Abstract
Targeted delivery of tumor-associated antigens to professional antigen-presenting cells (APC) is being explored as a strategy to enhance the antitumoral activity of cancer vaccines. Here, we generated a cell-based system for continuous in vivo production of a CTLA-4-ErbB2 fusion protein as a therapeutic vaccine. The chimeric CTLA-4-ErbB2 molecule contains the extracellular domain of CTLA-4 for specific targeting to costimulatory B7 molecules on the surface of APC, genetically fused to residues 1–222 of human ErbB2 (HER2) as an antigenic determinant. In wild-type BALB/c mice, inoculation of syngeneic epithelial cells continuously secreting the CTLA-4-ErbB2 fusion vaccine in the vicinity of subcutaneously growing ErbB2-expressing renal cell carcinomas resulted in the rejection of established tumors, accompanied by the induction of ErbB2-specific antibodies and cytotoxic T cells. In contrast, treatment with CTLA-4-ErbB2 vaccine-secreting producer cells alone was insufficient to induce tumor rejection in ErbB2-transgenic WAP-Her-2 F1 mice, which are characterized by pronounced immunological tolerance to the human self-antigen. When CTLA-4-ErbB2 producer cells were modified to additionally secrete interleukin (IL)-15, antigen-specific antitumoral activity of the vaccine in WAP-Her-2 F1 mice was restored, documented by an increase in survival, and marked inhibition of the growth of established ErbB2-expressing, but not antigen-negative tumors. Our results demonstrate that continuous in vivo expression of an APC-targeted ErbB2 fusion protein results in antigen-specific immune responses and antitumoral activity in tumor-bearing hosts, which is augmented by the pleiotropic cytokine IL-15. This provides a rationale for further development of this approach for specific cancer immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1215-7) contains supplementary material, which is available to authorized users.
Keywords: Cancer vaccine, ErbB2/HER2, ErbB2-transgenic mice, IL-15, CTLA-4
Introduction
Therapeutic cancer vaccines aim to induce effective immune responses specific for antigens selectively expressed by the tumor [1]. Professional antigen-presenting cells (APC) such as dendritic cells (DC) play a key role in the initiation and regulation of primary immune responses, and targeted delivery of tumor-associated antigens to DC is being explored as a strategy to induce or enhance anti-tumor immunity [2–5]. Ex vivo loading of APC requires extensive handling and manipulation of patient-derived cells. As an alternative, fusion proteins are being developed that can be directly applied in vivo and selectively target tumor-associated antigens to APC for uptake, processing, and presentation as peptide epitopes in complex with MHC [2, 3].
One such approach for targeted delivery of the tumor-associated self-antigen ErbB2 (HER2/neu) is based on a chimeric CTLA-4-ErbB2 molecule that contains the extracellular domain of CTLA-4 for specific binding to costimulatory B7 molecules on APC, genetically fused to a fragment of ErbB2 as an antigenic determinant [6, 7]. ErbB2 is a member of the epidermal growth factor receptor family of receptor tyrosine kinases and is overexpressed by many tumors of epithelial origin. ErbB2 transmits important growth and survival signals and is directly involved in tumor pathogenesis [8]. The humanized ErbB2-specific antibody trastuzumab and the tyrosine kinase inhibitor lapatinib are in clinical use for the treatment of ErbB2 overexpressing breast cancers [9]. Nevertheless, not all patients with tumors expressing high ErbB2 levels responded to treatment with these targeted therapeutics. Furthermore, in a significant proportion of patients, initial responses were followed by the development of resistance [10]. Cancer vaccines that aim at the initiation or enhancement of endogenous ErbB2-specific immune responses may offer a valuable treatment alternative, and peptide- and cell-based ErbB2 vaccines as well as vaccine combination therapies are being investigated in clinical studies [11–13].
ErbB2-transgenic mouse models have been invaluable for the evaluation of experimental ErbB2-specific cancer vaccines [14, 15]. We previously showed that intramuscular injection of a DNA construct for in vivo production of the APC-targeted CTLA-4-ErbB2 fusion protein induced humoral and cellular ErbB2-specific immune responses in BALB/c mice and protected vaccinated animals against subsequent challenge with murine tumor cells expressing human ErbB2. In immunotolerant BALB-neuT mice, a DNA vaccine encoding a similar CTLA-4 fusion with a fragment of the rat ErbB2 homolog Neu delayed the onset of spontaneous Neu-driven mammary carcinomas [7]. To enhance the activity of the CTLA-4-ErbB2 vaccine in a therapeutic setting, here we generated a cell-based system for continuous in vivo delivery of the APC-targeted molecule and investigated its effects against established tumors.
In wild-type BALB/c mice, inoculation of cells continuously secreting the CTLA-4-ErbB2 fusion vaccine resulted in the rejection of established ErbB2-expressing renal cell carcinomas, accompanied by the induction of ErbB2-specific antibodies and cytotoxic T cells (CTL). In ErbB2-transgenic WAP-Her-2 F1 mice [16], treatment with vaccine-secreting cells alone was insufficient to induce tumor rejection. To increase efficacy, we modified CTLA-4-ErbB2 producer cells to additionally secrete interleukin (IL)-15 as an adjuvant. IL-15 is a pleiotropic cytokine with multiple activities [17], which has been shown to enhance the ability of DC to stimulate antigen-specific CD8+ T cells [18–20]. In contrast to the initial vaccine, the modified vaccine-producing and IL-15-secreting cells displayed potent and antigen-specific therapeutic activity also in tumor-bearing, immunotolerant WAP-Her-2 F1 mice, documented by marked inhibition of tumor growth and an increase in survival.
Materials and methods
Mice and cell lines
BALB/c and C57BL/6 mice were from Harlan Laboratories (Horst, the Netherlands). WAP-Her-2 mice (C57BL/6 background) were maintained as described [15]. BALB/c × C57BL/6 WAP-Her-2 (WAP-Her-2 F1) mice were obtained by crossing female BALB/c with male WAP-Her-2 mice [16]. Transgene-positive animals were identified by PCR [15]. HC11 murine mammary epithelial cells [21], Renca-lacZ and Renca-lacZ/ErbB2 murine renal carcinoma cells [22], CTLL-2 murine cytotoxic T cells (ATCC), and human Raji B-cell lymphoma cells (ATCC) were cultured in RPMI 1640 (Invitrogen, Karlsruhe, Germany). 293T cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen). All media were supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, in addition containing 0.2 mg/ml Zeocin (Renca-lacZ and HC11 transfectants), 0.2 mg/ml Zeocin and 0.5 mg/ml G418 (Renca-lacZ/ErbB2) or 50 IU/ml IL-2 (CTLL-2).
Generation of vaccine-producing cells
HC11/CTLA-4-ErbB2222, HC11/CTLA-4-NY-ESO-1 and HC11/CTLA-4 single-cell clones were obtained by transfection of HC11 cells with pSecTag2-CTLA-4-ErbB2222, pSecTag2-CTLA-4-NY-ESO-1 or pSecTag2-CTLA-4 [7] using Fugene 6 (Roche Diagnostics, Mannheim, Germany), selection of stable transfectants with Zeocin, and limiting dilution. HC11 derivatives secreting IL-15 were generated by lentiviral transduction. The lentiviral transfer plasmid pS-IL15-IEW is based on plasmid pHR’SIN-cPPT-SIEW (pSIEW) [23] and contains human IL-15 cDNA. For production of VSV-G pseudotyped vector particles, 293T cells were cotransfected with plasmid pS-IL15-IEW together with packaging and envelope plasmids pCMVΔR8.91 and pMD2.G [24] using a PEI transfection protocol [25]. Vector-containing supernatant was added to HC11/CTLA-4-ErbB2222 and HC11/CTLA-4-NY-ESO-1 cells in the presence of polybrene, cells were centrifuged at 32°C for 90 min at 1,800×g and incubated overnight at 37°C before replacing the medium with regular growth medium. Transduced cells expressing the vector-encoded eGFP reporter gene were identified by flow cytometry using a FACSCalibur flow cytometer (BD Biosciences, Heidelberg, Germany). Data were analyzed with CELLQuest Pro software (BD Biosciences).
Binding assays and analysis of IL-15 expression
Binding of CTLA-4 fusion proteins from HC11 culture supernatants to B7-expressing Raji cells was determined by flow cytometry with Myc-tag-specific monoclonal antibody (mAb) 9E10 (Sigma-Aldrich, Deisenhofen, Germany), NY-ESO-1-specific mAb D8.38 [26], or ErbB2-specific mAb FRP5 [27], followed by PE-conjugated goat anti-mouse antibody (Dianova, Hamburg, Germany). For immunofluorescence microscopy, Raji cells were incubated with cleared HC11/CTLA-4-ErbB2222 culture supernatant for 1 h on ice. Bound CTLA-4-ErbB2222 protein was detected with mAb 9E10, followed by Alexa Fluor 488-coupled anti-mouse antibody (Invitrogen, Karlsruhe, Germany). Samples were analyzed with a Leica TCS SL laser scanning microscope (Leica Mikrosysteme, Bensheim, Germany). For detection of IL-15, IL-15Rα-positive Raji cells were incubated with cleared HC11/CTLA-4-ErbB2222/IL-15 or HC11/CTLA-4-NY-ESO-1/IL-15 culture supernatants for 1 h on ice, followed by anti-hIL-15 mAb 34559 (R&D Systems, Wiesbaden, Germany), PE-labeled goat anti-mouse antibody, and flow cytometric analysis. IL-15 in culture supernatants collected after 4 days of culture of 2 × 105 cells in 4 ml of medium was quantified in triplicate samples using a human IL-15 ELISA kit (eBioscience, Frankfurt am Main, Germany). To confirm bioactivity, IL-15/IL-2-dependent CTLL-2 cells (1 × 103/well) were seeded in 96-well plates and tested in triplicates for their proliferative response to culture supernatants from HC11/CTLA-4-ErbB2222 or HC11/CTLA-4-ErbB2222/IL-15 cells. Viability and proliferation of cells were analyzed in colorimetric WST-1 assays (Roche Diagnostics).
Therapeutic vaccination
Anesthetized 6–7-week-old female BALB/c or WAP-Her-2 F1 mice were inoculated subcutaneously (s.c.) with 6 × 105 Renca-lacZ/ErbB2 or Renca-lacZ cells in 100 μl DBPS (Invitrogen) into both flanks (BALB/c) or the interscapular region (WAP-Her-2 F1). At day 4, after tumor cell injection, animals were treated by peritumoral injection of 6 × 104 vaccine-producing HC11 cells in 100 μl DPBS. Treatment was repeated at days 11 and 18. Tumor growth was monitored by measuring two perpendicular tumor diameters with a caliper twice a week. Long-term protection was investigated by re-challenge of surviving animals by injection of 5 × 105 Renca-lacZ/ErbB2 tumor cells into the lateral tail vein 60 days after initial tumor cell inoculation. Four weeks later, mice were killed, lungs were excised, and tumor nodules on the lung surface were visualized by X-Gal staining and counted [22]. For depletion of CD4+ or CD8+ T-cell subsets [6], WAP-Her-2 F1 mice received intraperitoneal injections of anti-CD4 mAb YTS191 or anti-CD8α mAb YTS169 at days 3, 7, 10, 14, 17, 21, and 24 after tumor cell inoculation. Successful depletion was confirmed for each mouse by flow cytometric analysis of blood samples with FITC-conjugated anti-CD4 mAb RM4-4 and eFluor450-conjugated anti-CD8 mAb 53–6.7 (both from eBioscience). Therapeutic vaccination of CD4- or CD8-depleted animals was performed, and tumor growth was followed as described above. All animal experiments were approved by the appropriate government committee. Mice were killed when they showed distress or latest when tumor diameters reached 12–13 mm.
Analysis of antibody and T-cell responses
Tumor-bearing mice were vaccinated as described above. Peripheral blood was collected from the orbital sinus at days 18 or 25. Levels of ErbB2-specific antibodies were determined by incubation of Renca-lacZ/ErbB2 and Renca-lacZ cells with 1:100 or 1:20 diluted mouse sera, followed by PE-conjugated goat anti-mouse antibody and flow cytometric analysis. Pre-immune sera served as controls. T-cell responses were evaluated in in vivo cytotoxicity assays [7]. Fourteen days after the last vaccination of tumor-bearing animals, splenocytes from naïve BALB/c mice or non-transgenic littermates of WAP-Her-2 F1 mice were fluorescently labeled with either 0.5 (CFSElow) or 5 μM (CFSEhigh) carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen). CFSEhigh splenocytes were pulsed with 15 μg/ml of synthetic ErbB2 peptide TYLPTNASL [28] (PANATecs, Tübingen, Germany). CFSElow splenocytes were left untreated (BALB/c) or pulsed with control peptide KVPRNQDWL [29] (WAP-Her-2 F1). Equal amounts of CFSE-labeled cells from both populations were mixed, and a total of 2 × 107 cells were injected into the lateral tail vein of recipient mice. Two days later, animals were killed, splenocytes were isolated, and CFSE fluorescence was analyzed using a FACSCanto II flow cytometer (BD Biosciences). Data were analyzed with BD FACSDiva software, and the percentage of specific target cell killing was calculated as described [7].
Statistical analysis
Differences in tumor growth kinetics were evaluated by one-way ANOVA followed by Tukey–Kramer test. Differences in survival were analyzed using the log-rank test. In other experiments, differences between values from two groups were evaluated using the two-tailed unpaired Student’s t test. P values <0.05 were considered significant. Statistical calculations were done using Prism 5 software (GraphPad Software, La Jolla, CA).
Results
Generation of producer cells for continuous expression of APC-targeted fusion proteins
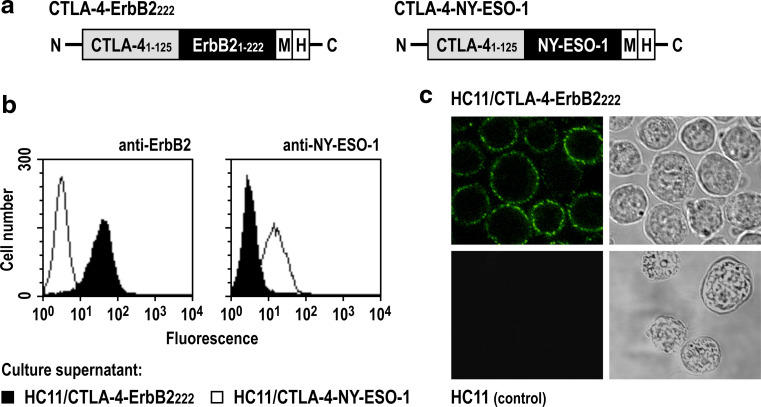
Vaccine-producing cells were generated by stable transfection of BALB/c-derived HC11 mammary epithelial cells with plasmid pSecTag2-CTLA-4-ErbB2222 that encodes under the control of a CMV promoter a fusion of the extracellular domain of human CTLA-4 and the N-terminal part of human ErbB2 (ErbB2222) (Fig. 1a, left). Similar cell lines were generated which carry plasmids pSecTag2-CTLA-4-NY-ESO-1 or pSecTag2-CTLA-4 that encode a fusion of CTLA-4 with the unrelated NY-ESO-1 tumor antigen (Fig. 1a, right) or the unfused CTLA-4 fragment. Continuous expression and secretion of the CTLA-4 proteins via an immunoglobulin κ-chain signal peptide provided by the vector backbone were confirmed by immunoblot analysis of HC11 culture supernatants (data not shown). Specific binding of CTLA-4-ErbB2222 and CTLA-4-NY-ESO-1 proteins from culture supernatants to B7-expressing Raji B-cell lymphoma cells was verified by flow cytometric analysis and confocal laser scanning microscopy (Fig. 1b, c). The ability of HC11/CTLA-4-ErbB2222 cells to induce ErbB2-specific antibody and T-cell responses was confirmed upon injection of naïve BALB/c mice with vaccine-producing cells in in vivo cytotoxicity experiments and by flow cytometric analysis of sera (see Supplementary Fig. 1 available on-line).
Fig. 1.
Vaccine-producing HC11 cells secrete functional CTLA-4 fusion proteins. a The CTLA-4-ErbB2222 fusion protein and the CTLA-4-NY-ESO-1 control molecule consist of amino acid residues 1–125 of human CTLA-4, amino acid residues 1–222 of human ErbB2 or full-length human NY-ESO-1, and C-terminal Myc (M) and polyhistidine (His) tags. b Binding of CTLA-4 fusion proteins from culture supernatants of HC11/CTLA-4-ErbB2222 or HC11/CTLA-4-NY-ESO-1 cells to B7 expressing Raji cells was verified by flow cytometry using ErbB2-specific and NY-ESO-1-specific antibodies. c Binding of CTLA-4-ErbB2222 from HC11/CTLA-4-ErbB2222 culture supernatant to the surface of Raji cells was confirmed by confocal laser scanning microscopy detecting the fusion protein with Myc-tag-specific antibody. Supernatant from HC11 wild-type cells served as control
Therapeutic vaccination of BALB/c mice induces regression of ErbB2-expressing tumors
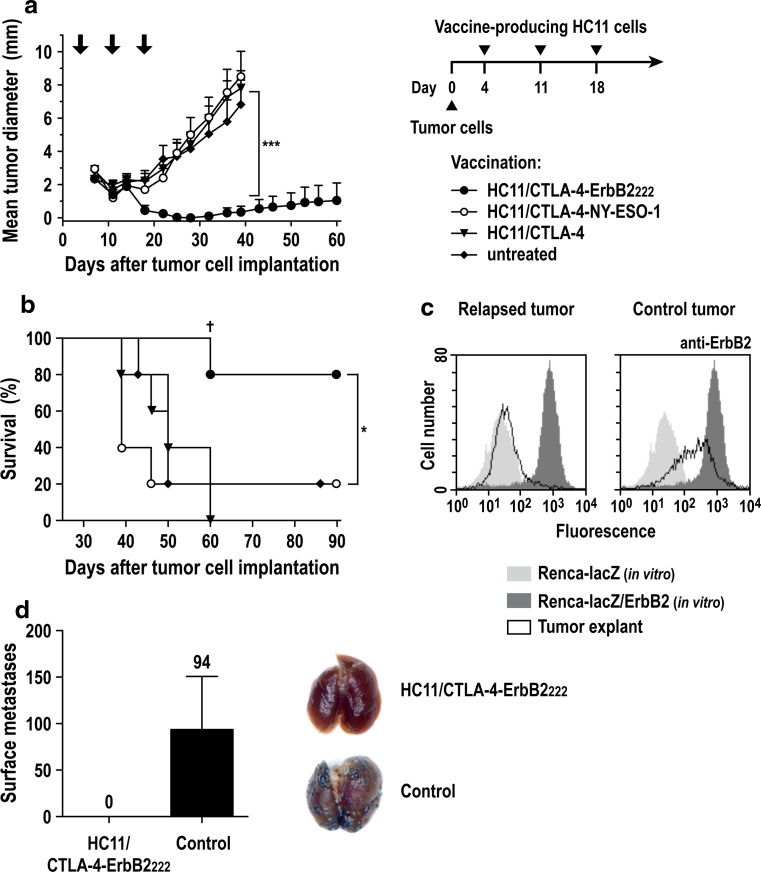
Next we investigated the effects of treatment with vaccine-producing HC11/CTLA-4-ErbB2222 cells on tumor growth using murine Renca-lacZ/ErbB2 renal carcinoma cells as a transplantable tumor model. These cells express high levels of human ErbB2 and grow as subcutaneous tumors in immunocompetent BALB/c mice [22, 30]. Animals were injected s.c. into both flanks with Renca-lacZ/ErbB2 cells, resulting in the formation of palpable tumors 4 days post-inoculation. Then, animals were treated by peritumoral injection of HC11/CTLA-4-ErbB2222 cells. Treatment was repeated at days 11 and 18, and tumor growth was followed for 90 days (Fig. 2a, right). Control animals received HC11/CTLA-4-NY-ESO-1 or HC11/CTLA-4 cells or were left untreated. All mice in the HC11/CTLA-4-ErbB2222 group responded to treatment, resulting in tumor rejection within 25 days after initiation of treatment (Fig. 2a). One of the tumors in the HC11/CTLA-4-ErbB2222 treatment group relapsed at the initial tumor site at day 32 (Fig. 2a, b). Ex vivo analysis of cells from the recurring tumor revealed a complete loss of ErbB2 surface expression, while tumor cells explanted from control animals displayed only a slight reduction in ErbB2 expression (Fig. 2c). The other animals in the HC11/CTLA-4-ErbB2222-treated group remained tumor-free until the end of the observation period (Fig. 2b). We also observed spontaneous tumor rejection in one animal each from the untreated and HC11/CTLA-4-NY-ESO-1-treated control groups, but not in mice treated with HC11/CTLA-4 cells (Fig. 2b).
Fig. 2.
Therapeutic vaccination of tumor-bearing BALB/c mice with HC11/CTLA-4-ErbB2222 cells. a Renca-lacZ/ErbB2 renal carcinoma cells were injected s.c. into both flanks of BALB/c mice (5 animals/group). At day 4, HC11/CTLA-4-ErbB2222, HC11/CTLA-4-NY-ESO-1, or HC11/CTLA-4 control cells were injected s.c. in the vicinity of each tumor. Treatments were repeated at days 11 and 18 (indicated by arrows). Mean tumor diameters ± SEM are shown; ***p < 0.001. b Survival of mice from the experiment shown in (a); *p < 0.05. One of the tumors in the HC11/CTLA-4-ErbB2222-treated group relapsed at day 32 (†). c The relapsed tumor was excised at day 60, and cells from the tumor explant were analyzed for ErbB2 expression by flow cytometry in comparison with Renca-lacZ/ErbB2 and ErbB2-negative Renca-lacZ cells from in vitro culture (left panel). Renca-lacZ/ErbB2 cells derived from an untreated tumor served as control (right panel). d Long-term protection of vaccinated animals. In a separate experiment, HC11/CTLA-4-ErbB2222-treated mice that had rejected s.c. Renca-lacZ/ErbB2 tumors after HC11/CTLA-4-ErbB2222 treatment were re-challenged by i.v. injection of Renca-lacZ/ErbB2 cells 2 months after initial tumor inoculation (n = 4). Four weeks later, pulmonary tumor nodules were visualized by X-Gal staining. Development of experimental metastasis in naïve animals is shown for comparison (n = 3). The mean number of surface metastases ± SEM is indicated (left). Representative images of X-Gal stained lungs are shown (right)
Therapeutic vaccination with HC11/CTLA-4-ErbB2222 cells was also successful if treatment was begun at later time points after tumor cell inoculation. Injection of HC11/CTLA-4-ErbB2222 cells at days 7, 14, and 21 or 10, 17, and 24 both resulted in complete rejection of established Renca-lacZ/ErbB2 tumors in 40% of the cases (see Supplementary Fig. 2 available on-line). To assess long-term protection of treated animals, in a separate experiment, mice cured by administration of HC11/CTLA-4-ErbB2222 cells were systemically rechallenged by intravenous injection of Renca-lacZ/ErbB2 tumor cells 2 months after initial tumor inoculation. Whereas pulmonary tumor nodules developed in naïve mice included as a control, no tumors were detected in lungs of animals that had already rejected initial s.c. Renca-lacZ/ErbB2 tumors upon treatment with HC11/CTLA-4-ErbB2222 cells (Fig. 2d).
Therapeutic vaccination of tumor-bearing mice induces ErbB2-specific immune responses
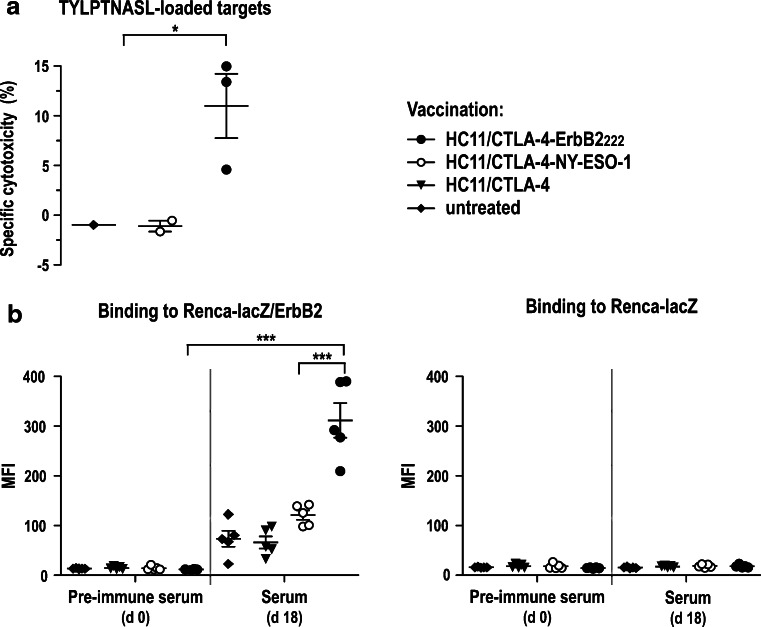
To analyze the immune responses induced by vaccination, tumor-bearing BALB/c mice were treated with HC11/CTLA-4-ErbB2222 cells at days 4, 11, and 18 as described above. Two weeks after the last treatment in vivo cytotoxicity assays were performed to assess ErbB2-specific CTL responses. Thereby, significant ErbB2-specific cytolytic activity was observed in HC11/CTLA-4-ErbB2222-treated animals, resulting in the elimination of up to 15% of adoptively transferred donor splenocytes pulsed with the H-2Kd-restricted ErbB2 peptide TYLPTNASL (residues 63–71 of the ErbB2 precursor protein) [28]. No ErbB2-specific cytotoxicity was observed in HC11/CTLA-4-NY-ESO-1-treated or untreated controls (Fig. 3a). We also investigated the induction of ErbB2-specific antibodies by analyzing sera taken at day 18 of the treatment schedule. Weak binding of serum antibodies from HC11/CTLA-4-NY-ESO-1- or HC11/CTLA-4-treated and untreated animals to ErbB2-expressing cells indicated induction of low levels of ErbB2-specific antibodies alone by exposure of BALB/c mice to Renca-lacZ/ErbB2 tumors. In contrast, markedly increased levels of ErbB2-specific antibodies were found in diluted sera from HC11/CTLA-4-ErbB2222-treated mice (Fig. 3b, left). No humoral activity against ErbB2-negative parental Renca-lacZ cells was observed in any of the treatment groups, confirming specificity of the antibody response for ErbB2 (Fig. 3b, right).
Fig. 3.
Immune responses induced by treatment with vaccine-secreting cells. a ErbB2-specific CTL responses. Tumor-bearing BALB/c mice were treated with HC11/CTLA-4-ErbB2222 cells as described in Fig. 2a. Two weeks later, in vivo cytotoxicity assays were performed as described in the methods section. The percentage of specific target cell killing was determined 48 h after injection of donor splenocytes pulsed with the H-2Kd-restricted ErbB2 peptide TYLPTNASL. HC11/CTLA-4-NY-ESO-1-treated and untreated animals served as controls. Values for individual animals and mean values ± SEM are shown; *p < 0.05. b ErbB2-specific antibody responses. At day 18 of the treatment schedule, peripheral blood was collected from mice treated as indicated (5 animals/group), and 1:100 dilutions of sera were analyzed for antibodies binding to Renca-lacZ/ErbB2 (left) or ErbB2-negative Renca-lacZ cells (right). Pre-immune sera served as controls. Mean fluorescence intensities (MFI) for sera from individual animals and mean values ± SEM are shown; ***p < 0.001
Effects of therapeutic vaccination in immunologically tolerant mice
Human ErbB2 represents a xenogenic antigen in BALB/c, but is a self-antigen in ErbB2-transgenic WAP-Her-2 mice [15]. To assess therapeutic activity of vaccine-producing HC11/CTLA-4-ErbB2222 cells in this model, we generated WAP-Her-2 F1 animals by crossing WAP-Her-2 mice on C57BL/6 background with wild-type BALB/c as described [16]. These animals displayed pronounced immunological tolerance toward human ErbB2 indicated by much more rapid growth of Renca-lacZ/ErbB2 tumors when compared with non-transgenic littermates and the absence of ErbB2-specific antibodies in untreated, Renca-lacZ/ErbB2 tumor-bearing WAP-Her-2 F1 mice (see Supplementary Fig. 3 available on-line).
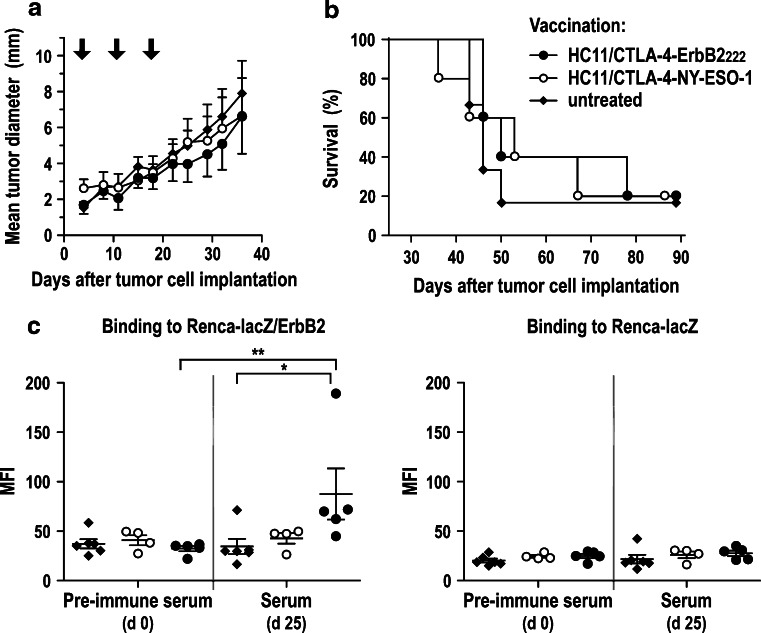
WAP-Her-2 F1 mice were inoculated s.c. with Renca-lacZ/ErbB2 cells in the interscapular region. At day 4, HC11/CTLA-4-ErbB2222 cells were injected, and treatment was repeated at days 11 and 18 as described above. Control animals received HC11/CTLA-4-NY-ESO-1 cells or were left untreated. In contrast to the effects observed in BALB/c wild-type mice, treatment with HC11/CTLA-4-ErbB2222 cells did not affect the growth of established Renca-lacZ/ErbB2 tumors in WAP-Her-2 F1 animals, resulting in tumor growth kinetics and overall survival rates very similar to those in control mice (Fig. 4a, b). Analysis of humoral immune responses revealed a modest induction of ErbB2-specific antibodies in HC11/CTLA-4-ErbB2222-treated, but not in HC11/CTLA-4-NY-ESO-1-treated or untreated animals (Fig. 4c).
Fig. 4.
Therapeutic vaccination of tumor-bearing WAP-Her-2 F1 mice. a Renca-lacZ/ErbB2 tumor cells were injected s.c. into the interscapular region of ErbB2-transgenic mice. At day 4, HC11/CTLA-4-ErbB2222 (n = 5) or HC11/CTLA-4-NY-ESO-1 cells (n = 5) were injected s.c. in the tumor vicinity, or animals were left untreated (n = 6). Treatments were repeated at days 11 and 18. Mice were monitored for 90 days. Mean tumor diameters ± SEM are shown for the time period when all animals in the respective groups were still alive. b Survival of mice from the experiment shown in (a). c ErbB2-specific antibody responses. At day 25 of the treatment schedule, peripheral blood was collected from tumor-bearing mice treated with HC11/CTLA-4-ErbB2222 (n = 5) or HC11/CTLA-4-NY-ESO-1 cells (n = 4), or untreated animals (n = 6), and 1:20 dilutions of sera were analyzed for presence of ErbB2-specific antibodies as described in Fig. 3b; **p < 0.01; *p < 0.05
Co-expression of IL-15 restores therapeutic activity of vaccine-producing cells in immunologically tolerant animals
We chose co-treatment with the pleiotropic cytokine IL-15 as an adjuvant to enhance the therapeutic activity of vaccination with HC11/CTLA-4-ErbB2222 cells. In the absence of IL-15Rα chain, IL-15 has a short in vivo half-life requiring high doses to achieve functional responses [31, 32]. For continuous production of IL-15 in the tumor vicinity, we modified HC11/CTLA-4-ErbB2222 and HC11/CTLA-4-NY-ESO-1 cells by lentiviral transduction to express IL-15 in addition to the APC-targeted vaccines. Transduced HC11/CTLA-4-ErbB2222/IL-15 and HC11/CTLA-4-NY-ESO-1/IL-15 cells secreted high and comparable amounts of IL-15 into the culture supernatant, which was functional and bound to IL-15Rα chain-expressing Raji cells. Biological activity of human IL-15 from HC11 culture supernatants in a murine system was confirmed in proliferation assays with IL-2/IL-15-dependent CTLL-2 cells (see Supplementary Fig. 4 available on-line).
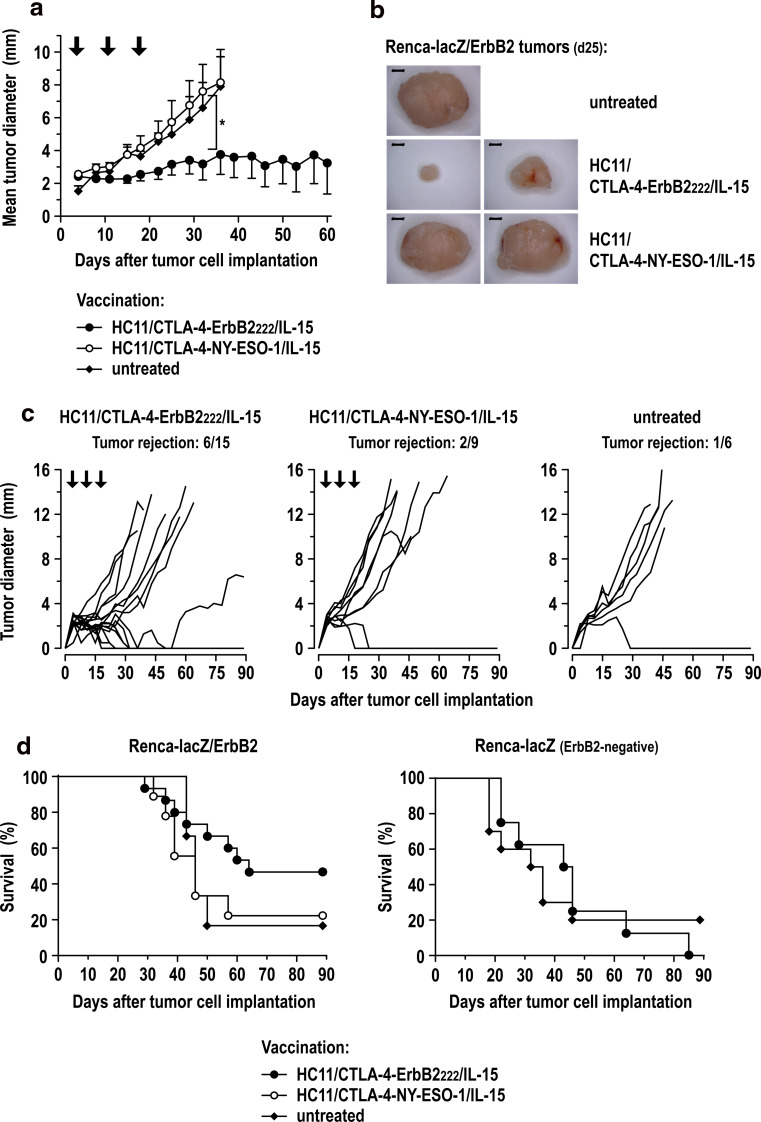
Next we analyzed the therapeutic activity of vaccine- and IL-15-producing cells in WAP-Her-2 F1 mice as described above for the initial HC11/CTLA-4-ErbB2222 vaccine. In contrast to the parental cells, treatment with HC11/CTLA-4-ErbB2222/IL-15 cells resulted in markedly delayed growth of established Renca-lacZ/ErbB2 tumors (Fig. 5a, b) and tumor rejection in 6 of 15 animals (Fig. 5c). Induction of antigen-specific immunity was confirmed in control experiments with the irrelevant NY-ESO-1 vaccine. Thereby, treatment with HC11/CTLA-4-NY-ESO-1/IL-15 cells secreting similar amounts of IL-15 had no effect on the growth of Renca-lacZ/ErbB2 tumors (Fig. 5a–c). Furthermore, therapeutic vaccination with HC11/CTLA-4-ErbB2222/IL-15 cells extended overall survival in comparison with HC11/CTLA-4-NY-ESO-1/IL-15-treated and untreated control groups (Fig. 5d, left), while it had no effect on the survival of mice carrying ErbB2-negative Renca-lacZ tumors (Fig. 5d, right).
Fig. 5.
Therapeutic vaccination of tumor-bearing WAP-Her-2 F1 mice with HC11/CTLA-4-ErbB2222/IL-15 cells. a At day 4, after Renca-lacZ/ErbB2 tumor cell inoculation, animals were treated with HC11/CTLA-4-ErbB2222/IL-15 (n = 15) or HC11/CTLA-4-NY-ESO-1/IL-15 cells (n = 9) or were left untreated (n = 6). Treatments were repeated at days 11 and 18. Mean tumor diameters ± SEM are shown; *p < 0.05. b Representative images of tumors excised at day 25 of the treatment schedule from a separate experiment performed as described in (a). Size bars indicate 2 mm. c Growth kinetics of individual Renca-lacZ/ErbB2 tumors in WAP-Her-2 F1 mice. Each line represents growth kinetics of the tumor of an individual animal from the experiment in (a). The fraction of tumor-free animals at day 90 is indicated. d Survival of WAP-Her-2 F1 mice after therapeutic vaccination with HC11/CTLA-4-ErbB2222/IL-15 cells. Left panel: survival of mice from the experiment described in (a) inoculated with Renca-lacZ/ErbB2 tumor cells and treated with HC11/CTLA-4-ErbB2222/IL-15 or HC11/CTLA-4-NY-ESO-1/IL-15 cells, or left untreated. Right panel: survival of WAP-Her-2 F1 mice inoculated with ErbB2-negative Renca-lacZ tumor cells and treated with HC11/CTLA-4-ErbB2222/IL-15 cells (n = 8) or left untreated (n = 10)
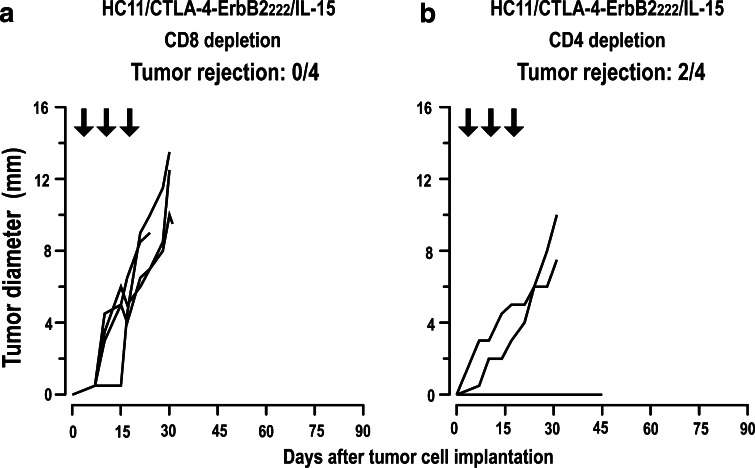
Analysis of humoral immune responses did not reveal induction of ErbB2-specific antibodies by HC11/CTLA-4-ErbB2222/IL-15 treatment (see Supplementary Fig. 5a available on-line), suggesting that inclusion of IL-15 in the vaccine shifted the induced immune response toward cellular effectors. Indeed, depletion of CD8+ T cells during HC11/CTLA-4-ErbB2222/IL-15 treatment impeded tumor rejection (Fig. 6a), indicating that CTL induced by vaccination with HC11/CTLA-4-ErbB2222/IL-15 cells are important for the therapeutic effect in WAP-Her-2 F1 mice. The CTL induced by vaccination of immunotolerant animals most likely recognize subdominant ErbB2 epitopes, since we did not observe increased cytotoxicity in HC11/CTLA-4-ErbB2222/IL-15-treated mice against splenocytes pulsed with the immunodominant ErbB2 peptide TYLPTNASL (see Supplementary Fig. 5b available on-line). In contrast to CD8 depletion, depletion of CD4+ T cells did not considerably alter the success of therapeutic vaccination, with tumor rejection still observed in half of the animals (Fig. 6b). These data demonstrate that antitumoral activity of the HC11/CTLA-4-ErbB2222/IL-15 vaccine in immunotolerant ErbB2-transgenic mice is not only due to the presence of IL-15 alone, but depends also on CD8+ T-cell-mediated, antigen-specific immunity induced by the APC-targeted CTLA-4-ErbB2222 molecule.
Fig. 6.
Depletion of T-cell subsets. WAP-Her-2 F1 mice were inoculated with Renca-lacZ/ErbB2 tumor cells and treated with HC11/CTLA-4-ErbB2222/IL-15 as described in Fig. 5a. CD8+ (a) or CD4+ T cells (b) were depleted with anti-CD8 or anti-CD4 antibodies at days 3, 7, 10, 14, 17, 21, and 24. Success of depletion was controlled for each mouse by flow cytometry (data not shown). Each line represents growth kinetics of the tumor of an individual animal (n = 4). The fraction of tumor-free animals at day 45 is indicated
Discussion
Targeting of tumor antigens to DC via fusion to cytokines or antibodies that recognize receptors on the DC surface facilitates more efficient ex vivo loading with antigen and can directly guide antigen to the APC relevant for cross-priming of tumor-specific T cells in vivo [2–4, 33]. We employed the soluble extracellular domain of human CTLA-4 to selectively deliver a fragment of the tumor-associated ErbB2 antigen to APC, which express the B7 molecules CD80 and CD86 on their surface [6, 7]. Human CTLA-4 interacts functionally with murine B7 proteins, enabling evaluation of CTLA-4-containing vaccine constructs in murine models [6, 34]. To continuously provide the chimeric CTLA-4-ErbB2222 fusion vaccine in the tumor vicinity, here, we utilized genetically modified epithelial cells expressing CTLA-4-ErbB2222 in secreted form. Peritumoral injection of these cells resulted in the induction of strong ErbB2-specific antibody and more moderate CTL responses in tumor-bearing BALB/c mice, and rejection of s.c. growing ErbB2-expressing renal cell carcinomas. Importantly, potent antitumoral activity and tumor rejection were retained if treatment with HC11/CTLA-4-ErbB2222 cells was initiated at later time points after tumor inoculation (see supplementary materials available on-line). In vivo production of the CTLA-4 domain itself had neither tumor-inhibiting nor tumor-promoting activity, demonstrated by similar tumor growth kinetics in untreated animals and mice treated with HC11/CTLA-4 or HC11/CTLA-4-NY-ESO-1 cells that secrete an irrelevant APC-targeted vaccine.
We chose HC11 mammary epithelial cells for in vivo expression and secretion of the CTLA-4-ErbB2222 fusion vaccine, since the secretory pathway in such cells appears less restrictive for heterologous proteins than other cultured cell types [35]. Immortalized HC11 cells are of BALB/c origin [21] and are non-tumorigenic unless transformed by an ectopically expressed oncogene [36, 37]. We found that these cells persist at the injection site for several weeks without significant expansion (data not shown). Hence, HC11/CTLA-4-ErbB2222 and HC11/CTLA-4-ErbB2222/IL-15 cells can locally provide the APC-targeted vaccine and adjuvant IL-15 for an extended time period in vivo, overcoming restrictions otherwise imposed by the short in vivo half-lives of these molecules.
In tumor-bearing BALB/c mice, vaccination with HC11/CTLA-4-ErbB2222 cells primarily induced an ErbB2-specific antibody response likely responsible for the observed inhibition of tumor growth. In other models, ErbB2-specific vaccination was shown to induce anti-ErbB2 antibodies that modulate ErbB2 expression and signaling [38, 39]. Likewise, ErbB2-specific antibodies induced by treatment with HC11/CTLA-4-ErbB2222 cells may effectively downmodulate ErbB2 signal transduction as observed for mixtures of monoclonal antibodies directed to different ErbB2 epitopes [40]. Nevertheless, ErbB2 signaling is not critical for tumorigenicity of Renca-lacZ/ErbB2 cells. This is indicated by similar tumor formation of parental ErbB2-negative Renca-lacZ cells and the observed vaccination-induced outgrowth of an ErbB2-negative tumor variant in one animal initially inoculated with Renca-lacZ/ErbB2 tumors. Hence, antitumoral activity of ErbB2-specific antibodies induced by treatment with HC11/CTLA-4-ErbB2222 cells is likely attributable to a combination of direct and indirect mechanisms that include antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and FcR-mediated effector functions [41–43].
Unlike treatment outcome in BALB/c mice, therapeutic vaccination with HC11/CTLA-4-ErbB2222 cells in the absence of adjuvant was not effective in ErbB2-transgenic WAP-Her-2 F1 animals. WAP-Her-2 mice are a well-established model for the evaluation of immune reactivity to human ErbB2 [15]. Likewise, WAP-Her-2 F1 mice of BALB/c × C57BL/6 mixed background demonstrate pronounced immunological tolerance toward human ErbB2 [16]. In WAP-Her-2 F1 mice, treatment with HC11/CTLA-4-ErbB2222 cells alone resulted only in a modest induction of ErbB2-specific antibodies that were not sufficient to mediate measurable antitumor effects. This may also have been affected by the strain background. Radkevich-Brown et al. [44] demonstrated that ErbB2 DNA vaccination induced significantly more pronounced humoral and cellular immune responses in ErbB2-transgenic mice of BALB/c background than in animals of BALB/c × C57BL/6 mixed background.
Modification of the cellular HC11/CTLA-4-ErbB2222 vaccine to additionally secrete the immunostimulatory cytokine IL-15 as an adjuvant augmented its activity and restored potent antitumoral effects in ErbB2-transgenic WAP-Her-2 F1 mice. This resulted in tumor rejection in a large proportion of the animals and extended overall survival. Thereby, the amount of IL-15 secreted by genetically modified HC11 cells did not affect systemic IL-15 levels. We did not detect free IL-15 in serum and urine of HC11/CTLA-4-ErbB2222/IL-15- and HC11/CTLA-4-NY-ESO-1/IL-15-treated animals (data not shown). The effector mechanisms induced by HC11/CTLA-4-ErbB2222/IL-15-treatment in WAP-Her-2 F1 were different from those triggered by the initial HC11/CTLA-4-ErbB2222 cells. We did not observe induction of measurable levels of ErbB2-specific antibodies, suggesting that in the presence of IL-15 cellular immune effectors were predominantly triggered.
IL-15 promotes activation of antigen-specific CD8+ T cells by DC [18–20] and may be able to revert tolerant T cells to become effectors [45]. While we did not detect the activation of systemic CTL reactive with the immunodominant H-2Kd-restricted ErbB2 peptide TYLPTNASL [28] in WAP-Her-2 F1, this does not exclude the presence of locally restricted TYLPTNASL-specific T cells and/or vaccination-induced activation of CTL that recognize subdominant ErbB2 epitopes. Indeed, tumor rejection was dependent on CD8+ T cells as indicated by the inability of animals to reject Renca-lacZ/ErbB2 cells if CD8+ cells were depleted during vaccination. Similar to the situation in Neu-transgenic BALB-neuT mice, in WAP-Her-2 F1 high affinity TYLPTNASL-specific T cells may be deleted by central tolerance mechanisms [46]. Adjuvant IL-15 can enhance vaccine responses to both dominant and subdominant tumor antigens [47], which is possibly of importance for tumor rejection in our model. While IL-15-stimulated innate effector mechanisms such as natural killer (NK) cells may contribute to antitumoral activity induced by HC11/CTLA-4-ErbB2222/IL-15-treatment in WAP-Her-2 F1 mice, tumor rejection in the ErbB2-transgenic animals was mostly dependent on antigen-specific immunity induced by the APC-targeted CTLA-4-ErbB2222 vaccine. In control experiments therapeutic vaccination with HC11/CTLA-4-ErbB2222/IL-15 cells had no effect on the growth of ErbB2-negative Renca-lacZ tumors, and treatment with HC11/CTLA-4-NY-ESO-1/IL-15 cells had no effect on the growth of Renca-lacZ/ErbB2 tumors.
Taken together, our results demonstrate that therapeutic vaccination of tumor-bearing hosts by injection of cells that facilitate continuous in vivo production of an APC-targeted vaccine can result in pronounced antigen-specific immunity and tumor rejection. In vivo produced IL-15 can further augment antitumoral activity of the APC-targeted vaccine in an immunologically tolerant background. Hence, we anticipate that similar strategies, for example based on irradiated whole tumor cell vaccines expressing and secreting APC-targeted molecules accompanied by broadly active immunomodulators, could complement existing approaches with recombinant cytokine- or antibody-antigen fusion proteins targeted to DC surface receptors [2–4, 33]. In contrast to the latter, such genetically modified tumor cell vaccines could more easily serve as a delivery system for multiple targeted tumor antigens and immunomodulatory molecules, which may broaden and further enhance the induced anti-tumor immune response. This may be relevant for effective cancer immunotherapy in tumor patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (DFG) GRK1172 and the LOEWE-Schwerpunkt Onkogene Signaltransduktion Frankfurt (OSF). We thank Dr. Christiane Sahm, Georg-Speyer-Haus, for lentiviral transfer vector pS-IL15-IEW; Dr. Giulio C. Spagnoli, University of Basel, for NY-ESO-1-specific mAb D8.38; Dr. Cristina Mastini, University of Turin for help with the set-up of WAP-Her-2 mice, the staff of the Georg-Speyer-Haus animal facility, for maintaining the transgenic mouse colony; Dr. Kurt Schönfeld, Georg-Speyer-Haus, for helpful suggestions and establishment of depletion experiments; and Mrs. Annemarie Schimpf, Mrs. Barbara Uherek, and Mr. Thorsten Geyer, Georg-Speyer-Haus, for excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Pejawar-Gaddy S, Finn OJ. Cancer vaccines: accomplishments and challenges. Crit Rev Oncol Hematol. 2008;67:93–102. doi: 10.1016/j.critrevonc.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Keler T, He L, Ramakrishna V, Champion B. Antibody-targeted vaccines. Oncogene. 2007;26:3758–3767. doi: 10.1038/sj.onc.1210375. [DOI] [PubMed] [Google Scholar]
- 3.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 4.Romani N, Thurnher M, Idoyaga J, Steinman RM, Flacher V. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol Cell Biol. 2010;88:424–430. doi: 10.1038/icb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohrbach F, Weth R, Kursar M, Sloots A, Mittrücker HW, Wels WS. Targeted delivery of the ErbB2/HER2 tumor antigen to professional APCs results in effective antitumor immunity. J Immunol. 2005;174:5481–5489. doi: 10.4049/jimmunol.174.9.5481. [DOI] [PubMed] [Google Scholar]
- 7.Sloots A, Mastini C, Rohrbach F, Weth R, Curcio C, Burkhardt U, Jäger E, Forni G, Cavallo F, Wels WS. DNA vaccines targeting tumor antigens to B7 molecules on antigen-presenting cells induce protective antitumor immunity and delay onset of HER-2/Neu-driven mammary carcinoma. Clin Cancer Res. 2008;14:6933–6943. doi: 10.1158/1078-0432.CCR-08-1257. [DOI] [PubMed] [Google Scholar]
- 8.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 10.Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 11.Peoples GE, Holmes JP, Hueman MT, Mittendorf EA, Amin A, Khoo S, Dehqanzada ZA, Gurney JM, Woll MM, Ryan GB, Storrer CE, Craig D, Ioannides CG, Ponniah S. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2008;14:797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 12.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA, dela Rosa C, Tietje K, Link J, Waisman J, Salazar LG. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, Jaffee EM. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C, Bouchard P, Wolf S, Modesti A, Musiani P, Lollini PL, Colombo MP, Forni G. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589–596. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piechocki MP, Ho YS, Pilon S, Wei WZ. Human ErbB-2 (Her-2) transgenic mice: a model system for testing Her-2 based vaccines. J Immunol. 2003;171:5787–5794. doi: 10.4049/jimmunol.171.11.5787. [DOI] [PubMed] [Google Scholar]
- 16.Jacob JB, Quaglino E, Radkevich-Brown O, Jones RF, Piechocki MP, Reyes JD, Weise A, Amici A, Wei WZ. Combining human and rat sequences in her-2 DNA vaccines blunts immune tolerance and drives antitumor immunity. Cancer Res. 2010;70:119–128. doi: 10.1158/0008-5472.CAN-09-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J Immunol. 2002;169:4928–4935. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- 19.Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol. 2004;34:66–73. doi: 10.1002/eji.200324567. [DOI] [PubMed] [Google Scholar]
- 20.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 21.Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988;7:2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurer-Gebhard M, Schmidt M, Azemar M, Altenschmidt U, Stöcklin E, Wels W, Groner B. Systemic treatment with a recombinant erbB-2 receptor-specific tumor toxin efficiently reduces pulmonary metastases in mice injected with genetically modified carcinoma cells. Cancer Res. 1998;58:2661–2666. [PubMed] [Google Scholar]
- 23.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M, Thrasher AJ. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 24.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 25.Sloots A, Wels WS. Recombinant derivatives of the human high-mobility group protein HMGB2 mediate efficient nonviral gene delivery. FEBS J. 2005;272:4221–4236. doi: 10.1111/j.1742-4658.2005.04834.x. [DOI] [PubMed] [Google Scholar]
- 26.Schultz-Thater E, Noppen C, Gudat F, Durmuller U, Zajac P, Kocher T, Heberer M, Spagnoli GC. NY-ESO-1 tumour associated antigen is a cytoplasmic protein detectable by specific monoclonal antibodies in cell lines and clinical specimens. Br J Cancer. 2000;83:204–208. doi: 10.1054/bjoc.2000.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harwerth IM, Wels W, Marte BM, Hynes NE. Monoclonal antibodies against the extracellular domain of the erbB-2 receptor function as partial ligand agonists. J Biol Chem. 1992;267:15160–15167. [PubMed] [Google Scholar]
- 28.Nagata Y, Furugen R, Hiasa A, Ikeda H, Ohta N, Furukawa K, Nakamura H, Furukawa K, Kanematsu T, Shiku H. Peptides derived from a wild-type murine proto-oncogene c-erbB-2/HER2/neu can induce CTL and tumor suppression in syngeneic hosts. J Immunol. 1997;159:1336–1343. [PubMed] [Google Scholar]
- 29.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth A, Rohrbach F, Weth R, Frisch B, Schuber F, Wels WS. Induction of effective and antigen-specific antitumour immunity by a liposomal ErbB2/HER2 peptide-based vaccination construct. Br J Cancer. 2005;92:1421–1429. doi: 10.1038/sj.bjc.6602526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, Yao Z, Dubois S, Ju W, Muller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci USA. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 34.Freeman GJ, Borriello F, Hodes RJ, Reiser H, Gribben JG, Ng JW, Kim J, Goldberg JM, Hathcock K, Laszlo G, et al. Murine B7–2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993;178:2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler SP, O’Sickey TK, Lord ST, Lubon H, Gwazdauskas FC, Velander WH. Secretion of recombinant human fibrinogen by the murine mammary gland. Transgenic Res. 2004;13:437–450. doi: 10.1007/s11248-004-9589-8. [DOI] [PubMed] [Google Scholar]
- 36.Hynes NE, Taverna D, Harwerth IM, Ciardiello F, Salomon DS, Yamamoto T, Groner B. Epidermal growth factor receptor, but not c-erbB-2, activation prevents lactogenic hormone induction of the beta-casein gene in mouse mammary epithelial cells. Mol Cell Biol. 1990;10:4027–4034. doi: 10.1128/mcb.10.8.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt R, Wong AM, Hynes NE. Mammary glands reconstituted with Neu/ErbB2 transformed HC11 cells provide a novel orthotopic tumor model for testing anti-cancer agents. Oncogene. 2001;20:5459–5465. doi: 10.1038/sj.onc.1204709. [DOI] [PubMed] [Google Scholar]
- 38.Steel JC, Ramlogan CA, Yu P, Sakai Y, Forni G, Waldmann TA, Morris JC. Interleukin-15 and its receptor augment dendritic cell vaccination against the neu oncogene through the induction of antibodies partially independent of CD4 help. Cancer Res. 2010;70:1072–1081. doi: 10.1158/0008-5472.CAN-09-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porzia A, Lanzardo S, Citti A, Cavallo F, Forni G, Santoni A, Galandrini R, Paolini R. Attenuation of PI3 K/Akt-mediated tumorigenic signals through PTEN activation by DNA vaccine-induced anti-ErbB2 antibodies. J Immunol. 2010;184:4170–4177. doi: 10.4049/jimmunol.0903375. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Kasus T, Schechter B, Lavi S, Yarden Y, Sela M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc Natl Acad Sci USA. 2009;106:3294–3299. doi: 10.1073/pnas.0812059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiridon CI, Ghetie MA, Uhr J, Marches R, Li JL, Shen GL, Vitetta ES. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8:1720–1730. [PubMed] [Google Scholar]
- 42.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 43.Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, Huang LQ, Murata S, Sgouros G, Emens LA, Reilly RT, Jaffee EM. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–1711. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radkevich-Brown O, Jacob J, Kershaw M, Wei WZ. Genetic regulation of the response to Her-2 DNA vaccination in human Her-2 transgenic mice. Cancer Res. 2009;69:212–218. doi: 10.1158/0008-5472.CAN-08-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 46.Rolla S, Nicolo C, Malinarich S, Orsini M, Forni G, Cavallo F, Ria F. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J Immunol. 2006;177:7626–7633. doi: 10.4049/jimmunol.177.11.7626. [DOI] [PubMed] [Google Scholar]
- 47.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.