Abstract
CD39 is an ectoenzyme, present on different immune cell subsets, which mediates immunosuppressive functions catalyzing ATP degradation. It is not known whether CD39 is expressed and implicated in the activity of CD8+ regulatory T lymphocytes (Treg). In this study, CD39 expression and function was analyzed in both CD8+ and CD4+CD25hi Treg from the peripheral blood of healthy donors as well as from tumor specimens. CD39 was found expressed by both CD8+ (from the majority of healthy donors and tumor patients) and CD4+CD25hi Treg, and CD39 expression correlated with suppression activity mediated by CD8+ Treg. Importantly, CD39 counteraction remarkably inhibited the suppression activity of CD8+ Treg (both from peripheral blood and tumor microenvironment) suggesting that CD39-mediated inhibition constitutes a prevalent hallmark of their function. Collectively, these findings, unveiling a new mechanism of action for CD8+ Treg, provide new knowledge on intratumoral molecular pathways related to tumor immune escape, which could be exploited in the future for designing new biological tools for anticancer immune intervention.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1392-z) contains supplementary material, which is available to authorized users.
Keywords: CD39, CD8+ Treg, Tumor immune escape, Tolerance
Introduction
CD39 is an ectoenzyme, present at the surface of different immune cells such as B, NK, and T lymphocytes, macrophages and dendritic cells that catalyzes conversion of extracellular ATP or ADP to AMP [1, 2]. Thus, it depletes the milieu and deprives cells from ATP. Moreover, acting in concert with the CD73 molecule, another ectoenzyme expressed by lymphocytes which dephosphorylates AMP into adenosine [3], it enriches the microenvironment with adenosine. While ATP is a potent activator of immune cells and inflammation, mimicking the effects of a danger signal, adenosine mediates immunosuppression inhibiting secretion of inflammatory cytokines and superoxide as well as desensitizing chemokine receptors [4]. Hence, CD39 exerts potent immunosuppressive effects at the same time decreasing ATP concentration and increasing adenosine levels in the extracellular compartment. Accordingly, CD39 has been found expressed on CD4+CD25hi regulatory T cells (Treg) and involved in their regulatory activity [5, 6].
Treg constitute a complex network of T cell subtypes which regulate effector immune responses. They are fundamental for immune homeostasis since: (a) guarantee peripheral tolerance inhibiting autoimmune responses; (b) impede the onset of hyperacute inflammatory processes shutting down immune reactions (and related tissue inflammation) after elimination of the triggering antigen/pathogen; (c) control chronic immune-related inflammatory processes thus limiting the extent of tissue damage [7]. Several Treg subpopulations have been identified so far. Although Treg subsets belonging to the CD4+ T cell compartment have been those more extensively studied in the last decade, growing interest is presently devoted to CD8+ Treg subsets. Notably, the first identified regulatory T cell subpopulation consisted in CD8+ T suppressor lymphocytes, as they were called at that time [8]. Indeed, after a long period in which these cells were looked with skepticism by immunologists [9], in the recent years, a wide array of CD8+ Treg subpopulations have been characterized both in mice and humans [10]. These cells, through several mechanisms, are able to regulate peripheral tolerance [11] and, when altered, appear to be pathogenetically involved in autoimmune diseases [10]. Interestingly, CD8+ Treg, as well as CD4+CD25hi Treg [12, 13], may infiltrate cancer likely contributing to tumor immune escape [14–19]. Importantly, the level of tumor infiltration by Treg negatively correlates with prognosis [20–24]. This is explained by their capacity to inhibit effector immune responses including tumor-specific reactions [25]. Subsequently, Treg tumor infiltration is considered a main cancer pathogenic factor, a concept supported by the observation that tumors themselves favor the enrichment of tumor microenvironment with Treg through in situ generation and/or homing [14, 26]. Tumor-infiltrating Treg mediate inhibition of tumor-specific immune responses through different mechanisms [25, 27, 28].
Recently, CD39 intratumoral expression was found related to tumor growth and dissemination [29], and the molecule was demonstrated at the surface of tumor-infiltrating CD4+ Treg, suggesting its possible involvement in intratumoral Treg activity [30]. Actually, it is not known whether CD39 is also expressed by tumor-infiltrating CD8+ Treg and which functional relevance has its expression by the different Treg subsets. In order to clarify these issues, Treg from peripheral blood of healthy donors as well as from tumor specimens, belonging to both the CD4+ and the CD8+ T cell subsets, were phenotypically and functionally analyzed in relation to the expression of the CD39 molecule.
Materials and methods
Subjects
Peripheral blood samples were collected from 13 healthy subjects.
Tumor samples were collected from a series of patients affected with renal, bladder or colorectal cancer. To avoid the risk of impairing the diagnostic value of surgically excised material, only small (≈5-mm diameter size) tissue specimens were collected, although this choice limited in some way the possibility to perform a more comprehensive array of immunological analyses.
The study was approved by the local ethical committee, and all patients and controls enrolled in the study provided their informed written consent.
Monoclonal antibodies (mAb) and immunofluorescence analyses
Cell expression of membrane antigens was analyzed by immunofluorescence incubating the cells (1 × 105 lymphocytes in 100 μl of PBS) with specific mAbs at 4 °C for 30 min in the dark. The following mAbs were used: phycoerythrin (PE) or fluorescein isothiocynate (FITC)-conjugated anti-CD127, allophycocianin (APC)-cyanin (Cy) 7-conjugated anti-CD3 (e-Biosciences, San Diego, CA), FITC-conjugated anti-CD25 (Miltenyi, Bergisch Gladbach, Germany), PE-Cy7 or eFluor 450-conjugated anti-CD8 (Biolegend, San Diego, CA or e-Biosciences, San Diego, CA, respectively), Peridinin Chlorophyll Protein Complex-cyanin 5.5 (PerCP-Cy5.5)-conjugated anti-CD28 (eBioscience), APC-conjugated anti-CD39 (Serotec, MorphoSys US Inc), FITC-conjugated anti-CD103 (Becton–Dickinson, BD Biosciences, San Josè CA), PE-conjugated anti-NKG2A (IL, Milan, Italy), FITC-conjugated anti-HLA-G (Exbio Praha a.s., Vestec, CZ), PE-conjugated anti-LAG3 (Bioss Inc., Vobum, MA), PE-conjugated anti-CD122 (BD Biosciences, San Josè CA), FITC-conjugated anti-PD1 (e-Biosciences, San Diego, CA), PE-conjugated anti-TGFβ (BD Biosciences, San Josè CA). Fluorochrome-conjugated isotype matched Abs were used as controls (BD Biosciences, San Josè CA). After staining procedures, the cells were acquired and analyzed by a FACSCanto flow cytometer (BD Biosciences, San Josè CA) using FACSDIVA software (BD Biosciences). Concerning the experiments in which the mean fluorescence intensity (MFI) of CD39 antigen expression was evaluated, data are expressed as CD39+ cell MFI divided by CD39- cell MFI in order to minimize the effects of interassay variations.
Analysis of IDO-1 expression by RT-PCR
Total RNA was extracted from frozen samples by using Omnizol reagent (EuroClone, United Kingdom, UK) according to the producer’s instructions and eluted in 50 μl H2O RNAase free. This RNA solution was treated with 6 U DNase I (Promega, Madison, WI, USA). Reverse transcription was carried out on 500 ng of total RNA using the Superscript II Reverse Transcriptase kit (Life Technologies, Carlsbad, CA). The following oligonucleotide pairs were used (sense and antisense, respectively): IDO-1, 5′ CTCAGTTCCTCCAGGACATG-3′ and 5′-TAAATCAGTGCCTCCAGTTCC-3′; GADPH, 5′- GGCATCCTGGGCTACACTGA-3′ and 5′- TGGTGGTCCAGGGGTCTT-3′. Classical RT-PCR amplification was undertaken with DreamTaq DNA polymerase (Fermentas, Amherst NY, USA). The cycling conditions were 5 min at 96 °C, 37 cycles of 45 s at 94 °C, 45 s at 57 °C, 1 min at 72 °C, with a final extension of 10 min at 72 °C. IDO-1 PCR product was confirmed by sequence analysis using the ABI BigDye Terminator Ready Reaction Mix (Life Technologies, Carlsbad, CA) and analyzed on an ABI 3130XL Genetic Analyzer (Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol.
Purification of CD8+ and CD4+CD25+ T lymphocytes
Peripheral blood mononuclear cells (PBMC) were purified from heparinized blood samples from healthy controls by centrifugation on Ficoll-Hypaque gradient (Biochrom AG, Berlin, Germany) for 30 min at 1,800 rpm.
Lymphocytes from surgical specimens were purified filtering minced tissues using a sterile cell strainer (Falcon, BD Biosciences, San Josè CA) and running the collected cells on Ficoll gradient. The total number of recovered lymphocytes ranged from 1 × 106 to 6 × 106 cells in the different samples. CD8+ T cells were isolated by sequential cycles of cell sorting on magnetic beads using microbeads conjugated with mAb specific for the CD8 antigen (Dynal CD8 positive isolation kit, Invitrogen by Life Technologies Ltd., Paisley, UK) following the manufacturer’s instructions. When the amount of purified cells made it possible, intratumoral CD8+ Treg were further isolated by cytometric cell sorting. To this aim, purified CD8+ T lymphocytes were labeled with PE-Cy7 or eFluor 450-conjugated anti-CD8 mAb, PE-conjugated anti-CD127 mAb, PerCP-Cy5.5-conjugated anti-CD28 mAb, and APC-conjugated anti-CD39 mAb and CD8+ CD28-CD127-CD39+ Treg were sorted using a FACSAria cytometer (BD Biosciences). The CD4+CD25+ regulatory T cell isolation kit, human (Miltenyi Biotech, Bergisch Gladbach, Germany) was used for purification of CD4+CD25+ T cells. The purity of sorted cells was ≥95 % as demonstrated by flow cytometric analysis.
Generation of CD8+ Treg from PBMC
CD8+ Treg were generated as described [31]. Briefly, purified CD8+ T lymphocytes (2 × 105 cells/well) resuspended in culture medium consisting of RPMI 1640 culture medium (Gibco by Life Technologies Ltd., Paisley, UK) added with 10 % fetal calf serum (Invitrogen by Life Technologies Ltd., Paisley, UK) were incubated with 20 U/ml of IL-2 (Proleukin, Eurocetus, Amsterdam, The Netherlands) and 10 ng/ml of IL-10 (PeproTech, Rocky Hill, NJ, USA) or 5 ng/ml of TGFβ (PeproTech, Rocky Hill, NJ, USA) in 96-well flat bottomed plates in 96-well flat-bottom plates (Corning Life Sciences, Amsterdam, The Netherlands) at 37 °C for 7 days.
At the end of the incubation, the cells were collected, washed, counted, and used as suppressors in a proliferation suppression assay.
Proliferation suppression assay
The suppression activity was evaluated by monitoring the inhibition of dye dilution in PBMC from healthy donors stained before the test with carboxyfluorescein succinimidyl ester (CFSE) (5 μM) (Molecular Probes, Invitrogen). After staining, the cells were pulsed with the anti-CD3 UCTH-1 mAb (5 μg/ml, BD Bioscience) and cultured for 5 days in a 96-well flat bottomed plate (1 × 105 cells/well) in the presence (or not) of the following suppressive T cell subpopulations (1 × 105 cells/well): (a) ex vivo generated CD8+ Treg from peripheral blood of healthy donors; (b) CD4+CD25hi Treg purified from the peripheral blood of healthy donors; (c) CD8+ T cells purified from tumor specimens; (d) CD4+CD25hi Treg purified from tumor specimens. In some experiments, CD8+ Treg or intratumoral CD8+ T cells were transfected with a CD39-specific silencing RNA (siRNA) before being tested for their suppression activity. In other experiments, suppression assays were performed in the presence or not of the ecto-ATPase inhibitor diethyl-β-γ-dibromomethylene-D-adenosine-5′-triphosphate trisodium salt hydrate (ARL67156) (250 μMol) (Tocris Bioscience, Bristol, United Kingdom) and/or of the neutralizing anti-IL10 23738 mAb (10 μg/ml) (R&D Systems, Minneapolis, USA), in order to counteract the inhibitory activities of CD39 or IL10, respectively. The mouse IgG2b isotypic control (R&D Systems, Minneapolis, USA) was used for the experiments with the anti-IL10 mAb. Then, the samples were washed in PBS and acquired by flow cytometer. The dead cells were excluded from analysis by adding 7-aminoactinomycin D (Becton–Dickinson, BD Biosciences, San Josè CA) before acquisition.
CD39 gene silencing
SASI_Hs02_00318598 CD39-specific siRNA (Sigma Aldrich s.r.l., Milan, Italy) was used for silencing CD39 gene expression on CD8+ Treg. The sequences of sense and antisense strands of CD39-specific siRNA were 5′-CUAUGUCUUCCUCAUGGUU-3′ and 5′-AACCAUGAGGAAGACAUAG-3′, respectively. In order to make the silencing activity more efficient, single strands of siRNA were annealed, according to the manufacturer’s recommendation. Single strands of siRNA were annealed in an annealing buffer, containing 10 mM Tris, pH 7.5, and 20 mM NaCl in RNAase free water, heated to 95 °C for 1 min, then cooled and annealed at room temperature for 12–16 h. The siRNA were precipitated and resuspended in RNAase free water. To monitor annealing, RNAs were run in a 2 % agarose gel in TAE buffer and stained with SYBR safe. T cells (4 × 105) were transfected with 5 μg of CD39-specific siRNA using the liposomal transfection reagent (Roche Applied Science, Monza, Italy) following the manufacturer’s instructions. Cells were cultured for 24 h in culture medium; cells treated with the liposomal transfection reagent without the CD39-specific siRNA were used as control. CD39 gene silencing by siRNA on Treg was validated by cytofluorimetric analysis.
Statistical analysis
Statistically significant differences between mean values were analyzed by the Mann–Whitney test for nonparametric values or by the paired t-test. Correlations between variables were analyzed by Spearman correlation test. Differences were considered statistically significant when P < 0.05. The statistical analyses were performed using the GraphPad Prism 4.0 Software (GraphPad Software, Inc, La Jolla, CA).
Results
CD8+ Treg generated from healthy donors express CD39
No data are so far available relatively to the expression and eventual functional role of the CD39 molecule in CD8+ Treg. In order to clarify this issue, the expression of CD39 was correlated with the suppression activity of CD8+ or CD4+CD25hi Treg from healthy subjects. To this aim, CD8+ Treg (named non-antigen specific CD8+ Treg in previous work [31]) were generated from circulating CD8+ T cells using either IL10 or TGFβ as inducer cytokine; CD4+CD25hi were purified from the peripheral blood. Both CD8+ Treg, phenotypically characterized to be CD8+ CD28-CD127lo [32] (and not expressing any of the following markers: FoxP3 [32], NKG2a, PD1, CD103, CD122, HLA-G, IDO, LAG3, TGFβ) (Online resource, Supplementary Figure 1), and CD4+CD25hi Treg showed the presence of the CD39 antigen with a similar extent of percentage expression, ranging from 2 to 92 % for CD8+ Treg and 8–100 % for CD4+CD25hi Treg (Table 1, Fig. 1a, b). Notably, CD8+ Treg generated in the presence of either IL10 or TGFβ showed comparable amount of CD39 expression (Online resource, Supplementary Figure 2).
Table 1.
Expression of CD39 molecule on Treg from healthy subjects
| Subject # | %CD39+/CD8+ Treg | %CD39+/CD4+CD25hi Treg |
|---|---|---|
| 1 | 62 | 38 |
| 2 | 30 | 52 |
| 3 | 92 | 76 |
| 4 | 4 | 100 |
| 5 | 2 | 61 |
| 6 | 90 | 49 |
| 7 | 30 | 70 |
| 8 | 7 | 74 |
| 9 | 6 | 14 |
| 10 | 6 | 8 |
| 11 | 50 | nd |
| 12 | 35 | nd |
| 13 | 40 | nd |
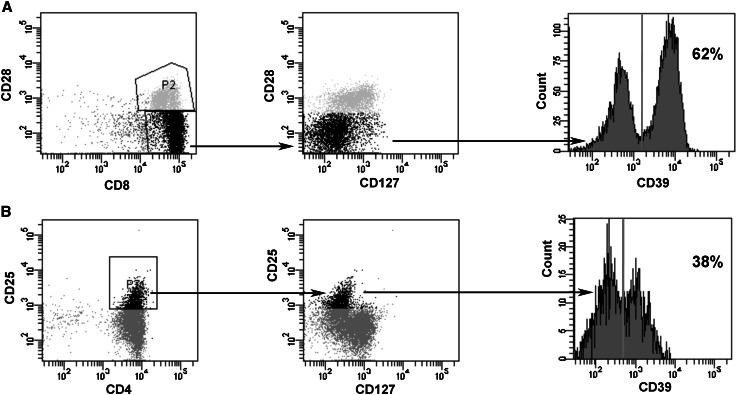
Fig. 1.
Phenotypes of CD8+ Treg (a) and CD4+CD25hi Treg (b) from a healthy subject (donor # 1). CD8+ Treg were generated in vitro from purified CD8+ T lymphocytes; CD4+CD25+ Treg were directly purified from the peripheral blood. Shown data are representative of the CD8+ and CD4+CD25hi Treg phenotypic characterization performed with cells from all the other donors. In the dotplots Treg populations are in black. The percentages of CD39+ Treg are indicated in the histograms
CD39 is involved in the suppression activity of CD8+ Treg generated from healthy donors
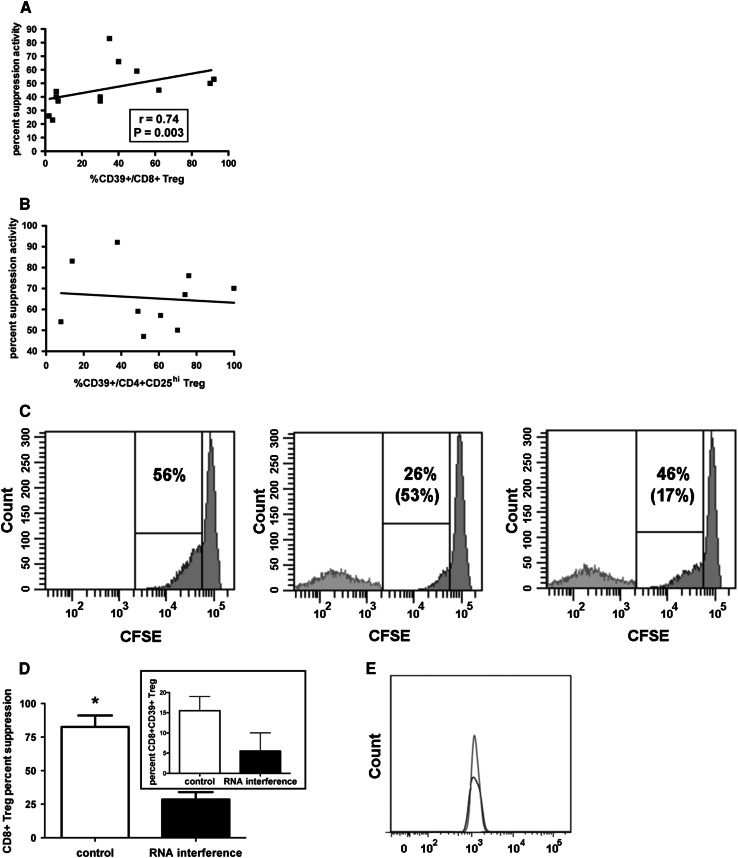
In order to verify whether the CD39 molecule plays any role on CD8+ Treg activity, the suppressive function of CD8+ Treg from 13 healthy donors was correlated with the relative percentage of CD39 expression. Interestingly, a positive correlation was observed between the two variables (Fig. 2a), suggesting a strict relationship between them. The same analysis performed on CD4+CD25hi Treg from the peripheral blood of 10 healthy donors did not show any significant correlation (Fig. 2b).
Fig. 2.
Relationship between suppression activity and CD39 expression in Treg from healthy subjects. CD8+ Treg were generated in vitro from purified CD8+ T lymphocytes; CD4+CD25+ Treg were directly purified from the peripheral blood. a Correlation between percent suppression activity and percent expression of CD39 on CD8+ Treg from 13 healthy subjects; b Correlation between percent suppressive activity and percent expression of CD39 on CD4+CD25hi Treg from 10 healthy subjects; c Experiment of suppression activity by CD8+ Treg treated or not with the SASI_Hs02_00318598 CD39-specific siRNA performed with cells from donor # 3. The histograms refer to the proliferation activity of a responder PBMC stimulated with an anti-CD3 mAb cultured without CD8+ Treg (left panel), with CD8+ Treg (middle panel), or with CD8+ Treg treated with the inhibitory CD39-specific siRNA for 24 h before the test (right panel). The percentages of proliferating cells are shown in all the three panels while the percentages of suppression activity are indicated between parentheses in the middle and right panel, respectively. The experiment is representative of all the experiments performed with cells from the other donors. d Counteraction of CD8+ Treg suppression activity by a CD39-specific interfering RNA. In vitro generated CD8+ Treg were transfected or not with the SASI_Hs02_00318598 CD39-specific siRNA for 24 h before being tested for their suppression activity (big panel). The percentage of CD8+ CD28-CD127loCD39+ Treg in control cells and in cells treated with the CD39-specific siRNA is shown in the insert. The results are expressed as mean ± SD of data from 4 independent experiments performed with cells from donors # 1, 2, 3, 8, respectively. *: P = 0.04. e Histogram showing CD39 expression on control CD8+ Treg untreated (dark gray) or treated with the liposomal transfection reagent in the absence of the CD39 specific siRNA (light gray)
Although a full characterization of CD8+ Treg would have required their purification ex vivo from blood samples of healthy donors, this was not possible due to the very low frequency of these cells in the circulation (Online resource, Supplementary Figure 3, panel A).
In order to define the relevance of CD39-dependent activity on CD8+ Treg global function, experiments were performed using a specific siRNA as CD39 inhibitor. Figure 2c, d demonstrates that downmodulation of CD39 expression on CD8+ Treg by RNA interfering (shown in the insert of Fig. 2d) induced a remarkable (>50 %) reduction of the suppressive function. Notably, siRNA-transfected CD8+ Treg did not show any variation of other tested membrane antigen expression (CD8, CD28, CD127) with respect to untreated cells or to cells treated with the liposomal transfection reagent only, demonstrating the CD39 specificity of siRNA effect (not shown). Moreover, CD39 expression was comparable on CD8+ Treg treated or not with the liposomal transfection reagent without the CD39-specific siRNA, again showing that the CD39 downmodulation is strictly a siRNA-mediated event (Fig. 2e).
CD39 is expressed on intratumoral CD8+ Treg and mediates suppression activity
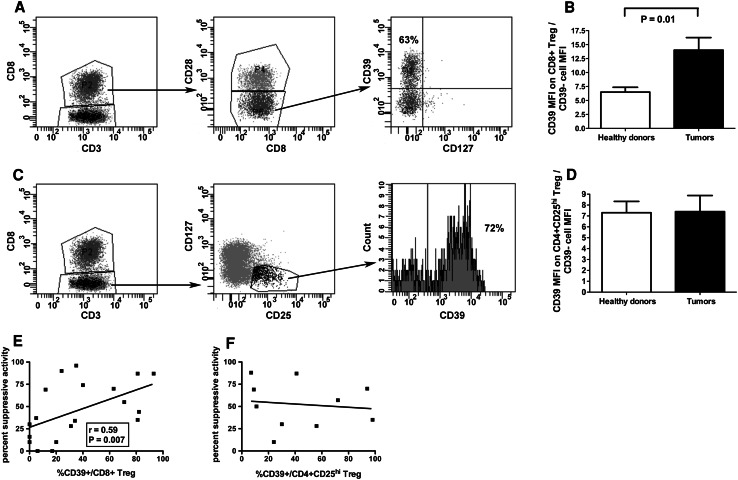
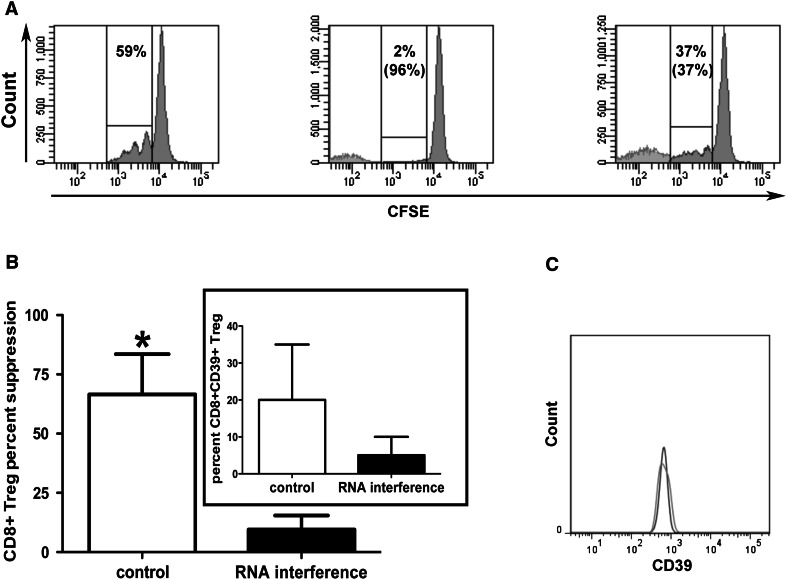
Since CD8+ Treg heavily infiltrate human cancers [14–19], it is of great interest to identify any mechanism used by these cells to induce intratumoral suppressive functions that may hamper tumor-specific immune responses. The characterization of the CD39 molecule as one relevant mediator of CD8+ Treg activity prompted an analysis on tumor-infiltrating CD8+ Treg in order to verify whether CD39-dependent inhibitory function is also exploited by these cells. Hence, the frequencies of CD8+ Treg were comparatively measured in the peripheral blood of healthy subjects and cancer patients. Interestingly, in our series of cancer patients, the percentage of circulating CD8+ CD28-CD127loCD39+Treg was significantly higher than that observed in healthy subjects (Online resource, Supplementary Figure 3, panels A, B and C). In order to verify whether CD8+ CD28-CD127loCD39+Treg were present and functional at the tumor site, intratumoral CD8+ Treg were analyzed from a series of surgical tumor specimens. To achieve comparative information concerning CD4+CD25hi Treg, intratumoral CD4+CD25hi Treg were also considered. In 28 out of 33 cases (84 %), intratumoral CD8+ Treg showed CD39 expression with a range of percentage expression comparable to that of CD8+ Treg generated from the peripheral blood of healthy donors, and with a molecular density per cell (as assessed by the measurement of the specific MFI) higher than that detected in the corresponding cells from healthy donors (Table 2, Fig. 3a, b). Importantly, the frequency of intratumoral CD8+ CD28-CD127loCD39+ Treg significantly correlated with the tumor stage as assessed by TNM classification (Online resource, Supplementary Figure 4). When intratumoral CD4+CD25hi Treg were analyzed, no differences between CD39-specific MFI on cells from healthy donors and tumor specimens were observed (Fig. 3c, d). These data suggest a possible involvement of CD39 in the regulatory activity exerted by each Treg subset within the intratumoral microenvironment. A significant correlation between the percentages of CD39 expression on intratumoral CD8+ Treg, but not on CD4+CD25hi Treg, and their relative suppression activities was detected (Fig. 3e, f) indicating a strict relationship between CD8+ Treg function and the expression of the CD39 molecule. To confirm the involvement of CD39 in mediating the suppression activity of tumor-infiltrating CD8+ Treg, the regulatory activity of CD8+ T cells purified from tumor specimens was also tested after treatment with a CD39-specific siRNA. Indeed, Fig. 4 shows that CD39 downmodulation greatly decreased the extent of suppression activity of tumor-infiltrating CD8+ Treg. These data were further confirmed when CD8+ CD28-CD127loCD39+ Treg were isolated by cytometer cell sorting from specimens of patients #22, #24, and #33 and tested for their suppression activity. Importantly, these cells showed a remarkable suppression activity in all analyzed cases (Online resource, Supplementary Figure 5, panels A, B and C). This was not the case for CD8+ CD28-CD127loCD39- that exerted remarkable suppression activity only in 1 out of 3 cases (Online resource, Supplementary Figure 5, panel D). Interestingly, the suppression activity was mainly dependent on soluble factors since observed also when the experiments were performed in a transwell system (Online resource 5, panel B). In order to define the relative contribution to CD8+ Treg function of CD39 activity compared with IL10 secretion (an already validated mechanism mediating CD8+ Treg suppression) [14], experiments were performed in which CD8+ Treg function was analyzed in cells treated with specific inhibitors of IL10, CD39, or both molecules. Interestingly, different efficacy of each inhibitor and of their combination was detected among samples from different cancer patients (Table 3 and Online resource, Supplementary Figure 6).
Table 2.
Tumor details and relative percentage expression of CD39 on intratumoral CD8+ and CD4+CD25hi Treg
| Patient # | Tumor hystology | Stage TNM | %CD39+/CD8+ Treg | %CD39+/CD4+CD25hi Treg |
|---|---|---|---|---|
| 1 | Renal cell carcinoma | 1B | 20 | nd* |
| 2 | Bladder carcinoma | In situ | 6 | nd |
| 3 | Renal cell carcinoma | 2B | 31 | 56 |
| 4 | Renal liposarcoma | 1 | 12 | 9 |
| 5 | Renal cell carcinoma | 1B | 0 | 30 |
| 6 | Renal cell carcinoma | 2 | 0 | 24 |
| 7 | Colonrectal adenocarcinoma | 3 | 0 | nd |
| 8 | Colonrectal adenocarcinoma | 3 | 71 | 94 |
| 9 | Renal cell carcinoma | 1 | 5 | 11 |
| 10 | Renal cell carcinoma | 1A | 35 | 7 |
| 11 | Renal cell carcinoma | 1B | 17 | nd |
| 12 | Renal cell carcinoma | 2 | 63 | 72 |
| 13 | Renal cell carcinoma | 2B | 93 | 98 |
| 14 | Renal cell carcinoma | 3 | 81 | 41 |
| 15 | Renal cell carcinoma | 3A | 34 | nd |
| 16 | Renal cell carcinoma | 1B | 24 | nd |
| 17 | Renal cell carcinoma | 3A | 40 | nd |
| 18 | Renal cell carcinoma | 3A | 23 | 80 |
| 19 | Renal cell carcinoma | 3A | 43 | 84 |
| 20 | Renal cell carcinoma | 1B | 0 | 33 |
| 21 | Renal cell carcinoma | 2 | 5.7 | 85 |
| 22 | Renal cell carcinoma | 3A | 82 | 55 |
| 23 | Renal cell carcinoma | 1B | 7.5 | 23 |
| 24 | Renal cell carcinoma | 3A | 81 | 98 |
| 25 | Bladder carcinoma | 1A | 6.3 | 71 |
| 26 | Bladder carcinoma | 1 | 0 | 0 |
| 27 | Bladder carcinoma | 2 | 95 | 85 |
| 28 | Bladder carcinoma | 3A | 52 | 74 |
| 29 | Bladder carcinoma | 3 | 42 | 62 |
| 30 | Bladder carcinoma | In situ | 14 | 100 |
| 31 | Bladder carcinoma | In situ | 3.6 | 87 |
| 32 | Bladder carcinoma | In situ | 20 | 82 |
| 33 | Bladder carcinoma | 3 | 25 | 33 |
* Not done due to lack of enough cells or sample unavailable
Table 3.
Suppression activity of intratumoral CD8+ T cells from different tumor patients in the presence or not of CD39 and/or IL10 inhibitors
| Patient # | Culture conditions | |||
|---|---|---|---|---|
| No inhibitors | CD39 inhibitiona | IL10 inhibitionb | CD39 + IL10 inhibition | |
| 15 | 34* | 11 | 25 | 25 |
| 16 | 90 | 76 | 76 | 59 |
| 17 | 74 | 0 | 36 | 0 |
* Data are expressed as percent suppression activity
aCD39 inhibition was achieved performing the assay in the presence of the ecto-ATPase inhibitor ARL67156 (250 μMol). No significant differences of the basal proliferative activity of anti-CD3 mAb-stimulated PBMC were observed in the presence or absence of ARL67156, ruling out the possibility of a direct activity of the inhibitor on the proliferating T cells
bIL10 inhibition was achieved by performing the assay in the presence of the neutralizing anti-IL10 23,738 mAb (10 μg/ml). No significant differences of the basal proliferative activity of anti-CD3 mAb-stimulated PBMC were observed in the presence or absence of the anti-IL10 mAb, ruling out the possibility of a direct activity of the inhibitor on the proliferating T cells. Moreover, in the presence of the isotypic control mAb, the percentages of suppression were 35, 89, and 77 % relatively to patients # 15, 16, and 17, respectively
Fig. 3.
CD39 expression on intratumoral Treg subsets. a and c Phenotypic analyses on tumor-infiltrating CD8+ (a) and CD4+CD25hi (c) Treg purified from tumor specimens of patient # 12. Shown data are representative of the CD8+ and CD4+CD25hi Treg phenotypic characterization performed with cells from all the other tumor specimens. The percentages of CD39+ Treg are indicated. b Comparison of CD39 MFI between CD8+ Treg ex vivo generated from the peripheral blood of healthy donors and CD8+ Treg purified from tumor specimens; data are expressed as MFI on CD8+ Treg divided by MFI of CD39- cells in order to minimize the effects of interassay variations. d Comparison of CD39 MFI between CD4+CD25hi Treg from the peripheral blood of healthy donors and CD4+CD25hi Treg purified from tumor specimens; data are expressed as MFI on CD4+CD25hi Treg divided by MFI of CD39- cells in order to minimize the effects of interassay variations. e Correlation between percent suppression activity and CD39 percent expression of CD8+ Treg from tumor specimens of all 19 patients enrolled in the study; f Correlation between percent suppression activity and CD39 percent expression of CD4+CD25hi Treg from tumor specimens of patients # 3, 4, 5, 6, 8, 9, 10, 12, 13, 14
Fig. 4.
CD39 expression on intratumoral Treg subsets and its relationship with their relative function. a Suppression activity of CD8+ Treg treated or not with the SASI_Hs02_00318598 CD39-specific siRNA: the experiment was performed with cells from patient # 12, and it is representative of all the experiments performed with cells from the other patients. The histograms refer to the proliferation activity of a responder PBMC (from one healthy donor) stimulated with an anti-CD3 mAb cultured without CD8+ Treg (left panel), with CD8+ Treg (middle panel), or with CD8+ Treg treated with the inhibitory CD39-specific siRNA for 24 h before the test (right panel). A control sample was also prepared culturing the anti-CD3 mAb-stimulated PBMC with CD8+ Treg treated with the liposomal transfection reagent used to transfect the cells with the siRNA but without the siRNA itself: in all the experiments, the suppression activity of these cells was comparable to that of untreated CD8+ Treg (not shown). The percentages of proliferating cells are shown in all the three panels while the percentages of suppression activity are indicated between parentheses in the middle and right panel, respectively. b Counteraction of CD8+ Treg suppression activity by a CD39-specific siRNA. CD8+ T lymphocytes purified from tumor specimens were transfected or not with the SASI_Hs02_00318598 CD39-specific siRNA 24 h before being tested for their suppression activity. The efficient downmodulation of the CD39 expression is shown in the insert. The results are expressed as mean ± SD of data from 4 independent experiments performed with cells from patients # 8, 9, 10, 12, respectively. c Histogram showing CD39 expression on control CD8+ Treg untreated (dark gray) or treated with the liposomal transfection reagent in the absence of the CD39 specific siRNA (light gray)
Discussion
The results of our study show that: (a) CD39 is frequently expressed by CD8+ Treg and is strictly involved in CD8+ Treg suppression activity; (b) CD39-dependent suppression is a main mechanism of action of tumor-infiltrating CD8+ Treg.
CD39 acts as immunomodulator by triggering the metabolic pathway that degrades exogenous ATP leading to adenosine production. Hence, at the same time, it deprives cells of an important energy source and favors the formation of a potent inhibitor of effector function. On this basis, it is not surprising that CD39 is expressed by CD4+CD25hi Treg where it has been previously shown to be linked with Foxp3 expression [32] and related to their inhibitory function [5, 6]. Considering that Treg are also present within the CD8+ T cell subpopulation, we searched for CD39 expression and functional relevance on CD8+ Treg. CD8+ Treg constitute an intricate cellular network, characterized by a wide array of subtypes differing each other for phenotypic and functional features (reviewed in [10]). We focused our attention on one specific CD8+ Treg subpopulation, previously called “non-antigen specific CD8+ Treg”, because these cells have already been found in tumor microenvironment [14]. In the previous studies, we found that these cells are characterized phenotypically by their lack of CD25, CD28, CD127, CCR7, and Foxp3 expression, and by the presence on their surface of CD45RA antigen [31, 32], thus suggesting that these cells are terminally differentiated T lymphocytes [33] not belonging to the population of Foxp3-dependent, natural Treg [34]. Although their generation may be mediated by both IL10 and TGFβ, as shown here, functionally these cells are strictly IL10-dependent, since their activity requires this cytokine to occur [31]. Indeed, they mediate their inhibitory signals through soluble factors, as demonstrated by the fact that their activity is unaltered when exerted in transwell systems [31]. IL10 is not the only player since CD8+ Treg function is hampered by counteraction of other molecules such as IFNγ and IL6 [35]. It is noteworthy to consider that all these cytokines may also have activatory, pro-inflammatory functions mainly triggering cells of innate immunity [36–38], suggesting that the role of CD8+ Treg is not only to suppress, but also to fine tune the homeostasis between innate and adoptive immunity.
Here, we enrich the knowledge on this Treg subset by demonstrating that CD39 may be also a relevant molecule for their function, notwithstanding the lack of Foxp3 expression [32]. It is likely that molecular pathways other than Foxp3 drive CD39 expression in this type of CD8+ Treg, and their identification will be the focus of future work. Importantly, CD39 percentage expression correlated with the functional activity of CD8+ Treg, an unprecedented finding replicated in both cells from healthy donors and tumor specimens. This suggests that CD39 activity on CD8+ CD28-CD127loCD39+ Treg may have a dominant functional role. Accordingly, our findings highlight that the cell subset constituted by CD8+ CD28-CD127lo T cells not expressing CD39 may have different functional behavior in different subjects, in the sense that these cells may act or not as Treg. This observation, together with the consideration that CD8+ CD28-CD127loCD39+ T cells invariably exert suppression activity, suggests that CD39 likely identifies a homogeneous subset with regulatory function among the CD8+ CD28-CD127lo T cells. The relevance of the identification of this particular subpopulation of Treg is suggested by the correlation that the level of their intratumoral infiltrate shows with tumor stage. This observation, associated with the finding of high frequency of these cells in the peripheral blood of cancer patients with respect to healthy subjects, suggests that their active recruitment at the tumor site might have a pathogenic role.
The discovery that CD39 is involved in CD8+ Treg suppression activity indicates that these cells may use several, potentially complementary pathways of immune regulation. Whether all these pathways act at the same time or vicariate each other in relationship with microenvironmental input has to be elucidated. It is also possible that the redundancy of inhibitory mediators expressed by these cells could be related to their specific activity on different cell subsets (as it is the case of CD4+ and CD8+ T lymphocytes that are both inhibited by CD8+ CD28-CD127loCD39+ Treg). On this basis, it will be of interest in the next studies to analyze the activity of CD8+ CD28-CD127loCD39+ Treg on other cell types involved in the immune response, such as DC, monocytes and NK lymphocytes, correlating the eventual inhibitory effects with the specific molecular mediator. Our data show that IL10 or CD39 inhibitors had variable efficacy in modulating the regulatory activity of CD8+ CD28-CD127loCD39+ Treg from different tumor patients. This observation highlights the importance of the characterization of intratumoral regulatory pathways (and molecules) in order to fully understand the leading mechanisms of immune escape at play in individual patients. Indeed, each of these mechanisms may represent a useful target for immune modulation in anti-tumor immunotherapy, provided that it is taken into consideration the immunobiology of the single tumor and that specific immune intervention(s) is tailored to patient requirements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported in part by a grant from Compagnia di San Paolo entitled “Immunoterapia anti-tumorale: analisi d’efficacia dei principali protocolli tradizionali d’immunizzazione e validazione dell’efficacia terapeutica dell’inibizione dell’interleuchina 10 nel trattamento del melanoma” and in part by a PRIN grant from MIUR entitled “Immunoterapia anti-tumorale operata attraverso l’inibizione dei circuiti regolatori citochino-dipendenti”. We thank Prof. A. Bacigalupo, Centro Cellule Staminali e Terapie Cellulari, IRCSS Azienda Ospedaliera Universitaria-San Martino IST, for allowing us to work in the Facs sorting facility.
Conflict of interest
The authors declare that they do not have conflict of interest.
Footnotes
Alessia Parodi and Florinda Battaglia contributed equally to this work.
References
- 1.Maliszewski CR, Delespesse GJ, Schoenborn MA, Armitage RJ, Fanslow WC, Nakajima T, Baker E, Sutherland GR, Poindexter K, Birks C. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol. 1994;153:3574–3583. [PubMed] [Google Scholar]
- 2.Mizumoto N, Kumamoto T, Robson SC, Sévigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 3.Airas L, Hellman J, Salmi M, Bono P, Puurunen T, Smith DJ, Jalkanen S. CD73 is involved in lymphocyte binding to the endothelium: characterization of lymphocyte-vascular adhesion protein 2 identifies it as CD73. J Exp Med. 1995;182:1603–1608. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 5.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 6.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 8.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 9.Moller G. Do suppressor T cells exist? Scand. J Immunol. 1988;27:247–250. doi: 10.1111/j.1365-3083.1988.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 10.Filaci G, Fenoglio D, Indiveri F. CD8(+) T regulatory/suppressor cells and their relationships with autoreactivity and autoimmunity. Autoimmunity. 2011;44:51–57. doi: 10.3109/08916931003782171. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin Immunol. 2011;23:446–452. doi: 10.1016/j.smim.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 13.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F. T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 15.Wang RF. CD8+ regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol. 2008;69:811–814. doi: 10.1016/j.humimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 16.Andersen MH, Sørensen RB, Brimnes MK, Svane IM, Becker JC, Straten P. Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J Clin Invest. 2009;119:2245–2256. doi: 10.1172/JCI38739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarnicki AG, Lysaght J, Todryk S, Mills KM. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 18.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 19.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+ CD25+ Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 20.Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE. 2011;6:e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liotta F, Gacci M, Frosali F, Querci V, Vittori G, Lapini A, Santarlasci V, Serni S, Cosmi L, Maggi L, Angeli R, Mazzinghi B, Romagnani P, Maggi E, Carini M, Romagnani S, Annunziato F. Frequency of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int. 2010;107:1500–1506. doi: 10.1111/j.1464-410X.2010.09555.x. [DOI] [PubMed] [Google Scholar]
- 22.Raghavan S, Quiding-Järbrink M. Regulatory T cells in gastrointestinal tumors. Expert Rev Gastroenterol Hepatol. 2011;5:489–501. doi: 10.1586/egh.11.44. [DOI] [PubMed] [Google Scholar]
- 23.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K, Ueoka H. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 27.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic selftolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 29.Künzli BM, Bernlochner MI, Rath S, Käser S, Csizmadia E, Enjyoji K, Cowan P, d’Apice A, Dwyer K, Rosenberg R, Perren A, Friess H, Maurer CA, Robson SC. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;7:231–241. doi: 10.1007/s11302-011-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandapathil M, Whiteside TL. Targeting human inducible regulatory T cells (Tr1) in patients with cancer: blocking of adenosine-prostaglandin E(2) cooperation. Expert Opin Biol Ther. 2011;11:1203–1214. doi: 10.1517/14712598.2011.581225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, Brenci S, Contini P, Olive D, Ghio M, Setti M, Accolla RS, Puppo F, Indiveri F. Non-antigen specific CD8+ T suppressor lymphocytes originate from CD8+ CD28- T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65:142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Fenoglio D, Ferrera F, Fravega M, Balestra P, Battaglia F, Proietti M, Andrei C, Olive D, Antonio LC, Indiveri F, Filaci G. Advancements on phenotypic and functional characterization of non-antigen-specific CD8+ CD28- regulatory T cells. Hum Immunol. 2008;69:745–750. doi: 10.1016/j.humimm.2008.08.282. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, Setti M, Puppo F, Indiveri F. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166:6452–6457. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 36.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM The dual role of IL-10. Trends Immunol. 2003;24:36–43. doi: 10.1016/S1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 37.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 38.Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.