Abstract
Recent findings show that immune cells constitute a large fraction of the tumor microenvironment and that they modulate tumor progression. Clinical data indicate that chronic inflammation is present at tumor sites and that IL-4, in particular, is upregulated. Thus, we tested whether IL-4 neutralization would affect tumor immunity. Current results demonstrate that the administration of a neutralizing antibody against IL-4 enhances anti-tumor immunity and delays tumor progression. IL-4 blockade also alters inflammation in the tumor microenvironment, reducing the generation of both immunosuppressive M2 macrophages and myeloid-derived suppressor cells, and enhancing tumor-specific cytotoxic T lymphocytes. In addition, IL-4 blockade improves the response to anti-OX40 Ab or CpG oligodeoxynucleotide immunotherapies. These findings suggest that IL-4 affects anti-tumor immunity and constitutes an attractive therapeutic target to reduce immune suppression in the tumor microenvironment, thus enhancing the efficacy of cancer therapy.
Keywords: IL-4, Macrophages, CD8, CpG ODN, Anti-OX40 Ab
Introduction
The goal of cancer immunotherapy is to elicit tumor-specific immunity capable of controlling or eliminating the tumor. However, active CTL induction, such as administration of cancer vaccines, is considered insufficient to elicit a durable cancer control and cure. In this context, an established tumor creates an immunosuppressive tumor microenvironment that inhibits the activity of cytotoxic T and NK cells that are critical for cancer elimination [1, 2]. Thus, strategies to reprogram the tumor microenvironment are being explored to improve the generation and activity of tumoricidal CD8 T cells. In addition, therapeutic options to overcome the barriers of the suppressive microenvironment and to support T cell infiltration and function in tumors are required.
Established tumors create an immunosuppressive microenvironment that contains regulatory T cells and various CD11b+ myeloid cells, including myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM) [1, 2]. These immune cells can also promote tumor growth and metastatic dissemination [3, 4]. Evidence from clinical studies suggests that high numbers of myeloid cells in the tumor correlate with poor overall survival in patients with cancer [5–10]. TAM and MDSC constitute most of these tumor-infiltrating leukocytes and are key contributors to the immunosuppressive milieu that protects tumors from elimination, therefore making them attractive targets for cancer immunotherapy.
Previous studies demonstrated two distinct types of polarized macrophages: classically activated (M1) and alternatively activated (M2) macrophage phenotypes [3, 11]. M2 macrophages are generated in Th2-biased inflammatory conditions or in the process of normal wound healing, and they express characteristic markers such as Retnla, Chi3l3, IL-10, and arginase 1 (Arg 1) [11, 12]. By contrast, M1 macrophages protect the host against infectious pathogens. In tumors, the infiltrating macrophages are considered to be of the M2 macrophage phenotype, which creates an immunosuppressive microenvironment for tumor progression [11, 12]. On the contrary, M1 macrophages do play an anti-tumor role.
Increased levels of IL-4 are commonly detected in the tumors of animals and patients with multiples types of primary and metastatic cancers [13–20]. Clinical evidence suggests that IL-4 can act as a tumor-promoting cytokine. This is consistent with studies in animal models that illustrate that IL-4 can facilitate tumor growth, and that blockade of IL-4 signaling can significantly delay cancer cell proliferation [18–22]. We and others previously documented that IL-4 was produced by CD4 T cells in tumor microenvironments [18, 21]. In particular, we demonstrated that T follicular helper (Tfh) cells are a major source of IL-4 in the tumor microenvironment [18]. CNS2 knockout mice, which have a selective deficiency of IL-4 in Tfh cells, but not in Th2 cells, displayed severely impaired expression and production of IL-4 in tumors and tumor-draining LN.
In this study, we further explored the effects of IL-4 blockade on the anti-tumor immunity and the immunosuppressive environment at the tumor site, to which myeloid cells are believed to contribute. The administration of a neutralizing antibody against IL-4 enhances anti-tumor immunity and delays tumor progression, thus having a dramatic impact on the local immune milieu. IL-4 blockade also alters the function of macrophages, reducing the generation of immunosuppressive M2 macrophages, and furthermore, synergistically augmenting cancer immunotherapies. Therefore, we propose the pharmacological targeting of IL-4, alone or in combination with other immunotherapies, as a suitable treatment for patients with cancer.
Materials and methods
Animals and tumor cell lines
BALB/c mice were obtained from Japan SLC (Hamamatsu, Japan) and studied at 6–10 weeks of age. All studies were approved by the Institutional Committee for the Use and Care of Laboratory Animals of Tohoku University. The murine 4T1 mammary carcinoma cell line and the CT26 colon cancer cell line were obtained from the American Type Culture Collection (Manassas, VA).
Reagents
Hybridomas for anti-IL-4 (11B11) and anti-OX40 Abs were injected intraperitoneally (i.p.) into nude mice to produce ascites. The IgG fraction from the ascites was purified using a ProteinG Midi kit (Bio-Rad, Hercules, CA), according to the instructions of the manufacturer. Isotype controls (anti-HRP, Rat IgG1) were purchased from BioXcell (West Lebanon, NH). Phosphorothioate oligodeoxynucleotides (ODN) were synthesized at BEX Co. Ltd. (Tokyo, Japan). The following ODN were used: CpG ODN (5′-GCTAGACGTTAGCGT-3′).
In vivo tumor studies
Mice were injected subcutaneously (s.c.) with 105 viable tumor cells. Tumor volumes were calculated using following formula: (length × width × height)/2. Tumor-bearing mice were divided into groups, depending on the tumor volume, to be averaged before the treatment. To deplete CD8+ cells, mice were injected i.p. with 500 µg rat anti-mouse CD8 (53.6.72) Ab (BioXCell). The metastatic 4T1 breast tumor model and post-surgery method was described previously [23, 24]. Briefly, mice were challenged s.c. in the flank with 105 of 4T1 cells. Developed tumors were surgically removed at day 14 and monitored for survival.
Flow cytometry
Tumor cells for flow cytometry were prepared as previously described [18, 25]. Cells were washed with PBS and stained with fluorochrome-conjugated anti-CD45, anti-CD3, anti-CD4, anti-CD8, anti-Ly6g, anti-Ly6c, and/or anti-CD11b Abs for 30 min and fixed in fixation buffer for 10 min. All Abs were obtained from BD Pharmingen (San Diego, CA) or Biolegend (San Diego, CA). Stained cells were analyzed using a BD LSRFORTESSA (BD Pharmingen).
Preparation of cells
Tumors were digested in Liberase/DNase I solution for 30 min at 37 °C. Cells were washed with 2% FCS/PBS, stained with fluorochrome-conjugated Abs for 30 min at 4 °C, and sorted using BD FACSAria to isolate TAM, as defined by the following characteristics: CD45+, CD11b+, Ly6c−, and Ly6g−.
CD4+ or CD8+ T cells were isolated from spleen cells in syngeneic mice using positive selection of CD4+ or CD8+ MACS beads (Miltenyi Biotec, Auburn, CA) as recommended by the manufacturer.
T cell proliferation assay
T cell proliferation assay was performed as described previously [25]. Briefly, MACS-sorted CD4+ and CD8+ T cells were labeled with 0.3 mM 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE), according to the manufacturer’s instructions (eBioscience, San Diego, CA). T cells were stimulated with 1 µg of anti-CD3 and anti-CD28 Abs for 3 days. T cell proliferation was monitored by CFSE dilution using a BD LSRFORTESSA.
ELISpot and ELISA assays
ELISpot and ELISA assays were performed as described previously [23, 25]. Briefly, 96-well Immulon II plates (Millipore, Billerica, MA) for the ELISpot assay were previously coated with anti-IFN-gamma (IFNg) Ab (BD Biosciences). 5.0 × 105 LN cells/well were incubated at 37 °C with AH-1 peptide for 14 h. The plates were washed and treated with biotinylated anti-IFNg Ab (R&D Systems, MN) for 1 h. The plates were washed again and followed by streptavidin alkaline phosphatase. Plates were developed with 5-bromo-4-chloro-3-indolyl phosphatase solution (Sigma-Aldrich) in agarose (Sigma-Aldrich). The significant number of spots was determined by the statistical difference compared with the number of spots in unstimulated wells.
Culture supernatants were used to determine concentrations of IFNg or IL-4 using the ELISA assay, according to the manufacturer’s recommendation. Paired anti-IFNg and anti-IL-4 Abs were purchased from BD Biosciences. Plates were developed with ABTS Peroxidase Substrate (KPL, Gaithersburg, MD).
Real-time RT-PCR
Real-time RT-PCR was performed as described previously [18]. Briefly, total RNA was extracted from cells using the TRIzol reagent (Life Technologies, Carlsbad, CA) and reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR was conducted on cDNA using TaqMan probes and the TaqMan Gene Expression Master Mix kit (Applied Biosystems, Foster City, CA). Relative mRNA expression levels were calculated independently after correcting for GAPDH expression.
Statistical analysis
A two-sided unpaired Student’s t test was used to analyze tumor growth and cellular responses. p values <0.05 were considered statistically significant.
Results
IL-4 neutralization slows tumor growth
Increased levels of IL-4 have been reported in the tumor sites of patients with various cancers [13–20]. To examine the role of IL-4 in tumor growth, BALB/c mice were injected s.c. with CT26 colon or 4T1 breast tumor cells, and treated i.p. with anti-IL-4 Ab (11B11) or control antibody on days 12, 17, and 22. These tumor cells grew to form detectable masses (approximately 3–5 mm in diameter) 12 days after being injected into the flanks of syngeneic mice. As shown in Fig. 1a, tumor growth was significantly delayed in anti-IL4 Ab-treated mice, whereas control Ab had no effect.
Fig. 1.
Effects of IL-4 neutralization on tumor growth and tumor-specific IFNg production. a CT26 colon cancer cells (1.0 × 105) or 4T1 breast cancer cells (1.0 × 105) were injected s.c. into BALB/c mice. On days 12, 17, and 22, mice were injected i.p. with 500 µg of anti-IL-4 Ab (11B11) or 500 µg of control Ab, and the tumor size was monitored. Data represent the mean ± SEM of 6–10 mice per group from two independent experiments. b BALB/c mice were challenged s.c. in the flank with 4T1 cells (1.0 × 105). Developed tumors were surgically removed at day 14 and mice were monitored for survival. After surgical resection, mice were treated with 500 µg of anti-IL-4 Ab on days 14, 19, 24 and 29. Data show survival (N = 19; untreated, N = 15; anti-IL-4 Ab-treated group). c Tumor-draining LN from CT26 tumor-bearing mice were isolated on day 24, re-stimulated ex vivo with 1 µg/ml AH-1 peptide, and monitored for IFNg secretion using the ELISpot assay. Results represent the mean ± SD of five mice per group from two independent experiments. d CT26 colon cancer cells (1.0 × 105) were injected s.c. into BALB/c mice. On days 12, 17, and 22, mice were injected i.p. with anti-CD8 Ab (Ly2) and/or anti-IL-4 Ab, and the tumor size was monitored. Data represent the mean ± SEM of 6–10 mice per group from two independent experiments. *p < 0.05 compared with the untreated group. **p < 0.01 compared with the untreated group. ***p < 0.001 compared with the untreated group
Next, we examined the ability of anti-IL-4 Ab to prevent metastasis after the surgical removal of the implanted tumor. Implanted 4T1 cells can generate spontaneous metastasis to the lung, liver, and lymph nodes [23, 24]. After surgical resection, mice were treated with anti-IL-4 Ab on days 14, 19, 24 and 29. As shown in Fig. 1b, untreated mice were uniformly lethal within 2 months. In contrast, the treatment of anti-IL-4 Ab significantly reduced the lethality and prolonged their life.
To clarify the mechanism by which IL-4 neutralizing Ab slows tumor growth and prolongs survival, cells were isolated from the draining LN of CT26 tumor-bearing mice on day 24. The cells were cultured ex vivo with the AH-1 peptide, which is a CD8-restricted epitope expressed by CT26. LN cells from untreated tumor-bearing mice responded significantly to stimulation from the AH-1 peptide, compared with unstimulated LN cells, by secreting IFNg. This was not observed in the presence of the SIIFEKL peptide as a negative control peptide (Fig. 1c and unpublished data). Similarly, LN cells from tumor-free mice displayed no response when cultured with the AH-1 peptide (Fig. 1c). LN cells from tumor-bearing mice treated with anti-IL-4 Ab responded strongly to AH-1 stimulation, whereas those treated with control Ab displayed no activity (Fig. 1c). These groups all responded similarly to the stimulation of concanavalin A as a positive control (unpublished data). These findings suggested that the administration of anti-IL-4-neutralizing Ab enhanced the number of tumor-specific CD8 T cells in tumor-draining LN. To evaluate the relevance of these T cells in vivo, mice that had been challenged with tumor cells and treated with the anti-IL-4 Ab were injected with anti-CD8 Ab to deplete CD8 T cells. As seen in Fig. 1d, protection was abrogated by the depletion of CD8 T cells, indicating that CD8 T cells play a critical role in IL-4-depleted tumor immunity.
To gain further insight into the mechanism underlying this effect, the fraction of CD4 or CD8 T cells infiltrating the tumor was analyzed by flow cytometry. Consistent with the enhancement of anti-tumor immunity observed in anti-IL-4 Ab-treated mice, treatment with anti-IL-4 Ab significantly increased the fraction of CD8 and CD4 T cells infiltrating the tumor (Fig. 2a). Similar results were observed in the 4T1 model (unpublished data). These findings suggest that IL-4 blockade enhanced anti-tumor immunity and slowed tumor growth.
Fig. 2.
Effect of IL-4 neutralization on infiltrated immune cells and gene expression in tumors. Mice were treated as described in Fig. 1. a Tumors were removed from CT26 tumor-bearing mice on day 26, and the number of tumor-infiltrating CD45, CD4, CD8 cells, MDSC (CD11b+, Ly6c+, Gr-1int) and TAM (CD11b+, Ly6c–, Ly6g–) were determined by flow cytometry. b mRNA expression of tumors from CT26 tumor-bearing mice was analyzed on day 26. Results were evaluated independently for each mouse, and data represent the means + SD of 5–8 mice per group from two independent experiments. *p < 0.05 when compared with untreated group. **p < 0.01 when compared with untreated group. ***p < 0.01 when compared with untreated group
IL-4 neutralization alters the tumor microenvironment
Treatment with anti-IL-4 Ab led to significant changes in the frequency of tumor infiltration by CD4 and CD8 T cells and tumor-specific CD8 T cells in LN. To evaluate molecular mediators associated with their immunological functions, the mRNA expression of effector and immunosuppressive cytokines in the tumor was evaluated by real-time RT-PCR. Treatment with anti-IL-4 Ab had no effect on the expression of IL-4 itself (Fig. 2b). Consistent with the increase in CD8 T cells at the tumor site, the expression of IFNg, IL-12, and Granzyme B (GZMB) was significantly increased in anti-IL-4 Ab-treated tumors (Fig. 2b). No effect was observed on the expression of Foxp3, which is known as a regulatory T cell (Treg cell)-specific transcription factor. In addition, angiogenic factors were evaluated. The expressions of vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) were significantly reduced in anti-IL-4 Ab-treated tumors (Fig. 2b).
It is well established that immature myeloid cells and macrophages accumulate at tumor sites [1]. These cells are considered important markers of immune suppression, as they suppress the tumoricidal activity of CTL and NK cells. The ability of myeloid cells to suppress T cell activation is linked to their metabolism of l-arginine via Arg 1 and the release of inducible nitric oxide synthase [3, 4]. As presented in Fig. 2b, the expression of Arg 1 was significantly reduced in anti-IL-4 Ab-treated tumors. TAM express characteristic M2 markers such as Retnla (Fizz-1) and Chi3l3 [3]. Gene expression analysis revealed that those M2 markers were significantly downregulated in anti-IL-4 Ab-treated tumors (Fig. 2b).
IL-4 neutralization decreases the T cell-suppressive activity of TAM
IL-4 blockade significantly reduced levels of the TAM markers and the immunosuppressive factor Arg 1 in tumors. Thus, the frequency of TAM or MDSC in tumors was evaluated by flow cytometry. Contrary to expectations, the frequencies of these myeloid cells in tumors from anti-IL-4-treated mice were comparable to those in the untreated groups (Fig. 2a). IL-4 blockade did not reduce the accumulation of TAM and MDSC in tumors (Fig. 2a).
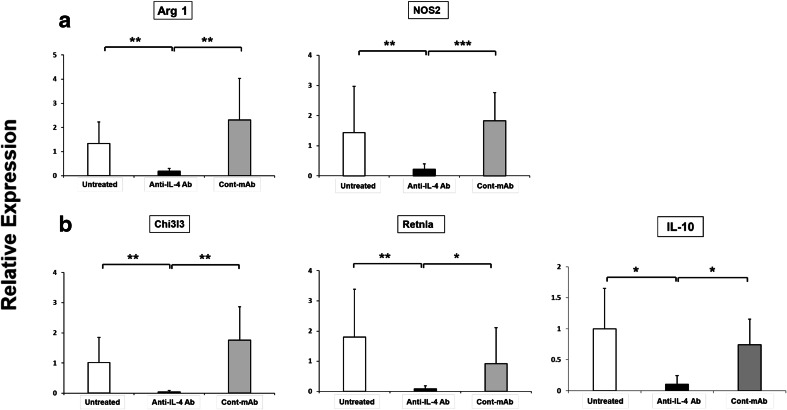
To characterize TAM (CD11b+, Ly6c−, Ly6g−) and MDSC (CD11b+, Ly6cHigh, Ly6g−) in tumors from IL-4-depleted mice, these cells were sorted by flow cytometry and analyzed for gene expression by real-time RT-PCR. To study the impact of systemic IL-4 neutralization on the functional properties of MDSC, gene expression was examined in tumor-infiltrating MDSC. Of interest, the expression of Arg 1 was severely reduced in MDSC from IL-4-depleted mice, whereas treatment with control Ab had no effect on MDSC (Fig. 3a). Similarly, nitric oxide synthase 2 (NOS2) expression was also significantly reduced in MDSC from IL-4-depleted mice (Fig. 3a).
Fig. 3.
Effect of IL-4 neutralization on gene expression in MDSC and TAM. Mice were treated as described in Fig. 1. On day 26, a tumor-infiltrating MDSC (mMDSC: CD45+, CD11b+, Ly6c+, Ly6g−) were purified by flow cytometry and analyzed for mRNA levels of Arg 1 and NOS2 by real-time quantitative RT-PCR. b TAM (CD45+, CD11b+, Ly6c−, Ly6g−) were also purified by flow cytometry and analyzed for mRNA levels of Chi3l3, Retnla and IL-10 by real-time quantitative RT-PCR on day 26. Results were evaluated independently for each mouse, and data represent the means + SD of five mice per group. *p < 0.05 when compared with control Ab group. **p < 0.01 when compared with untreated or control Ab group. ***p < 0.001 when compared with control Ab group
As shown in Fig. 3b, the expression of M2 markers, such as Chi3l3, Retnla, and IL-10, was significantly reduced in TAM from IL-4-depleted mice. These cells also displayed severely decreased expression of Arg 1 (data not shown). These results suggest that IL-4 blockade did not change the frequency of myeloid cells in tumors, but it altered their characteristics and activation status.
It is well established that MDSC and TAM suppress the proliferation and functional activity of Ab-stimulated T cells [1–4]. To examine the effect of IL-4 neutralization on this inhibitory activity, TAM were isolated from tumors by flow cytometry. Those cells were mixed with CD4 or CD8 T cells and stimulated with anti-CD3 and anti-CD28 Abs. These T cells proliferated in response to anti-CD3/anti-CD28 Abs stimulation. Consistent with previous reports, this proliferation was almost completely inhibited when TAM were added to the culture (Fig. 4a, b). However, this ability to suppress T cell proliferation was abrogated in TAM from IL-4-depleted tumors. By contrast, no reduction in suppressive activity was observed when TAM from control Ab-treated tumors were added (Fig. 4a, b). These findings suggest that the function of immunosuppressive myeloid cells in the tumor microenvironment is significantly reduced when IL-4 is depleted in tumor-bearing mice.
Fig. 4.
Effect of IL-4 neutralization on the suppressive activity of TAM. Mice were treated as described in Fig. 1. On day 26, tumor-infiltrating macrophages, were purified by flow cytometry based on the pattern of surface marker expression (TAM: CD45+, CD11b+, Ly6c−, Ly6g−). T cells were separated and purified by MACS and labeled with CFSE, stimulated with 0.5 ug/ml anti-CD3/CD28 Abs, and cultured with TAM (T cells:TAM = 1:0.3). CD4 and CD8 T cells proliferation was monitored by CFSE dilution on day 3. a Representative example and b mean + SD of five mice per group from three independent experiments are shown. ***p < 0.001 when compared with anti-IL-4 Ab-treated group
Next, cytokine production and the characterization of induced-CD4 T cells were evaluated by real-time RT-PCR and the ELISA assay in response to anti-CD3/anti-CD28 Abs stimulation following incubation with TAM. CD4 T cells, stimulated with anti-CD3 and anti-CD28 Abs, produced both IFNg and IL-4. TAM from untreated mice moderately inhibited the production of IFNg, but not IL-4 (Fig. 5a, b). Of interest, anti-IL4-treated TAM significantly enhanced the production of IFNg, but not IL-4, indicating the shift in the Th1 differentiation. Next, the expressions of T-bet and GATA3 in CD4 T cells were evaluated. As shown in Fig. 5c, d, the expressions of T-bet and GATA3 were significantly increased by anti-CD3/-CD28 Abs stimulation without TAM. Untreated TAM did not increase those expressions. Anti-IL-4-treated TAM increased the expression of T-bet, but not GATA3, indicating the induction of Th1 differentiation. Taken together, these results suggested that IL-4 blocked TAM-supported Th1 differentiation in vitro, indicating the possession of M1 macrophage functions.
Fig. 5.
Effect of CD4 T cell differentiation by TAM in vitro. Cells were cultured for 3 days as described in Fig. 4. a, b Production of IFNg and IL-4 in culture supernatants were assessed by ELISA. c, d Cells cultured for 3 days were analyzed for mRNA levels of T-bet and GATA3 by real-time RT-PCR. Results represent the mean + SD of results from three independent TAM preparations. *p < 0.05
IL-4 neutralization enhances the efficacy of immunotherapy
IL-4 blockade slowed tumor growth and altered the tumor microenvironment (reducing the generation of immunosuppressive M2 macrophages while enhancing the generation of M1 macrophages and tumor-specific CTL). However, the administration of anti-IL-4 Ab could not eliminate a tumor completely. Based on the increased CD8 T cell infiltration in tumors, we reasoned that efficacy might be enhanced in combination with CpG ODN or anti-OX40 Ab treatment, which both enhance anti-tumor immunity. It is well established that stimulation via CpG ODN or anti-OX40 Ab treatment leads to T cell activation [26–29]. Therapies utilizing CpG ODN or anti-OX40 Ab have been found to present anti-tumor effects in a number of model systems [26–29]. Thus, the combination of CpG ODN or anti-OX40 plus anti-IL-4 Abs was analyzed. To reveal the effects of combination therapies, suboptimal doses of CpG ODN or anti-OX40 Ab, as determined in previous reports or preliminary studies, were used in this study, which by themselves did not cause the marked inhibition of tumor growth. Systemic (intraperitoneal) treatment with CpG ODN or anti-OX40 Ab tended to slow tumor growth, albeit insignificantly. By contrast, animals treated with a combination of CpG ODN and anti-IL-4 Ab did not exhibit an increase in tumor size (Fig. 6a). The combination synergistically inhibited tumor growth compared to CpG or anti-IL-4 Ab treatment alone. The combination of anti-OX40 Ab and anti-IL-4 Ab also synergistically inhibited tumor growth, similar to the combination of CpG ODN and anti-IL-4 Ab (Fig. 6b). These findings suggest that IL-4 neutralization enhances the anti-tumor immunity of cancer immunotherapy.
Fig. 6.
Combination treatment with anti-IL-4 Ab and CpG ODN or anti-OX-40 Ab has synergistic anti-tumor effects. CT26 colon cancer cells (1.0 × 105) were injected s.c. into BALB/c mice. Mice were injected i.p. with 500 µg of anti-IL-4 Ab on days 12, 17, and 22 plus a 25 µg of CpG ODN on days 13 and 18 or b 250 µg of anti-OX40 Ab on days 13 and 18; tumor size was monitored. Data represent the mean + SEM of 8–10 mice per group from two independent experiments. c Tumor-draining LN from CT26 tumor-bearing mice were isolated on day 24, re-stimulated ex vivo with 1 µg/ml AH-1 peptide, and monitored for IFNg secretion by the ELISpot assay. Results represent the mean ± SD of 5–7 mice per group from two independent experiments. CT26 tumors were removed on day 22, and the number of tumor-infiltrating CD45+ cells that expressed CD4 and CD8 was determined by flow cytometry. d Representative results from one mouse per group and e mean ± SD from five independently analyzed mice per group, showing CD4- and CD8-expressing cells as a percentage of all cells (including tumor cells). *p < 0.05, **p < 0.01, ***p < 0.005
For example, the combination of CpG ODN and anti-IL-4 Ab significantly enhanced the number of IFNg-producing cells in tumor-draining LN compared to CpG or anti-IL-4 Ab treatment alone (Fig. 6c). Tumor-infiltrating CD8 T cells were profoundly associated with improved host survival. Therefore, the effects of combination treatments on the frequency of tumoricidal cells were analyzed. Treatment with anti-IL-4 Ab or CpG ODN alone increased the frequency of CD4 and CD8 T cells infiltrating the tumor site by threefold compared to the frequency for untreated controls (p < 0.01; Fig. 2a). This increase was magnified in mice treated with a combination of these two agents. The combination synergistically increased both CD4 and CD8 T cell numbers by >eightfold (Fig. 6d, e).
Discussion
Clinical evidence suggests that IL-4 can act as a tumor-promoting molecule, as it is found at high levels in multiple types of human primary and metastatic cancers [13–17]. Thus, we investigated the anti-tumor effect of IL-4 blockade using a neutralizing anti-IL-4 Ab in animal models. Consistent with previous reports, IL-4 blockade slowed tumor growth and prolonged survival in multiple tumor models (Fig. 1a, b). The effect of IL-4 neutralization on tumor growth was dependent on the increased presence of CD8 T cells in tumors, as their selective depletion abrogated the improved outcome (Fig. 1d). The current findings extended these observations by illustrating that the induction of tumor-specific CTL and the expression of genes associated with CTL responses, such as IFNg, GZMB, and IL-12, were increased in tumors from IL-4-depleted mice (Figs. 1c, 2b). Furthermore, IL-4 neutralization reduced the ability of tumor-infiltrating macrophages to inhibit T cells, leading to increased numbers of tumor-infiltrating CD8 T cells (Figs. 2a, 3, 4). Finally, these effects allowed the augmentation of cancer immunotherapies, such as treatment with CpG ODN or anti-OX40 Ab (Fig. 6a, b).
Recent reports demonstrated that IL-4 can suppress tumor immunity in several manners. IL-4, a typical Th2 cytokine, can downregulate the development of Th1 inflammation and anti-tumor response and directly act on CD8 T cells to render them non-cytotoxic [30]. The current findings confirmed these data by demonstrating that IL-4 neutralization enhances anti-tumor immunity, specifically increasing the numbers of tumor-specific CD8 T cells in LN and tumor-infiltrating CD8 T cells (Figs. 1c, 2a). IL-4 also induces the production of other anti-inflammatory cytokines and supports the generation of immunosuppressive cells of the myeloid lineage [4, 11, 12, 31]. In this context, the current findings demonstrate that IL-4 blockade induced dramatic changes of the tumor microenvironment, resulting in the downregulation of Th2-biased genes and the upregulation of Th1-biased genes (Fig. 2b).
Consistent with current data, several reports have shown that the administration of anti-IL-4 antibody marginally, but significantly, slowed tumor growth or prolonged survival [19–21]. Denardo et al. demonstrated that the treatment of anti-IL-4 Ab significantly reduced the tumor metastasis in lung tissue of MMTV-PyMT mice, and prevented the activation of TAM [21]. In addition to the effect of immune functions, IL-4 promotes tumor growth by mediating an increased proliferation and survival, and protecting them from apoptosis through the IL-4 receptor on the tumor cells directly [20, 32, 33]. Prokopchuk et al. showed that IL-4 enhances proliferation of human pancreatic cancer cells through the activation of MAP Kinase [32]. Venmar et al. has shown that the knockdown of the IL-4 receptor resulted in significantly attenuated lung metastasis in the 4T1 model through the inhibition of Erk1/2, Akt, and mTOR activation [33]. IL-4 signaling has also been demonstrated to function in the protection of tumor cells from apoptosis by increasing anti-apoptosis proteins [20]. These data indicate that IL-4 in tumors directly and indirectly induce tumor development and progression. In contrast, the direct effect of IL-4 signaling on CT26 tumor cells could be excluded from the current results since the CT26 cells did not express IL-4Ra (CD124) [34].
It is well established that IL-4 promotes the differentiation of M2 macrophages and upregulation of Arg 1 activity, which increases the suppressive function of MDSC and TAM [3, 4, 11, 12]. The current studies examined the functional characteristics and nature of the tumor-infiltrating macrophages. IL-4 blockade abrogated the inhibitory activity of T cells in TAM, but it did not affect the population of tumor-infiltrating myeloid cells (Figs. 2a, 4). Consistent with this activity, expression of the M2 markers, Retnla and Fizz-1, was severely reduced in tumors from IL-4-depleted mice (Fig. 3a, b). Furthermore, anti-IL4-treated TAM significantly enhanced the production of IFNg and the expression of T-bet in CD4 T cells in vitro, indicating a shifting to M1 macrophage functions (Fig. 5a–d). Thus, IL-4-depleted myeloid cells changed their functional properties, but not their population. This, in turn, facilitated the expansion and infiltration of cytotoxic CD8 T cells, and thus supported CD8 T cell-mediated tumor regression.
Monocytic MDSC also promote the expansion of regulatory T cells that downregulate tumor-specific immunity [35]. Current results showed that no effect was observed on the expression of Foxp3 in the bulk of the tumor (Fig. 2b). However, the expression of Foxp3 was significantly reduced in CD4 T cells from anti-IL-4-treated animals due to the increased-number of CD4 T cells in the tumor (unpublished data). The production of ROS by granulocytic MDSC (gMDSC) also contributes to the suppression of T cells. However, IL-4 blockade had no effect on the number of gMDSC or on the expression of ROS (unpublished data). Unfortunately, we were unable to observe whether tumor-infiltrating MDSC inhibited the proliferation of T cells since it was technically difficult to isolate enough MDSC from the tumor, and the viability of those cells was very low (unpublished data).
The existence of myeloid cells, such as macrophages and/or MDSC, in multiple types of human cancers correlates with poor clinical outcomes, as well as the enhancement of angiogenesis and promotion of tumor growth and metastasis [12, 21, 36–38]. In this context, the current findings illustrated that IL-4 blockade severely reduced the expression of VEGF and EGF in tumors (Fig. 2b). VEGF is known as a major contributor to angiogenesis and is released by TAM [36]. Its levels correlate with the density of myeloid cells in several types of human cancers [36–38]. EGF is also expressed by TAM. DeNardo et al. reported that the activation of TAM by IL-4 significantly augments the induction of EGF expression in TAM by CSF-1 derived from malignant cells, and the IL-4-enhanced EGF/CSF-1 paracrine loop contributes to the invasive behavior of cancer metastasis [21]. The targeting of TAM appears to be a promising approach for cancer therapy. Thus, multiple strategies are being pursued to reduce their activities. The successful reduction in macrophage numbers by CSF-1R blockade is associated with an improvement in tumor-specific immunity [39]. Clinical trials evaluating the efficacy of CSF-1R antagonists in several types of solid tumors are ongoing. Another possible strategy for reducing TAM numbers is to manipulate the TAM phenotype or re-polarize macrophages toward M1 [25, 40, 41].
The current studies demonstrated that IL-4 blockade altered the tumor microenvironment, increasing the number of T cells in tumors and decreasing the suppressive function of macrophages, but this treatment did not shrink tumors (Fig. 1a). These findings led us to examine combination treatments with another immunotherapy that enhances the activity of CTL. Our findings demonstrated that IL-4–neutralizing Ab, in combination with immunotherapeutic agents (CpG ODN or anti-OX40 Ab), delivered significantly inhibited tumor growth, accompanied by synergistically increased T cell infiltration; effects that were not achieved by the administration of each treatment alone (Fig. 6).
The current studies observed that mice were generally tolerant of large doses of anti-IL-4 Ab (11B11), with no evidence of toxicity observed at doses up to 50 mg•kg−1. Previous reports using anti-IL-4 Ab (11b11) demonstrated that 25–50 mg•kg−1 of antibody was treated to neutralize endogenous IL-4 [19–21, 42, 43]. Those reports did not observe the toxicity of anti-IL-4 Ab. In clinical studies, a humanized anti-IL-4 mAb (pascolizumab) has been developed as a potential therapeutic agent for allergic diseases [44]. Although phase I/II of the clinical trials in human asthmatics did not demonstrate clinical efficacy, there were no significant adverse reactions reported [44]. As a promising safety profile of IL-4 neutralization in humans is well established, these findings support the use of anti-IL-4 Ab for cancer immunotherapy.
In our studies of anti-IL-4 Ab administration, treatment was associated with dramatic changes in the tumor microenvironment, including a shift from a Th2- to Th1-polarized microenvironment, increases in tumor-infiltrating T cells, and decreases in the suppressive activity of TAM. The features of IL-4 blockade suggest that they may be useful in combination with other cancer treatments, including radiation and chemotherapy. Recent advances in cancer immunotherapies, such as immune checkpoint inhibitors, have resulted in impressive clinical benefits for some cancers [45]. Multiple strategies are currently being pursued to reduce their suppressive activities, and therefore macrophages in tumors should be considered a target of cancer immunotherapy. These encouraging results support the continued development of macrophage-targeted therapy and clinical testing of IL-4 blockade as adjuncts to cancer immunotherapy.
Abbreviations
- Arg 1
Arginase 1
- CFSE
Carboxyfluorescein succinimidyl ester
- EGF
Epidermal growth factor
- GZMB
Granzyme B
- IFNg
IFN-gamma
- MDSC
Myeloid-derived suppressor cells
- NOS2
Nitric oxide synthase 2
- ODN
Oligodeoxynucleotides
- TAM
Tumor-associated macrophages
- Tfh cells
T follicular helper cells
- Treg cell
Regulatory T cell
- VEGF
Vascular endothelial growth factor
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interests.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant No. 25430103 and 16K07106, Japan.
References
- 1.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 2.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6(8):613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 3.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60(10):2632–2635. [PubMed] [Google Scholar]
- 6.Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5(5):1107–1113. [PubMed] [Google Scholar]
- 7.Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17(3):445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 8.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56(20):4625–4629. [PubMed] [Google Scholar]
- 9.Lee AH, Happerfield LC, Bobrow LG, Millis RR. Angiogenesis and inflammation in invasive carcinoma of the breast. J Clin Pathol. 1997;50(8):669–673. doi: 10.1136/jcp.50.8.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol. 2000;7(7):263–269. doi: 10.1046/j.1442-2042.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang HW, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: priming for protumoral functions. Cell Cycle. 2010;9(24):4824–4835. doi: 10.4161/cc.9.24.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, Tanaka Y, Zurawski S, Zurawski G, Bover L, Liu YJ, Banchereau J, Palucka AK. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208(3):479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN, Melanoma Study Group of the Mayo Clinic Cancer C Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15(6):1931–1939. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Wu Y, Su Z, Amoah Barnie P, Jiao Z, Bie Q, Lu L, Wang S, Xu H. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PLoS One. 2014;9(8):e104453. doi: 10.1371/journal.pone.0104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baier PK, Wolff-Vorbeck G, Eggstein S, Baumgartner U, Hopt UT. Cytokine expression in colon carcinoma. Anticancer Res. 2005;25(3B):2135–2139. [PubMed] [Google Scholar]
- 17.Li J, Wang Z, Mao K, Guo X. Clinical significance of serum T helper 1/T helper 2 cytokine shift in patients with non-small cell lung cancer. Oncol Lett. 2014;8(4):1682–1686. doi: 10.3892/ol.2014.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirota H, Klinman DM, Ito SE, Ito H, Kubo M, Ishioka C. IL4 from T follicular helper cells downregulates antitumor immunity. Cancer Immunol Res. 2017;5(1):61–71. doi: 10.1158/2326-6066.CIR-16-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1(4):389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Jiang J, Wang Z, Zhang J, Xiao M, Wang C, Lu Y, Qin Z. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008;68(21):8687–8694. doi: 10.1158/0008-5472.CAN-08-0449. [DOI] [PubMed] [Google Scholar]
- 21.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 23.Shirota H, Klinman DM. CpG-conjugated apoptotic tumor cells elicit potent tumor-specific immunity. Cancer Immunol Immunother. 2011;60(5):659–669. doi: 10.1007/s00262-011-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60:2710–2715. [PubMed] [Google Scholar]
- 25.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188(4):1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4(6):420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 28.Shirota H, Tross D, Klinman DM. CpG oligonucleotides as cancer vaccine adjuvants. Vaccines (Basel) 2015;3(2):390–407. doi: 10.3390/vaccines3020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirota H, Klinman DM. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines. 2014;13(2):299–312. doi: 10.1586/14760584.2014.863715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villacres MC, Bergmann CC. Enhanced cytotoxic T cell activity in IL-4-deficient mice. J Immunol. 1999;162(5):2663–2670. [PubMed] [Google Scholar]
- 31.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92(5):921–928. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venmar KT, Carter KJ, Hwang DG, Dozier EA, Fingleton B (2014) IL4 receptor ILR4α regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res 74(16):4329–4340. doi:10.0008-5472.CAN-14-0093 [DOI] [PMC free article] [PubMed]
- 34.Yang CY, Liu HW, Tsai YC, Tseng JY, Liang SC, Chen CY, Lian WN, Wei MC, Lu M, Lu RH, Lin CH, Jiang JK. Interleukin-4 receptor-targeted liposomal doxorubicin as a model for enhancing cellular uptake and antitumor efficacy in murine colorectal cancer. Cancer Biol Ther. 2015;16(11):1641–1650. doi: 10.1080/15384047.2015.1095397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valkovic T, Dobrila F, Melato M, Sasso F, Rizzardi C, Jonjic N. Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch. 2002;440(6):583–588. doi: 10.1007/s004280100458. [DOI] [PubMed] [Google Scholar]
- 38.Shieh YS, Hung YJ, Hsieh CB, Chen JS, Chou KC, Liu SY. Tumor-associated macrophage correlated with angiogenesis and progression of mucoepidermoid carcinoma of salivary glands. Ann Surg Oncol. 2009;16(3):751–760. doi: 10.1245/s10434-008-0259-6. [DOI] [PubMed] [Google Scholar]
- 39.Ries CH, Hoves S, Cannarile MA, Ruttinger D. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Curr Opin Pharmacol. 2015;23:45–51. doi: 10.1016/j.coph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, O’Garra A, Trinchieri G, Caux C. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196(4):541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang CY, Zen K. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. 2012;4(5):341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 42.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-polarized CD4+ T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res. 2015;3(5):518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi T, Ueki T, Sasaki Y, Kajiwara T, Li B, Moriyama N, Kawabe K. Th2-like response and antitumor effect of anti-interleukin-4 mAb in mice bearing renal cell carcinoma. Cancer Immunol Immunother. 1997;43(6):375–381. doi: 10.1007/s002620050347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tourangeau LM, Kavanaugh A, Wasserman SI. The role of monoclonal antibodies in the treatment of severe asthma. Ther Adv Respir Dis. 2011;5(3):183–194. doi: 10.1177/1753465811400489. [DOI] [PubMed] [Google Scholar]
- 45.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]