Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive human neoplasms, having extremely poor prognosis with a 5-year survival rate of <1 % and a median survival of 6 months. In contrast to other malignancies, pancreatic cancer is highly resistant to chemotherapy and targeted therapy. Therefore, new treatment options are urgently needed to improve the survival of patients with PDAC. Based on our data showing that patients with higher CD8+ T cell tumour infiltration exhibited prolonged overall and disease-free survival compared to patients with lower or without CD8+ T cell tumour infiltration, we suggested that immunotherapy could be a promising treatment option for PDAC. However, clinical data from the chemoradioimmunotherapy with interferon-α (IFN) trial did not point to an improved efficiency of chemoradiation combined with IFN as compared to chemoradiotherapy alone, suggesting an important role of the immune suppression induced by PDAC and/or unspecific immune stimulation. In support of this hypothesis, we found that the PDAC patients and experimental mice had an increased number of regulatory T cells and myeloid-derived suppressor cells. These results allowed us to conclude that PDAC provokes not only an anti-tumour immune response, but also strong immune suppression. Thus, we supposed that new immunotherapeutical strategies should involve not only stimulation of the immune system of PDAC patients, but also exert control over the tumour immune suppressive milieu.
Keywords: Anticancer immunity, Immune suppression, Immunotherapy, Pancreatic adenocarcinoma, CITIM 2013
Pancreatic adenocarcinoma (PDAC)
Patients with carcinoma of the exocrine pancreas have an especially poor prognosis with a 5-year survival rate of <1 % and a median survival of 4–6 months. At the time of PDAC diagnosis, about 40 % of patients have a locally advanced unresectable disease and approximately 40 % an advanced metastatic disease [1]. Even after surgical intervention with a curative intention, recurrence is common and a majority of patients develop distant metastasis. After resection, the 5-year survival rate is in specialized centres at best 15 % without, or 25 % with adjuvant chemotherapy. Patients with a metastatic disease are usually treated with chemotherapy that is minimally effective [2].
The reasons for this poor prognosis are PDACs early dissemination, lack of early specific symptoms and its late diagnosis [3]. A growing PDAC can take more than 15 years to metastasize, during which patients are generally asymptomatic. When the disease finally becomes symptomatic, it is usually too late for surgery. In contrast to other malignancies, PDAC is highly resistant to chemotherapy and targeted therapy. The molecular mechanisms that determine the treatment resistance are poorly understood. In recent years, it has been demonstrated that a number of different proteins are involved in the therapy resistance [4]. Invasion and metastasis formation are the most important steps in pancreatic malignancy and can be triggered by stroma cells [5]. Therefore, the understanding of the processes of chemoresistance and metastasis is crucial for the development of new strategies for the treatment and prevention of PDAC progression.
The current management of PDAC is prescribed by the tumour stage, comorbidities and performance status of patients [2]. Neoadjuvant therapy has been found to be of potential benefit for patients with locally advanced, potentially unresectable PDAC. After neoadjuvant chemoradiation or chemotherapy alone, R0/R1 resection can be achieved in up to 40 % of patients with locally advanced, unresectable PDAC [6].
Adjuvant chemotherapy based on gemcitabine or 5-fluorouracil showed a significant increase in the overall and disease-free survival of PDAC patients [7, 8]. Currently, the use of single-agent gemcitabine [9] or modulated 5-fluorouracil therapy [10] is the standard treatment for advanced pancreatic cancer. However, the clinical impact of these chemotherapeutics remains modest, owing to a high degree of inherent and acquired chemoresistance. Recently, published data from the FOLFIRINOX chemotherapeutic trial (oxaliplatin, irinotecan, leucovorin and 5-fluorouracil) showed a significant survival advantage compared to gemcitabine (11.1 vs. 6.8 months). However, this trial was associated with increased toxicity, making FOLFIRINOX suitable only for patients with a good performance status [11]. Several biological agents targeting tumour-related signalling cascades have been tested. Erlotinib, a small molecule inhibitor of the EGF receptor tyrosine kinase, is the only biological agent to date that has recently been shown to offer, combined with gemcitabine, a marginal survival benefit for patients with advanced PDAC [12]. Several other biological agents have proven ineffective.
New treatment options are urgently needed to improve the survival rate of patients with PDAC. This need motivates oncologists to search for novel approaches such as immunotherapy, including specific (T cells, monoclonal antibodies, etc.) and unspecific (cytokines and NK cells) approaches to treating PDAC patients. However, before applying immunotherapy, it is useful to understand the role of the immune system in anti-tumour defence, in general and in the case of PDAC.
PDAC activates the anti-tumour immune response
It is now generally accepted that a number of solid tumours are capable of activating the anti-tumour immune response. Malignant melanoma represents the best example of such malignancies (for review, see [13]). The main immunogenic features of malignant melanoma are (a) naturally occurring spontaneous tumour regression by some melanoma patients; (b) the tumour infiltration with CD8+ T cells; (c) the expression of tumour antigens [in particular, cancer–testis antigens (CTA)]; (d) the circulation of tumour-antigen-specific T cells and/or antibodies in the patients’ peripheral blood; (e) immunological and sometimes clinical response to unspecific and specific immunotherapy. These features allow for the conclusion that malignant melanoma is a highly immunogenic tumour.
Could these “immune features” also be recognized for PDAC? In the case of this malignancy, it is extremely complicated to register a spontaneous tumour regression due to tumour localization. However, some anecdotal cases of such regression have been reported [14]. Unfortunately, it remains unclear whether the anti-cancer immune response took part in these cases or did not.
Concerning CD8+ T cells, their accumulation correlates well with the survival of patients with different types of cancer [15]. These data firstly support the hypothesis that the immune system protects the organism against growing tumour. Secondly, tumour infiltration with CD8+ T cells can serve as an independent surrogate marker for cancer patient survival. With regard to PDAC, an earlier work of Ryschich et al. [16] and a study by Fukunaga et al. [17] showed that CD8+ T cell accumulation in human PDAC tumours is associated with the longer survival of patients. With another cohort of R0-/R1-resected PDAC patients, we performed a similar analysis that also demonstrated a survival benefit for patients with high CD8+ T cell accumulation in tumours (Bazhin et al., unpublished data). Additionally, in an orthotopic murine model of PDAC, we saw a high accumulation of CD8+ T cells in mouse tumours (Karakhanova et al., unpublished data). Molecular control of CD8+ T cell recruitment was reported to involve the leucocyte counter-receptor LFA-1 and possibly its ligand ICAM-1 [18]. Thus, the CD8+ T cell accumulation in PDAC can be recognized not as an epiphenomenon, but can instead be associated with patients’ survival as a phenomenon, highlighting the importance of the anti-tumour immune response in PDAC.
Expression of cancer antigens is a hallmark of melanoma cells. In particular, it concerns CTA, which were discovered in this malignancy (for review, see [19]). Meanwhile, such antigen expression has been found not only in melanoma but also in other malignancies, including PDAC, MAGE-A1, MAGE-A3, MAGE-A4, MAGE-A10, LAGE-1, NY-ESO-1, SCP-1, SSX-2, SSX-4 and HERV-K-MEL, which were found in many studies to be expressed in tumours of PDAC patients [20, 21]. In addition, tumour-associated and mutated antigens, which are not exclusively expressed in cancer cells, have been found in PDAC, including WT1, MUC1, hTERT, survivin, CEA, HER-2/neu, k-RAS, p53, PNLIPRP2 and MIA [22].
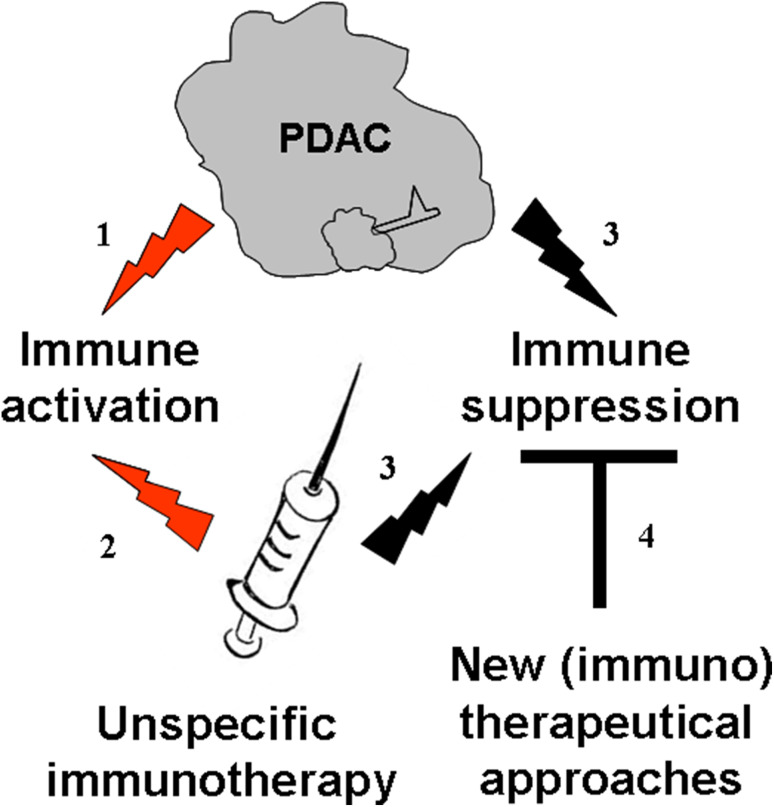
Being expressed in tumour cells, CTA induce cellular and/or humoral immune responses, which in turn leads to the generation of CTA-specific T cells or antibodies in the peripheral blood of patients with melanoma and some other cancers [23]. Recently, seromic profiling identified 29 preferentially immunogenic proteins in serum of PDAC patients [24]. Could the presence of an antibody against CTA and other tumour antigens be beneficial for PDAC patients? The answer is definitively ‘yes’. For example, Heller et al. [22] showed that PDAC patients with the MIA antibody had a better survival rate then the antibody-negative patients. To a larger extent, authors of the seromic work discriminated a set of antibodies for good and bad prognosis [24]. Thus, these data present evidence that the PDAC indeed induces an anti-cancer immune response (Fig. 1, path 1); this immune activation is associated with a survival benefit for PDAC patients.
Fig. 1.
Two immune faces of pancreatic adenocarcinoma: possible implication for immunotherapy. PDAC (path 1) as well as unspecific immunotherapy (path 2) activates immune system of the PDAC patients. Concomitant immune suppression arm is also activated (path 3). We supposed that new immunotherapeutical strategies should involve not only a stimulation of the immune system of PDAC patients, but also control over the tumour immune suppressive milieu (path 4). The black arrows conduct to the immune suppression, and the red ones to the immune activation
Based on our data showing that patients with higher CD8+ T cell tumour infiltration exhibited prolonged overall and disease-free survival as compared to patients with lower or without CD8+ T cell tumour infiltration, we suggested that PDAC patients could be promising candidates for immunotherapy.
PDAC patients treated with IFN show systemic activation of the immune system, which correlates with better survival
With regard to the above-mentioned hypothesis and based on research conducted at the Virginia Mason Clinic that published data of a phase II trial, where chemoradiotherapy was combined with interferon-α2b (IFN) showing an overall 5-year survival of 55 % [25], a randomized, open, controlled, prospective, multi-centre phase III trial (CapRi-trial) was carried out at our department to confirm the results of the Virginia Mason scheme [26]. A total of 110 Men and women with biopsy-proven completely resected (R0 or R1) PDAC of the pancreatic head were randomized in the trial. Patients in study arm A (radiochemoimmunotherapy) were treated with 5-fluorouracil, cisplatin and IFN. External beam radiation was given concurrently with chemotherapy. Patients in study arm B (chemotherapy) were treated with bolus injection of folinic acid followed by bolus injection of 5-fluorouracil.
Treatment with the low-dose of IFN alone led to an increase in neutrophils, monocytes (as well as their activated forms), dendritic cells (DC) and to an enhanced NK cell-mediated cytotoxicity, while subsequent chemoradioimmunotherapy increased the amount of neutrophils, monocytes (including activated ones), CD4+ T cells, CD8+ T cells and DC. Additionally, this treatment induced an increase in the number of effector memory CD8+ T cells and of the CD8+/CD4+ ratio (Bazhin et al., unpublished data). In addition, in the Panc02 orthotopic mouse model of PDAC, an increase in the CD8+ T cells frequencies in tumours following the IFN treatment was also recorded (Karakhanova et al., unpublished data).
A correlation analysis of immunological parameters with patient survival rates revealed that high lymphocyte frequencies present even before the therapy positively correlated with a better disease-free condition and overall survival. In addition, an increase in effector/effector memory CD8+ T cells after IFN injection positively correlated with patient outcomes during the therapy (Bazhin et al., unpublished data).
These data secure conclusive evidence that IFN as an unspecific therapeutic indeed activates the immune systems of PDAC patients (Fig. 1, path 2), which correlates favourably with patient outcomes.
PDAC triggers immunosuppression
However, the clinical data from the CapRi-trial did not point to the improved efficiency of the chemoradiation combined with IFN as compared to chemoradiotherapy alone [27], suggesting an important role of the immune suppression induced by PDAC and/or by unspecific immune stimulation. In general, immune suppression is manifested in two different ways. Firstly, immune suppressive cells such as regulatory (FoxP3+CD25+) T cells (Treg) and myeloid-derived suppressor cells (MDSC) are involved in cancer-induced immune suppression. Secondly, surface regulatory molecules like CTLA-4 and B7-H1 are recognized as crucial players in the immune suppressive milieu.
Treg inhibit a wide variety of physiological and pathological immune responses against self, foreign and tumour antigens [28]. In cancer patients and preclinical mouse models, Treg accumulate in the tumour tissue, where they dampen anti-tumour immune responses [29]. MDSC represent another group of immune cells with suppressive functions. In tumour-bearing hosts, MDSC comprise a heterogeneous population of immature myeloid cells that are precursors of DC, macrophages and granulocytes [30]. CTLA-4 molecules were shown to regulate T cell activation. The importance of CTLA-4 was demonstrated by the lethal systemic immune hyperactivation phenotype of Ctla4-knockout mice (for review, see [31]). The regulatory cell surface protein B7-H1 modulated the immune response not only in a healthy state, but also in cancer states [32]. B7-H, together with another regulatory molecule B7-DC, acts as a ligand for the PD-1 receptor [33], responding for the regulation of T cell activation.
In support of our hypothesis that PDAC triggers immunosuppression (Fig. 1, path 3), we found that PDAC patients have an increased number of Treg and MDSC in their peripheral blood (Karakhanova et al., unpublished data). In the orthotopic Panc02 model of PDAC, we demonstrated that the tumours of these mice were highly infiltrated with Treg. Remarkably, these cells exhibited the effector/memory phenotype, suggesting their enhanced suppressive activity and higher proliferation capacity [34]. Also, a high amount of MDSC was detected in the tumours of this mouse model (Karakhanova et al., unpublished data).
In human PDAC, the B7-H1 expression was upregulated and correlated strongly with poor patient prognosis [35]. We showed in vivo and in vitro the expression of B7-H1 on PDAC tumour cells and tumour-infiltrated leucocytes. It should be noted that this expression can be upregulated both in vivo and in vitro on all cell types investigated (Karakhanova et al., unpublished data). With respect to CTLA-4, a decrease in the number of CD4+ T cells expressing this immunosuppressive molecule is associated with better and disease-free survival of patients from the CapRi study (Bazhin et al., unpublished data).
These results allowed us to conclude that PDAC provokes not only an anti-tumour immune response, but also strong immune suppression through the accumulation of immunosuppressive cells and the upregulation of immunosuppressive molecules.
Future concepts of PDAC immunotherapy
Recent development in cancer immunotherapy comprises various strategies to specifically activate anti-tumour immune responses, such as an expansion and adoptive transfer of tumour-reactive T cells, vaccination with tumour-specific antigens and concomitant targeting of inhibitory pathways and immune suppressive cells [36]. Assuming that PDAC affects both arms of the immune response—immune activation and immune suppression—and taking into account that unspecific immunotherapeutical approaches also lead as to the stimulation of immune suppression, we hypothesized that future concepts of PDAC immunotherapy should be designed based on the elimination of immune suppression (Fig. 1, path 4) [37]. The next phase is to try and establish (a) how we can reduce the immunosuppressive effects of PDAC and (b) whether this reduction could improve survival.
Depletion of Treg with antibodies against CD25 or folate receptor 4 significantly augmented anti-tumour immune responses in several preclinical mouse models, including PDAC [38–41]. However, as these markers are also expressed on activated CD4+FoxP3- and CD8+ T cells, such treatments may concomitantly diminish the effector arm of anti-tumour immunity [42, 43]. Furthermore, a MDSC depletion strategy using antibodies remains elusive, because of the high heterogeneity of this cell population. Recently, (low-dose) chemotherapy emerged as a promising approach for selective Treg and MDSC depletion. For instance, administration of low-dose cyclophosphamide selectively decreased the presence of Treg in cancer patients and tumour-bearing animals [44], while gemcitabine in a therapeutic dose-depleted MDSC [45].
In the PDAC mouse model, we showed that the administration of low-dose gemcitabine leads to a preferential depletion of proliferating Treg; this depletion manifested in the prolonged survival of tumour-bearing mice without any cytotoxic effect on the tumour [34]. Recently, in the ret transgenic mouse model of spontaneous malignant melanoma, we demonstrated that the phosphodiesterase-5 inhibitor sildenafil inhibited MDSC suppressive activity, restored T cell activation and improved the survival of melanoma-bearing mice [46]. Having been motivated by these results, we also applied sildenafil in the orthotopic murine model of PDAC. In this model, sildenafil was able to deplete MDSC in mouse tumours. As a result, we documented the improved survival of PDAC-bearing mice treated with sildenafil (Karakhanova et al., unpublished data).
Inhibition of cell surface molecules with specific monoclonal antibodies is the way to eliminate the immunosuppressive effects of such molecules as CTLA-4 and B7-H1. The history of ipilimumab has demonstrated the clinical benefit of such antibodies [47]. Besides, two clinical trials have been performed testing ipilimumab (human anti-CTLA-4 antibody) in patients with metastatic and local advanced PDAC [48, 49]. In the trial [48] with 3.0 mg/kg ipilimumab, Royal et al. did not detect responses to therapy by strict response evaluation criteria in solid tumours (RECIST), but there were patients with tumour regression that was immunologically mediated by the CTLA-4 antibody, if immune RECIST [50] were applied.
In respect of B7-H1, using the Panc02 mouse orthotopical model of PDAC, we found that blocking the B7-H1 molecule with a specific monoclonal antibody against B7-H1 improved the survival of tumour-bearing mice. This increase in the anti-tumour immune response was achieved through the stimulation of effector CD8 T cells and via the Treg inhibition (Karakhanova et al., unpublished data).
Conclusions
Taken together, PDAC activates the anti-cancer immune response both in patients and in tumour-bearing mice. At the same time, PDAC triggers the immune suppressive arm of the immune system, which has negative effects on the anti-cancer immune response. Both anti-cancer immunity and immune suppression can be modulated by an additional, unspecific stimulation of the immune system. We supposed that new immunotherapeutical strategies should involve not only stimulation of the immune system of PDAC patients, but also control of the tumour immune suppressive milieu.
Acknowledgments
This work was supported by Else Kröner Fresenius-Stiftung (2010.A124) to Prof. A. V. Bazhin and Prof. V. Umansky. We would like to acknowledge Prof. Eduard Ryschich for his kind assistance with immunohistology.
Conflict of interest
All authors do not have any conflict of interest.
Abbreviations
- CTA
Cancer-testis antigens
- IFN
Interferon-α
- MDSC
Myeloid-derived suppressor cells
- PDAC
Pancreatic adenocarcinoma
- RECIST
Response evaluation criteria in solid tumours
- Treg
Regulatory T cells
References
- 1.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326(7):455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62(2):317–326. doi: 10.1136/gutjnl-2012-303588. [DOI] [PubMed] [Google Scholar]
- 3.Rosty C, Goggins M. Early detection of pancreatic carcinoma. Hematol Oncol Clin North Am. 2002;16(1):37–52. doi: 10.1016/S0889-8588(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 4.Ischenko I, Seeliger H, Jauch KW, Bruns CJ. Metastatic activity and chemotherapy resistance in human pancreatic cancer—influence of cancer stem cells. Surgery. 2009;146(3):430–434. doi: 10.1016/j.surg.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strobel O, Berens V, Hinz U, Hartwig W, Hackert T, Bergmann F, Debus J, Jager D, Buchler MW, Werner J. Resection after neoadjuvant therapy for locally advanced, “unresectable” pancreatic cancer. Surgery. 2012;152(3 Suppl 1):S33–S42. doi: 10.1016/j.surg.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Olah A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Buchler MW. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 9.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Stocken DD, Tudur Smith C, Bassi C, Ghaneh P, Owen E, Moore M, Padbury R, Doi R, Smith D, Buchler MW. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 2009;100(2):246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 12.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 13.Parmiani G, Castelli C, Santinami M, Rivoltini L. Melanoma immunology: past, present and future. Curr Opin Oncol. 2007;19(2):121–127. doi: 10.1097/CCO.0b013e32801497d7. [DOI] [PubMed] [Google Scholar]
- 14.Herreros-Villanueva M, Hijona E, Cosme A, Bujanda L. Spontaneous regression of pancreatic cancer: real or a misdiagnosis? World J Gastroenterol. 2012;18(23):2902–2908. doi: 10.3748/wjg.v18.i23.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 16.Ryschich E, Notzel T, Hinz U, Autschbach F, Ferguson J, Simon I, Weitz J, Frohlich B, Klar E, Buchler MW, Schmidt J. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res. 2005;11(2 Pt 1):498–504. [PubMed] [Google Scholar]
- 17.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Takeichi T, Mocevicius P, Deduchovas O, Salnikova O, Castro-Santa E, Buchler MW, Schmidt J, Ryschich E. alphaL beta2 integrin is indispensable for CD8+ T-cell recruitment in experimental pancreatic and hepatocellular cancer. Int J Cancer. 2012;130(9):2067–2076. doi: 10.1002/ijc.26223. [DOI] [PubMed] [Google Scholar]
- 19.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18(6):267–268. doi: 10.1016/S0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 20.Wadle A, Kubuschok B, Imig J, Wuellner B, Wittig C, Zwick C, Mischo A, Waetzig K, Romeike BF, Lindemann W, Schilling M, Pfreundschuh M, Renner C. Serological immune response to cancer testis antigens in patients with pancreatic cancer. Int J Cancer. 2006;119(1):117–125. doi: 10.1002/ijc.21744. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz-Winnenthal FH, Galindo-Escobedo LV, Rimoldi D, Geng W, Romero P, Koch M, Weitz J, Krempien R, Niethammer AG, Beckhove P, Buchler MW, Z’Graggen K. Potential target antigens for immunotherapy in human pancreatic cancer. Cancer Lett. 2007;252(2):290–298. doi: 10.1016/j.canlet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Heller A, Zornig I, Muller T, Giorgadze K, Frei C, Giese T, Bergmann F, Schmidt J, Werner J, Buchler MW, Jaeger D, Giese NA. Immunogenicity of SEREX-identified antigens and disease outcome in pancreatic cancer. Cancer Immunol Immunother. 2010;59(9):1389–1400. doi: 10.1007/s00262-010-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazhin AV, Wiedemann N, Schnolzer M, Schadendorf D, Eichmuller SB. Expression of GAGE family proteins in malignant melanoma. Cancer Lett. 2007;251(2):258–267. doi: 10.1016/j.canlet.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Gnjatic S, Ritter E, Buchler MW, Giese NA, Brors B, Frei C, Murray A, Halama N, Zornig I, Chen YT, Andrews C, Ritter G, Old LJ, Odunsi K, Jager D. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci USA. 2010;107(11):5088–5093. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185(5):476–480. doi: 10.1016/S0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 26.Knaebel HP, Marten A, Schmidt J, Hoffmann K, Seiler C, Lindel K, Schmitz-Winnenthal H, Fritz S, Herrmann T, Goldschmidt H, Krempien R, Mansmann U, Debus J, Diehl V, Buchler MW. Phase III trial of postoperative cisplatin, interferon alpha-2b, and 5-FU combined with external radiation treatment versus 5-FU alone for patients with resected pancreatic adenocarcinoma—CapRI: study protocol [ISRCTN62866759] BMC cancer. 2005;5:37. doi: 10.1186/1471-2407-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T, Bartsch D, Klein J, Mansmann U, Jager D, Capussotti L, Kunz R, Buchler MW. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012;30(33):4077–4083. doi: 10.1200/JCO.2011.38.2960. [DOI] [PubMed] [Google Scholar]
- 28.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2010;126(7):1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121(5):734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133(1):98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 35.Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, Büchler MW, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268(1):98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 37.Bazhin AV, Bayry J, Umansky V, Werner J, Karakhanova S. Overcoming immunosuppression as a new immunotherapeutic approach against pancreatic cancer. Oncoimmunology. 2013;2(9):e25736. doi: 10.4161/onci.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 39.Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC. Depletion of CD4+ CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol. 2006;13(9):1252–1258. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27(1):145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs C, Duewell P, Heckelsmiller K, Wei J, Bauernfeind F, Ellermeier J, Kisser U, Bauer CA, Dauer M, Eigler A, Maraskovsky E, Endres S, Schnurr M. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int J Cancer. 2011;128(4):897–907. doi: 10.1002/ijc.25399. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto T, Yanagimoto H, Satoi S, Toyokawa H, Hirooka S, Yamaki S, Yui R, Yamao J, Kim S, Kwon AH. Circulating CD4+ CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas. 2012;41(3):409–415. doi: 10.1097/MPA.0b013e3182373a66. [DOI] [PubMed] [Google Scholar]
- 43.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205(9):2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 45.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9(7–8):900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, Umansky V. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci USA. 2011;108(41):17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr, Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]