Abstract
The attenuated vaccinia virus, modified vaccinia Ankara, has been engineered to deliver the tumor antigen 5T4 (TroVax®). Here, we report results from a randomized open-label phase II trial in castration-resistant prostate cancer patients in which TroVax was administered in combination with docetaxel and compared against docetaxel alone. The aim was to recruit 80 patients (40 per arm), but the study was terminated early due to recruitment challenges. Therefore, this paper reports the comparative safety and immunological and clinical efficacy in 25 patients, 12 of whom were treated with TroVax plus docetaxel and 13 with docetaxel alone. 5T4-specific immune responses were monitored throughout the study. Clinical responses were assessed by measuring changes in tumor burden by CT and bone scan and by quantifying PSA concentrations. TroVax was well tolerated in all patients. Of 10 immunologically evaluable patients, 6 mounted 5T4-specific antibody responses. Patients treated with TroVax plus docetaxel showed a greater median progression-free survival of 9.67 months compared with 5.10 months for patients on the docetaxel alone arm (P = 0.097; HR = 0.31; 95 % CI 0.08–1.24). Importantly, a pre-treatment biomarker previously demonstrated to predict 5T4 immune response and treatment benefit showed a strong association with 5T4 antibody response and a statistically significant association with progression-free survival in patients treated with TroVax plus docetaxel, but not docetaxel alone.
Keywords: Castration-resistant prostate cancer, Cancer vaccine, 5T4 tumor antigen, Biomarker, Docetaxel
Introduction
Prostate cancer is the most common solid tumor malignancy in men in the United States and was estimated to account for 241,740 new cases and 28,170 deaths in 2012 [1]. Approximately 70 % of subjects will have metastases at some time during the course of their disease [2]. Androgen deprivation is the standard therapy for metastatic prostate cancer and achieves temporary tumor control or regression in 80–85 % of subjects [3–6]. Despite hormonal therapy, virtually all subjects with metastatic prostate cancer ultimately develop progressive disease [7, 8], and therefore, the management of castration-resistant prostate cancer (CRPC) remains a significant clinical challenge.
A new era in prostate cancer chemotherapy was introduced in 2004 when docetaxel became the first FDA-approved chemotherapeutic agent for metastatic CRPC [9]. In a pivotal phase III study, docetaxel plus prednisone given every 3 weeks resulted in 2.4 months increase in overall survival (OS) compared with mitoxantrone plus prednisone [10]. Since 2010, five additional agents have been shown to increase median overall survival (OS) of metastatic CRPC patients, four of which induced tumor regression and increased disease-free survival by directly targeting the proliferative potential of prostate cancer cells. Notably, in 2010, Sipuleucel-T became the first adaptive immunotherapy approved by the FDA for the treatment for solid tumors and more specifically for the treatment for metastatic CRPC. Sipuleucel-T is an autologous dendritic cell vaccine based on loading the patients dendritic cells ex vivo with a fusion protein (PA2024) comprised of the prostatic acid phosphatase (PAP) and granulocyte–macrophage colony-stimulating factor (GM-CSF). The FDA approval was based on results from a phase III trial in minimally symptomatic or asymptomatic metastatic CRPC patients which demonstrated a 22.5 % relative reduction in the risk of death (HR 0.775; 95 % CI 0.61–0.98; P = 0.03) and a 4.1-month improvement in median OS (25.8 vs 21.7 months) [11]. Unlike most other cancer therapies, adaptive immunotherapy does not target the tumor cells or their proliferative potential directly, but instead engages the adaptive immune response which takes time to be fully established. Therefore, the results of the Sipuleucel-T phase III trials answered pivotal questions regarding the efficacy of adaptive immunotherapy and distinct clinical aspects of its antitumor effect: (1) stimulation of the immune system can extend survival to a similar extent to the direct targeting of tumor cells with chemotherapy or hormonal agents, (2) toxicity is very low and generally does not compromise quality of life, (3) subsequent use of docetaxel was not associated with increased toxicity or lack of immunological response. Conversely, it is unknown whether Sipuleucel-T would synergize with docetaxel or hormonal agents. A different type of vaccine in late development is Prostvac which utilizes 2 viral vectors—Vaccinia and fowlpox—to carry a modified PSA gene and 3 co-immunostimulatory molecules to maximize immune stimulation and response. Based on the encouraging results of a randomized phase II trial in patients with metastatic CRPC showing a median overall survival of 8.5 months [12], a pivotal phase III study is ongoing.
TroVax is another viral vector-based vaccine which utilizes a modified vaccinia Ankara (MVA) to deliver the 5T4 tumor associated antigen. 5T4 is a 72-kDa protein and is expressed at the cell surface on the placenta and a wide range of human carcinomas including prostate, renal, and colorectal cancers, but rarely on normal tissues [13–15]. The restricted expression of 5T4 on normal tissues and its high prevalence on many common human carcinomas make it an attractive target for cancer immunotherapy.
TroVax has been tested in ten phase I, II, and III clinical studies in prostate [16], colorectal [17–20], and renal [21–25] cancer patients and shown to be safe and well tolerated both as a monotherapy and in combination with various standard treatment modalities. Furthermore, 5T4-specific immune responses were induced in the majority of patients and shown to correlate with clinical benefit. However, a phase III trial in renal cancer patients failed to show a significant increase in overall survival, but did lead to the identification of a pre-treatment biomarker which predicted both the magnitude of the induced 5T4 antibody response and treatment benefit (enhanced survival of TroVax-treated patients compared with placebo-treated patients) [26, 27]. Preliminary data demonstrated that RCC patients enrolled into the phase III trial who had normal pre-treatment levels of hemoglobin, platelets, and monocytes showed a survival advantage in favor of the TroVax arm [25]. Subsequent univariate analyses demonstrated that pre-treatment levels of hemoglobin and platelets were the best positive (hemoglobin) or negative (platelets) predictors of the induced 5T4 antibody response [26]. Finally, multivariate analyses demonstrated that pre-treatment MCHC (hemoglobin/hematocrit) and platelet levels were the strongest predictors of treatment benefit (MCHC) or lack of benefit (platelets) [27]. Based upon these observations, we aimed to enrich for patients who were more likely to benefit from treatment with a cancer vaccine by excluding patients with low hemoglobin, high platelets, and high monocytes (factors which are all readily measured as part of standard testing). While not claiming that one or all of these factors are causally related to the induction of a 5T4 immune response or treatment benefit, we believe that these factors are associated with an inflammatory environment and that such an immune milieu may not be optimal for vaccine efficacy. Thus, one key aim of this study was to prospectively test the ability of the pre-treatment biomarker (identified in renal cancer patients) to predict 5T4-specific antibody response and clinical benefit in patients with CRPC.
Theoretically, a combinatorial approach using cytotoxic chemotherapy alongside immunotherapy is an attractive option since the former can cause rapid tumor de-bulking, while the latter can induce long-term immunological memory and control micro-metastatic disease [28]. In addition, it has been demonstrated that taxane-based chemotherapy can be administered safely with vaccination and elicited a beneficial immunomodulatory effects including stimulation of cytokine production, induction of T-cell-specific response and T-cell infiltration of tumor cells [29–31]. In a mouse model, docetaxel administered post-vaccination also resulted in enhanced immunogenicity and efficacy [31]. Based on these data, we hypothesized that the combination of TroVax plus docetaxel may be complementary or even synergistic compared with docetaxel alone and would increase progression-free survival (PFS) of subjects with CRPC due to (1) de-bulking by the cytotoxic agent resulting in smaller disease burden which may be more readily controlled by the adaptive immune response, (2) potential immunomodulatory effects of docetaxel resulting in enhanced immune responses post-vaccination, and (3) release of tumor antigens (including 5T4) due to treatment with docetaxel resulting in boosting of antigen-specific immune responses.
Patients and methods
Patient characteristics
This study aimed to enroll 80 male subjects aged ≥ 18 years with progressive CRPC. Subjects had to be taxane-naïve with an ECOG score of 0 or 1 and life expectancy ≥ 6 months and received no prior systemic chemotherapy treatment. Subjects must have had evidence of disease progression concomitant with surgical or medical castration, as demonstrated by PSA progression, new bone disease and/or progression of measurable disease, and have adequate bone marrow function (an absolute lymphocyte count of ≥500/μL, absolute neutrophil count of ≥1,200/μL, platelet count ≥10,000/μL). Key exclusion criteria included presence of brain metastases, history of allergic response to vaccinia vaccinations, egg proteins or neomycin, and positive for HIV or hepatitis B or C.
Furthermore, based upon observations in previous clinical trials and the identification of a biomarker predictive of treatment benefit, this study aimed to enrich for patients who were more likely to benefit from treatment with TroVax; this was achieved by excluding patients who had low hemoglobin levels (<11 g/dL initially, then relaxed to <10 g/dL by protocol amendment) and high levels of platelets (>400 × 109/mL) and monocytes (>0.8 × 109/mL).
Clinical trial design
This phase II trial was a randomized, open-label study in taxane-naïve subjects with metastatic CRPC comparing TroVax plus docetaxel to docetaxel alone. Subjects randomized to the TroVax plus docetaxel arm received a single injection of 1 × 109 tissue culture infectious dose (TCID50) of TroVax on weeks 1 (day 1), 2 (day 10), and 4 (day 22) followed by an injection every 3 weeks (weeks 7, 10, and 13) and a further injection every 6 weeks (weeks 19, 25, 31, and 37). The first three doses of TroVax were administered prior to the subject receiving docetaxel. Subsequent TroVax injections were delivered on day 1 of the appropriate docetaxel cycle, 2 h prior to the chemotherapy administration. Docetaxel was administered every 3 weeks starting at week 4 (day 22) following the completion of the first three TroVax injections. Subjects received docetaxel at a dose of 75 mg/m2 on day 1 of each cycle (1 cycle = 3 weeks). Subjects randomized to receive docetaxel alone were treated at a dose of 75 mg/m2 on day 1 of each cycle (1 cycle = 3 weeks); treatment started at week 1. Subjects on both treatment arms received a maximum of 10 docetaxel infusions over the course of the study.
Statistical analysis
The primary objective of the study was to establish whether the incidence of progression-free survival (PFS), defined by the absence of progression (assessed by PCWG2 criteria; [33]) at week 37 in the TroVax plus docetaxel treatment arm, was higher than the incidence in the docetaxel alone treatment arm. A sample size of 40 subjects per group was estimated to have a power of at least 80 % to detect a statistically significant improvement in the response rate in the TroVax plus docetaxel arm of 30 % or more using one-sided tests of significance at the 10 % level using Fisher’s exact test. Secondary endpoints included PFS, overall survival (OS), PSA response, RECIST response, and analysis of the ability of a previously identified pre-treatment biomarker to predict 5T4 antibody response and treatment benefit.
Assessment of disease status was undertaken by repeat radiographic studies using RECIST criteria every 12 weeks, in addition to PSA sampling every 3 weeks.
PFS was summarized by use of Kaplan–Meier methodology. The Log-Rank test was used for curve comparison and to report P values. Hazard ratios (TroVax plus docetaxel versus docetaxel alone) were calculated by Cox regression. Correlation coefficients were calculated by the Spearman method.
Measurement of humoral responses
5T4 and MVA-specific antibody responses were determined using a validated semi-quantitative ELISA. Polyclonal plasma, known to be positive for both 5T4 and MVA antibodies, was used as a standard curve for each assay. The standard curves for each ELISA were assigned a nominal value of 5T4 or MVA antibody relative units (RU) and were titrated from 200 to 1.56 RU. A cut-point was established for each assay by analyzing 5T4 and MVA-specific antibody levels in plasma recovered from 50 healthy donors. Cut-points of 11.17 RU and 5.00 RU were established for 5T4 and MVA, respectively. Variation in the level of 5T4 and MVA antibody levels was assessed in cancer patients who had not received any 5T4- or MVA-targeted therapies. A 1.54-fold increase in 5T4 antibody and a 1.76-fold increase in MVA antibody were established as the level at which a 1 % false positive rate could be expected. A positive response was reported if the post-TroVax antibody levels exceeded the cut-point and the increase, relative to the baseline, exceeded the pre-determined fold increase for each antigen.
Results
Patient characteristics
The study initiated in October 2010 with the aim of enrolling 80 patients at multiple centers in the US. However, changes in the treatment landscape for prostate cancer in the US resulted in slow patient recruitment which unfortunately led to the premature closure of the study in October 2012 when 25 patients had been randomized (12 on the TroVax plus docetaxel arm and 13 on the docetaxel alone arm). Patient characteristics are detailed in Table 1.
Table 1.
The number of patients in each arm of the study and details their demographics and disease characteristics
| Characteristics | TroVax + Docetaxel | Docetaxel alone |
|---|---|---|
| Total number of patients | 12 | 13 |
| Race/ethnicity | ||
| Caucasian | 10 | 10 |
| African–American | 0 | 2 |
| Hispanic | 2 | 1 |
| Age (years) | ||
| Median | 73 | 72 |
| Mean | 71.8 | 72.8 |
| Range | 61–85 | 58–84 |
| Sites of metastatic disease | ||
| A (Rising PSA no detectable metastatic disease) | 1 | 1 |
| B (Nodal spread, no bone/visceral disease) | 0 | 0 |
| C (Bone disease ± nodal disease, no visceral) | 8 | 9 |
| D (Visceral metastases) | 3 | 3 |
| Gleason score | ||
| Median | 7 | 7 |
| Range | 5–9 | 6–10 |
| PSA (ng/mL) | ||
| Mean | 224.6 | 373.8 |
| Range | 5.2–1642.8 | 3.1–1455.6 |
| ECOG performance status | ||
| 0 | 8 | 8 |
| 1 | 4 | 5 |
Safety
The most common TEAEs (those in at least 10 % of one or both treatment arms) are shown in Table 2. The most common individual event in the TroVax plus docetaxel group was fatigue, which was reported for eight subjects (72.7 %). Fatigue was also the most common individual event in the docetaxel alone group (seven subjects, 53.8 %). In summary, TroVax was well tolerated in combination with docetaxel, and there was no clear and consistent evidence of increased toxicity of docetaxel in combination, although the incidence of docetaxel-related hematological toxicities was higher in the TroVax plus docetaxel arm than the docetaxel alone arm, but the numbers are too small to draw any statistical conclusions.
Table 2.
Treatment emergent adverse events (TEAEs) in at least 10 % of subjects
| Adverse event | Docetaxel alone | TroVax and docetaxel | ||
|---|---|---|---|---|
| Events | Subjects (N = 13) | Events | Subjects (N = 11) | |
| Any TEAE | 138 | 11 (84.6 %) | 192 | 11 (100.0 %) |
| Blood and lymphatic system disorders | 7 | 4 (30.8 %) | 38 | 8 (72.7 %) |
| Anemia | 0 | 0 | 6 | 4 (36.4 %) |
| Leukopenia | 0 | 0 | 10 | 3 (27.3 %) |
| Neutropenia | 6 | 3 (23.1 %) | 21 | 6 (54.5 %) |
| Cardiac disorders | 2 | 2 (15.4 %) | 2 | 2 (18.2 %) |
| Tachycardia | 2 | 2 (15.4 %) | 1 | 1 (9.1 %) |
| Gastrointestinal disorders | 37 | 8 (61.5 %) | 31 | 10 (90.9 %) |
| Abdominal pain | 2 | 2 (15.4 %) | 0 | 0 |
| Constipation | 6 | 5 (38.5 %) | 6 | 5 (45.5 %) |
| Diarrhea | 12 | 5 (38.5 %) | 8 | 5 (45.5 %) |
| Nausea | 10 | 4 (30.8 %) | 6 | 5 (45.5 %) |
| Vomiting | 3 | 2 (15.4 %) | 2 | 2 (18.2 %) |
| General disorders and administration site conditions | 24 | 9 (69.2 %) | 24 | 9 (81.8 %) |
| Fatigue | 10 | 7 (53.8 %) | 11 | 8 (72.7 %) |
| Pain | 7 | 5 (38.5 %) | 6 | 3 (27.3 %) |
| Infections and infestations | 3 | 3 (23.1 %) | 4 | 2 (18.2 %) |
| Herpes zoster | 0 | 0 | 2 | 2 (18.2 %) |
| Injury, poisoning, and procedural complications | 0 | 0 | 9 | 4 (36.4 %) |
| Fall | 0 | 0 | 3 | 2 (18.2 %) |
| Investigations | 5 | 4 (30.8 %) | 12 | 5 (45.5 %) |
| Weight decreased | 3 | 3 (23.1 %) | 2 | 2 (18.2 %) |
| Metabolism and nutrition disorders | 5 | 3 (23.1 %) | 7 | 5 (45.5 %) |
| Decreased appetite | 4 | 3 (23.1 %) | 3 | 3 (27.3 %) |
| Dehydration | 0 | 0 | 2 | 2 (18.2 %) |
| Musculoskeletal and connective tissue disorders | 9 | 6 (46.2 %) | 18 | 7 (63.6 %) |
| Arthralgia | 1 | 1 (7.7 %) | 4 | 4 (36.4 %) |
| Back pain | 6 | 4 (30.8 %) | 0 | 0 |
| Joint range of motion decreased | 0 | 0 | 2 | 2 (18.2 %) |
| Musculoskeletal pain | 1 | 1 (7.7 %) | 3 | 2 (18.2 %) |
| Myalgia | 0 | 0 | 3 | 3 (27.3 %) |
| Pain in extremity | 0 | 0 | 3 | 2 (18.2 %) |
| Nervous system disorders | 9 | 4 (30.8 %) | 7 | 5 (45.5 %) |
| Dizziness | 2 | 2 (15.4 %) | 0 | 0 |
| Neuropathy peripheral | 4 | 3 (23.1 %) | 1 | 1 (9.1 %) |
| Psychiatric disorders | 2 | 2 (15.4 %) | 8 | 4 (36.4 %) |
| Depression | 0 | 0 | 3 | 3 (27.3 %) |
| Insomnia | 0 | 0 | 4 | 3 (27.3 %) |
| Respiratory, thoracic, and mediastinal disorders | 13 | 5 (38.5 %) | 12 | 6 (54.5 %) |
| Cough | 2 | 2 (15.4 %) | 5 | 4 (36.4 %) |
| Dyspnoea | 6 | 2 (15.4 %) | 1 | 1 (9.1 %) |
| Skin and subcutaneous tissue disorders | 9 | 8 (61.5 %) | 7 | 6 (54.5 %) |
| Alopecia | 6 | 6 (46.2 %) | 5 | 5 (45.5 %) |
TroVax-induced antibody responses
Antibody responses against the 5T4 tumor antigen and the MVA viral vector were quantified at baseline and at weeks 4, 5, 8, 31, and 37 post-treatment (following the 2nd, 3rd, 4th, 9th, and 10th vaccinations, respectively); results are illustrated in Table 3. Of 10 patients who provided blood samples for immuno-monitoring, 6 mounted positive 5T4-specific antibody responses, while 9 mounted positive MVA-specific antibody responses. Of the 6 patients who mounted positive 5T4-specific antibody responses, only 3 showed a response during the period in which docetaxel was administered; another 3 did not sero-convert until following completion of chemotherapy. In contrast, MVA-specific antibody responses were detected following 2–3 TroVax injections (i.e., during the period in which docetaxel was administered).
Table 3.
5T4- and MVA-specific antibody responses in patients receiving TroVax plus docetaxel
| Patient ID | Screen | Week 4 | Week 5 | Week 8 | Week 31 | Week 37 | Response category |
|---|---|---|---|---|---|---|---|
| 5T4-specific antibody responses | |||||||
| 01/001 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | N/A | Negative |
| 01/003 | 5.92 | 5.92 | 6.90 | 8.46 | N/A | N/A | Negative |
| 06/001 | <5.00 | <5.00 | <5.00 | 5.13 | 22.98 | 15.43 | Positive |
| 06/002 | <5.00 | <5.00 | <5.00 | 8.90 | 7.69 | 19.24 | Positive |
| 06/008 | 5.60 | 6.68 | 17.99 | 21.23 | N/A | N/A | Positive |
| 06/011 | 27.71 | 21.60 | 20.72 | 22.65 | N/A | N/A | Negative |
| 06/012 | 12.25 | 11.89 | 12.30 | N/A | N/A | N/A | Negative |
| 07/001 | 7.73 | 7.80 | 7.98 | 7.58 | 10.05 | 13.13 | Positive |
| 07/004 | <5.00 | <5.00 | <5.00 | 14.08 | N/A | N/A | Positive |
| 07/008 | 11.45 | 16.27 | 37.79 | 85.36 | 33.14 | 51.39 | Positive |
| MVA-specific antibody responses | |||||||
| 01/001 | <5.00 | <5.00 | <5.00 | 6.22 | <5.00 | N/A | Negative |
| 01/003 | 6.43 | 83.91 | 61.82 | 46.50 | N/A | N/A | Positive |
| 06/001 | 11.96 | 143.35 | 88.80 | 48.18 | 33.65 | 56.79 | Positive |
| 06/002 | <5.00 | 179.02 | 123.91 | 99.70 | 18.77 | 20.24 | Positive |
| 06/008 | 6.69 | 78.69 | 101.58 | 51.09 | N/A | N/A | Positive |
| 06/011 | 13.29 | 93.34 | 77.64 | 66.00 | N/A | N/A | Positive |
| 06/012 | 12.43 | 50.18 | 42.70 | N/A | N/A | N/A | Positive |
| 07/001 | 7.62 | 42.17 | 37.80 | 41.36 | 34.98 | 40.85 | Positive |
| 07/004 | <5.00 | 7.34 | 12.65 | 33.02 | N/A | N/A | Positive |
| 07/008 | <5.00 | 53.22 | 47.67 | 60.13 | 51.45 | 65.04 | Positive |
Results are expressed in relative units (RU). Results tabulated in bold text and underlined represent positive antibody response relative to the pre-injection baseline. Response category indicates whether the patient mounted a positive antibody response at any time point post-treatment initiation. N/A indicates where samples were not available for analysis
Clinical efficacy
Disease progression was monitored throughout the trial by quantifying PSA levels every 3 weeks and by performing CT scans and bone scans every 12 weeks. Due to the premature termination of the study, resulting in reduced power, the primary efficacy objective of demonstrating an improvement in PFS at 37 weeks was not met.
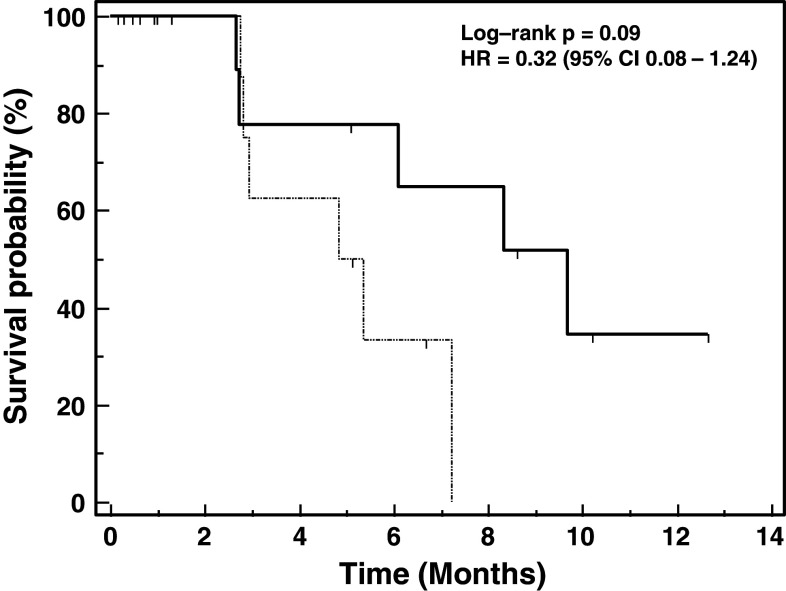
Figure 1 illustrates the progression-free survival of patients treated with TroVax plus docetaxel compared with patients treated with docetaxel alone. Patients treated with TroVax plus docetaxel showed a greater median PFS (9.67 months) compared with the docetaxel alone arm (5.10 months), the associated log-rank test being significant at the 10 % level (P = 0.097; HR = 0.31; 95 % CI 0.08–1.24). In total, 4 patients on the docetaxel alone treatment arm and 3 patients on the TroVax plus docetaxel treatment arm showed partial responses. Periods of disease stabilization were seen in 4 patients on the TroVax plus docetaxel treatment arm (≥13 weeks to >49 weeks) and 2 patients on the docetaxel alone treatment arm (≥25 weeks and ≥ 37weeks).
Fig. 1.
Progression-free survival. Kaplan–Meier plot of progression-free survival in patients treated with TroVax plus docetaxel (n = 12; solid line) or docetaxel alone (n = 13; dashed line)
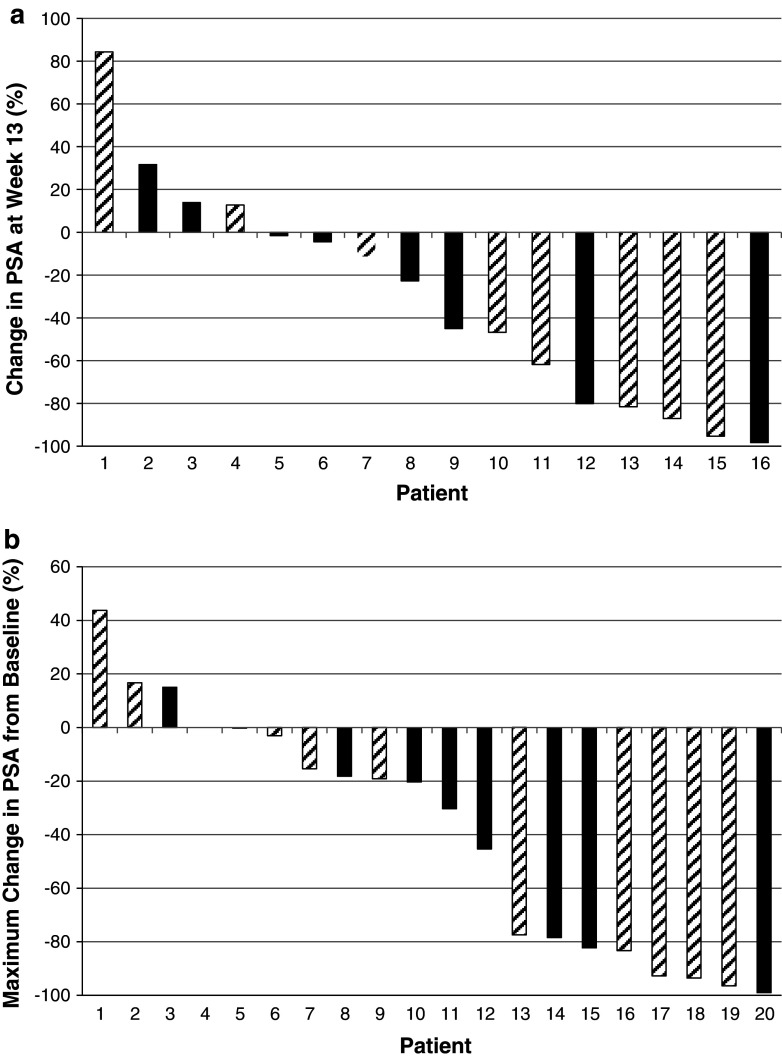
Serum PSA levels were quantified every 3 weeks. Waterfall plots showing the maximal percentage decrease at week 13 or at any time point post-treatment initiation are illustrated in Fig. 2a and b, respectively. There were no clear differences in the PSA profiles seen for patients enrolled into either treatment arm.
Fig. 2.
PSA waterfall plots. Figure 2a shows the percentage change in PSA at week 13 relative to baseline (screen) for all patients who had PSA data available at week 13 (n = 16). Figure 2b plots the maximal change in PSA levels at any time post-treatment initiation in all patients who had at least one post-treatment PSA measurement available (n = 20). Patients treated with docetaxel alone are shown as hashed bars; patients treated with TroVax plus docetaxel are shown in solid bars
Relationship between progression-free survival, 5T4-specific antibody response and a pre-treatment biomarker
Previously, a biomarker consisting of pre-treatment hemoglobin, hematocrit, and 5T4 antibody levels (immune response surrogate; IRS) has been shown to predict the magnitude of 5T4 antibody responses induced by TroVax and treatment benefit in renal cancer patients. This study aimed to validate the biomarker prospectively by demonstrating that it predicted both 5T4 antibody response and treatment benefit in CRPC patients. Due to the premature termination of this clinical trial, treatment benefit could only be assessed by analysis of disease progression since minimal survival data were available (at the time of study closure, 1 patient on the TroVax plus docetaxel arm and 1 patient on the docetaxel alone arm had passed away).
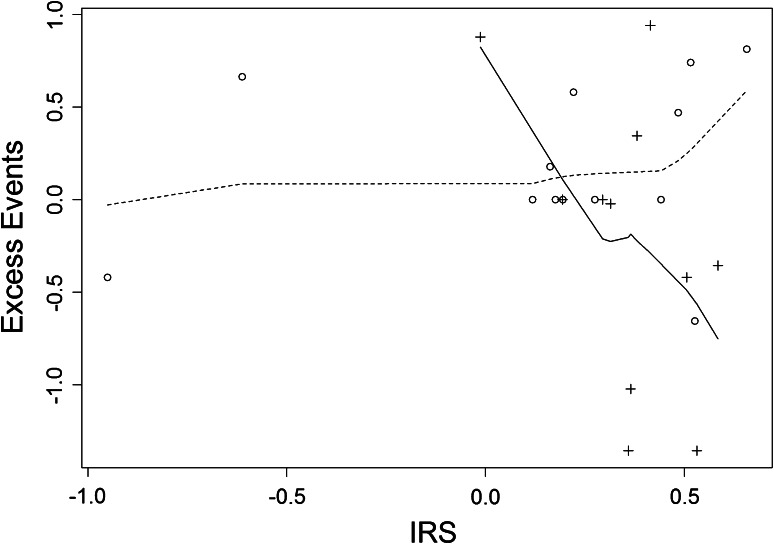
The pre-treatment biomarker score was calculated for each patient using the algorithm published previously [26] and used (1) as an explanatory variable in a proportional hazards model for PFS and (2) as an explanatory variable in a regression model for immune response. Overall, the IRS was not a significant predictor of PFS in all 25 patients (P = 0.73). However, there was a marked difference in the predictive ability of the IRS in the treatment groups separately: there was no association in the docetaxel alone arm (P = 0.631; higher IRS associated with worse PFS), while in the TroVax plus docetaxel arm, higher IRS predicted improved PFS (P = 0.044). The difference in predictive ability between the treatment arms is significant at P = 0.039 and is illustrated in an excess event plot in Fig. 3.
Fig. 3.
Association of the pre-treatment biomarker (immune response surrogate; IRS) with PFS. The figure shows a scatter plot of excess PFS events against IRS, with fitted smoothed prediction lines for the docetaxel alone arm (dotted line) and the TroVax plus docetaxel arm (solid line) separately
For the analyses reported here, antibody response was defined as the logarithm of the ratio of antibody level post-vaccination to that at baseline. Although the regression coefficient of the 4th vaccination antibody response on the IRS was 0.99 (very close to the 1.00 theoretically expected of an immune response surrogate), statistical significance over the 9 evaluable subjects in the TroVax plus docetaxel arm was not achieved. However, an exploratory analysis where the highest antibody response was used instead of the response after the 4th vaccination was more encouraging: the regression coefficient was 2.58 with significance probability 0.085.
Discussion
TroVax was well tolerated in combination with docetaxel, and there was no evidence of enhanced toxicity or decreased efficacy of docetaxel. However, there was some suggestion that the chemotherapy regimen did decrease both the frequency and the magnitude of 5T4-specific antibody responses with some patients only sero-converting very late in the vaccination time course following the completion of chemotherapy. In contrast, a previous study tested TroVax ± GM-CSF in CRPC patients previously treated with chemotherapy [16] and demonstrated that all 27 patients mounted a strong 5T4-specific antibody response after 2–3 TroVax injections. Although the study reported here did not compare TroVax alone versus TroVax plus docetaxel, the immune responses were generally of lower magnitude and frequency compared with previous clinical trials. Taken together, these observations suggest that the addition of docetaxel to TroVax may impact on the induction of antibody responses against 5T4. This result contrasts with reports from other groups who have demonstrated a synergistic effect between an immunotherapy and docetaxel in both pre-clinical [32] and clinical [31] settings. It is possible that the reason for the discrepancy is the relative timing of the administration of the vaccine and the chemotherapy. For example, results from a pre-clinical model demonstrated that administration of a vaccine 1 day before, 1 day after, or concurrent with docetaxel inhibited immune responses compared with administration of docetaxel >4 days post-vaccination which augmented antigen-specific immune responses [32]. In this study, TroVax was administered alone for the first 2 vaccinations but subsequently was given concurrently with docetaxel. Therefore, it is possible that the scheduling of TroVax relative to docetaxel and dexamethasone administration was not optimal in this study, and any future combinatorial studies would need to address the timing of treatment.
Any conclusions drawn from this clinical trial relating to efficacy need to be tempered due to the premature termination of the study and therefore the reduced number of patients treated and subsequent lack of statistical power. Despite this caveat, it is encouraging that patients treated with TroVax plus docetaxel showed an increase in PFS of >4 months compared with patients treated with docetaxel alone. Based upon observations from a previous phase III study in which renal cancer patients with a pre-treatment “inflammatory signature” appeared to receive little benefit from TroVax, we took an enrichment approach in this study by excluding patients with low hemoglobin levels and high platelet and monocyte levels. It is tempting to speculate that this enrichment approach was at least partially responsible for the enhanced PFS seen in the patients treated with TroVax, especially as historically cancer immunotherapy approaches have shown little or no impact on disease progression in prostate cancer patients [12, 33, 34].
One of the secondary objectives of this study was to prospectively validate the pre-treatment biomarker identified previously in renal cancer patients. Although the relatively small number of patients means that the results need to be interpreted with caution, it was encouraging that the biomarker was associated with the magnitude of the induced 5T4 antibody response and showed a significant association with PFS. This result was particularly surprising because the biomarker was identified in renal cancer patients treated with TroVax plus either IL-2, IFN-α, or sunitinib and was shown to be a predictor of patient survival, rather than PFS. Several potential biomarkers predictive of clinical or immunological efficacy of cancer immunotherapies have been reported, but to our knowledge, this is the first to be “validated” prospectively. Of the candidate biomarkers identified, a common theme may be emerging with most markers being reflective of a general inflammatory state of the patient [35] and likely to be impacted by disease stage such that patients with later stage disease and larger tumor burden have sub-optimal levels of the biomarker. This fits with the general consensus that cancer vaccines are most likely to show efficacy in patients with earlier stage disease.
Future clinical studies aim to demonstrate that the pre-treatment biomarker is applicable in patients with colorectal cancer, ovarian cancer, and mesothelioma.
Conflict of interest
Richard Harrop and Daniel Blount wish to disclose that they are employees of Oxford BioMedica, the manufacturer and developer of TroVax®. Franklin Chu, Nashat Gabrail, Sandy Srinivas, and Anna Ferrari have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Gittes RF. Carcinoma of the prostate. N Engl J Med. 1991;324(4):236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- 3.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA, Goodman PJ. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321(7):419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Kelly WK. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol. 1993;11(8):1566–1572. doi: 10.1200/JCO.1993.11.8.1566. [DOI] [PubMed] [Google Scholar]
- 5.Small EJ, Srinivas S. The antiandrogen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer. 1995;76(8):1428–1434. doi: 10.1002/1097-0142(19951015)76:8<1428::AID-CNCR2820760820>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Schellhammer PF, Venner P, Haas GP, Small EJ, Nieh PT, Seabaugh DR, Patterson AL, Klein E, Wajsman Z, Furr B, Chen Y, Kolvenbag GJ. Prostate specific antigen decreases after withdrawal of antiandrogen therapy with bicalutamide or flutamide in patients receiving combined androgen blockade. J Urol. 1997;157(5):1731–1735. doi: 10.1016/S0022-5347(01)64846-8. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Mazumdar M, Kelly WK. Clinical trials in relapsed prostate cancer: defining the target. J Natl Cancer Inst. 1996;88(22):1623–1634. doi: 10.1093/jnci/88.22.1623. [DOI] [PubMed] [Google Scholar]
- 8.Small EJ, Vogelzang NJ. Second-line hormonal therapy for advanced prostate cancer: a shifting paradigm. J Clin Oncol. 1997;15(1):382–388. doi: 10.1200/JCO.1997.15.1.382. [DOI] [PubMed] [Google Scholar]
- 9.Dagher R, Li N, Abraham S, Rahman A, Sridhara R, Pazdur R. Approval summary: docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin Cancer Res. 2004;10(24):8147–8151. doi: 10.1158/1078-0432.CCR-04-1402. [DOI] [PubMed] [Google Scholar]
- 10.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 11.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 12.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990;61:89–95. doi: 10.1038/bjc.1990.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzynska T, Marsh PJ, Schofield PF, Roberts SA, Myers KA, Stern PL. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69:899–902. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damelin M, Geles KG, Follettie MT, Yuan P, Baxter M, Golas J, DiJoseph JF, Karnoub M, Huang S, Diesl V, Behrens C, Choe SE, Rios C, Gruzas J, Sridharan L, Dougher M, Kunz A, Hamann PR, Evans D, Armellino D, Khandke K, Marquette K, Tchistiakova L, Boghaert ER, Abraham RT, Wistuba II, Zhou BB. Delineation of a cellular hierarchy in lung cancer reveals an oncofetal antigen expressed on tumor-initiating cells. Cancer Res. 2011;71:4236–4246. doi: 10.1158/0008-5472.CAN-10-3919. [DOI] [PubMed] [Google Scholar]
- 16.Amato RJ, Drury N, Naylor S, Jac J, Saxena S, Cao A, Hernandez-McClain J, Harrop R. Vaccination of prostate cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother. 2008;31:577–585. doi: 10.1097/CJI.0b013e31817deafd. [DOI] [PubMed] [Google Scholar]
- 17.Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, Carroll MW. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 18.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls J, Wassan H, Habib N, Anthoney A. Vaccination of colorectal cancer patients with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487–4494. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 19.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven N, Hawkins RE. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–986. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkord E, Dangoor A, Drury NL, Harrop R, Burt DJ, Drijfhout JW, Hamer C, Andrews D, Naylor S, Sherlock D, Hawkins RE, Stern PL. An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother. 2008;31:820–829. doi: 10.1097/CJI.0b013e3181876ab3. [DOI] [PubMed] [Google Scholar]
- 21.Amato RJ, Shingler W, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin Cancer Res. 2008;14:7504–7510. doi: 10.1158/1078-0432.CCR-08-0668. [DOI] [PubMed] [Google Scholar]
- 22.Amato RJ, Shingler W, Goonewardena M, de Belin J, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother. 2009;32:765–772. doi: 10.1097/CJI.0b013e3181ace876. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins RE, Macdermott C, Shablak A, Hamer C, Thistlethwaite F, Drury NL, Chikoti P, Shingler W, Naylor S, Harrop R. Vaccination of patients with metastatic renal cancer with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside interferon-alpha. J Immunother. 2009;32:424–429. doi: 10.1097/CJI.0b013e31819d297e. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman HL, Taback B, Sherman W, Kim DW, Shingler WH, Moroziewicz D, DeRaffele G, Mitcham J, Carroll MW, Harrop R, Naylor S, Kim-Schulze S. Phase II trial of modified vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med. 2009;7:2. doi: 10.1186/1479-5876-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, McDonald M, Eastty S, Shingler WH, de Belin J, Goonewardena M, Naylor S, Harrop R. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010;16:5539–5547. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 26.Harrop R, Shingler WH, McDonald M, Treasure P, Amato RJ, Hawkins RE, Kaufman HL, de Belin J, Kelleher M, Goonewardena M, Naylor S. MVA–5T4-induced immune responses are an early marker of efficacy in renal cancer patients. Cancer Immunol Immunother. 2011;60:829–837. doi: 10.1007/s00262-011-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrop R, Treasure P, de Belin J, Kelleher M, Bolton G, Naylor S, Shingler WH. Analysis of pre-treatment markers predictive of treatment benefit for the therapeutic cancer vaccine MVA–5T4 (TroVax) Cancer Immunol Immunother. 2012;61(12):2283–2294. doi: 10.1007/s00262-012-1302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake CG (2012) Combination immunotherapy approaches. Ann Oncol (Suppl 8):viii41–viii46. doi:10.1093/annonc/mds262 [DOI] [PMC free article] [PubMed]
- 29.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49(4–5):181–185. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason K, Staab A, Hunter N, McBride W, Petersen S, Terry N, Milas L. Enhancement of tumor radioresponse by docetaxel: involvement of immune system. Int J Oncol. 2001;18(3):599–606. doi: 10.3892/ijo.18.3.599. [DOI] [PubMed] [Google Scholar]
- 31.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumour activity: effects of docetaxel on immune enhancement. Clin Can Res. 2008;14(11):3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M, Prostate Cancer Clinical Trials Working Group Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15(9):969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrop R. Cancer vaccines: identification of biomarkers predictive of clinical efficacy. Hum Vaccin Immunother. 2013;9(4):1–5. doi: 10.4161/hv.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]