Abstract
Background
Lung cancer is the leading cause for cancer-related mortality and morbidity, and the survival of late-stage non-small cell lung cancer (NSCLC) remains poor. We hereby evaluate conventional chemotherapy followed by immunotherapy using dendritic cells and cytokine-induced killer cells in the treatment for late stage of NSCLC.
Methods
Twenty-eight untreated patients suffered from IIIB to IV NSCLC were enrolled in the study between August 2004 and October 2005, and all received four courses of vinorelbine–platinum (NP) chemotherapy. Fourteen of them received conventional NP chemotherapy followed by vaccinated with CEA (605–613) peptide-pulsed autologous dendritic cells and CIK cells. Vaccination was repeated at 30-day intervals for 4 cycles. The adverse effects, time to progression (TTP), and overall survival (OS) in each group were evaluated.
Results
The adverse effect as a result of chemoimmunotherapy was mild and tolerable. Rash, acne, and pruritus were more frequent in the chemoimmunotherapy group than in the chemotherapy group (64.2% vs. 7.1%, P = 0.004). Non-infectious fever was more frequent in the chemoimmunotherapy group than in the chemotherapy group (71.4% vs. 21.4% P = 0.02). Less grade 3/4 fatigue was observed in patients receiving chemoimmunotherapy: 7.1% versus 57.1% in chemotherapy group, P = 0.01. Compared with patients in chemotherapy group, time to progression in chemoimmunotherapy significantly prolonged, with the median improved from 5.2 months (95% CI: 3.3–6.0) to 6.9 months (95% CI: 5.0–8.8) (P = 0.03). The 1-, 2-, and 5-year survival rates were 64.3, 49, and 21.0%, respectively in chemoimmunotherapy group. Overall survival rate showed no statistically difference between two groups (P = 0.18).
Conclusions
Chemoimmunotherapy could alleviate adverse effects of conventional chemotherapy and prolong survival for patients with late-stage NSCLC.
Keywords: Chemoimmunotherapy, Dendritic cell, Cytokine-induced killer cell, Non-small cell lung cancer
Introduction
Lung cancer is one of the most common cancers today, and non-small cell lung cancer (NSCLC) is a leading cause of cancer-related fatalities [1]. It is often diagnosed at an advanced stage that is only amenable to palliative therapy [2]. Chemotherapy is the current standard treatment for advanced stage NSCLC. The median survival time of patients with stage IIIB and stage IV disease is about 1 year [3]. The toxicity profiles of chemotherapy include gastrointestinal, marrow-suppressive, and infective disorders. The limited efficacy and moderate toxicity of chemotherapy for lung cancer have justified the development of novel therapeutic modalities [3].
Among various approaches, immunotherapy has become one promising strategy to provide clinical benefits for these patients. Immunotherapy, based on the knowledge that the immune system can distinguish cancerous cells from normal cells, may represent one new approach with low toxicity and high specificity. Increasing evidence suggests that immunotherapy can increase survival and quality of life of patients suffered from lung cancer [4, 5].
Dendritic cells (DCs) are the most potent antigen-presenting cells in the body, and they are responsible for the initiation of both innate and adoptive immune responses. Vaccination of DCs pulsed with tumor-associated antigen (TAAs) induces protective immune responses. Several studies have shown that specific CTLs can be generated by human DCs pulsed with TAAs in cancer patients. Some studies employed preidentified TAA or synthetic peptides containing CTL epitopes of known TAA as the source of antigens. Carcinoembryonic antigen (CEA) is an 18-kDa glycoprotein. It is widely used as a serological marker of lung cancer because of its overexpression in the later. These findings, together with the high prevalence of CEA in NSCLC, seem to make it possible for CEA peptide-pulsed DC vaccination to induce specific immune response [6–8]. We therefore selected CEA as the antigen of choice for active immunotherapy and used CEA605–613, restricted with HLA-A2, for loading onto DC.
Cytokine-induced killer (CIK) cells are cytotoxic lymphocytes generated from peripheral lymphocytes by a cytokine cocktail including anti-CD3 monoclonal antibody. They possess an enhanced cytotoxicity and a higher proliferation than lymphokine-activated killer (LAK) cells [9]. Several studies show that interactions between DC and CIK cells caused changes in the surface molecule expression of both populations, led to an increase in IL-12 secretion, and rendered an improved cytotoxic activity [10–12]. Therefore, DC combining with CIK may have a major impact on immunotherapeutic protocols for patients with cancer.
Preclinical data accumulated in recent years suggest that the combination of immunotherapy with chemotherapy might provide significant benefit. However, to date, there is no data to evaluate clinical efficacy of conventional chemotherapy followed by DC/CIK immunotherapy in patients suffered from late-stage NSCLC.
The initial results of this study provide clinical evidence supporting the hypothesis that the combination of DC/CIK immunotherapy with chemotherapy, called chemoimmunotherapy, could result in benefit for cancer patients with advanced disease.
Patients and methods
We performed a paired study to compare the clinical outcomes of patients with stage IIIB–IV receiving the combination of chemotherapy and immunotherapy containing CEA-pulsed dendritic cells (DC) and cytokine-induced killer cells (CIK) (chemoimmunotherapy) and chemotherapy alone. Patients had histologically proven non-small cell lung cancer (NSCLC) according to the WHO criteria. Participants were required to have elevated CEA level in peripheral blood, ranging from 6 to 394 ng/ml (cutoff value: 5 ng/ml). Mononuclear cells (MNC) obtained from patients were phenotyped for HLA-A2 allele by flow cytometry. Patients who are HLA-A2 positive were eligible for this clinical trial. Each patient in the study had an Eastern Cooperative Oncology Group performance status score ≤2. All patients had adequate bone marrow, liver, and renal functions. Patients who had metastatic disease in the central nervous system, autoimmune disease, or active acute or chronic infection were excluded. Between August 2004 and October 2005, 28 patients were enrolled in this study. Patients were randomized in the chemoimmunotherapy group or chemotherapy group. Patients were monitored for progression of their tumor by computed tomography every 4 weeks. CEA level was measured before and after treatment using chemiluminescence-based technique. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria v.2.0 grading system. This study was approved by the ethical committee of Shanghai Chest Hospital, and written informed consents were obtained from all the patients before enrollment. The primary end point was the assessment of toxicity. The secondary end points were the time of progression (TTP) and overall survival (OS).
Treatments
Fourteen patients in the chemotherapy group received 4 cycles of vinorelbine with platinum (NP) chemotherapy. In each cycle, vinorelbine (25 mg/m2) was given at day 1, day 8, and cisplatin (75 mg/m2) was given at day 1. For the chemoimmunotherapy group, peripheral blood was used for the preparation of DC/CIK before each chemotherapy. DC/CIK was administrated intravenously after NP was given in the same way as in the chemotherapy group. Patients in the chemoimmunotherapy group totally received four treatments for DC/CIK at intervals of 1 month. Clinical examinations of these patients were performed by oncology specialists weekly or biweekly, including a complete blood count, chest computed tomography scan, and liver and kidney function examinations.
Preparation of DCs
Peripheral blood mononuclear cells (PBMC) from a 100-ml blood specimen were enriched by density gradient centrifugation with Ficoll-Paque. PBMC were resuspended in ex vivo with 1% autologous heat-inactivated serum, plated at a concentration of 5 × 106 cells/ml, and allowed to adhere to 10-cm2 dishes. Non-adherent cells were removed after 4 h at 37°C in a humidified incubator for the CIK cells preparation, and the adherent cells were cultured at 37°C for 7 days in ex vivo supplemented with 1% heat-inactivated autologous serum in the presence of 1,000 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leukomax; Novartis International AG, Basel, Switzerland), 500 units/ml recombinant human interleukin (IL-4) (GMP-grade, Strathmann Biotec AG, Hannover, Germany).
Peptide loading
The CEA peptide (YLSGANLNL; CEA605–613) was from Boya Biotech Company (Shanghai, China) with at least 95% HPLC-grade purity. All preparations were tested to be free of endotoxins. On day 8, DC were incubated with the CEA peptide at a concentration of 10 μg/ml for 12 h. DC were then harvested, resuspended at concentration of 1 × 106/ml in 0.9% normal saline, and administrated intravenously.
Generation of cytokine-induced killer cells
Non-adherent PBMC were prepared and grown in ex vivo medium containing 1,000 units/ml of human recombinant interferon (IFN-γ) (Boehringer, Mannheim, Germany) added on day 0. After 24 h of incubation, 50 ng/ml of antibody against CD3 (Orthoclone OKT 3; Cilag GmbH, Sulzbach, Germany) was added. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and subcultured every 3 days in fresh complete medium containing 1,000 U/ml IL-2 at 3 × 106 cells/ml. On day 9, CIK cells resuspended in 0.9% normal saline were administrated intravenously.
Statistical analyses
The result of CEA level was expressed as mean ± SD. Results were analyzed by paired t test. The comparisons of clinical characteristics and severity of treatment-related toxicity were performed using Fisher exact test. Time to disease progression (TTP) was calculated from the date of initiation of treatment to the date of disease progression or death. Overall survival (OS) was the time between the first day of treatment and the date of death or the date on which patients were last known to be alive. Correlations of the methods of treatment with TTP and OS were determined with the Kaplan–Meier method and its associate log-rank statistic. Differences were considered statistically significant when P < 0.05 (two-tailed).
Results
Patients and treatment administration
Between August 2004 and October 2005, 28 patients were enrolled in this study. The demographic and pathologic characteristics of 28 patients were depicted in Table 1. Patients treated with chemoimmunotherapy in this study(6 men and 8 women) had a median age of 50 (range 39–68), and patients treated with chemotherapy (7 men and 7 women) had a median age of 48 (range 40–65). The most common histological NSCLC subtype in chemoimmunotherapy was adenocarcinoma: 71.4% versus 78.5% in chemotherapy group. Most of patients in each group had stage IV disease (71.4%).
Table 1.
Patient characteristics in two groups
| Characteristics | Chemoimmunotherapy | Chemotherapy |
|---|---|---|
| Number | 14 | 14 |
| Sex | ||
| Male | 6 | 7 |
| Female | 8 | 7 |
| ECOG performance status | ||
| 0 | 2 | 1 |
| 1 | 4 | 6 |
| 21 | 8 | 7 |
| Stage statue | ||
| IIIB (T 4 alone) | 1 | 1 |
| IIIB (N3 alone) | 1 | 2 |
| IIIB (T4 N3 both) | 2 | 1 |
| IV | 10 | 10 |
| Tumor histology | ||
| Adenocarcinoma | 10 | 11 |
| Squamous cell | 3 | 3 |
| Large cell | 1 | 0 |
Characteristics of chemoimmunotherapy
In the chemoimmunotherapy group (Table 2), the average number of DC injected was 8.1 ± 2.5 (106)and that of CIK injected was 13.3 ± 3.5 (108). Serum CEA level was considered evaluated when the value increased 25% of the baseline. CEA level decreased 25% of the baseline was defined decreasement. Otherwise, it was defined stable. In chemoimmunotherapy group, CEA level decreased in 3 patients (21.4%), while it was stable in 9 patients (64.2%). Grade 1–3 skin toxicity occurred in 9 patients (64.2%), and grade 1–4 non-infective fever toxicity occurred in 10 patients (71.4%).
Table 2.
Chemoimmunotherapy of patients
| Patient | Average number of DC administrated (106) | Average number of CIK administrated (108) | Serum CEA | Toxicity of skin (°) | Non-infective fever (°) |
|---|---|---|---|---|---|
| 1 | 11.5 ± 1.9 | 18.9 ± 2.3 | ↑ | 0 | I |
| 2 | 6.5 ± 0.98 | 12.5 ± 2.5 | ↓ | I | III |
| 3 | 7.6 ± 1.7 | 11.3 ± 0.8 | → | II | I |
| 4 | 12.25 ± 2.8 | 11.2 ± 2.2 | → | 0 | 0 |
| 5 | 5.4 ± 2.5 | 9.9 ± 0.4 | → | 0 | I |
| 6 | 6.6 ± 0.85 | 19.4 ± 0.8 | → | II | 0 |
| 7 | 7.5 ± 3.3 | 10.8 ± 0.9 | → | III | 0 |
| 8 | 8.2 ± 0.8 | 16.3 ± 2.2 | → | I | I |
| 9 | 5.5 ± 2.5 | 17.7 ± 0.6 | → | II | 0 |
| 10 | 10.5 ± 0.7 | 13.8 ± 1.9 | ↓ | III | II |
| 11 | 8.9 ± 0.7 | 9.7 ± 0.4 | ↑ | 0 | I |
| 12 | 8.2 ± 1.0 | 10.4 ± 0.9 | ↓ | I | IV |
| 13 | 6.7 ± 0.9 | 10.9 ± 1.1 | → | 0 | III |
| 14 | 10.4 ± 2.6 | 11.8 ± 1.2 | → | II | II |
The average number of immune cells and adverse event in each patient of chemoimmunotherapy group were shown. CEA level decreased in 3 patients (21.4%), while it was stable in 9 patients (64.2%)
Adverse event
Severe treatment-induced hematological toxicities including grade 3/4 anemia (28.5% vs. 42.8%, P = 0.69) and grade 3/4 leucopenia (71.4% vs. 92.8%, P = 0.32) occurred more frequently in the chemotherapy only group. With regard to non-haemotologic toxicities, nausea events (64.2% vs. 92.8% P = 0.16) were significantly more frequent in the chemotherapy group. Rash, acne, and pruritus were more frequent in the chemoimmunotherapy group than in the NP group (64.2% vs. 7.1%, P = 0.004). Non-infectious fever was more frequent in the chemoimmunotherapy group than the NP group (71.4% vs. 21.4% P = 0.02). Less grade 3/4 fatigue was observed in patients receiving chemoimmunotherapy: 7.1% versus 57.1% in NP group, P = 0.01. One patient (7%) in chemotherapy group experienced febrile neutropenia.
Time to progression and overall survival
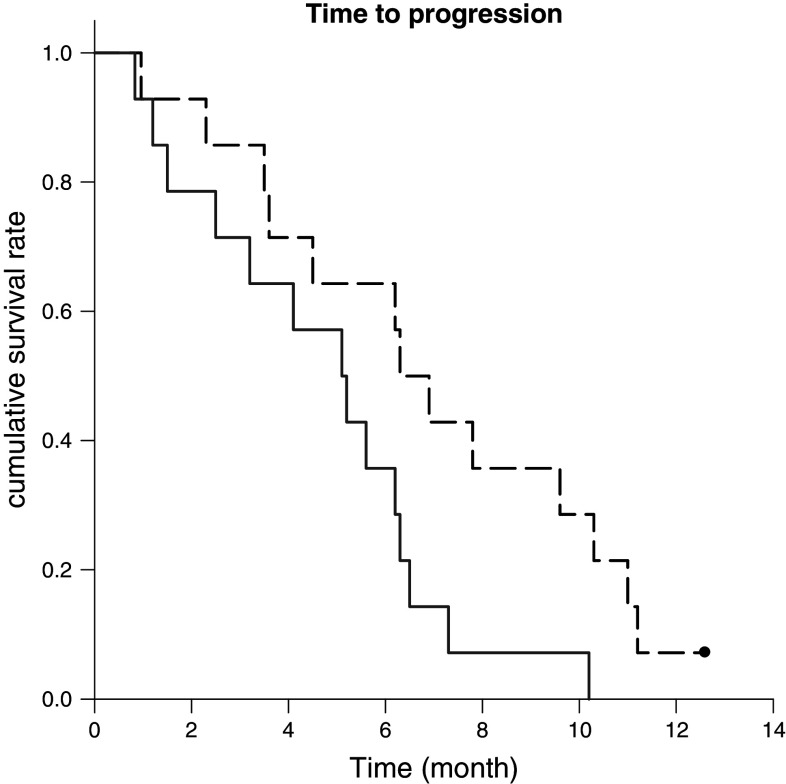
All 28 eligible patients were assessed for TTP and OS. At the time of the analysis, there were 25 deaths and 3 patients confirmed alive. The median time to progression of patients in chemoimmunotherapy is 6.9 months (95% CI: 5.0–8.8) and 5.2 months (95% CI: 3.3–6.0) in the chemotherapy group. (P = 0.03) (Fig. 1).
Fig. 1.
Time to progression in chemoimmunotherapy group showed statistically improvement (long dash) compared with chemotherapy only (solid dash) (P = 0.03). The median time to progression is 6.9 (95% CI: 5.0–8.8) and 5.2 (95% CI: 3.3–6.0), respectively
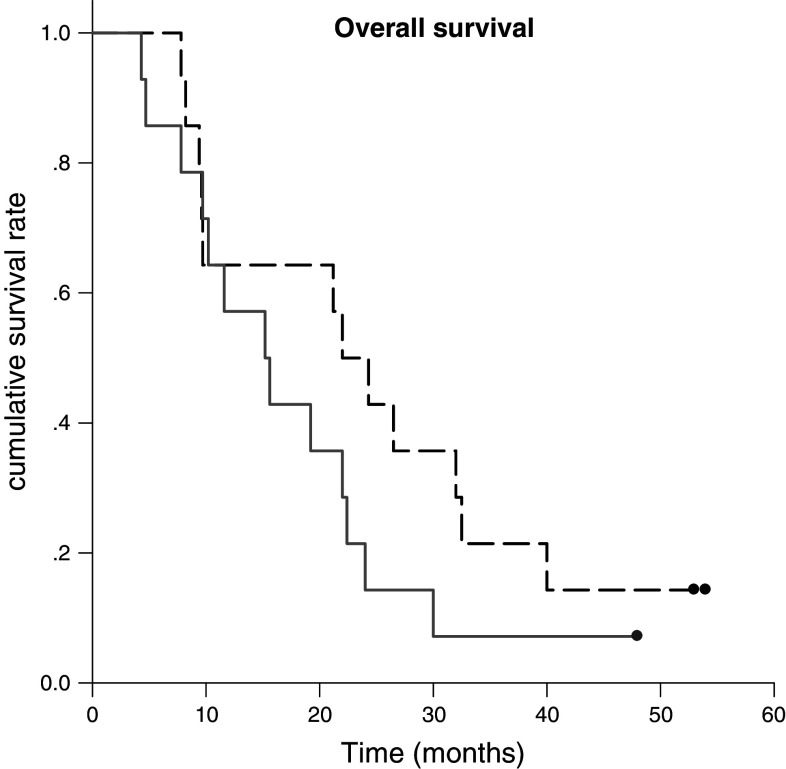
The 1-, 2-, and 5-year survival rates in chemoimmunotherapy were 64.3, 49.8, and 21%, respectively. Overall survival showed numeric improvement in chemoimmunotherapy, but there is no statistical difference. (P = 0.18), (Fig. 2).
Fig. 2.
The 1-, 2-, and 5-year survival rates in the chemoimmunotherapy was 64.3, 49.8, and 21.0%, respectively. Overall survival showed no statistically difference between chemotherapy group (solid dash) and chemoimmunotherapy group (long dash). (P = 0.18)
Discussion
Increasing clinical studies suggest that immunotherapy may represent another solution to improving cancer treatment. It is generally accepted that the advantage of CIKs lies in its specificity with the ability to directly lyse tumor cell and to secrete IFN-γ and TNF-α.
Dendritic cells, known as the most powerful antigen-presenting cells, have been under intense investigation as components of antitumor vaccines, specifically as a delivery mode for tumor antigens. Identification and selection of appropriate antigens for DC loading is very important in the development of DC-based immunotherapy.
Here, we launch an open-label phase I/II clinical trial to compare chemoimmunotherapy containing CEA-pulsed DC and CIK with conventional chemotherapy.
A major aim of this study was to examine the safety and feasibility of the combination treatment using DC/CIK vaccination and conventional chemotherapy. The combination therapy was well tolerated, and it appeared that adverse effects commonly seen in the conventional chemotherapy were less likely to occur in the chemoimmunotherapy group, although there was no evidence of statistical significance. Furthermore, chemoimmunotherapy relieved fatigue commonly seen in the conventional chemotherapy. Skin toxicity and non-infective fever were observed in the course of chemoimmunotherapy. However, they were generally mild, transient, and manageable with supportive care.
To further assess the clinical efficacy of chemoimmunotherapy, we evaluated the time to progression and the overall survival rate. We found that chemoimmunotherapy can prolong time to progression significantly from 5.2 to 6.9 months in patients with late-stage NSCLC. This finding indicates that DC/CIK immunization could synergize with chemotherapy to provide potent systemic antitumor activity. There are a number of potential mechanisms that could explain the observed effect. Firstly, cell death following chemotherapy may have a profound influence on the subsequent immune response. Secondly, chemotherapy depletes regulatory T cells, potentially enhancing immune responses. Thirdly, post-chemotherapy immune system reconstitution may provide a unique opportunity for therapeutic intervention by shaping the repertoire toward reactivity to tumor antigens. Finally, chemotherapy might change the cytokine profiling in DC toward their immunostimulating potential. In our opinion, the reduction in adverse effects by DC/CIK immunization may play an important role in improving the overall anticancer activity. Other possibilities are currently under investigation.
It is important to point out that in our preliminary study with relative small sample size, we recruited only 28 patients. Further, studies are required to rigorously test this treatment in the setting of a multi-center, randomized clinically trial.
In conclusion, our study provided the direct clinical evidence supporting the notion that the combination of DC-CIK vaccination and conventional chemotherapy can alleviate the toxicity profiles and prolong time to progression in late-stage NSCLC. Our data align well with the theory by Dr. Shurin [13–15] that cancer vaccines may be most effective in close combination with other treatments, specifically, chemotherapy rather than as a single modality. This concept may have board implications for the further development of new vaccine strategies.
Acknowledgments
This research was supported by Nature Science Foundation of Shanghai, China (No.: 10ZR1428200).
Footnotes
Runbo Zhong and Jiajun Teng contributed equally to this work.
References
- 1.Jemal A, Tivare RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Sun JM, Park JO, Won YW, Kim JH, Yun J, Lee J, Park YH, Ahn JS, Ahn MJ, Park K. Who are less likely to receive subsequent chemotherapy beyond first-line therapy for advanced non-small cell lung cancer? Implications for selection of patients for maintenance therapy. J Thorac Oncol. 2010;5(4):540–545. doi: 10.1097/JTO.0b013e3181d3504d. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Kelly RJ, Gulley JL, Giaccone G. Targeting the immune system in non-small-cell lung cancer: bridging the gap between promising concept and therapeutic reality. Clin Lung Cancer. 2010;11(4):228–237. doi: 10.3816/CLC.2010.n.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gérard C, Debruyne C. Immunotherapy in the landscape of new targeted treatments for non-small cell lung cancer. Mol Oncol. 2009;3(5–6):409–424. doi: 10.1016/j.molonc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cranmer LD, Trevor KT, Hersh EM. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother. 2004;53:275–306. doi: 10.1007/s00262-003-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I. Vaccines for solid tumours. Lancet Oncol. 2004;5:681–689. doi: 10.1016/S1470-2045(04)01610-9. [DOI] [PubMed] [Google Scholar]
- 8.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ming S, Bing Z, Zi-Rong T, et al. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2004;10(8):1146–1151. doi: 10.3748/wjg.v10.i8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui L, Changli W, Jinpu Y, et al. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery. Cytotherapy. 2009;11(8):1076–1083. doi: 10.3109/14653240903121252. [DOI] [PubMed] [Google Scholar]
- 11.Susan EA, Jose RG, Martin S, et al. Dendritic cells pulsed with CEA peptide induced CEA-specific CTL with restricted TCR repertoire. J Immunother. 1998;21(1):17–26. doi: 10.1097/00002371-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Marten A, Ziske C, Schottker B, et al. Interactions between dendritic cells and cytokine-induced killer cells lead to an activation of both populations. J Immunother. 2001;24(6):502–510. doi: 10.1097/00002371-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183(1):137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, Shurin GV. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13(18 Pt 1):5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]