Abstract
Active immunotherapy and cancer vaccines that promote host antitumor immune responses promise to be effective and less toxic alternatives to current cytotoxic drugs for the treatment of cancer. However, the success of tumor immunotherapeutics and vaccines is dependent on identifying approaches for circumventing the immunosuppressive effects of regulatory T (Treg) cells induced by the growing tumor and by immunotherapeutic molecules, including Toll-like receptor (TLR) agonists. Here, we show that tumors secrete high concentrations of active TGF-β1, a cytokine that can convert naive T cells into Foxp3+ Treg cells. Silencing TGF-β1 mRNA using small interfering RNA (siRNA) in tumor cells inhibited active TGF-β1 production in vitro and restrained their growth in vivo. Prophylactic but not therapeutic administration of TGF-β1 siRNA reduced the growth of CT26 tumors in vivo. Furthermore, suppressing TGF-β1 expression at the site of a tumor, using siRNA before, during and after therapeutic administration of a TLR-activated antigen-pulsed dendritic cell vaccine significantly reduced the growth of B16 melanoma in mice. The protective effect of co-administering TGF-β1 siRNA with the DC vaccine was associated with suppression of CD25+Foxp3+ and CD25+IL-10+ T cells and enhancement of tumor infiltrating CD4 and CD8 T cells. Our findings suggest that transient suppression of TGF-β1 may be a promising approach for enhancing the efficacy of tumor vaccines in humans.
Keywords: Tumor immunity, Regulatory T cells, TGF-β, Dendritic cell vaccine
Introduction
Active immunotherapy has the potential to be a less toxic and more effective approach than current cytotoxic drugs for treating malignancies. The majority of immunotherapeutic and vaccine strategies in development for the treatment of cancer involve manipulating the cooperation between the innate and adaptive immunity to promote effective cytotoxic T-lymphocyte (CTL) and Th1 response against the tumor [1]. Dendritic cells (DC) are key antigen presenting cells and play a crucial role in directing adaptive immune responses, consequently DC have been targeted in the development of cancer vaccines [2]. DC pulsed with tumor antigens and stimulated to mature with cytokines or immunomodulatory molecules such as TLR agonists can induce Th1 responses in vitro. However, this has not always translated to effective immunotherapy when transferred in vivo.
One explanation for the relatively modest success with active immunotherapeutics and vaccine approaches to cancer is the relatively high prevalence of regulatory T (Treg) cells infiltrating the tumor. CD4+CD25+ natural Treg cells which constitutively express Foxp3 are ordinarily a beneficial component of a healthy immune system, playing a critical role in maintaining self-tolerance and preventing the development of autoimmune diseases. However, Treg cells are also induced by infection and tumors where they can be beneficial to the host in preventing immunopathology, but also subvert host immunity to pathogens and tumors [3, 4]. In addition to natural Treg cells, Treg cells can be induced under the influence of IL-10 and TGF-β, and these inducible Treg cells, which secrete further IL-10 and TGF-β, suppress protective immune responses against tumors [5].
Patients with different cancers have increased numbers of Tregs cells [6]. Tumors have adapted to produce and secrete molecules such as VEGF, PGE-2, and TGF-β which maintain DC in an immature state and induce differentiation of Treg cells from naïve T cells [7]. Production of TGF-β by tumors appears to be an evasion strategy to ensure tumor survival [8]. TGF-β can act directly on naive CD4+ T cells to induce Foxp3 expression, converting them to a Treg phenotype [9, 10]. Approaches which target Treg cells and break self-tolerance also have considerable potential for enhancing the efficacy of vaccines or active immunotherapeutics against tumors.
Depletion of Treg cells has already been shown to promote antitumor immune responses in vivo in mouse models [11, 12]. Furthermore, blockade of CTLA4, constitutively expressed on Treg cells, using the humanized monoclonal antibodies ipilimumab and tremelimumab has shown some efficacy against a range of human cancers [1]. Furthermore, ipilimumab and tremelimumab have been assessed in the clinic in combination with peptide or DC vaccines [13, 14]. The cell-based cancer vaccine Provenge has already licensed for human use [15], but there is considerable scope for improved efficacy by attenuation of regulatory responses using a number of approaches, including blockade of CTLA4 or TGF-β. However, persistent suppression of CTLA4 can be associated with the development of colitis and autoimmune diseases [16]. Alternatively transient suppression of TGF-β1 and consequently Treg responses may allow development of antitumor immune responses in vivo, especially when administered with a tumor vaccine. Small interfering RNAs (siRNA) oligos are an effective means of silencing gene expression and have been assessed in clinical trials as therapies for respiratory disease and macular degeneration [17]. Here, we have used siRNA to silence the expression of tumor-derived TGF-β1. We demonstrate that silencing TGF-β1 mRNA suppresses Treg cell induction and significantly enhances antitumor immunity induced with a DC vaccine in a poorly immunogenic tumor model.
Materials and methods
siRNA oligos
siRNA oligos specific for TGF-β1 were purchased from AMBION, (Austin, Texas) siRNA ID 187280 with the sequence CCAAGGAGACGGAAUACAGtt. This sequence was designed and generated by AMBION using stringent algorithms to eliminate off-target effects and spans exon 3 and 4 in the transcript starting at nucleotide 1494 (to nuc 1512). The TGF-β1 siRNA is specific for mouse and does not knockdown human TGF-β1. The non-specific control siRNA (RISC-FREE) was purchased from Dharmacon, (Lafayette, CO).
Mice
Six to eight week old C57BL/6 and BALB/c female mice were purchased from Harlan Laboratories, (Oxen, UK). Animal experiments and maintenance were approved and regulated by the university ethics committee and the Irish Department of Health.
Tumor models
The B16F10 murine melanoma and CT26 colon carcinoma cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and used for tumor induction in C57BL/6 and BALB/c mice, respectively, by subcutaneously (s.c.) administration into the flank. Tumor growth was recorded every 2–3 days and animals were killed when tumors measured 15 mm in diameter. Tumor volume (V) was calculated as V = (π/6) (d12 × d2) where d1 is the shortest diameter measurement.
siRNA transfection
CT26 cells were transfected with siRNA (100 nM) using oligofectamine (Invitrogen) according to manufacturer’s protocol. Supernatants were removed on indicated days to determine TGF-β1 concentrations by ELISA (R&D Systems, catalog number DY1679).
DC tumor vaccine
Murine bone marrow-derived immature DCs were generated as described [18]. DC were loaded at a 1:1 ratio with heat-shocked (43°C for 1 h), γ-irradiated (200 Gy) (hs/irr) tumor cells followed by stimulation with CpG (5 μg/ml) for 18 h. DC were washed and injected (5 × 105) s.c. into the tumor site days 3, 10, and 17 after tumor inoculation. For siRNA treatments, 5 μg of siRNA was complexed with oligofectamine and administered s.c. in a volume of 100 μl.
Treg cell conversion assay
CD4+CD25− cells were purified from the spleens of naive mice using MACS cell sorter. Cells were cultured with plate-bound α-CD3 (2 μg/ml, BD) soluble α-CD28 (BD) and 20 U/ml IL-2 (BD) and where indicated recombinant TGF-β1 (5 ng/ml; R&D Systems).
Flow cytometric analysis
For conversion assay, cells were stained with antibodies specific for CD4, CD25, and Foxp3 after 7 days incubation and analyzed by FACS. Tumors were removed on day 21 and single-cell suspensions were prepared. Cells were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 2 h and then brefeldin A (5 μg/ml) was added for further 4 h at 37°C. Cells were stained with antibodies to CD4 or CD8 (eBioscience). Cells were then fixed and permeablized, then incubated with anti-IL-10 or anti-Foxp3 (eBioscience). Immunofluorescence was analyzed using a CyAn (Dakocytomation) with FloJo (Stanford University) software.
Statistical analysis
Statistical analysis was performed using GraphPad Instat. Anova or Student’s t test was used to compare statistical differences of means between groups.
Results
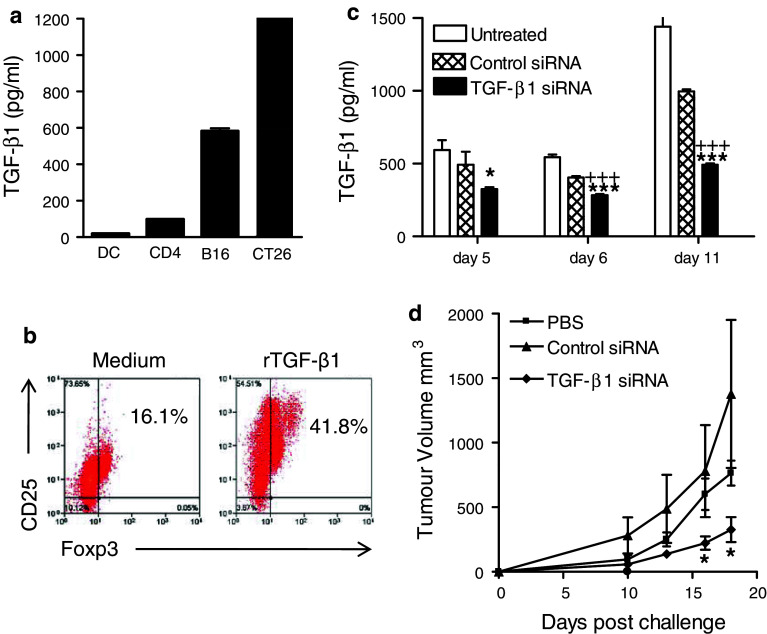
We first examined the production of TGF-β1 by two tumor cell lines in vitro relative to other cell types. CT26 and B16 tumor cells secreted high concentrations of TGF-β1 when compared with DC and CD4 T cells (Fig. 1a). We next examined the capacity of TGF-β1 to convert naive T cells to Treg cells in vitro. Naive CD4+ T cells were purified and incubated with recombinant TGF-β1 and IL-2 for 7 days and analyzed for Foxp3 and CD25 expression. Foxp3 expression was upregulated in TGF-β1-treated cells, indicating their conversion to a Treg phenotype (Fig. 1b).
Fig. 1.
Gene silencing of TGF-β1 in tumor cells inhibits Treg cell conversion and growth of tumor cells in vivo. a DC, CD4+ T cells, or tumor cells (B16 and CT26) were cultured for 24 h, and TGF-β1 was quantified in the supernatants by ELISA. b Naive murine CD4+CD25− cells were cultured at 5 × 105 cells/ml in anti-CD3-coated wells in medium containing IL-2 and soluble anti-CD28 with or without 5 ng/ml of recombinant TGF-β1. After 7 days, cells were analyzed by FACS for the expression of Foxp3 and CD25 after gating on CD4. c siRNA specific for TGF-β1 (100 nM) or a negative control non-specific siRNA were transfected into CT26 cells using oligofectamine. Supernatants were removed at the indicated times and TGF-β was quantified by ELISA. *P < 0.05, ***P < 0.001 versus untreated; +++ P < 0.001 versus control siRNA. d CT26 cells were transfected in vitro with siRNA specific for TGF-β1 or control siRNA (TGF-β1 siRNA reduced TGF-β1 protein expression by 45% after 5 days and 66% after 9 days in transfected tumors cells compared with control tumor cells) and then injected s.c. into BALB/c mice 5 days after transfection. Tumor growth was monitored over a 20-day period. *P < 0.05 versus PBS or control siRNA
To investigate the effect of tumor-derived TGF-β1 on tumor growth, we used siRNA to silence expression of the gene in the CT26 cells. The TGF siRNA was specific for mouse TGF-β1, and this was chosen because recombinant TGF-β1 induces conversion of naïve CD4 T cells to Foxp3 positive Treg cells in vitro. Expression of TGF-β1 protein was significantly reduced in siRNA-transfected CT26 cells compared with untransfected cells and this persisted for 11 days (Fig. 1c). The effect of reducing TGF-β1 expression on tumor growth in vivo was assessed by inoculating BALB/c mice s.c. with CT26 cells (2x105) transfected in vitro with siRNA specific for TGF-β1. The rate of tumor growth was slower for CT26 cells transfected with TGF-β1 siRNA, when compared with those transfected with a control siRNA or untransfected cells (Fig. 1d).
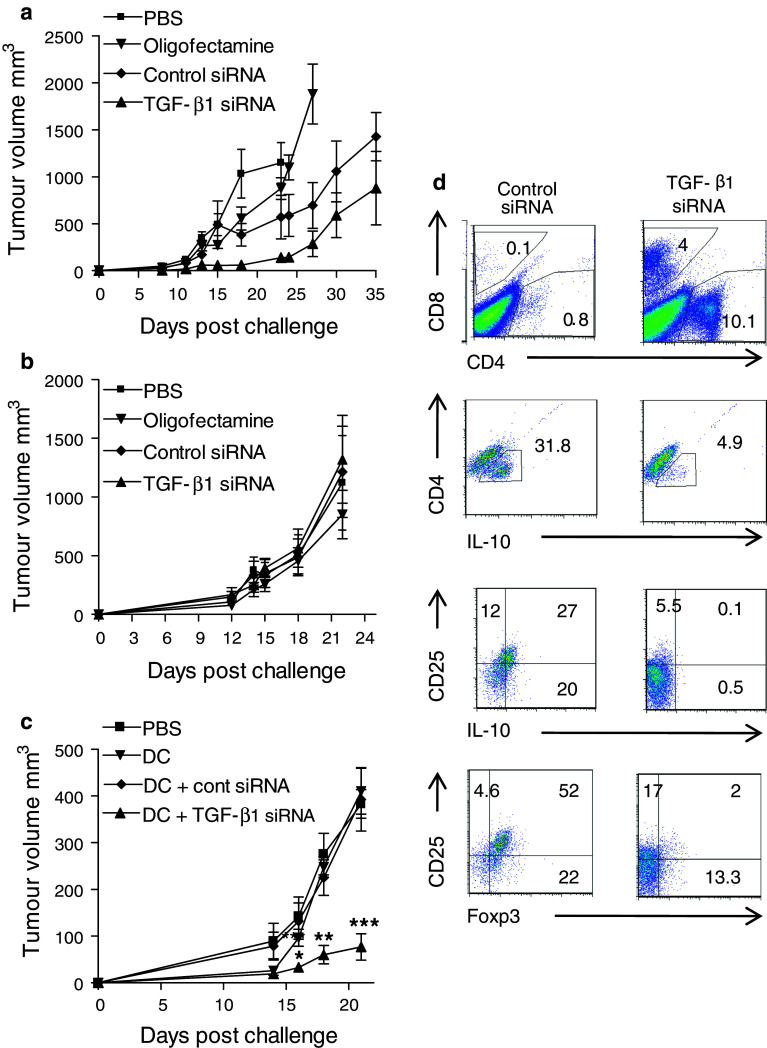
We next examined the possibility that silencing TGF-β1 in vivo might have potential to modify tumor growth. BALB/c mice were given injections of siRNA (5 μg) into the site of the tumor on day −5, −3, −1, 1, and 3 and CT26 cells were given on day 0. Mice treated with TGF-β1 siRNA had reduced tumor growth compared with mice treated with control siRNA (Fig. 2a). However, using a therapeutic approach, where mice were treated with siRNA every 2 days from day 6, TGF-β1 siRNA did not affect tumor growth (Fig. 2b).
Fig. 2.
Treatment with TGF-β1 siRNA suppresses Treg cell induction and enhances the efficacy of DC tumor vaccine. a BALB/c mice were injected with 2 × 105 CT26 colon carcinoma cells in the flank on day 0. Mice treated from days −5, −3, −1, 1, and 3 (a) or every 2 days from day 6 (b) with 5 μg of siRNA for TGF-β1 or control siRNA complexed with oligofectamine or with oligofectamine alone. Tumor growth was monitored. The experiment was terminated when tumors reached a diameter of 15 mM. c C57BL/6 mice were injected in the flank on day 0 with 2 × 105 cells B16F10 melanoma cells. Mice were treated on day 3, 10, and 17, with PBS or a DC vaccine, comprising 5 × 105 DC loaded with hs/irr B16F10 cells and stimulated with CpG, washed and injected into the site of the tumor. On day 2, 3, 4, 9, 10, 11, 16, 17, and 18, the relevant groups were also injected with 5 μg TGF-β1 or control siRNA into the tumor site. Growth was monitored over 21 days. *P < 0.05, **P < 0.01, and ***P < 0.001 versus PBS or DC vaccine with control siRNA. d Tumors were removed on day 21 and cells stimulated with PMA and ionomycin overnight were stained for CD4, CD8, CD25, IL-10, and Foxp3 and analyzed by FACS. Results presented are mean ± SD or representative FACS plots for 5–6 mice per group and are representative of 3 experiments
We examined the possibility that TGF-β1 siRNA might work therapeutically using a vaccine approach. We have reported that TLR-activated DC pulsed with hs/irr tumor cells can protect against the poorly immunogenic B16 tumor cells when IL-10 signaling is inhibited [19] therefore we used this model. DC loaded with B16 antigen and stimulated with CpG were injected s.c. 3, 10, and 17 days after challenge of mice with B16F10 cells. Mice were injected intratumorally with siRNA the day before, after and same day as the DC vaccine. The data demonstrate that administration of the DC vaccine alone had little effect on tumor growth (Fig. 2a). In contrast, administration of TGF-β1 siRNA significantly enhanced the efficacy of the DC vaccine; the tumor burden was significantly lower in mice given the DC vaccine in combination with TGF-β1 siRNA, compared with control siRNA (Fig. 2c).
We examined the influence of TGF-β1 silencing on the frequency of Treg cells in the tumor mass. Mice treated with the DC vaccine in combination with TGF-β1 siRNA had enhanced tumor infiltrating CD4 and CD8 T cells, but fewer CD25+Foxp3+ and CD25+IL-10+ T cells (Fig. 2d). This was a consistent finding between mice in each experimental group and across different experiments. Our findings demonstrate that TGF-β1 produced by or in response tumor cells can suppress antitumor immunity and compromise the efficacy of tumor vaccine and suggest that inhibition of Treg cell induction by silencing TGF-β1 is a promising approach for enhancing the efficacy of a tumor vaccine.
Discussion
In this study, we have demonstrated that suppression of Treg cell induction by targeting TGF-β1 using siRNA can enhance the efficacy of a DC vaccine against a poorly immunogenic tumor in mice. It is well documented that growing tumors create an immunosuppressive environment, which inhibits the generation of antitumor immunity [12]. Tumor cells secrete or induce production of immunosuppressive molecules, including the cytokines TGF-β and IL-10, which inhibit the function of T cells or innate immune cells, including DC. DC purified from tumor-bearing mice have defective function, including abnormal differentiation, maturation, and migration [20]. Furthermore, growing tumors can recruit, induce, and convert Treg cells. The detrimental effect of Treg cells on tumor immunity was clearly demonstrated through the demonstration that depletion of CD25+ cells can reduce tumor growth in mice [12, 21]. We have previously reported that active immunotherapy using TLR ligands or tumor-derived HSP60 can promote the induction of Treg cells as well as protective Th1 cells [19, 22]. This is a major obstacle in the induction of antitumor immune response and has highlighted the importance of identifying methods for overcoming tolerance and immunosuppression in the development of immunotherapies against cancer [23].
TGF-β is involved in or influences a number of cell processes, including cellular proliferation, survival, differentiation, and apoptosis [24]. It is secreted by tumor cells, supporting their growth and survival [25]. TGF-β also has anti-inflammatory properties and is secreted by certain populations of Treg cells and, at least in part, mediates their immunosuppressive function. TGF-β-secreting DC and T cells are found at tumor sites, where it is thought that they function to suppress effector T cell responses, thus promoting and maintaining tumor growth [26]. Therefore, targeting TGF-β has the potential for enhancing antitumor immunity. In this study, we used siRNA specific for TGF-β1. All three TGF-β isoforms have been implicated in tumor regulation but TGFβ1 has a particular role in suppressing tumor immunity through the conversion of CD4 cells to a Treg phenotype. Our approach was to improve vaccine efficacy through attenuation of immunosuppression by converted Treg cells and is therefore more specific than previous studies using anti-TGF-β antibodies that block all three isoforms. Since TGF-β has important functions independent of its pro-tumor role, strategies that transiently remove its tumor-promoting effect may be safer than those that completely block this cytokine or Treg cells in general. For this reason, siRNA may be a more attractive approach for blocking TGF-β. Furthermore, the use of liposomes to deliver the siRNA oligos enhances uptake by phagocytic cells, which should help to target macrophages and DC, the key APC for driving T cell responses.
Recent studies have shown that cell-based vaccines have promise for the treatment of cancer, with the recent licensing of Provenge for prostate cancer [15]. The advantage of the DC vaccine approach is that the DC is activated in vitro away from the immunosuppressive environment of the growing tumor. However, in a therapeutic setting, tumor-induced Treg cells and TGF-β may still compromise the capacity of the DC vaccine to promote effective antitumor immunity. The present study shows that this can be circumvented by transiently silencing TGF-β1 immediately before and after injection of the DC vaccine. We found that a vaccine comprising of DC pulsed with hs/irr tumor cells and activated with the TLR9 agonist CpG had only marginal therapeutic efficacy against B16 tumors in mice. In contrast, administration of TGF-β1 siRNA significantly enhanced the efficacy of the DC vaccine. The reduction in tumor growth was associated with an expansion of CD4 and CD8 T cells, but a reduction in CD4+CD25+Foxp3+ T cells infiltrating the tumors. Infiltrating DC are detectable in the human and murine tumors and these have regulatory phenotype, including TGF-β expression, and promote the induction of Treg cells [27] (unpublished observations). The DC therapy approach, which employs DC activated in vitro with a TLR agonist, when combined with silencing TGF-β1 expression in vivo circumvented the induction of Treg cells and allowed the development of protective effector T cell responses.
The TGF-β1 siRNA used in this study was delivered using a liposome-based delivery system. We found this to be an effective approach for targeting siRNA to tumor cell and DC, which are major sources of the TGF-β1 that contribute to immunosuppression in cancer. While these reagents may not be directly translatable to clinical studies, a number of approaches have been employed for delivery of siRNA in humans, including direct injection of naked siRNA locally in eye disease [17] or systemic delivery of siRNA-containing nanoparticles for tumor therapy [28]. Methods have been developed to improve safety and guide delivery to specific cellular targets. This together with the fact that siRNA can be designed to target any gene makes the likelihood of siRNA being widely used in therapeutics a real prospect in the very near future. The method of combining siRNA with a DC vaccine reported here has the potential to be used in combination with any vaccine that is negatively affected by Treg cells generated through the influence of TGF-β1 and is therefore broadly applicable to the field of vaccines in general.
Acknowledgments
We thank Andrew Jarnicki for his help with the initiation of this study. This work was supported by a Science Foundation Ireland (SF) PI grant (06/IN.1/B87) to Kingston Mills.
Conflict of interest
Kingston Mills is a co-founder and shareholder in Opsona Therapeutics Ltd and TriMod Therapeutics Ltd, University start-up companies involved in the development of immunotherapeutics. None of the authors have a financial relationship with SFI who sponsored the research.
References
- 1.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58(3):317–324. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4(11):841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 4.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 5.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 6.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage -small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 7.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 8.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178(5):2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172(9):5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 11.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32(11):3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Jarnicki AG, Lysaght J, Todryk S, Mills KHG. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cell. J Immunol. 2006;177(2):896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 13.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, Yan L, Targan S, Solomon J, Nichol G, Yellin M, Weber JS. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17(4):896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribas A, Comin-Anduix B, Chmielowski B, Jalil J, de la Rocha P, McCannel TA, Ochoa MT, Seja E, Villanueva A, Oseguera DK, Straatsma BR, Cochran AJ, Glaspy JA, Hui L, Marincola FM, Wang E, Economou JS, Gomez-Navarro J. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15(19):6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lares MR, Rossi JJ, Ouellet DL. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 2010;28(11):570–579. doi: 10.1016/j.tibtech.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins SC, Lavelle EC, McCann C, Keogh B, McNeela E, Byrne P, O’Gorman B, Jarnicki A, McGuirk P, Mills KH. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J Immunol. 2003;171(6):3119–3127. doi: 10.4049/jimmunol.171.6.3119. [DOI] [PubMed] [Google Scholar]
- 19.Jarnicki AG, Conroy H, Brereton C, Donnelly G, Toomey D, Walsh K, Sweeney C, Leavy O, Fletcher J, Lavelle EC, Dunne P, Mills KH. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180(6):3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170(1):101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 21.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 22.Toomey D, Conroy H, Jarnicki AG, Higgins SC, Sutton C, Mills KH. Therapeutic vaccination with dendritic cells pulsed with tumor-derived Hsp70 and a COX-2 inhibitor induces protective immunity against B16 melanoma. Vaccine. 2008;26(27–28):3540–3549. doi: 10.1016/j.vaccine.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21(2):166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS, Berger WH. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987;47(3):707–712. [PubMed] [Google Scholar]
- 26.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 27.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121(4):1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]