Abstract
Immunosuppression in tumor microenvironments critically affects the success of cancer immunotherapy. Here, we focused on the role of interleukin (IL)-6/signal transducer and activator of transcription (STAT3) signaling cascade in immune regulation by human dendritic cells (DCs). IL-6-conditioned monocyte-derived DCs (MoDCs) impaired the presenting ability of cancer-related antigens. Interferon (IFN)-γ production attenuated by CD4+ T cells co-cultured with IL-6-conditioned MoDCs corresponded with decreased DC IL-12p70 production. Human leukocyte antigen (HLA)-DR and CD86 expression was significantly reduced in CD11b+CD11c+ cells obtained from peripheral blood mononuclear cells (PBMCs) of healthy donors by IL-6 treatment and was STAT3 dependent. Arginase-1 (ARG1), lysosomal protease, cathepsin L (CTSL), and cyclooxygenase-2 (COX2) were involved in the reduction of surface HLA-DR expression. Gene expressions of ARG1, CTSL, COX2, and IL6 were higher in tumor-infiltrating CD11b+CD11c+ cells compared with PBMCs isolated from colorectal cancer patients. Expression of surface HLA-DR and CD86 on CD11b+CD11c+ cells was down-regulated, and T cell-stimulating ability was attenuated compared with PBMCs, suggesting that an immunosuppressive phenotype might be induced by IL-6, ARG1, CTSL, and COX2 in tumor sites of colorectal cancer patients. There was a relationship between HLA-DR expression levels in tumor tissues and the size of CD4+ T and CD8+ T cell compartments. Our findings indicate that IL-6 causes a dysfunction in human DCs that activates cancer antigen-specific Th cells, suggesting that blocking the IL-6/STAT3 signaling pathway might be a promising strategy to improve cancer immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1791-4) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cells, Antigen presentation, HLA class II, IL-12, Helper T cells
Introduction
Dysfunctional immune responses in the tumor microenvironment are a critical issue affecting the development of cancer immunotherapies. Immunosuppressive cytokines such as interleukin (IL)-10 and transforming growth factor (TGF)-β produced at high levels in tissues containing tumors block the functions of anti-tumor effector T cells. Myeloid-derived suppressor cells (MDSCs), induced from immature myeloid cells in tumor microenvironments, also block anti-tumor immunity [1–3]. To develop more effective cancer treatments, immune dysfunction in cancer patients needs to be overcome.
Recently, the effectiveness of immunocheckpoint therapy using anti-programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), and/or cytotoxic T lymphocyte-associated protein 4 (CTLA-4) antibodies was reported in solid tumors [4–6]. These results indicate that the removal of blocking signals can restore lymphocyte anti-tumoral functions.
Dendritic cells (DCs) are antigen-presenting cells that induce antigen-specific immune responses through the activation of CD4+ T and CD8+ T cells. In cancer patients, DCs expressing HLA class I, class II, and co-stimulatory molecules on the cell surface are crucial for inducing cancer-related antigen-specific helper T (Th) cells and cytotoxic T lymphocytes (CTLs) [7–10]. Correct regulation of DC function in tumors is important for the induction of anti-tumor immunity.
IL-6 is a pleiotropic cytokine that has a variety of effects on cells and tissues. It is produced by many different cells, including immune cells, fibroblasts, endothelial cells, and tumor cells [11, 12]. IL-6 binds to the IL-6 receptor (IL-6R), and this IL-6/IL-6R complex associates with the signal-transducing membrane protein, glycoprotein 130 (gp130), inducing its dimerization to initiate IL-6 signaling. Dimerization of gp130 is followed by the rapid activation of the Janus kinase (Jak) family and several signaling pathways, including phosphoinositide 3-kinase (PI3K)/extracellular signal-related kinase (ERK)/mitogen-activated protein kinase (MAPK) and STAT3. STAT3 activation induces many effector genes that are involved in cell growth, differentiation, and survival.
We previously showed that IL-6 signaling suppressed major histocompatibility complex (MHC) class II expression on murine DCs via STAT3 activation and was attenuated in CD4+ T cell-mediated immune responses [13, 14]. Furthermore, we demonstrated that administration of a monoclonal antibody (mAb) against IL-6R enhanced T cell responses and inhibited tumor growth in vivo [15]. In mice with tumors, IL-6 suppressed CD4+ T cell-mediated immunity through the down-regulation of MHC class II via enhanced arginase activity in DCs [16]. Administration of arginase-1 inhibitors, nor-NOHA or l-arginine, blocked the reduction of MHC class II levels in DCs during tumorigenesis. Furthermore, the injection of nor-NOHA at peri-tumor sites enhanced CD4+ T cell responses, resulting in an inhibition of tumor growth. IL-6-mediated STAT3 activation and the subsequent reduction in MHC class II expression in DCs appear to be a critical mechanism for inducing immune system dysfunction in cancer. Blocking IL-6/STAT3 signaling cascades might therefore be a promising approach to overcoming dysfunctional anti-tumor immunity.
In the current study, we focused on the IL-6/STAT3 signaling pathway in human DCs. We report the effects of IL-6 on the antigen-presenting ability of DCs in T cell-mediated anti-tumor immunity of colorectal cancer patients.
Materials and methods
Antibodies and reagents
APC-conjugated antihuman CD11c (3.9), APC-Cy7-conjugated CD4 (SK3), APC-Cy7-conjugated CD8 (RPA-T8), PE-conjugated HLA-DR (G46-6), FITC-conjugated antihuman HLA-A/B/C (G46-2.6), PE-conjugated CD80 (L307.4), PE-conjugated CD86 (2331, FUN-1) mAbs, PE-conjugated mouse IgG1κ isotype control, and FITC-conjugated mouse IgG1κ isotype control were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Control rat IgG was purchased from MP Biomedicals (Santa Ana CA, USA). 7-Amino-actinomycin D (7AAD) was purchased from Beckman Coulter (Brea, CA, USA). Recombinant human granulocyte macrophage colony-stimulating factor (GM-CSF) and human IL-4 were purchased from PeproTech Inc. (Rocky Hill, NJ, USA). Recombinant human IL-6 was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). A specific inhibitor of STAT3, 6-nitrobenzo[b]thiophene-1,1-dioxide, a COX2 inhibitor (sc-58125), and the arginase inhibitor (Nw-hydroxy-l-arginine; nor-NOHA) were purchased from Calbiochem (San Diego, CA, USA). Dimethyl sulfoxide (DMSO), which was used as a solvent, was purchased from Wako Pure Chemical Industries, Ltd. Antihuman CD3 mAb (OKT3) was purchased from BioLegend (San Diego, CA, USA), and antihuman IL-12 mAb (C8.6) was purchased from BD Biosciences. Antihuman CD4, CD8, and CD14 microbeads were purchased from Miltenyi Biotec K.K. (Bergisch Gladbach, Germany). We used AIM-V (Invitrogen, Carlsbad, CA, USA) without serum for cell culture.
Informed consent
Research protocols involving human subjects were approved by the institutional review board of Hokkaido University Graduate School of Medicine and the Institute for Genetic Medicine. Written informed consent was obtained from each patient or healthy donor.
Generation of MoDCs and IL-6-conditioned MoDCs
We obtained PBMCs from healthy donors using Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) gradient centrifugation. CD14+ cells, separated from PBMCs of healthy donors by magnetic cell sorting, were seeded in 12-well culture dishes (4 × 105 cells/well). MoDCs were induced from CD14+ cells by culturing in the presence of recombinant human IL-4 (50 ng/mL) and GM-CSF (50 ng/mL) for 7 days. Recombinant human IL-6 (50 ng/mL) was added at day 6. MoDCs cultured in the presence of IL-6 for 24 h were designated IL-6-conditioned DCs.
Preparation of CD4+ T cells from PBMCs of healthy donors
CD4+ T cells were sorted by magnetic cell sorting from the PBMCs of healthy donors. The isolated CD4+ T cells (1 × 105 cells) were co-cultured with autologous MoDCs (1 × 104 cells) for cytokine production assays. Purities of the isolated CD4+ T cells were >95 %.
Induction of cancer antigen-specific Th cells
We cultured PBMCs (2 × 106 cells) in the presence of survivin-derived peptide (EHKKHSSGCAFLSVKKQFEELTLGEFLKLDRERAKNKIAK, 5 µM) [17], or 6-transmembrane epithelial antigen of prostate (STEAP)-derived peptides (SLLLGTIHALIFAWN, 5 µM and QFVWYTPPTFMIAVF, 5 µM) [18] for 7 days. PBMCs were then treated with a streptococcus-derived anticancer immunotherapeutic agent, OK-432 (0.1 KE/ml), mitomycin-treated autologous MoDCs (1 × 105 cells), the same peptide that was previously used for stimulation, and IL-2 (10 IU/ml) for 7 days. Cells were re-stimulated and cultured for another 7 days. At day 21, the specificity of induced CD4+ T cells for each antigen was evaluated by co-culture with MoDCs in the presence of the corresponding antigen (Supplemental Fig. 1). For cytokine production assays, antigen-specific CD4+ T cells (5 × 104) were co-cultured with MoDCs or IL-6-conditioned MoDCs (5 × 103) in the presence of cancer antigen peptides or control peptides for 24 h.
Preparation of DCs and co-culture with autologous T cells from colorectal cancer patients
TILs and PBMCs were collected from patients with colorectal cancers. All colorectal cancer tissues were immediately minced using scissors after surgical resection and digested with 1 mg/mL collagenase (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 30 min. Single-cell suspensions were obtained by passage through a 100-µm nylon cell strainer (BD Biosciences). Surface HLA-DR and CD86 expression of 7AAD−CD45+CD11b+CD11c+ cells from PBMCs and TILs was evaluated by flow cytometry (Supplemental Fig. 2). For polymerase chain reaction (PCR) analysis and co-culture with T cells, 7AAD−CD45+CD11b+CD11c+ cells from PBMCs or TILs were isolated using a fluorescence-activated cell sorting (FACS) system. Autologous CD4+ and CD8+ T cells were sorted from PBMCs of colorectal cancer patients for T cell proliferation assays. Purities of the isolated 7AAD−CD45+CD11b+CD11c+ cells, CD4+ T, and CD8+ T cells were >95 %. CD4+ and CD8+ T cells were pre-incubated in carboxyfluorescein succinimidyl ester (CFSE) (5 nM) at room temperature for 15 min. After washing with phosphate-buffered saline (PBS), CFSE-labeled CD4+ and CD8+ T cells (1 × 105 cells) were co-cultured with CD11b+CD11c+ cells (5 × 104 cells) from PBMCs or TILs in the presence of an anti-CD3 mAb (OKT3, 2 µg/mL). 7AAD−CD45+CD11b+CD11c+ cells were added to T cells (1 × 105 cells) at different ratios (1:2–1:8) to measure IFN-γ production. The level of IFN-γ in culture supernatants was measured at 24 h by enzyme-linked immunosorbent assay (ELISA). Proliferation of CD4+ and CD8+ T cells was evaluated at 72 h by flow cytometry.
PCR analysis
Total RNA was extracted from human DCs or 7AAD−CD45+CD11b+CD11c+ cells derived from PBMCs and TILs with an RNeasy Mini kit (Qiagen, Hilden, Germany). First-strand cDNAs were synthesized using 1 µg of total RNA, oligo (dT) (Invitrogen), and Superscript III reverse transcriptase (Invitrogen). Genes encoding human IL6, COX2, CTSL, ARG1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were amplified in a LightCycler (Roche, Indianapolis, IN, USA). The primer sequences and numbers of universal probes used in this study were as follows: IL6 (forward: 5′-caggagcccagctatgaact-3′, reverse: 5′-gaaggcagcaggcaacac-3′, universal probe: #7), COX2 (forward: 5′-cttcacgcatcagtttttcaag-3′, reverse: 5′-tcaccgtaaatatgatttaagtccac-3′, universal probe: #23), CTSL (forward: 5′-gcaaggatgagtgtaggattca-3′, reverse: 5′-gggagggcagttgaggac-3′, universal probe: #75), ARG1 (forward: 5′-tggcagaagtcaagaagaacg-3′, reverse: 5′-atgcttccaattgccaaact-3′, universal probe: #64), and GAPDH (forward: 5′-agccacatcgctcagacac-3′, reverse: 5′-gcccaatacgaccaaatcc-3′, universal probe: #60). Sample signals were normalized to the reference gene GAPDH using the ∆∆C t method (∆C t = ∆C tsample − ∆C treference).
Flow cytometry
Surface expression of HLA-A/B/C, HLA-DR, CD80, and CD86 was evaluated with a FACSCantoII™ (BD Biosciences), and results were analyzed with FlowJo software (Tree Star, Ashland, OR, USA). The mean fluorescence intensity (MFI) ratio (sample ∆MFI (specific marker MFI − isotype control MFI)/control sample ∆MFI × 100) or the median FI ratio (sample ∆median FI (specific marker median FI − isotype control median FI)/control sample ∆MFI × 100) was calculated for samples. A FACSAriaII™ (BD Biosciences) was used for the isolation of CD11b+CD11c+ DCs from PBMCs and TILs of colorectal cancer patients.
ELISA
We determined IFN-γ, IL-12p70, and IL-10 levels in culture supernatants using OptEIA™ human IFN-γ, IL-12p70, and IL-10 ELISA kits (BD Biosciences), respectively, according to the manufacturer’s instructions. Total and active TGF-β levels in culture supernatants were determined by using a Quantikine™ human TGF-β1 Immunoassay kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.
Immunohistochemistry (IHC)
Normal and cancer tissues were obtained from colorectal surgical specimens (n = 27). Patient information is summarized in Supplemental Table 1. Colon specimens were fixed in formalin and embedded in paraffin. After deparaffinization, antigen retrieval was conducted by boiling for 10 min in 0.01 M citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3 % (v/v) hydrogen peroxide for 15 min. After pre-incubation with blocking I buffer (Nacalai Tesque Inc., Kyoto, Japan) for 10 min, slides were incubated with mouse antihuman HLA-DR α-chain mAb (clone TAL.1B5, Dako, Glostrup, Denmark), mouse antihuman CD4 mAb (clone 4B12, Novocastra Laboratories Ltd., Newcastle Upon Tyne, UK), mouse antihuman CD8 mAb (clone 1A5, Novocastra Laboratories Ltd.), or a polyclonal rabbit antihuman IL-6 (Rockland Immunochemicals, Inc., Gilbertsville, PA, USA) antibody overnight at 4 °C. Sections were then incubated for 30 min with horseradish peroxidase (HrP)-labeled anti-mouse IgG polyclonal antibody (Nichirei Biosciences Inc., Tokyo, Japan) or an HrP-labeled goat anti-rabbit antibody (Dako). Positive signals were visualized using 3-3′-diaminobenzidine-4HCL (DAB). Sections were counterstained with Mayer’s hematoxylin.
Statistical analysis
Statistical significance was evaluated using the Student’s t test for two groups of data. In some experiments, the results were evaluated by Dunnett’s posttest (vs. control group). A P value less than 0.05 was considered statistically significant.
Results
IL-6 attenuates DC-mediated activation of cancer-related antigen-specific CD4+ T cells
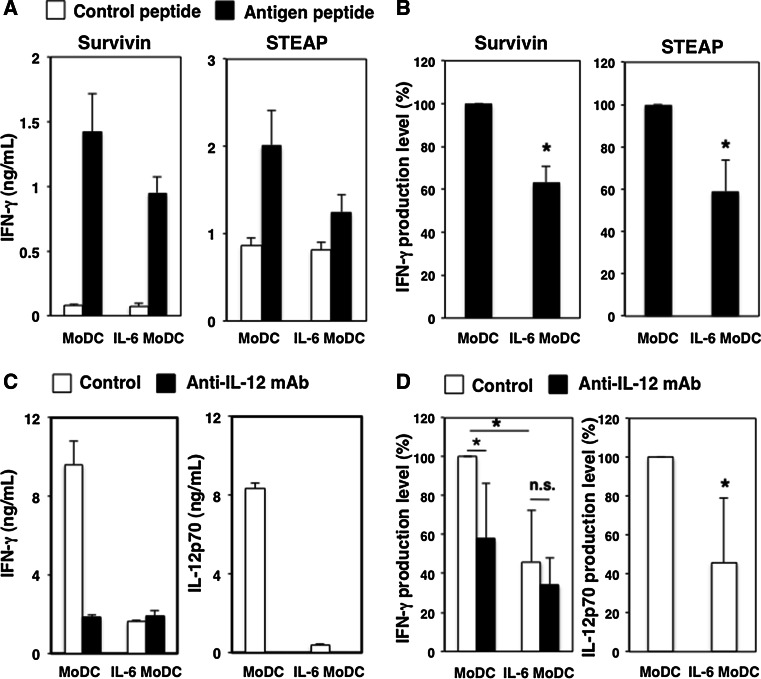
We first investigated whether IL-6 influenced the antigen-presenting ability of human MoDCs. Cancer-related antigen, survivin- or STEAP-specific CD4+ T cells were co-cultured with MoDCs or IL-6-conditioned MoDCs in the presence of each antigen peptide. IFN-γ production by survivin- and STEAP-specific CD4+ T cells was significantly decreased after co-culturing with IL-6-conditioned MoDCs compared with control MoDCs (Fig. 1a, b). We confirmed that IFN-γ production by antigen-specific CD4+ T cells was inhibited in the presence of anti-HLA-DP/DQ/DR mAbs (Supplemental Fig. 3). These findings suggest that IL-6 attenuates the DC-mediated activation of cancer-related antigen-specific CD4+ T cells.
Fig. 1.
IL-6 impairs the DC-mediated activation of IFN-γ-producing CD4+ T cells. a Survivin- or STEAP-specific CD4+ T cells (5 × 104) were co-cultured with MoDCs or IL-6-conditioned DCs (5 × 103) in the presence of cancer antigen peptides or control peptides for 24 h. IFN-γ productions were determined by ELISA. Four independent experiments were conducted, and similar results were confirmed for each experiment. The mean and standard deviation (SD) of representative data are presented. b Percentages of IFN-γ production levels are indicated. Data are mean ± SD (n = 4). *P < 0.05 versus Control. c CD4+ T cells (1 × 105) were co-cultured with control MoDCs or IL-6-conditioned MoDCs (1 × 104) in the presence of anti-CD3 mAb (2 µg/mL) for 24 h. A neutralizing anti-IL-12 mAb or control Ab (2 µg/mL) was added to cultures. IFN-γ and IL-12p70 productions were evaluated by ELISA. Five independent experiments were conducted, and similar results were confirmed for each experiment. The mean and SD of representative data are presented. d Percentages of IFN-γ and IL-12p70 production levels are indicated. Data are mean ± SD (n = 5). *P < 0.05 versus Control. n.s. not significant
IL-6 reduces IFN-γ production by CD4+ T cells
Next, CD4+ T cells, isolated from healthy donors, were cultured with MoDCs or IL-6-conditioned MoDCs in the presence of an agonistic anti-CD3 mAb. IFN-γ production by CD4+ T cells co-cultured with IL-6-conditioned MoDCs was significantly decreased compared with cells co-cultured with MoDCs (Fig. 1c, d). We further confirmed that IFN-γ production by anti-CD3 mAb-stimulated CD4+ T cells was reduced when they were co-cultured with IL-6-conditioned BDCA1+ DCs from PBMCs of healthy donors (Supplemental Fig. 4). However, the effects of IL-6 on mature type BDCA1+ DCs were lower than for MoDCs. In addition, we confirmed that the HLA-DR expression of BDCA+ DCs from blood was not down-regulated by IL-6 treatment (data not shown). Therefore, these data suggest that IL-6 suppresses the maturation of MoDCs as well as T cell activation and cytokine production.
Additionally, we found that IL-12p70 production in culture supernatants from IL-6-conditioned DCs was significantly reduced. We confirmed that IFN-γ production by CD4+ T cells co-cultured with MoDCs was decreased to levels similar to those for IL-6-conditioned DCs in the presence of neutralizing anti-IL-12 mAb (Fig. 1c, d). These data suggest that IL-6 attenuates the induction of Th1 cells by decreasing IL-12p70 production by DCs.
IL-6-mediated STAT3 activation reduces the surface expression of HLA class II and CD86
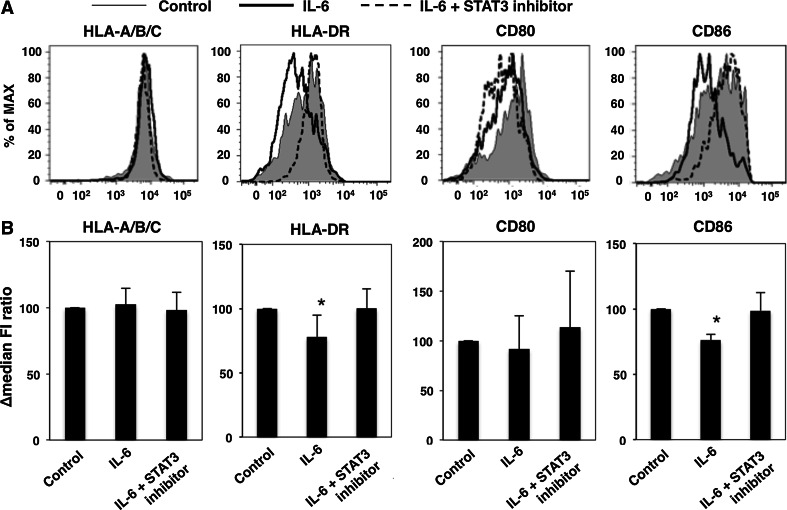
We investigated whether IL-6 contributed to the surface expression levels of HLA class I, HLA class II, and co-stimulatory molecules on human DCs. HLA-DR and CD86 expression on CD11b+CD11c+ cells was significantly reduced following treatment with IL-6. The expression of HLA-A/B/C and CD80 was not altered. The reduction of HLA-DR and CD86 expression levels was blocked by the addition of a STAT3 inhibitor (Fig. 2a, b). These findings suggest that IL-6/STAT3 signaling in human DCs may suppress the surface expression levels of HLA class II and CD86 molecules.
Fig. 2.
IL-6 down-regulates HLA class II and CD86 expression levels in CD11b+CD11c+ cells via STAT3-dependent mechanisms. Adherent cells from PBMCs of healthy donors were stimulated with IL-6 in the presence of a STAT3 inhibitor or DMSO, and surface expression levels of HLA-A/B/C, HLA-DR, CD80, and CD86 on immature CD11b+CD11c+ cells were evaluated by flow cytometry. a Representative profiles. b ∆median FI ratios are presented as mean and SD (n = 5). *P < 0.05 versus Control
COX2, lysosomal protease, and arginase activities are involved in the IL-6-mediated down-regulation of surface HLA-DR expression levels
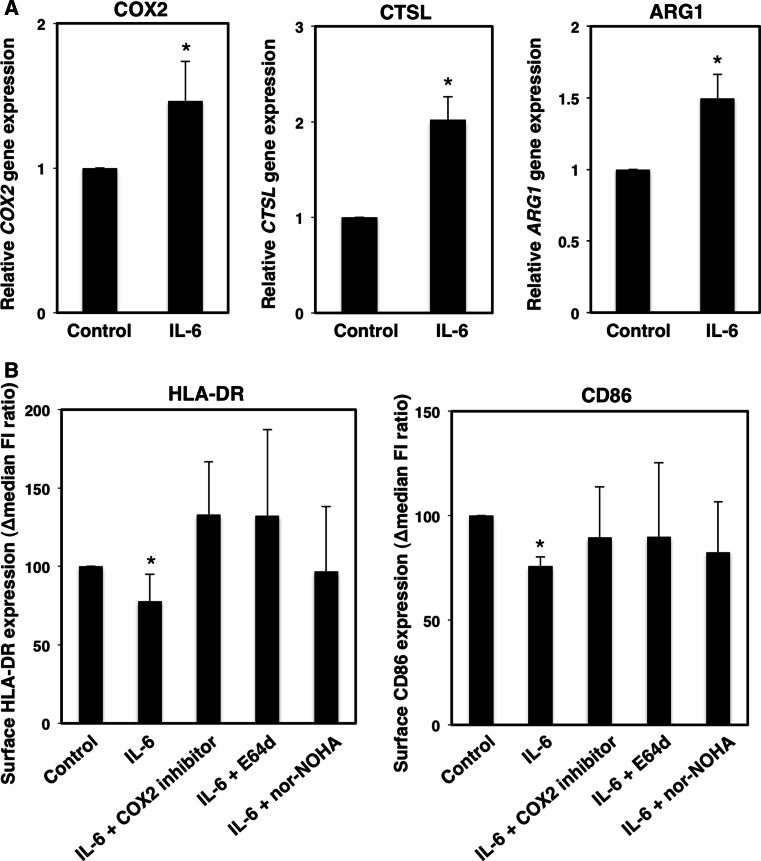
We further examined the ability of IL-6-induced effector molecules to decrease HLA class II and CD86 expression in human DCs. IL-6 stimulation induced the expression of COX2, CTSL, and ARG1 genes in CD11b+CD11c+ cells. (Figure 3a) We confirmed that IL-6 induced a down-regulation of HLA-DR expression that was restored by the addition of COX2, lysosomal protease, or arginase inhibitors. The COX2 inhibitor partially restored the surface expression levels of CD86 (Fig. 3b). These data indicate that the IL-6/STAT3 signaling might negatively regulate HLA-DR expression levels on human DCs through the activation of COX2, lysosomal protease, and arginase.
Fig. 3.
COX2, CTPL, and ARG1 gene expression is related to the down-regulation of surface HLA-DR expression levels. Adherent cells collected from PBMCs of healthy donors were stimulated with IL-6. a Expression of COX2, CTPL, and ARG1 genes was evaluated by quantitative PCR. The mean and SD for expression levels of each gene are presented (n = 3). *P < 0.05 versus Control. b Surface expression levels of HLA-DR and CD86 on CD11b+CD11c+ cells following IL-6 stimulation in the presence of a COX-2 inhibitor (sc-58125; 10 µg/mL), a lysosomal protease inhibitor (E64d; 20 nM), or an arginase inhibitor (nor-NOHA; 3 µg/mL) were evaluated by flow cytometry. The ∆median FI ratio is presented as the mean and SD (n = 5). *P < 0.05 versus IL-6
Down-regulation of surface expression levels of HLA-DR and CD86 on CD11b+CD11c+ cells in tumor tissues from colorectal cancer patients
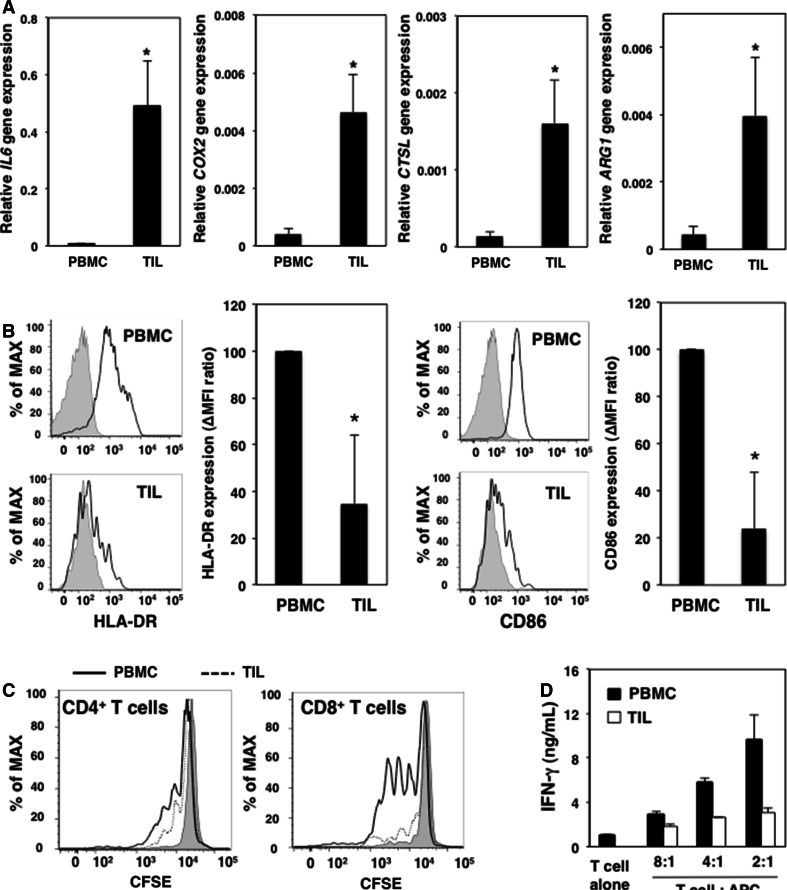
We next investigated whether tumor microenvironments suppressed the function of CD11b+CD11c+ cells in cancer patients. We examined the expression levels of various genes in CD11b+CD11c+ cells isolated from the tumor tissues and PBMCs of colorectal cancer patients. Expression of the IL6, COX2, CTSL, and ARG1 genes in CD11b+CD11c+ cells from TILs was greater than that in PBMCs from colorectal cancer patients (Fig. 4a). We confirmed that tumor-infiltrating CD11b+CD11c+ cells were an IL-6-producing population in the tumor microenvironment. HLA-DR and CD86 expression levels on CD11b+CD11c+ cells from TILs were lower compared with those from PBMCs of colorectal cancer patients (Fig. 4b).
Fig. 4.
Maturation and T cell-stimulating ability of CD11b+CD11c+ cells are reduced in the tumor microenvironments of colorectal cancer patients. 7AAD−CD45+CD11b+CD11c+ cells derived from PBMCs and TILs were collected. a Expression levels of IL6, COX2, CTSL, and ARG1 genes in CD11b+CD11c+ cells from PBMCs and TILs were evaluated by quantitative PCR. Representative data are shown as the mean and SE of relative gene expression levels. b HLA-DR and CD86 expression levels on the surface of CD11b+CD11c+ cells from PBMCs and TILs were evaluated by flow cytometry. Representative profiles are shown with ∆MFI ratios presented as mean and SD (n = 8). *P < 0.05 versus CD11b+CD11c+ cells from PBMCs. c CFSE-labeled CD4+ and CD8+ T cells (1 × 105) were stimulated with an anti-CD3 mAb in the presence of CD11b+CD11c+ cells (5 × 104) from PBMCs or TILs for 72 h. Proliferation of T cells in the presence of CD11b+CD11c+ cells from PBMCs (solid line) or TILs (dotted line) was evaluated by flow cytometry. Three independent experiments were conducted on samples from colorectal cancer patients. Representative profiles are shown. d IFN-γ production by CD4+ and CD8+ T cells (1 × 105) co-cultured with CD11b+CD11c+ cells (0, 1.25 × 104, 2.5 × 104, or 5 × 104) from PBMCs or TILs was measured by ELISA. Three independent experiments were conducted on samples from colorectal cancer patients, and similar results were confirmed for each experiment. Representative data are shown
Tumor-infiltrating CD11b+CD11c+ cells have impaired T cell-stimulating functions
We further examined the T cell-stimulating ability of tumor-infiltrating DCs. The proliferation of CD4+ and CD8+ T cells was reduced in the presence of CD11b+CD11c+ cells from TILs compared with those from PBMCs (Fig. 4c). IFN-γ production by T cells was attenuated in the presence of CD11b+CD11c+ cells from TILs, and increased production was dependent on the number of cells added (Fig. 4d). These data suggested that the tumor microenvironment impaired the T cell-stimulating ability of tumor-infiltrating antigen-presenting cells such as DCs in colorectal cancer patients.
HLA-DR expression levels are related to the infiltration of CD4+ and CD8+ T cells at tumor sites
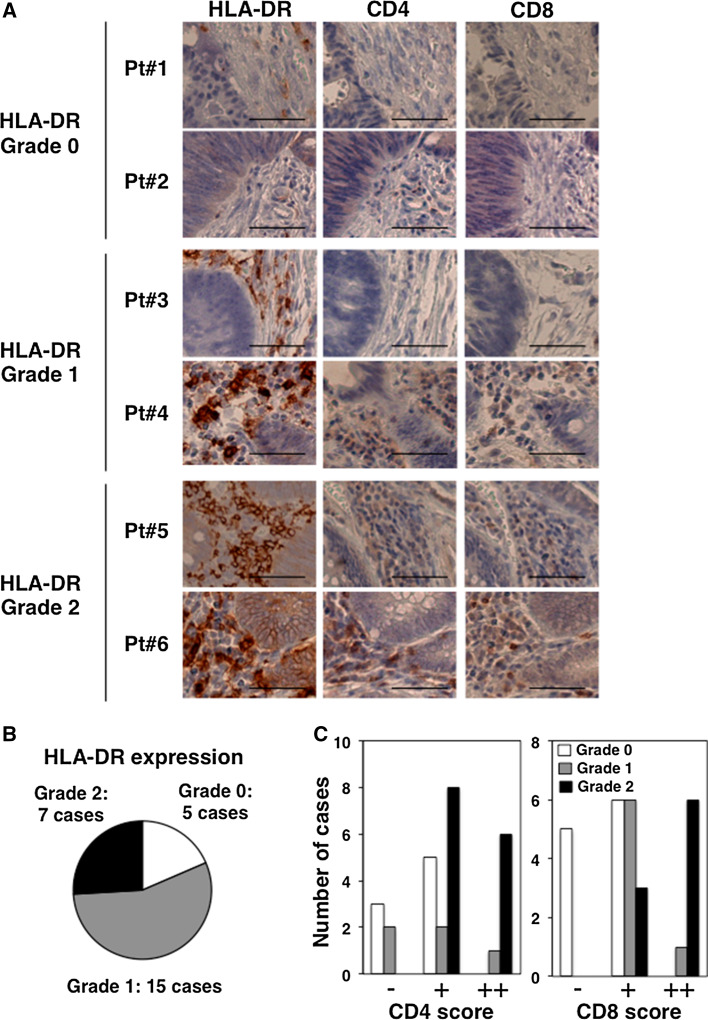
Finally, HLA-DR expression levels and the frequency of infiltrating CD4+ and CD8+ T cells were investigated in the tumor microenvironment of colorectal cancer patients. In this study, we observed infiltrating CD4+ and CD8+ T cells in colorectal tumor tissues with high HLA-DR expression levels (Fig. 5a). We evaluated the HLA-DR expression levels and frequencies of CD4+ and CD8+ T cells in tumor sites (Fig. 5b, c). HLA-DR expression levels in tumor tissues were closely related to the size of the CD4+ T and CD8+ T cell compartments in colorectal cancer patients.
Fig. 5.
HLA-DR expression levels correlated with the frequency of CD4+ and CD8+ T cells at tumor sites in colorectal cancer patients. Tumors were collected from colorectal surgery specimens (n = 27). a Immunohistochemistry was used to detect the expressions of HLA-DR, CD4, and CD8. Subjects were classified as grade 0–2 based on HLA-DR expression levels. Representative images (×200 magnification) of samples from six patients (Pt#1-6) with grade 0–2 HLA-DR expressions are shown. Scale bars indicate 200 µm. b The numbers of cases with HLA-DR grade 0–2 are shown. c Levels of CD4 and CD8 expression in patient tissues with HLA-DR grade 0–2 expression were classified as absent, faint (−), moderate (+), and high (++). The numbers of cases for CD4 and CD8 scores are shown
Discussion
Patients with advanced cancer are considered immunocompromised. Therefore, improving the immune status of cancer patients is required for effective cancer immunotherapies. Prospective and retrospective studies have shown that serum IL-6 levels are related to tumor stage and size, metastasis, survival of colon cancer patients [19], chemotherapy efficacy for advanced pancreatic cancer [20], advanced-stage and metastasis-related morbidity of breast cancer [21], and the efficacy of personalized peptide vaccination for advanced biliary tract cancer [22]. Results from these studies suggest that IL-6 levels in cancer are not simply useful as promising prognostic biomarkers, but are also related to tumorigenesis and anti-tumor immune responses. The precise mechanism by which IL-6 modulates anti-tumor responses in systemic and tumor microenvironments remains to be elucidated.
In the current study, IL-6-conditioned MoDCs attenuated IFN-γ production by cancer-related antigen-specific effector CD4+ T cells. We confirmed that IFN-γ production by CD4+ T cells was significantly reduced when they were co-cultured with IL-6-conditioned DCs (Fig. 1a, b). This finding suggests that IL-6 suppresses the DC-mediated induction of Th1 cells. We also observed that IL-12p70 production by DCs was also reduced in parallel with decreased IFN-γ production by CD4+ T cells (Fig. 1c, d).
It was previously reported that STAT3 regulated NF-κB recruitment to the IL-12p40 promoter in murine DCs [23] and that STAT3 inhibited IL-12p35 gene expression in mice [24]. We found that pre-treatment of IL-6-conditioned MoDCs with a STAT3 inhibitor increased IL-12p70 production along with IFN-γ production by CD4+ T cells after T cell receptor stimulation (data not shown). These findings suggest that IL-6-dependent STAT3 activation in MoDCs might be involved in reducing IL-12p70 production in humans.
In general, it is well known that IL-10 and TGF-β cytokines suppress DC maturation. In this study, we confirmed that the production of IL-10 but not the active form of TGF-β was enhanced in the culture supernatants of IL-6-conditioned MoDCs (Supplemental Fig. 5). Therefore, IL-10 production by IL-6-conditioned MoDCs might be involved in the reduction of IL-12p70 and the subsequent production of IFN-γ by CD4+ T cells.
IL-12 activates STAT4 in CD4+ T cells, subsequently inducing IFN-γ secretion [25]. IL-12 is an important cytokine for Th1 immunity [26, 27], as it is essential for inducing fully activated CTLs in tumor-bearing hosts [28, 29]. IL-12p35−/− mice are more susceptible to tumor development following exposure to carcinogens compared with wild-type mice [30]. We reported that cancer antigen-derived peptides containing helper epitopes efficiently induced CTLs in vitro according to the helper function of antigen-specific CD4+ T cells [17]. In a clinical trial, a cancer peptide vaccine containing helper epitopes induced CTLs in an advanced cancer patient [31]. Blocking the IL-6/STAT3 signaling cascade might promote IL-12p70 production by DCs to induce cancer-specific Th1 cells in immunosuppressed cancer patients.
We observed that IL-6 stimulation reduced HLA-DR and CD86 expression levels on immature DCs derived from the PBMCs of healthy donors in a STAT3-dependent manner (Fig. 2). Arg1, lysosomal proteases, and COX2 were responsible for the down-regulation of HLA-DR on human DCs (Fig. 3). We previously demonstrated that arginine was required for the gene expression of MHC class II and arginase activation induced by IL-6 caused dysfunction of DCs in a tumor-bearing mouse model [16]. The IL-6-induced activation of lysosomal proteases reduced MHC class II αβ-dimer levels in murine DCs [13]. Previous reports indicated that activation of the COX2/PGE2 cascade inhibited the maturation of DCs [32, 33]. We speculated that these functional molecules might be involved in the mechanisms of IL-6-induced down-regulation of HLA class II in human DCs.
We observed that tumor-infiltrating CD11b+CD11c+ cells from colorectal cancer specimens had reduced surface expression levels of HLA-DR and CD86 and attenuated CD4+ and CD8+ T cell-stimulating abilities compared with CD11b+CD11c+ cells from the PBMCs of autologous patients (Fig. 4). We speculated that intratumoral DCs had a reduced ability to activate effector Th1 cells and CTLs in the tumor microenvironment.
In this study, we observed that IFN-γ production by T cells in the presence of CD11b+CD11c+ cells derived from TILs was higher at a 2:1 ratio (T cells-APC) than at an 8:1 ratio, although the number of CD11b+CD11c+ cells was greater at the 2:1 ratio (Fig. 4d). In these experiments, IFN-γ was produced by T cells co-cultured with CD11b+CD11c+ cells from TILs. Previous studies demonstrated that DCs induced from bone marrow in the presence of IFN-γ effectively activated T cells by enhancing their maturation. Therefore, we speculated that IFN-γ-conditioned CD11b+CD11c+ cells from TILs altered the maturation status during culture, resulting in the gain of T cell-stimulating ability in vitro.
IL-6 can be produced by various cells, including cancer cells, cancer-associated fibroblasts, and immune cells to cause chronic inflammation at tumor sites in cancer patients. We confirmed that the gene expression level of IL-6 was elevated in tumor-infiltrating CD11b+CD11c+ cells (Fig. 4a). This was an indicator that CD11b+CD11c+ cells might be a major IL-6-producing population in the tumor microenvironment of colorectal cancer patients. Gene expression levels of ARG1, CTSL, and COX2 were increased in tumor-infiltrating CD11b+CD11c+ cells. These results suggest that the inhibition of IL-6-induced activation of arginase, lysosomal proteases, and COX2 in tumor-infiltrating DCs might restore the down-regulation of surface HLA class II expression and effectively activate cancer-specific Th1 cells in the tumor microenvironment.
HLA class II expression levels were closely related to the invasion of tumor sites by CD4+ and CD8+ T cells. The presence of intratumoral T cells is considered a good prognostic factor in colorectal cancer [34–38]. Recent studies indicated that an elevated number of intratumoral T cells, elevated HLA class I expression levels, and STAT1 activation are beneficial prognostic biomarkers for colorectal cancer [39]. Additionally, PD-L1 expression by cancer cells was related to invasion by immune cells and the efficiency of anti-PD-L1 therapy [40]. These studies show that the presence of tumor-infiltrating T cells and adequate activation of effector T cells are required for cancer immunotherapy. We postulated that maintaining the antigen-presenting ability of DCs would be critical for the induction and activation of cancer antigen-specific T cells in tumor microenvironments. Therefore, a therapeutic strategy for improving the function of DCs will hopefully be effective for increasing the number of good responders to a cancer immunotherapy.
Recent clinical trials using a chimeric anti-IL-6 mAb were performed on patients with advanced solid tumors [41], B cell non-Hodgkin’s lymphoma, multiple myeloma, Castleman disease [42], metastatic castration-resistant prostate cancer [43], and ovarian cancer [44]. Results from these trials showed that these treatments were tolerated by patients. In addition, serum C-reactive protein levels were decreased, and anemia caused by chronic inflammation was improved following the administration of anti-IL-6 mAb. These results suggest that anti-IL-6 mAb might control the status of chronic inflammation in patients with advanced cancer. It is also suggested that anti-IL-6 mAb assists with recovering the function of tumor-infiltrating DCs through the inhibition of IL-6/STAT3 signaling in the tumor microenvironment. We expect that the administration of anti-IL-6 mAb combined with cancer immunotherapy, such as a peptide vaccine, might demonstrate greater clinical efficacy.
In summary, we found that IL-6 induced STAT3 activation and reduced the surface expression levels of HLA class II and CD86 molecules. Concurrently, IL-12p70 production by human DCs was reduced. This resulted in the subsequent suppression of T cell-mediated anti-tumor immunity. Therefore, IL-6 may be related to immunosuppression in cancer patients by causing DC dysfunction, suggesting that inhibition of the IL-6/STAT3 signaling pathway might be a promising strategy for improving cancer immunotherapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Ms. Ai Nishiuchi for her technical and secretarial assistance. This work was partially supported by a Grant-in-Aid for Scientific Research (25460584 to Hidemitsu Kitamura), a Research Fellowship for Young Scientists (251464 to Kentaro Sumida), a Grant-in-Aid for Translational Research Network Program from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), a Health and Labour Sciences Research Grant (11103410 to Hidemitsu Kitamura) from the Ministry of Health, Labour, and Welfare (MHLW), Japan, and by the Joint Research Program of the Institute for Genetic Medicine, Hokkaido University.
Abbreviations
- 7AAD
7-Amino-actinomycin D
- ARG1
Arginase-1
- CFSE
Carboxyfluorescein succinimidyl ester
- COX2
Cyclooxygenase-2
- CTL
Cytotoxic T lymphocyte
- CTSL
Cathepsin L
- DC
Dendritic cell
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme-linked immunosorbent assay
- ERK
Extracellular signal-related kinase
- FACS
Fluorescence-activated cell sorting
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- gp130
Glycoprotein 130
- HLA
Human leukocyte antigen
- IHC
Immunohistochemistry
- IL-6R
Interleukin-6 receptor
- IL
Interleukin
- JAK
Janus kinase
- mAb
Monoclonal antibody
- MAPK
Mitogen-activated protein kinase
- MDSC
Myeloid-derived suppressor cell
- Median FI
Median fluorescence intensity
- MFI
Mean fluorescence intensity
- MHC
Major histocompatibility complex
- MoDC
Monocyte-derived dendritic cell
- nor-NOHA
Nw-hydroxy-l-arginine
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death ligand 1
- PI3 K
Phosphoinositide 3-kinase
- SD
Standard deviation
- STAT3
Signal transducer and activator of transcription 3
- STEAP
6-transmembrane epithelial antigen of prostate
- TGF
Transforming growth factor
- Th
Helper T
- TIL
Tumor-infiltrating lymphocyte
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34:33–40. doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 11.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 12.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura H, Kamon H, Sawa SI, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa SI, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 15.Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K, Satoh T, Kitamura H, Nishimura T. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol. 2012;42:2060–2072. doi: 10.1002/eji.201142335. [DOI] [PubMed] [Google Scholar]
- 16.Narita Y, Kitamura H, Wakita D, Sumida K, Masuko K, Terada S, Nakano K, Nishimura T. The key role of IL-6-arginase cascade for inducing dendritic cell-dependent CD4(+) T cell dysfunction in tumor-bearing mice. J Immunol. 2013;190:812–820. doi: 10.4049/jimmunol.1103797. [DOI] [PubMed] [Google Scholar]
- 17.Ohtake J, Ohkuri T, Togashi Y, Kitamura H, Okuno K, Nishimura T. Identification of novel helper epitope peptides of Survivin cancer-associated antigen applicable to developing helper/killer-hybrid epitope long peptide cancer vaccine. Immunol Lett. 2014;161:20–30. doi: 10.1016/j.imlet.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi S, Kumai T, Matsuda Y, Aoki N, Sato S, Kimura K, Kitada M, Tateno M, Celis E, Kobayashi H. Six-transmembrane epithelial antigen of the prostate and enhancer of zeste homolog 2 as immunotherapeutic targets for lung cancer. J Transl Med. 2011;9:191. doi: 10.1186/1479-5876-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J Colorectal Dis. 2010;25:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 20.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, Higashi S, Kato H, Terao K, Ochiai A. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 2013;108:2063–2069. doi: 10.1038/bjc.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta N, Goswami B, Mittal P. Effect of standard anthracycline based neoadjuvant chemotherapy on circulating levels of serum IL-6 in patients of locally advanced carcinoma breast—a prospective study. Int J Surg. 2012;10:638–640. doi: 10.1016/j.ijsu.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Yoshitomi M, Yutani S, Matsueda S, Ioji T, Komatsu N, Shichijo S, Yamada A, Itoh K, Sasada T, Kinoshita H. Personalized peptide vaccination for advanced biliary tract cancer: IL-6, nutritional status and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med. 2012;3:463–469. doi: 10.3892/etm.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoentjen F. STAT3 regulates NF-B recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–696. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- 24.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morinobu A, Gadina M, Strober W, Visconti R, Fornace A, Montagna C, Feldman GM, Nishikomori R, O’Shea JJ. STAT4 serine phosphorylation is critical for IL-12-induced IFN-gamma production but not for cell proliferation. Proc Natl Acad Sci U S A. 2002;99:12281–12286. doi: 10.1073/pnas.182618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsich CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophage. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 27.Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 28.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, Ohkuri T, Homma S, Ohtake J, Wakita D, Togashi Y, Kitamura H, Todo S, Nishimura T. First clinical trial of cancer vaccine therapy with artificially synthesized helper/ killer-hybrid epitope long peptide of MAGE-A4 cancer antigen. Cancer Sci. 2012;103:150–153. doi: 10.1111/j.1349-7006.2011.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harizi H, Juzan M, Grosset C, Rashedi M, Gualde N. Dendritic cells issued in vitro from bone marrow produce PGE(2) that contributes to the immunomodulation induced by antigen-presenting cells. Cell Immunol. 2001;209:19–28. doi: 10.1006/cimm.2001.1785. [DOI] [PubMed] [Google Scholar]
- 33.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–5505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, Mantovani A, Roncalli M, Malesci A. CD3+ cells at the invasive margin of deeply invading (pT3–T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 35.Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012;147:366–372. doi: 10.1001/archsurg.2012.35. [DOI] [PubMed] [Google Scholar]
- 36.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, Danciu M, Bruneval P, Scripcariu V, Chevallier JM, Zinzindohoué F, Berger A, Galon J, Pagès F. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20(7):1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 38.Maby P, Tougeron D, Hamieh M, Mlecnik B, Kora H, Bindea G, Angell HK, Fredriksen T, Elie N, Fauquembergue E, Drouet A, Leprince J, Benichou J, Mauillon J, Le Pessot F, Sesboué R, Frebourg T, Galon J, Latouche JB. Correlation between density of CD8+ T cell infiltrates in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res. 2015;75(17):3446–3455. doi: 10.1158/0008-5472.CAN-14-3051. [DOI] [PubMed] [Google Scholar]
- 39.Simpson JAD, Al-Attar A, Watson NFS, Scholefield JH, Ilyas M, Durrant LG. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut. 2010;59:926–933. doi: 10.1136/gut.2009.194472. [DOI] [PubMed] [Google Scholar]
- 40.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C, Berger AE, Pardoll DM, Topalian SL. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21(17):3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S, Joly F, Ray-Coquard I, Dirix L, Machiels JP, Steven N, Reddy M, Hall B, Puchalski TA, Bandekar R, van de Velde H, Tromp B, Vermeulen J, Kurzrock R. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2192–2204. doi: 10.1158/1078-0432.CCR-13-2200. [DOI] [PubMed] [Google Scholar]
- 42.Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, Lentzsch S, Frank RC, Zweegman S, Wijermans PW, Orlowski RZ, Kranenburg B, Hall B, Casneuf T, Qin X, van de Velde H, Xie H, Thomas SK. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;161:357–366. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fizazi K, De Bono JS, Flechon A, Heidenreich A, Voog E, Davis NB, Qi M, Bandekar R, Vermeulen JT, Cornfeld M, Hudes GR. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, Singh N, Avril N, Cummings J, Rexhepaj E, Jirström K, Gallagher WM, Brennan DJ, McNeish IA, Balkwill FR. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.