Abstract
Purpose and experimental design
Although an increase in regulatory T cells (Tregs) is observed in tumor microenvironments, the underlying mechanism is not fully clarified. Since it was suggested that Tregs showed a lower sensitivity toward oxidative stress in comparison with conventional T cells, in the present study, we investigated the H2O2 production and apoptosis of Tregs in gastric and esophageal cancer tissues, employing flow cytometric analysis using fresh samples (n = 93) and immunohistochemical analysis (n = 203).
Results
The increased tumor-infiltrating Tregs coexisted with elevated H2O2 production according to disease progression. The grade of apoptosis in Tregs was less pronounced than that in conventional T cells, and there was a positive correlation between H2O2 production and the grade of apoptosis in conventional T cells, while there was no correlation between H2O2 production and the grade of apoptosis in Tregs. Moreover, Tregs were less sensitive to H2O2-induced apoptosis compared with conventional T cells in vitro.
Conclusions
We have demonstrated that the increased prevalence of tumor-infiltrating Tregs closely related to their lower sensitivity to H2O2-induced apoptosis.
Keywords: Foxp3, Regulatory T cells, Gastric cancer, Esophageal cancer, Hydrogen peroxide
Introduction
Regulatory T cells (Tregs) are one of the T-cell subsets, which have a functionally immunosuppressive property that inhibits effector cells from acting against the self in auto-immune diseases or tumor [1, 2].
Recently, many studies of human tumors have shown that the frequency of Tregs among tumor-infiltrating lymphocytes (TILs), tumor-draining regional lymph nodes, and peripheral blood lymphocytes (PBLs) is higher in cancer patients in comparison with their normal counterparts in several malignancies [3–5]. Of note, we previously showed that after patients underwent the curative resection of gastric cancer, the increased proportion of Tregs was significantly reduced, and the levels were almost equal to those in normal healthy donors [6]. These results strongly suggest that tumor-related factors induce and expand the Treg pool. However, the underlying mechanisms behind Treg accumulation within tumor microenvironments are not fully clarified. There have been several explanations describing the mechanisms behind the tumor microenvironment recruiting Tregs, including CCL22 or CCL17 [5, 7, 8], nitric oxide production, COX-2/PGE2 expression, or interleukin IL-10 [9, 10]. In addition, it has also been shown that Tregs are less sensitive to apoptosis induced by H2O2 in comparison with conventional CD4 T cells in vitro [11], and it is well known that tumor microenvironments produce significant amounts of reactive oxygen species such as H2O2 [12, 13]. These observations suggest that H2O2-induced apoptosis may be related to the selective enrichment of Tregs within tumor microenvironments. Thus, it is important to evaluate the balance between the recruitment and survival of Tregs, in order to clarify the regulation of Tregs within tumor microenvironments. However, to our knowledge, no previous report has described the relationship between H2O2 production and Treg accumulation within tumor microenvironments in human tumor specimens in vivo.
In the present study, we investigated the H2O2 production and apoptosis of Tregs in patients with gastric and esophageal cancer, employing flow cytometric analysis using fresh samples and immunohistochemical analysis.
Materials and methods
Patients
For flow cytometric analysis with fresh samples, 53 patients with gastric cancer (40 men and 13 women, 65.7 ± 9.9 years old, 40 intestinal type and 13 diffuse type according to the Lauren classification) and 40 patients with esophageal squamous cell carcinoma (ESCC) (37 men and 3 women, 69.5 ± 8.1 years old), who were operated on at the University of Yamanashi Hospital, were enrolled. Patients with gastric cancer were divided into 2 groups: those with early disease (n = 33) corresponding to stage I of the TNM classification for gastric cancer (UICC, 5th edition) and those with advanced disease (n = 20) corresponding to stages II (n = 7), III (n = 11), and IV (n = 2). Patients with ESCC were divided into 2 groups: those with early disease (n = 16) corresponding to stages 0 (n = 3) and I (n = 13) of the TNM classification for esophageal cancer (UICC, 5th edition) and those with advanced disease (n = 24) corresponding to stages II (n = 14), III (n = 8), and IV (n = 2).
For immunohistochemical analysis with formalin-embedded specimens, 108 gastric cancer (n = 51 in stage I, n = 25 in stage II, n = 14 in stage III, and n = 18 in stage IV) and 95 ESCC patients (n = 7 in stage 0, n = 12 in stage I, n = 35 in stage II, n = 31 in stage III, and n = 10 in stage IV) were enrolled in the present study. In gastric cancer, 79 patients were intestinal type and 29 were diffuse type, according to the Lauren classification.
This study was approved by the ethics committee of the University of Yamanashi, and written informed consent was obtained from all patients.
Cell preparation
Tumor tissue and normal gastric and esophageal mucosa (0.8–1.0 cm3) were isolated during surgery and homogenized by mechanical mincing with X-VIVO15 medium (CAMBREX, East Rutherford, NJ, USA). Subsequently, the single-cell suspension was purified by centrifugation with Ficoll-Paque Plus (Amersham Biosciences, Uppsala, Sweden).
CD4(+)CD25high and CD4(+)CD25(−)T cells were isolated from PBLs from healthy donors using magnetic bead separation, which is CD4-negative selection followed by CD25-positive selection using a Dynal® CD4(+)CD25(+) Tregs Kit (Invitrogen, Carlsbad, CA, USA).
Immunohistochemistry with 8OHdG-, Foxp3-, CD4-, and CD8-mAbs
8-hydroxy-2′-deoxyguanosine (8OHdG) staining was performed by using the avidin–biotin–peroxidase complex method (Vectastain ABC elite kit, Vector laboratories, Burlingame, CA, USA) [14]. Briefly, each paraffin section was dewaxed, followed by antigen retrieval with Target Retrieval Solution (10 mmol citrate buffer at pH 6.0, Dako, Glostrup, Denmark) with an autoclave (121 °C, 15 min), and we incubated sections for 20 min with diluted normal blocking serum. Then, anti-8OHdG-monoclonal-antibody (the Japan Institute for the Control of Aging, Fukuroi, Japan) was applied for 4 h at room temperature. Thereafter, the sections were incubated with 1 % H2O2 for 10 min, and peroxidase-linked secondary antibodies and diaminobenzidine were used to detect specific binding.
Foxp3 staining was performed by using the avidin–biotin–peroxidase complex method described by us [15]. Briefly, each paraffin section was dewaxed, followed by antigen retrieval with Epitope Retrieval Solution (10 mmol citrate buffer at pH 6.0, Dako) in a preheated water bath (98 °C, 40 min), and endogenous peroxidase was blocked by Chemmate Peroxidase Blocking Solution (Dako). Then, biotinylated anti-human Foxp3 antibody (diluted by PBS at 1:20, eBioscience, San Diego, CA, USA) was applied for 40 min at room temperature. Thereafter, the sections were incubated with streptavidin-conjugated horseradish peroxidase (Dako) for 10 min, followed by development with 3,3′-diaminobenzidine (Dako) for 5 min.
CD8- and CD4-staining was performed by using the avidin–biotin–peroxidase complex method. Briefly, each paraffin section was dewaxed, followed by antigen retrieval with Target Retrieval Solution (10 mmol citrate buffer at pH 6.0, Dako) with an autoclave (121 °C, 15 min). The sections were cooled at room temperature for 30 min. After washing with PBS, endogenous peroxidase was blocked with 3 % hydrogen peroxide. Next, after washing again with PBS, the sections were incubated with diluted normal blocking serum for 20 min and incubated with diluted anti-human-CD8-mAb (Clone C8/144B, Dako) or anti-human-CD4-mAb (Clone 4B12, Dako), overnight at 4 °C. Thereafter, peroxidase-linked secondary antibodies and diaminobenzidine were used to detect specific binding.
Quantitative evaluation of 8OHdG-, Foxp3-, CD4-, and CD8-positive cells was carried out using five randomly selected areas (0.8–1.5 mm2/sample) at a magnification of 400× by two observers (Y. M. and K. K.) in a blinded manner.
H2O2 production assay with flow cytometry
The production of H2O2 by both baseline and stimulated monocytes was assessed by the formation of intracellular 2′,7′-dichlorofluorescein (DCF) described by us [16, 17]. Cells in 200 μl of X-vivo were incubated with 5 μl of 1 mM 5-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acethyl ester (CM-H2DCFDA; Invitrogen) for 20 min at 37 °C in a shaking water bath shielded from the light in the presence or absence of phorbol myristate acetate (PMA; Wako pure chemicals, Osaka, Japan; final concentration, 0.5 μg/ml). The reaction was stopped by the addition of 100 μl of ice-cold PBS, and cells were stained with CD14-APC (Dako). The production of H2O2 was determined as the mean DCF fluorescent intensity of PMA-stimulated CD14(+) cells—mean DCF fluorescent intensity of non-stimulated CD14(+) cells.
Flow cytometric analysis for Tregs and apoptosis
To analyze the prevalence of Tregs, CD4(+)CD25(+)Foxp3(+) cells were evaluated using the Human Regulatory T-cell Staining Kit (eBioscience) according to the manufacturer’s protocol. Briefly, the single-cell suspension was stained using a cocktail of anti-CD4-FITC mAb and anti-CD25-APC mAb, and, then, after permeabilization, samples were blocked by normal rat serum and stained using anti-Foxp3-PE mAb or PE-conjugated rat IgG2a as an isotype control. Subsequently, the number of Foxp3(+) cells on the gating of CD4(+) cells was evaluated, and the frequency of Foxp3(+) Tregs was expressed as a percentage of the total CD4(+) cells.
For the analysis of apoptosis, freshly isolated cells derived from TILs were stained using anti-CD4-FITC mAb, anti-CD25-APC mAb, and 7-amino-actinomycin D (7-AAD) (BD, Franklin Lakes, NJ, USA).
H2O2 treatment in vitro
Lymphocytes were incubated with a physiological dose of H2O2 in AIM-V serum-free medium (Invitrogen) for 4 h, and subjected to apoptosis analysis with 7-AAD staining.
Statistical analysis
The non-paired Student’s t-test was used to examine the differences between the groups. Correlations between values were evaluated using non-parametric Spearman’s rank correlation. Findings were considered significant when p values were <0.05.
Results
Increased prevalence of Foxp3(+) cells in gastric and esophageal cancer
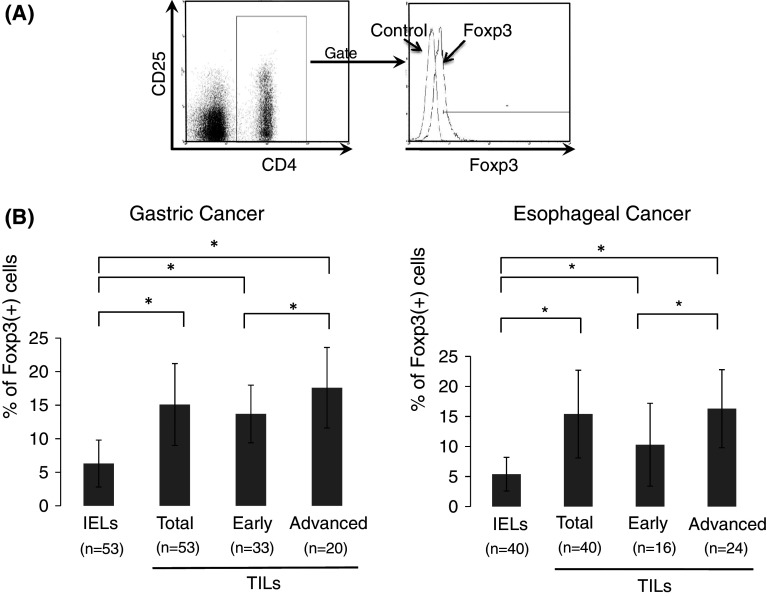
To analyze the prevalence of Tregs in freshly isolated samples, CD4(+)CD25(+)Foxp3(+) cells were evaluated by flow cytometry (Fig. 1a) and expressed as a percentage of the total CD4(+) cells. In all cases of gastric cancer (n = 53), the frequency of Tregs in TILs of gastric cancer was significantly higher than that in intraepithelial lymphocytes (IELs) of the normal gastric mucosa (mean ± SD: 15.1 ± 6.1 % vs. 6.3 ± 3.5 %, respectively, Fig. 1b). Furthermore, when patients with gastric cancer were divided into two groups: those with early disease (n = 33) corresponding to stage I and those with advanced disease corresponding to stages II, III, and IV (n = 20), there was a significant difference in the frequency of Tregs in TILs between patients with early and advanced disease (13.7 ± 4.3 % vs. 17.6 ± 6.0 %, respectively).
Fig. 1.
Increased prevalence of Foxp3(+) cells in gastric and esophageal cancer. CD4(+)CD25(+)Foxp3(+) cells are expressed as a percentage of the total CD4(+) cells analyzed by flow cytometry (a). In patients with gastric and esophageal cancer, the frequency of Tregs (%Foxp3) in tumor-infiltrating lymphocytes (TILs) and intraepithelial lymphocytes (IELs) of the normal mucosa is shown in b. Patients with gastric cancer were divided into two groups: those with early disease corresponding to stage I and those with advanced disease corresponding to stages II, III, and IV. Patients with esophageal cancer were divided into two groups: those with early disease corresponding to stages 0 and I and those with advanced disease corresponding to stages II, III, and IV. Data were analyzed using a non-paired Student’s t-test, and findings were considered significant when p values were <0.01 (*)
Similarly, in all cases of ESCC (n = 40), the frequency of Tregs in TILs was significantly higher than that in IELs of the normal esophageal mucosa (15.4 ± 7.3 % vs. 5.4 ± 2.8 %, respectively, Fig. 1b). Furthermore, when patients with ESCC were divided into two groups: those with early disease (n = 16) corresponding to stages 0 and I and those with advanced disease corresponding to stages II, III, and IV (n = 24), there was a significant difference in the frequency of Tregs in TILs between patients with early and advanced disease (10.3 ± 6.9 % vs. 16.3 ± 6.8 %, respectively). Thus, an increased prevalence of Tregs was observed in TILs of gastric cancer and ESCC, and their grades were elevated according to disease progression.
H2O2 production within tumor microenvironments
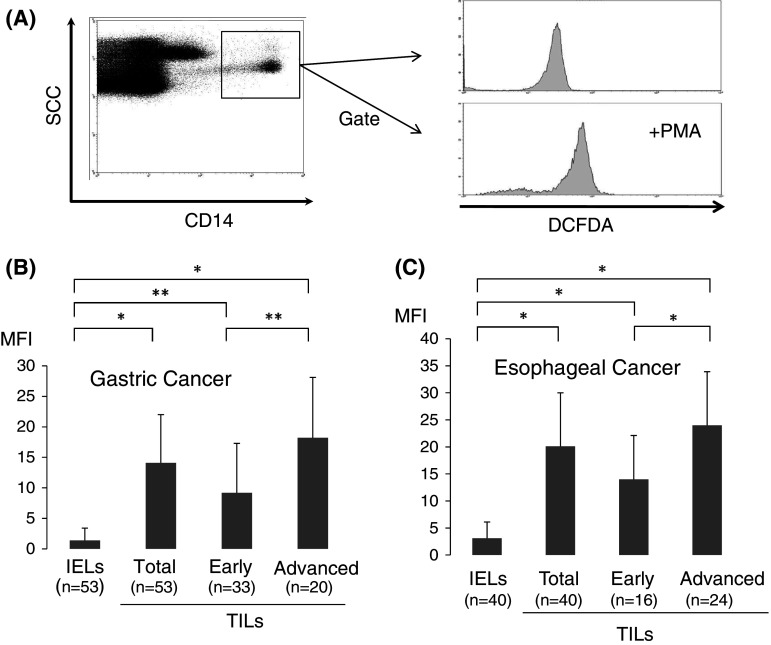
In order to evaluate the oxidative state in tumor microenvironments in freshly isolated samples, we evaluated the levels of H2O2 within tumor-infiltrating Mϕ with flow cytometric analysis employing the DCFDA/PE-CD14 technique [16, 17].
As shown in Fig. 2a, the production of H2O2 derived from tumor-infiltrating Mϕ can be evaluated quantitatively by flow cytometry. As a result, H2O2 production was significantly elevated in TILs [14.1 ± 7.9, mean fluorescence intensity (MFI)] of gastric cancer in comparison with IELs of the normal gastric mucosa (1.4 ± 2.0 MFI), and the grade of H2O2 production within TILs was more pronounced in advanced than in early disease of gastric cancer (Fig. 2b). Similarly, H2O2 production was significantly increased in TILs (20.1 ± 9.9 MFI) of ESCC in comparison with IELs of the normal esophageal mucosa (3.1 ± 3.0 MFI), and the grade of H2O2 production in tumor-infiltrating Mϕ was more pronounced in advanced than in early disease of ESCC (Fig. 2c). Thus, the levels of H2O2 production in tumor-infiltrating Mϕ were up-regulated according to disease progression.
Fig. 2.
H2O2 production within tumor microenvironments. The production of H2O2 derived from tumor-infiltrating Mϕ was quantitatively evaluated with flow cytometric analysis employing the DCFDA/PE-CD14 technique (a). H2O2 production, defined as the mean fluorescence intensity (MFI), is shown in TILs and IELs of gastric (b) and esophageal cancer (c). The definition of early and advanced disease of gastric and esophageal cancer is described in the legend of Fig. 1. Data were analyzed using a non-paired Student’s t-test, and findings were considered significant when p values were <0.01 (*). or < 0.05 (**)
Less apoptotic cells in CD4(+)CD25high regulatory T cells within tumor microenvironments
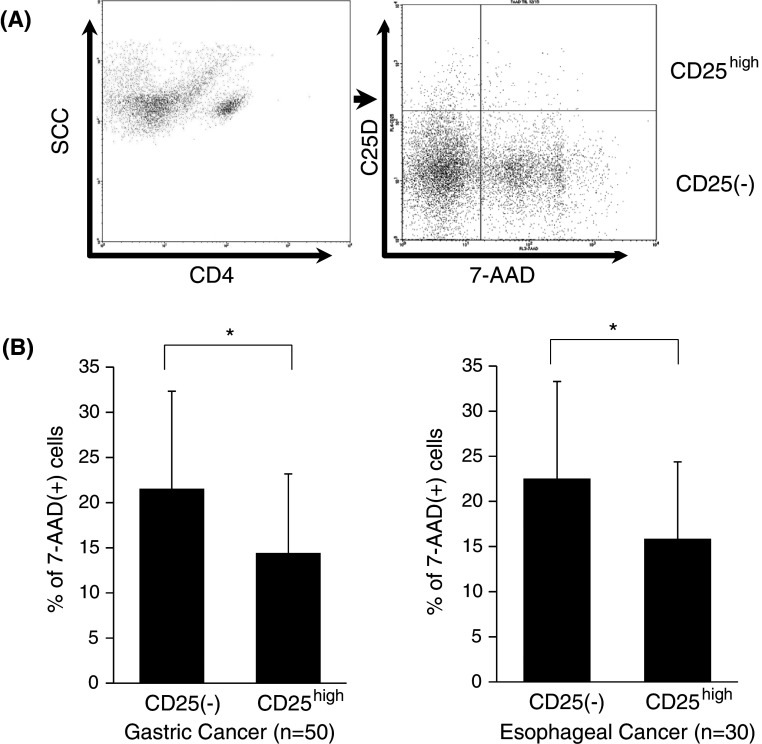
To further evaluate the relationship between the apoptosis of Tregs and H2O2 production in freshly isolated TILs, we investigated the proportion of apoptotic cells showing 7-AAD staining between CD4(+)CD25(−)T cells and CD4(+)CD25highTregs derived from freshly isolated TILs. Since intracellular Foxp3 staining leads to major artifacts regarding apoptotic analysis such as 7-AAD staining, CD4(+)CD25high T cells as a marker of Tregs were evaluated in this experiment (Fig. 3a). In our experimental setting, CD4(+)CD25high T cells comprised more than 93 % Foxp3(+) Tregs.
Fig. 3.
Reduced apoptotic cells in CD4(+)CD25high regulatory T cells. The proportion of apoptotic cells showing 7-AAD-positive staining between CD4(+)CD25(−)T cells and CD4(+)CD25highTregs was evaluated by flow cytometry (a). Summarized data derived from freshly isolated TILs in gastric and esophageal cancer are shown in b. Data were analyzed using a non-paired Student’s t-test, and findings were considered significant when p values were <0.01 (*)
As shown in Fig. 3b, the proportion of 7-AAD-positive apoptotic cells was significantly elevated in CD4(+)CD25(−)T cells compared with CD4(+)CD25highTregs in TILs derived from both gastric (21.5 ± 10.8 % vs. 14.4 ± 7.8 %, respectively) and esophageal (22.5 ± 10.8 % vs. 15.1 ± 8.1 %, respectively) cancer (Fig. 3b). Thus, less apoptotic cells were observed in Tregs in comparison with conventional CD4(+) T cells within tumor microenvironments.
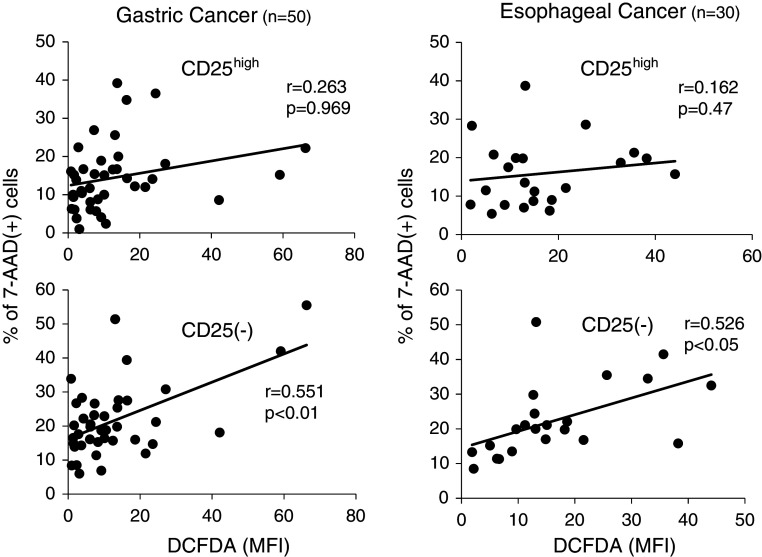
Furthermore, as shown in Fig. 4, the proportion of apoptotic cells in CD4(+)CD25(−)T cells was positively correlated with H2O2 production from tumor-infiltrating Mϕ both in gastric and esophageal cancer, while the proportion of apoptotic cells in CD4(+)CD25highTregs did not significantly correlated with H2O2 production. Thus, the grade of apoptosis in Tregs did not differ regardless of H2O2 production within tumor microenvironments, while the apoptosis of conventional CD4(+) T cells was up-regulated according to the increased H2O2 production.
Fig. 4.
Correlation of apoptotic cells with H2O2 production. The production of H2O2 derived from tumor-infiltrating Mϕ was evaluated quantitatively with flow cytometric analysis employing the DCFDA/PE-CD14 technique, as shown in Fig. 2. The proportion of apoptotic cells showing 7-AAD-positive staining was evaluated by flow cytometry. The correlation of apoptotic cells with H2O2 production was evaluated between CD4(+)CD25(−)T cells and CD4(+)CD25highTregs. Correlations between values were evaluated using non-parametric Spearman’s rank correlation
Oxidative stress and Foxp3(+) Tregs evaluated by immunohistochemistry
In order to obtain further supporting evidence that the oxidative state is related to Tregs apoptosis within tumor microenvironments, immunohistochemical analysis of the oxidative stress (anti-8OHdG) and the infiltrating grade of Tregs (anti-Foxp3), CD4 T cells, and CD8 T cells was performed in serial sections for larger cohorts of gastric (n = 108) and esophageal (n = 95) cancer.
Representative immunostainings for anti-8OHdG, anti-Foxp3, anti-CD4, and anti-CD8 mAb in serial sections are shown in Fig. 5a. The grade of oxidative stress (anti-8OHdG staining) was classified into 8OHdG-high groups or 8OHdG-low groups based on the median value of the counts of 8OHdG-positive cells within tumors. Of note, semiquantitative analysis indicated that the infiltrating grade of CD8 T cells was significantly down-regulated in the 8OHdG-high group compared with those in the 8OHdG-low group in both gastric (39.1 ± 10.2 vs. 59.5 ± 19.1 cells/field, respectively) and esophageal (41.9 ± 11.8 vs. 69.5 ± 20.1 cells/field, respectively) cancer (Fig. 5b). Also, infiltrating grade of CD4 T cells was significantly decreased in the 8OHdG-high group compared with those in the 8OHdG-low group in gastric cancer (65.2 ± 13.2 vs. 90.1 ± 23.1 cells/field, respectively). On the contrary, the infiltrating grade of Foxp3(+) Tregs did not differ regardless of the oxidative state in both gastric and esophageal cancer (Fig. 5b). Thus, the infiltrating grade of Tregs did not differ regardless of the oxidative state, while the infiltrating grade of conventional CD8(+) and CD4(+) T cells was down-regulated according to the increased oxidative stress.
Fig. 5.
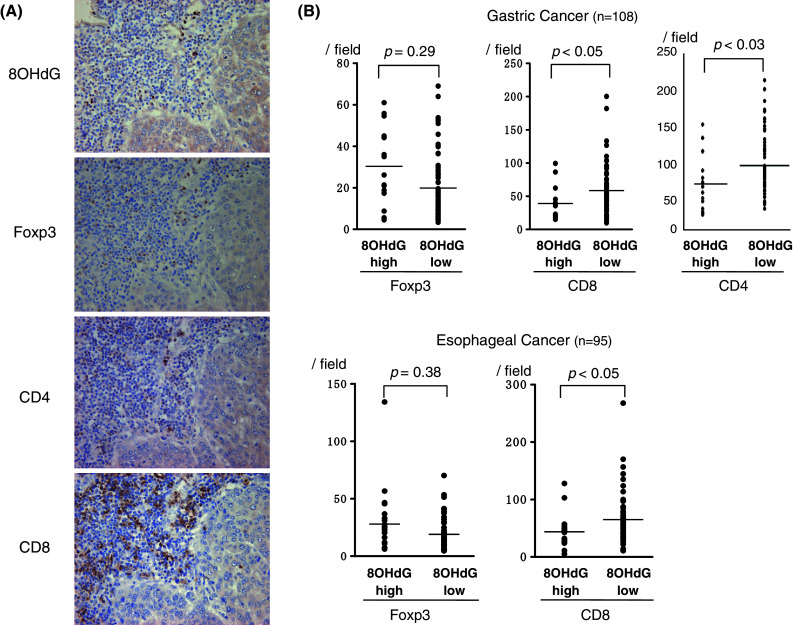
Oxidative stress and Foxp3(+) Tregs evaluated by immunohistochemistry. Representative immunostaining for anti-8OHdG, anti-Foxp3, anti-CD4, and anti-CD8 mAb in serial sections is shown in a. The grade of oxidative stress was classified into 8OHdG-high or 8OHdG-low groups based on the median value of the counts of 8OHdG-positive cells within tumors. Semiquantitative analysis indicated that the infiltrating grade of CD8(+) T cells was significantly down-regulated in the 8OHdG-high group compared with the 8OHdG-low group in both gastric and esophageal cancer (b). Also, the infiltrating grade of CD4(+) T cells was significantly decreased in the 8OHdG-high group compared with the 8OHdG-low group in gastric cancer (b). On the contrary, the infiltrating grade of Foxp3(+) Tregs did not differ regardless of the oxidative state in both gastric and esophageal cancer (b). Data were analyzed using a non-paired Student’s t-test
Lower sensitivity of Tregs to H2O2-induced apoptosis in vitro
CD4(+)CD25highTregs and CD4(+)CD25(−)T cells were separated by magnetic bead selection from PBLs in healthy donors (n = 10, Fig. 6a). CD4(+)CD25highTregs or CD4(+)CD25(−)T cells were incubated with AIM-V serum-free medium in the presence of H2O2 at the indicated physiological doses for 18 h and then subjected to apoptosis analysis with 7-AAD staining. Representative flow cytometric data indicated that CD4(+)CD25(−)T cells underwent apoptosis more progressively than CD4(+)CD25highTregs (Fig. 6b). Summarized data from 10 independent experiments from different donors revealed that there was a marked difference in the grade of apoptosis between CD4(+)CD25(−)T cells and CD4(+)CD25highTregs in response to H2O2 (Fig. 6c). Thus, Tregs are less sensitive to H2O2-induced apoptosis than conventional T cells.
Fig. 6.
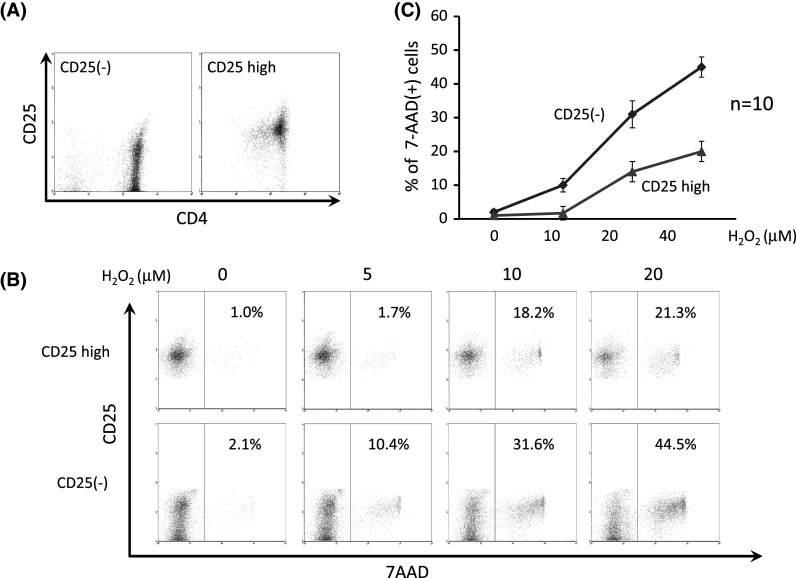
Lower sensitivity of Tregs to H2O2-induced apoptosis in vitro. CD4(+)CD25highTregs and CD4(+)CD25(−)T cells were separated by magnetic bead selection from PBLs in healthy donors (n = 10, a). CD4(+)CD25highTregs or CD4(+)CD25(−)T cells were incubated with H2O2 for 18 h at the indicated physiological doses and subjected to apoptosis analysis with 7-AAD staining. Representative flow cytometric data indicate that CD4(+)CD25(−)T cells underwent apoptosis more progressively than CD4(+)CD25highTregs (b). Summarized data from 10 independent experiments from different donors reveal that there is a marked difference in the grade of apoptosis between CD4(+)CD25(−)T cells and CD4(+)CD25highTregs in response to H2O2 (c)
Discussion
In the present study, we showed that the increased tumor-infiltrating Tregs were related to their lower sensitivity to H2O2-induced apoptosis within tumor microenvironments in comparison with conventional T cells. This is based on the following observations in gastric and esophageal cancer. First, increased tumor-infiltrating Tregs coexisted with elevated H2O2 production in tumor-infiltrating Mϕ according to the disease progression. Second, the grade of apoptosis in Tregs was less pronounced than in conventional T cells within tumor microenvironments, and there was a positive correlation between the oxidative stress and the grade of apoptosis in conventional T cells, while there was no correlation between the oxidative stress and the grade of apoptosis in Tregs. Third, Tregs were less sensitive to H2O2-induced apoptosis compared with conventional T cells in vitro. Taken together, the increased prevalence of Tregs within tumor microenvironments might be related to their lower sensitivity to H2O2-induced apoptosis.
In general, there have been several explanations describing the mechanisms behind the tumor microenvironment recruiting Tregs, including CCL22 or CCL17 through CCR4 [5, 7, 8], nitric oxide production, COX-2/PGE2 expression, or interleukin IL-10 [9, 10]. For example, Curiel et al. [5] reported that the CCL22 chemokine derived from a tumor induces the migration of Tregs through CCR4, which is a chemokine receptor for CCL22, and impairs antitumor immunity in ovarian cancer. Also, we showed that Tregs were able to migrate in response to CCL22 and CCL17 within tumor microenvironments of human tumors including gastric and esophageal cancer [7, 8]. Moreover, it has recently been shown that Tregs have functional VEGF receptor 2, and tumor-derived VEGF might recruit Tregs within tumor microenvironments [18]. Thus, it is likely that tumor microenvironment-derived factors can recruit Tregs into tumor microenvironments as chemoattractants.
It is well known that several shutdown mechanisms against T or NK cells act within tumor microenvironments, including oxidative stress–induced cell death [19, 20] or activation-induced cell death related to CD95 and CD95L interaction [21, 22]. With regard to oxidative stress-induced cell death, it was shown that T-cell subsets can differ in their susceptibility to oxidative stress-induced cell death, and naive CD4(+)CD25(−)/lowCD45RA(+) T cells showed a significantly greater resistance to H2O2 than memory-type T cells [23]. In addition, Tregs, especially of the naive CD45RA(+) type, were shown to exhibit reduced sensitivity to oxidative stress-induced cell death in comparison with conventional CD4(+)T cells and maintained their suppressive function in vitro [11]. These observations suggest that Tregs might indicate the different behaviors in response to oxidative stress within tumor microenvironments in comparison with conventional T cells, and it is desirable to evaluate the survival factors of Tregs in addition to the recruitment factors, in order to elucidate the regulation mechanisms of Tregs within tumor microenvironments.
To our knowledge, no previous report has described the relationship between oxidative stress and Treg accumulation within tumor microenvironments in human tumor specimens in vivo. In the present study, we confirmed using the freshly isolated and formalin-embedded tumor samples that the increased tumor-infiltrating Tregs are related to their lower sensitivity to H2O2-induced apoptosis within tumor microenvironments. Considering the previous in vitro data and our present in vivo data, Tregs can persist in the tumor microenvironment with their lower susceptibility to oxidative stress-induced cell death in comparison with conventional T cells, regardless of oxidative stress generated by tumor-associated macrophages, malignant cells, granulocytes, and myeloid-derived suppressor cells [19, 20]. Thus, Tregs with a lower susceptibility to oxidative stress may cause the relative enrichment of Tregs within tumor microenvironments, rather than the increased number of Tregs induced by the chemoattractive mechanisms. Furthermore, we have shown in the present study that the relative enrichment of Tregs within tumor microenvironments was observed in different histological types of tumor, which are gastric adenocarcinoma and esophageal squamous cell carcinoma, suggesting that this phenomenon may be a common feature that occurred in tumor microenvironment in general. Further study will be needed to support this concept. It is important to evaluate the balance between the recruitment and survival of Tregs, in order to clarify the regulation of Tregs within the tumor microenvironments.
There is increasing evidence explaining why Tregs show reduced sensitivity to oxidative stress-induced cell death. For example, the amount of thiols per cell, which is a major antioxidative molecule, was significantly greater in the Tregs population in comparison with conventional T cells [24]. Tregs contain high levels of catecholamines (dopamine, nor-epinephrine, and epinephrine), which confer increased resistance to oxidative stress [25]. Thus, these phenomena may be attributed to the high antioxidative capacity of Tregs.
In conclusion, we demonstrated that the increased tumor-infiltrating Tregs are closely related to their lower sensitivity to H2O2-induced apoptosis within tumor microenvironments of gastric and esophageal cancer. This phenomenon suggests that Tregs with a lower susceptibility to oxidative stress may cause the selective enrichment of Tregs within tumor microenvironments, in addition to the chemoattractive mechanisms. A better understanding of the underlying mechanism of Treg regulation, especially oxidative stress-related mechanisms, or a strategy for controlling Tregs may lead to a novel therapeutic strategy for cancer.
Acknowledgments
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflict of interest
There is no conflict of interest in the present study.
References
- 1.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 2.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 3.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 4.Siddiqui SA, Frigola X, Bonne-Annee S, Mercader M, Kuntz SM, Krambeck AE, et al. Tumor-infiltrating Foxp3−CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13:2075–2081. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 5.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 6.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama T, Kono K, Izawa S, Mizukami Y, Kawaguchi Y, Mimura K, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:422–429. doi: 10.1111/j.1442-2050.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 9.Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY. Nitric oxide induces CD4+CD25+Foxp3− regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc Natl Acad Sci USA. 2007;104:15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–8873. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 11.Mougiakakos D, Johansson CC, Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;113:3542–3545. doi: 10.1182/blood-2008-09-181040. [DOI] [PubMed] [Google Scholar]
- 12.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 13.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 14.Kondo S, Toyokuni S, Iwasa Y, Tanaka T, Onodera H, Hiai H, et al. Persistent oxidative stress in human colorectal carcinoma, but not in adenoma. Free Radic Biol Med. 1999;27:401–410. doi: 10.1016/S0891-5849(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 15.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, et al. Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer. 2008;98:148–153. doi: 10.1038/sj.bjc.6604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi A, Kono K, Ichihara F, Sugai H, Amemiya H, Iizuka H, et al. Macrophages in tumor-draining lymph node with different characteristics induce T-cell apoptosis in patients with advanced stage-gastric cancer. Int J Cancer. 2003;104:393–399. doi: 10.1002/ijc.10973. [DOI] [PubMed] [Google Scholar]
- 17.Izawa S, Kono K, Mimura K, Kawaguchi Y, Watanabe M, Maruyama T, et al. H2O2 production within tumor microenvironment inversely correlated with infiltration of CD56(dim) NK cells in gastric and esophageal cancer: possible mechanisms of NK cell dysfunction. Cancer Immunol Immunother. 2011;60:1801–1810. doi: 10.1007/s00262-011-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol. 2010;40:197–203. doi: 10.1002/eji.200939887. [DOI] [PubMed] [Google Scholar]
- 19.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 20.Malmberg KJ, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, et al. Inhibition of activated/memory (CD45RO(+)) T cells by oxidative stress associated with block of NF-kappaB activation. J Immunol. 2001;167:2595–2601. doi: 10.4049/jimmunol.167.5.2595. [DOI] [PubMed] [Google Scholar]
- 21.Klemke CD, Brenner D, Weiss EM, Schmidt M, Leverkus M, Gulow K, et al. Lack of T-cell receptor-induced signaling is crucial for CD95 ligand up-regulation and protects cutaneous T-cell lymphoma cells from activation-induced cell death. Cancer Res. 2009;69:4175–4183. doi: 10.1158/0008-5472.CAN-08-4631. [DOI] [PubMed] [Google Scholar]
- 22.Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15:70–79. doi: 10.1038/sj.cdd.4402274. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi A, Hanson MG, Norell HR, Havelka AM, Kono K, Malmberg KJ, et al. Preferential cell death of CD8+ effector memory (CCR7−CD45RA−) T cells by hydrogen peroxide-induced oxidative stress. J Immunol. 2005;174:6080–6087. doi: 10.4049/jimmunol.174.10.6080. [DOI] [PubMed] [Google Scholar]
- 24.Mougiakakos D, Johansson CC, Jitschin R, Bottcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 25.Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, et al. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]