood JM, Wojtaszek C, Feltquate D, Logan T (2008) Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J Clin Oncol 26 (May 20 suppl):abstr 3007
- 62.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 63.Lee J, Lee EN, Kim EY, Lee HJ, Park HJ, Sun CL, Lee SK, Joh JW, Lee KW, Kwon GY, Kim SJ. 4-1BB promotes long-term survival in skin allografts treated with anti-CD45RB and anti-CD40L monoclonal antibodies. Transplant Proc. 2005;37(1):123–125. doi: 10.1016/j.transproceed.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167(12):6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 66.Jensen SM, Maston LD, Gough MJ, Ruby CE, Redmond WL, Crittenden M, Li Y, Puri S, Poehlein CH, Morris N, Kovacsovics-Bankowski M, Moudgil T, Twitty C, Walker EB, Hu HM, Urba WJ, Weinberg AD, Curti B, Fox BA Signaling through OX40 enhances antitumor immunity. Semin Oncol 37(5):524–532. doi:10.1053/j.seminoncol.2010.09.013 [DOI] [PMC free article] [PubMed]
- 67.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O’Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870, 893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 68.Wolchok JD, Yang AS, Weber JS. Immune regulatory antibodies: are they the next advance? Cancer J. 2010;16(4):311–317. doi: 10.1097/PPO.0b013e3181eb3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16(12):3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 70.Giampietri A, Bonmassar A, Puccetti P, Circolo A, Goldin A, Bonmassar E. Drug-mediated increase of tumor immunogenicity in vivo for a new approach to experimental cancer immunotherapy. Cancer Res. 1981;41(2):681–687. [PubMed] [Google Scholar]
- 71.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L (2011) Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. doi:10.1007/s00281-011-0245-0 [DOI] [PubMed]
- 72.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95(7):896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, Fan AC, Yang Q, Braunstein L, Crosby E, Ryeom S, Felsher DW. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18(5):485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denardo D, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Hope SR, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Dis. 2011;1(1):OF52–OF65. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP, Greten TF. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103(2):205–211. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 77.Lohmann C, Muschaweckh A, Kirschnek S, Jennen L, Wagner H, Hacker G. Induction of tumor cell apoptosis or necrosis by conditional expression of cell death proteins: analysis of cell death pathways and in vitro immune stimulatory potential. J Immunol. 2009;182(8):4538–4546. doi: 10.4049/jimmunol.0803989. [DOI] [PubMed] [Google Scholar]
- 78.Hadrup SR, Toebes M, Rodenko B, Bakker AH, Egan DA, Ovaa H, Schumacher TN. High-throughput T-cell epitope discovery through MHC peptide exchange. Methods Mol Biol. 2009;524:383–405. doi: 10.1007/978-1-59745-450-6_28. [DOI] [PubMed] [Google Scholar]
- 79.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, Tsao H, Wargo JA. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70(13):5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 80.Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, Escuin-Ordinas H, Chmielowski B, Koya RC, Ribas A. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010;16(24):6040–6048. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang CC, Lai F, Thorne RF, Yang F, Liu H, Hersey P, Zhang XD (2011) MEK-Independent Survival of B-RAFV600E melanoma cells selected for resistance to apoptosis induced by the RAF inhibitor PLX4720. Clin Cancer Res. doi:10.1158/1078-0432.CCR-10-2225 [DOI] [PubMed]
- 82.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41(5):544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Markel G, Cohen-Sinai T, Besser MJ, Oved K, Itzhaki O, Seidman R, Fridman E, Treves AJ, Keisari Y, Dotan Z, Ramon J, Schachter J. Preclinical evaluation of adoptive cell therapy for patients with metastatic renal cell carcinoma. Anticancer Res. 2009;29(1):145–154. [PubMed] [Google Scholar]
- 86.Flaherty KT, McArthur G. BRAF, a target in melanoma: implications for solid tumor drug development. Cancer. 2010;116(21):4902–4913. doi: 10.1002/cncr.25261. [DOI] [PubMed] [Google Scholar]