Abstract
In our recent phase I trial, we demonstrated that the AE37 vaccine is safe and induces HER-2/neu-specific immunity in a heterogeneous population of HER-2/neu + prostate cancer patients. Herein, we tested whether one AE37 boost can induce long-lasting immunological memory in these patients. Twenty-three patients from the phase I study received one AE37 boost 6-month post-primary vaccinations. Local/systemic toxicities were evaluated following the booster injection. Immunological responses were monitored 1-month (long-term booster; LTB) and 3-year (long-term immunity; LTI) post-booster by delayed-type hypersensitivity, IFN-γ ELISPOT and proliferation assays. Regulatory T cell (Treg) frequencies, plasma transforming growth factor-β (TGF-β) and indoleamine 2,3-deoxygenase (IDO) activity levels were also determined at the same time points. The AE37 booster was safe and well tolerated. Immunological monitoring revealed vaccine-specific long-term immunity in most of the evaluated patients during both LTB and LTI, although individual levels of immunity during LTI were decreased compared with those measured 3 years earlier during LTB. This was paralleled with increased Tregs, TGF-β levels and IDO activity. One AE37 booster generated long-term immunological memory in HER-2/neu + prostate cancer patients, which was detectable 3 years later, albeit with a tendency to decline. Boosted patients had favorable clinical outcome in terms of overall and/or metastasis-free survival compared with historical groups with similar clinical characteristics at diagnosis. We suggest that more boosters and/or concomitant disarming of suppressor circuits may be necessary to sustain immunological memory, and therefore, further studies to optimize the AE37 booster schedule are warranted.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1461-3) contains supplementary material, which is available to authorized users.
Keywords: HER-2/neu, AE37 vaccine, Prostate cancer, Immunological memory
Introduction
Immunotherapy in the form of peptide-based vaccines is a promising modality for treating cancer. Tumor-associated antigens are proteins expressed by tumors capable of inducing specific tumor immunity. Sources of tumor-associated antigens that have been extensively studied in prostate cancer are prostate-specific antigen (PSA), prostatic acid phosphatase (PAP) and prostate-specific membrane antigen (PSMA) [1].
HER-2/neu (HER2) is a 185,000-kDa transmembrane glycoprotein that belongs to the HER family of epidermal growth factor receptors [2]. Majority of prostate cancer (PCa) patients (approx. 75 %) have tumors expressing HER2, and HER2 overexpression is found mainly in those patients who have become resistant to hormonal ablation [3–5]. Therefore, HER2 targeting has become an attractive treatment strategy for castrate-resistant PCa patients [6, 7].
AE36 is a HER2-derived peptide from the intracellular domain of the HER2 protein (HER2: 776–790, GVGSPYVSRLLGICL), which promiscuously binds to MHC class II molecules [8, 9]. Vaccination with the AE36 peptide has been shown by us and others to generate peptide-specific T cell immune responses in mice and to sensitize in vitro T lymphocytes from cancer patients [2, 10–13]. AE37 represents the AE36 native peptide with the addition of the four amino acid Ii-Key peptide (LRMK) moieties from the MHC class II-associated invariant chain (Ii) protein [2, 11]. The Ii-Key peptide considerably increases the binding potency and immunogenicity of MHC class II epitopes when covalently linked to a native helper epitope [10]. Ii-Key/MHC class II epitope hybrids have further been shown to enable direct extracellular charging of the MHC class II molecule by binding to its allosteric site and inducing a favorable conformational change in the epitope-binding groove, thus bypassing the need for intracellular antigen processing [2, 11]. Pre-clinical investigations in our laboratory revealed that mice immunized with the Ii-Key/AE36 hybrid (i.e., AE37) had enhanced proliferation of native peptide-recognizing CD4+ T cells, increased IFN-γ release, as well as enhancement of cytotoxic T cell (CTL) anti-tumor activity [13]. Moreover, we could demonstrate that patient-derived CD4+ T cells primed with AE37 provided a significantly stronger helper effect to autologous CD8+ T cells specific for a HER2 CTL epitope, as illustrated by either IFN-γ ELISPOT assays or specific autologous tumor cell lysis in vitro and in vivo in SCID mice [12].
In our previous phase I study, we showed that the AE37 vaccine is safe and can induce HER2-specific cellular immune responses in patients with prostate cancer [14]. Herein, we have attempted to determine whether these patients develop both in vivo and in vitro long-term immunological memory after one AE37 booster.
Patients and methods
Patient population
Of the 32 patients enrolled in our previous phase I trial (EudraCT 2006-003299-37; [14]), 23 received one AE37 booster 6-month post-primary vaccine regimen, following the approval of St Savas Institutional Review Board and written informed consent. Sixteen of these patients were available for reevaluation 3 years after booster and their clinical records are given in Table 1. At the time of their enrollment and just before receiving their first vaccination (January–July 2008), all reevaluated patients had castrate-sensitive (CS) non-metastatic (NM) disease except patient PR32 who had developed castrate-resistant (CR) metastatic (M) disease (Table 1). Patients were then clinically monitored as per standard of care. As of the writing of this paper (June 2013), 11 of these patients were still displaying CS/NM disease (receiving anti-androgen therapy (AAT)), and 4 had progressed either to CR/NM (n = 1; under treatment with second-line AAT) or to CS/M (n = 1) and CR/M stage (n = 2; free of chemotherapy at least 1 month prior to testing; Table 1). The patient with CR/M disease at enrollment was without radiographic progression and remained so as of the writing of this paper (ca 57 months; Table 1).
Table 1.
Clinical characteristics of patients who received one AE37 booster
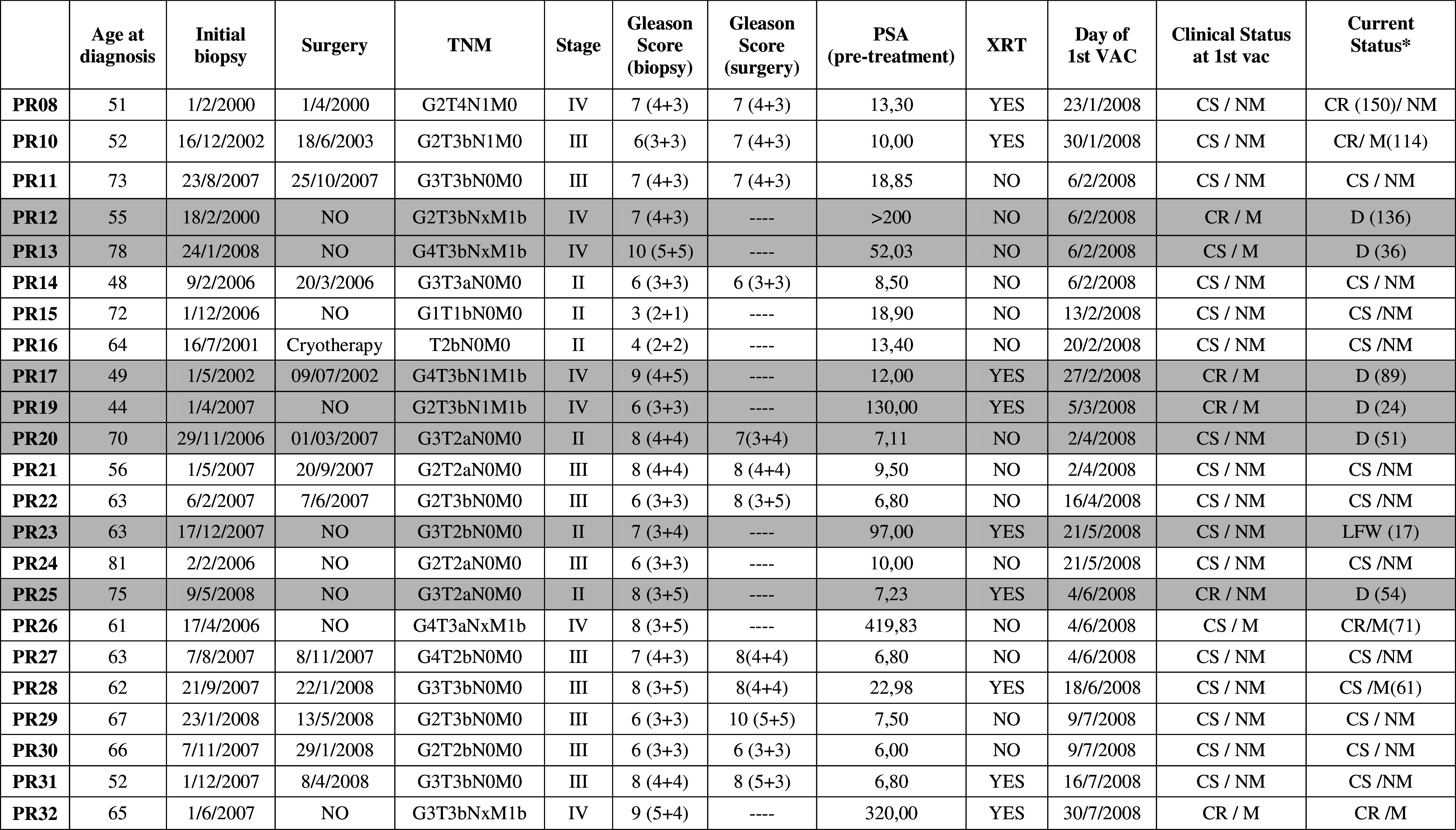
Gray background defines patients who were not tested at LTI
M metastatic, NM non-metastatic, CS castrate sensitive, CR castrate resistant, D death, LFW lost in follow-up
* Clinical status by June 2013. Numbers in parentheses represent months from initial biopsy to change in clinical status
Booster vaccine
The AE37 hybrid peptide (Ii-Key/HER-2/neu(776–790): Ac-LRMK GVGSPYVSRLLGICL-NH2) was produced in GMP grade by NeoMPS (San Diego, CA). AE37 mixed with GM-CSF (Bayer HealthCare Pharmaceuticals LLC, Seattle, WA) was administered as in the primary series [14].
Time points of immune monitoring
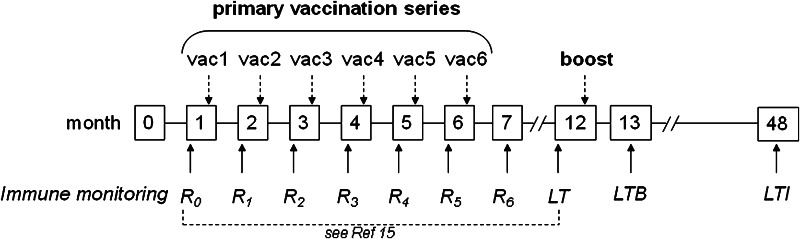
The time schedule for vaccinations and immunological assessments is shown in Fig. 1.
Fig. 1.
Time schedule for vaccinations and immune monitoring. The primary vaccination regimen consisted of 6 monthly injections [15]. Immune monitoring was performed before each vaccination (R0–R5) and 1 month after the last (6th) vaccination (R6). Long-term (LT) assessment was done 6 months after the last vaccination, and after this, 23 of the initially 32 patients enrolled in the phase I study [15] were offered a booster vaccination on the same day. Long-term booster (LTB) was performed 1 month later and long-term immunity (LTI), 3 years later
Delayed-type hypersensitivity
The DTH reaction was assessed and measured as described [14].
T cell proliferation assay
Proliferation assays were performed with thawed peripheral blood mononuclear cells (PBMCs) that were seeded at 2.5 × 105 cells/well in CTL medium (C.T.L. Europe GmbH, Germany), in 96-well plates in triplicates either in medium alone, or stimulated with 10 μg/ml final concentration of the native AE36 peptide. Stimulation with 2 μg/ml pokeweed mitogen (PWM; Sigma-Aldrich LLC, St Louis, MO) was used as a positive control. Proliferation was assessed on day 4, after loading the cells with 1 μCi/well of 3H-Thymidine (30–40 Ci/mmol, 1 mCi/ml, Amersham, Cardiff, United Kingdom) for the last 16 h of incubation. The stimulation index (SI) was calculated as the ratio of the average of test triplicate wells to the average of the medium control triplicates. A positive proliferative response was defined as a SI of at least 1.5 (i.e., 50 % above medium control).
ELISPOT assay
This was performed as described by us previously [14]. Patients were considered to have increased response if the mean number of specific spots (i.e., experimental spots minus medium control spots) at the time point tested (Rx) (i) after subtraction of mean number of specific spots at pre-vaccination (R0) was equal or greater than 10 (i.e., Rx minus R0 ≥ 10) and (ii) after division by the mean number of specific spots at (R0) was equal or greater than 1.5 (Rx/R0 ≥ 1.5).
Analysis of Tregs
This was performed by using anti-CD45-peridinin chlorophyll protein (PerCP), CD4 allophycocyanin (APC), CD25-fluorescein isothiocyanate (FITC) and CD127-phycoerythrin (PE) (all purchased from BD Biosciences, BD Europe) as reported by us recently [14].
Plasma human TGF-β determination
Alterations in blood circulating concentrations of TGF-β at the indicated time points were measured by using commercial kits as previously described [14].
Assessment of IDO activity
IDO activity was estimated by quantifying tryptophan (Trp) and its metabolite kynurenine (Kyn) similarly as described [15, 16]. Trp and Kyn were identified by reverse phase HPLC (LC 10 AvP system, Shimadzu, Duisburg, Germany equipped with a C18 ReprosilPur Basic® column, Dr Maisch GmbH, Entringen, Germany) and isocratic elution with 3 % ACN 0.05 % TFA, at a flow rate of 0.25 ml/min. Trp was detected by fluorescence (Exc. 285 nm/Em 405 nm) and Kyn with a UV/Vis detector at 360 nm [16].
To quantify both products, a series of 7–8 standards (range Trp: 5–100 μM and Kyn: 1.25–20 μM, both Sigma) were included in each experiment by diluting frozen stock solutions in human serum albumin 70 g/l (Biotest AG, Dreieich, Germany) in duplicates. Patient′s plasmas were tested in duplicates, and sera of three donors without cancer were also systematically included. Normalization was performed by spiking 3-nitro-l-tyrosinase at 0.1 mM (Sigma, detection 360 nm) as internal calibrator in all samples. Results were calculated from peak areas and are expressed as Kyn μM/Trp mM ratios (mean of duplicate measurements) [15].
Statistical analysis
GraphPad Prism version 5 software was used for statistical analysis of data. The two-tailed Wilcoxon matched pair t test with a 95 % confidence interval (CI) was used for statistical evaluation of patients at different time points. Statistically significant differences were considered when the p value was ≤0.05. Kaplan–Meier curves and log rank test were used for the evaluation of metastasis-free survival (MFS) and overall survival (OS).
Results
Toxicity
Following the booster injection, no systemic adverse events were reported. Local toxicity was mild, with all patients but one having only grade 1 symptoms consisting of a combination of erythema, edema and induration. This group included two patients who, in addition, developed an ulcer and one patient who also developed pruritus. One patient developed grade 2 symptoms consisting of erythema, edema, induration, ulcer and pruritus. After booster, 15 patients developed higher dermal reactions (DR) to AE37 as compared to the DR observed after the last (6th) vaccine (Supplementary Fig. S1); two patients developed weaker DR, whereas in the remainders, DR were found to be at almost similar levels (Fig. S1).
AE37 booster-induced immune response in vivo
DTH reactions
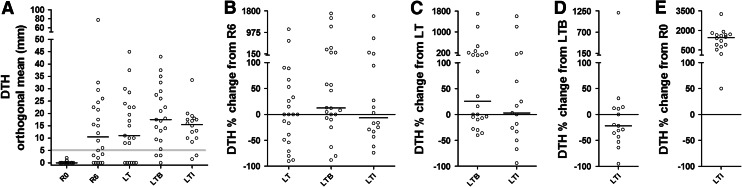
AE37 boost was given 6-month- post-primary vaccination regimen at long term (LT; see also Fig. 1), and DTH reactions in response to intradermal injection of AE36 were quantified at LTB and LTI (i.e., 1 month and 3 years later; see also Fig. 1). Of the 22 patients who were tested for DTH reaction to AE36 during LTB (with a median induration diameter (ID) of 17.25 mm range: 0–43 mm; mean ± SD: 18.09 ± 12.48), 18 (82 %) had DTH reactions >5 mm. Remarkably, 3 years later, during LTI, a similar percentage of the boosted patients developed >5 mm DTH reactions to the vaccine (14 of 16; 87.5 %) with a median ID of 15.50 mm for all assessed patients (range: 1.5–33.5 mm; mean ± SD: 14.50 ± 7.46) (p = 0.0516; Fig. 2a). At LT (i.e., 6-month post-primary vaccinations and before booster), 15 of 22 patients (68 %) had DTH reactions >5 mm with a median ID of 10.50 mm for the 22 patients (range: 0.0–45 mm; mean ± SD: 14.09 ± 13.29) (p = 0.7609 vs. LTI), whereas one-month post-primary vaccinations (R6), 14 of 23 patients (61 %) had similar DTH responses (median ID 12.00 mm) (range: 0.0–77.50 mm; mean ± SD: 14.54 ± 17.24) (p = 1.0000 vs. LTI; Fig. 2a). Thus, there were no significant changes in DTH reactions recorded at R6, LT and LTI, with slight, though significant increases during LTB. Figure 2b–e shows the magnitude levels (ML) of each individual’s DTH reactions at successive time points as a percent change of the ML of the DTH reaction developed by the same individual at a preceding time. Thus, the median ML (MML) of DTH during LTB was by 12.5 % higher compared to the corresponding levels at R6 (Fig. 2b). Similarly, the MML at LTB exceeded by 25.5 % those at LT (Fig. 2c), whereas at LTI, the MML decreased by 22 % compared to LTB (Fig. 2d). Despite this reduction, all patients exhibited increased DTH at LTI compared to R0 (Fig. 2e). Interestingly, one of the patients had a >5 mm DTH reaction only at LTI (13 mm) and one other developed similar DTH reactions at LTB and LTI (13 and 17 mm, respectively). Another patient who performed weakly at LT (8 mm) developed strong reactions during LTB and LTI (29 and 20 mm, respectively).
Fig. 2.
a DTH responses to AE36 at R0, R6 and LT (reported also in [15]), as well as after a single AE37 booster at LTB and LTI (herein). Cutoff point for positive reactions, 5 mm (faint horizontal line). b–e Show the magnitude level (ML) of each individual’s DTH reactions at successive time points as a percent change of the ML of the DTH reaction developed by the same individual at a preceding time point. Bars and circles represent medians and individual values, respectively
AE37 booster-induced immune responses in vitro
Proliferation assay
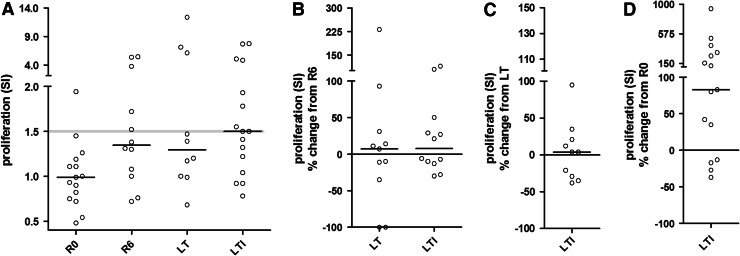
Median SI at LTI was 1.5 and ranged between 0.78 and 7.76 (mean ± SD: 2.57 ± 2.37), which was statistically non-significant as compared to LT (median SI: 1.30; range: 0.68–12.38; mean ± SD: 3.35 ± 3.92) (p = 0.6250; Fig. 3a). Statistical significance was reached for both LT and LTI versus R0 (p = 0.0547 and p = 0.0097, respectively; Fig. 3a). Remarkably, proliferative responses persisted at almost equal levels post-primary vaccinations (R6) and throughout the following 3.5-year period (LT and LTI). This is depicted in Fig. 3b, c where the MML of proliferation at R6, LT and LTI was found to be almost similar when compared to each other. MML at LTI were 83 % above those at R0 (Fig. 3d). All cultures were positive upon stimulation with PWM (data not shown).
Fig. 3.
Proliferative responses to AE36. a Individual SI at the indicated time points is depicted. Cutoff point for SI = 1.5 (faint horizontal line). b–d Show the ML of each individual’s proliferative responses at successive time points as a percent change of the ML of the SI developed by the same individual at a preceding time point. Bars and circles represent medians and individual values, respectively
AE36-specific IFN-γ secretion
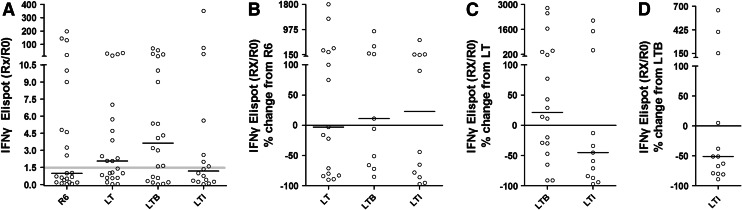
Median response at LTI (expressed as Rx/R0) was 1.18 and ranged between 0.03 and 351 (mean ± SD: 29.21 ± 87.81), which was statistically non-significant as compared to LT (median: 2.07; range: 0.03–35; mean ± SD: 6.33 ± 10.32) (p = 0.2243) and to LTB (median: 3.64; range: 0.01–70; mean ± SD: 10.86 ± 18.50) (p = 0.2769; Fig. 4a). Compared to IFN-γ spots measured at R0, 15 of 22 evaluated patients (68 %) developed increased responses at LTB (range of Rx/R0: 1.60–70; Fig. 4a) of whom 11 also had augmented IFN-γ responses compared with responses measured at LT (Fig. 4c). In 7 patients, responses decreased during LTB as compared to LT (Fig. 4c). Interestingly, during LTI, 7 of 16 evaluable patients (43.75 %) had 1.60- to 351-fold increased numbers of IFN-γ-secreting T cells above those from R0 (Fig. 4a), Moreover, 3 of these patients developed overall maximal immunity to AE36 at LTI (27-, 73- and 351-fold increases compared to R0; Fig. 4a). The MML of IFN-γ responses at LT, LTB and LTI remained similar compared to R6 (Fig. 4b). However, when compared to the MML at LT, only patients at LTB maintained almost similar levels of immunity (21.5 % median change), whereas the levels of IFN-γ responses at LTI considerably decreased (MML -45 % compared to LT) (Fig. 4c). Interestingly, three patients at LTI had higher levels of immunity to AE36 as compared with the median response at LTB (152, 401 and 654 % increase) and another 9 decreased (38–89 % decrease) so that the median levels of IFN-γ immunity at LTI were almost half as much compared to those at LTB (Fig. 4d).
Fig. 4.
IFN-γ ELISPOT responses to AE36. a The Rx/R0 ratios of specific spots at the indicated time points are depicted. Cutoff point: Rx/R0 = 1.5 (faint horizontal line). b–d Show the ML of each individual’s IFN-γ response at successive time points as a percent change of the ML of the Rx/R0 developed by the same individual at a preceding time point. Bars and circles represent medians and individual values, respectively
Regulatory T cells
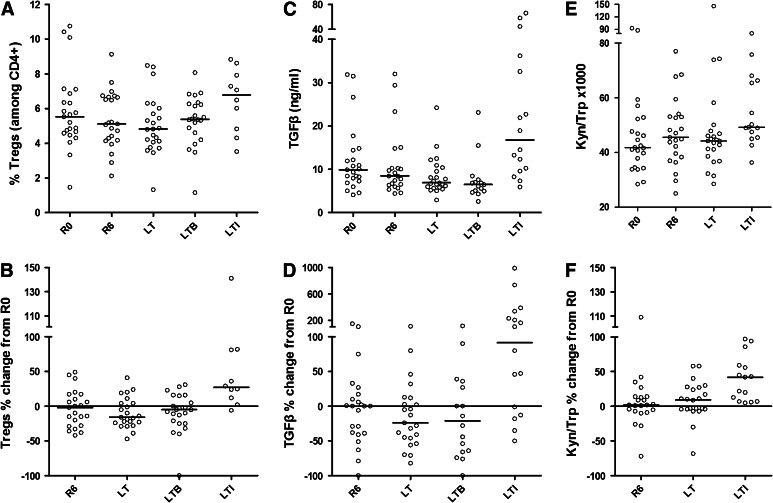
Tregs were defined as CD4+CD25highCD127low/− cells [15]. Post-primary vaccinations (R6), there was a slight decrease in this type of cell compared to pre-vaccination (median: 5.11 %; range: 2.12–9.13 %; mean ± SD: 5.34 ± 1.65 % at R6 vs. median: 5.53 %; range: 1.46–10.75 %; mean ± SD: 5.85 ± 2.2 % at R0), which became significant 6 months later at LT (median: 4.82 %; range: 1.32–8.48 %; mean ± SD: 5.07 ± 1.68 %; p = 0.0264 vs. R0) (Fig. 5a, b and [15]). Afterward, at LTB, the number of Tregs started to increase (mean ± SD: 5.32 ± 1.47 %; not statistically significant) and reached maximal values at LTI (mean ± SD: 6.49 ± 1.81; median: 6.78 %; range: 3.52–8.83 %) (p = 0.0117 vs. LTB and p = 0.0059 vs. R0; Fig. 5a). The median increase in Treg frequency at LTI was 27 % (range: −6 to 141 %; mean ± SD: 42.60 ± 45.39) compared to R0 (Fig. 5b).
Fig. 5.
a Circulating Tregs, c TGF-β plasma levels and e IDO activity measured as the kynurenine-to-tryptophan ratio (Kyn/Trp), determined at the indicated time points. b, d, f Show the ML of individual Treg frequencies b, TGF-β levels d and Kyn(μM)/Trp (mM) ratios as an assessment of IDO activity e at successive time points as a percent change of the corresponding ML at R0. Bars and circles represent medians and individual values, respectively
TGF-β levels in the plasma
There was a decrease in plasma TGF-β levels at R6 (median: 8.46 ng/mL; range: 4.42–31.99 ng/mL; mean ± SD: 10.98 ± 7.67 ng/mL) as compared to R0 (median: 9.87 ng/mL; range: 4.11–31.84 ng/mL; mean ± SD: 12.12 ± 7.85 ng/mL), (p = 0.28), which became statistically significant at LT (median: 6.87 ng/mL; range: 2.90–24.23 ng/mL; mean ± SD: 8.48 ± 4.47; p = 0.0217). At LTB, individual TGF-β levels remained slightly decreased compared to R0, without statistical significance (median: 6.47 ng/mL; range: 2.6–23 ng/mL; mean ± SD: 7.67 ± 5.14; p = 0.3575) (Fig. 5c, d). TGF-β levels in plasma paralleled circulating Treg frequencies at LTI at which time point they significantly increased (median: 16.74 ng/mL; range: 5.91–66.10 ng/mL; mean ± SD: 23.93 ± 18.66) and reached statistical significance compared to R0 (p = 0.0214) and LT or LTB (p < 0.002; Fig. 5c, d). The median increase in TGF-β levels at LTI compared to R0 was 91.5 % (range: −50 to 989 %; mean ± SD: 200.1 ± 292.8) (Fig. 5d).
IDO activity
We also evaluated IDO activity by measuring tryptophan and kynurenine in patients’ plasma samples and estimating Kyn/Trp levels. Values for tryptophan and kynurenine measured at R0, R6, LT and LTI are shown in Supplementary Figures S2A-S2D. Kyn/Trp levels were significantly higher at LTI (median: 49.19; range: 36.32–80.53; mean ± SD: 55.00 ± 13.01) as compared to R0 (median: 41.83; range: 28.40–92.83; mean ± SD: 46.38 ± 16.09) (p = 0.0004), R6 (median: 45.50; range: 25.00–76.99; mean ± SD: 47.31 ± 12.62) (p < 0.0001) or LT (median: 44.21; range: 28.50–146.40; mean ± SD: 49.58 ± 23.87) (p = 0.0181) (Figs. 5e, f). Kyn/Trp levels at LT were also higher, although not statistically significant, than those measured at R0 (p = 0.0803). The median Kyn/Trp levels at LTI exceeded the median Kyn/Trp value at R0 by almost 40 % (range: 5–97 %; mean ± SD: 40.13 ± 32.68) (Fig. 5f).
Clinical evaluation
Boosted patients were clinically monitored from first diagnosis to June 2013 as per the standard of care. Median follow-up was 73 months (mo) from diagnosis (range: 17–161 mo) and 60 months from the first vaccine (range 12–65 mo). Metastatic patients (n = 6) had a median OS of 89 months (83.33 % survived at 24 months and 66.67 % at 36 months) (Supplementary Fig.S3A). Time of death for four of them from diagnosis was as follows: 136 months for patient PR12 (40 months after his 1st vaccine), 36 months for PR13 (35 months from 1st vaccine), 89 months for PR17 (20 months from 1st vaccine) and 24 months for PR19 (13 months from 1st vaccine). The remainders, PR26 and PR32, are still alive, with 86 and 73 months from diagnosis (61 and 51 months from 1st vaccine), respectively. Two of the non-metastatic patients, PR20 and PR25, died from unrelated reasons (neurotoxic brain death and liver cirrhosis) at 51- and 54-month post-diagnosis (34 and 53 months from 1st vaccine), respectively.
Based on Gleason score, which is a strong prognostic risk factor for metastasis [17], we stratified our non-metastatic group of patients into intermediate (Gleason score 5–7; n = 9)- and high (Gleason score 8–10; n = 8)-risk subgroups and evaluated their time to metastasis. The proportion of non-metastatic patients, for both groups, at 5-year post-diagnosis was 100 % (median follow-up was 70.5 months; range 54–79, and 89 months; range 17–161 for the intermediate- and high-risk groups, respectively) (Supplementary Fig.S3B).
Discussion
In this study, we show that HER-2+ prostate cancer patients previously vaccinated with the AE37 vaccine, safely and effectively develop long-term immunity over time after a single vaccine booster. The immunological response rates measured at 4 years assessment post-initiation of primary vaccinations were found to be virtually similar to those measured after the primary vaccinations and 1 month after booster. Furthermore, in certain patients, immunological measurements at LTI were even higher than at previous time points. These findings suggest that one single AE37 boost may have contributed to the maintenance and, in some cases, improvement of the immunological response induced 3 years earlier during the primary vaccination cycle. Notwithstanding, the magnitude of the immunological responses in the majority of patients had a downward trend at LTI which was associated with increased levels of suppressor elements.
The small sample size as well as the heterogeneity of our boosted patient population does not allow conclusive clinical interpretations. Notwithstanding, it is noteworthy that the vast majority of these patients, based on Gleason score as a predictive risk factor for metastasis, had a most favorable outcome compared to historical groups. Thus, none of our patients belonging to the intermediate- or high-risk group for developing metastases did so, although according to standard criteria, the percent probability to remain free of metastasis at 5 years is about 40 % for patients with a Gleason score 8–10, and 73 % for patients with a Gleason score 5–7, which comes down to 62 % at 7 years [17]. This 100 % metastasis-free 5-year interval provides a compelling evidence for clinical benefit from the AE37 vaccine. This is also supported by the median OS of 89 months for our metastatic patients, which considerably exceeds that observed from historical group of patients [18–20].
The AE37 vaccine contains an immunogenic epitope of HER2 (i.e., the p776-790 15-mer; AE36) which is modified to enhance recognition by CD4+ T cells and also shows highly promiscuous binding to a series of MHC class II alleles with various affinities, as tested in binding and functional assays [2, 8, 9]. Long HLA class II-matched peptides such as AE36, which is contained in AE37, may be particularly suited for vaccination protocols by CD4+ T-helper cells that are known to interact extensively with other immune cells [21, 22]. We hypothesize that AE37-induced T-helper cells may engage dendritic cells at tumor sites, thereby presenting antigens from apoptotic tumor cells and inducing epitope spreading. We are addressing this issue in an ongoing studies on our long-term survivors from the phase I trial by using MHC class I dextramers and have identified T cell responses against PSA epitopes (unpublished). Moreover, according to prediction algorithms (SYFPEITHI), AE36 encompasses MHC class I-binding motifs, which renders it suitable for directly triggering, in addition to CD4+, CD8+ T cells as well. Indeed, during primary vaccinations, we could demonstrate increased percentage of both CD4+ and CD8+ T cells producing IFN-γ upon in vitro sensitization with AE36 [14]. We plan to synthesize the nonamers embedded within AE37 and then investigate retrospectively whether these can be recognized in vitro by CD8+ T cells from our AE37-vaccinated patients.
We have measured increased Treg frequencies at LTI. Actually, after an initial drop following the primary vaccination cycle, the number of circulating Tregs started to progressively increase and even exceeded pre-vaccination values 3-year post-AE37 boost. The potential for the induction of Tregs by cancer vaccines is a recognized issue. Functional Tregs specific for an HLA-DR-restricted epitope within a NY-ESO-1 peptide vaccine were expanded in the blood and tumor tissue of vaccinated patients [23]. Similarly, two other clinical studies have shown that tumor antigen-specific Tregs can be induced by cancer vaccines targeting either MAGE-A3 [24] or papillomavirus [25]. In contrast to these reports, other studies failed to detect an expansion of Tregs in response to vaccination [26–28], while in another report, these were even found to be decreased [29]. In all of these studies, patients having different types and stages of cancer were vaccinated against various tumor antigens, and therefore, it is likely that the effects of cancer vaccines on tumor antigen-specific Tregs vary depending on the vaccinating antigen and the nature as well as the stage of the cancer. While recent findings have demonstrated a favorable prognosis for cancer patients having increased frequencies of intratumoral Tregs [30], a survey of the literature suggests that finding intratumoral Tregs is of equivocal prognostic value [31]. It is unknown at present whether the increased frequencies of circulating Tregs at LTI could be exclusively interpreted as a harbinger of subsequent immune suppression. In prostate cancer, there is indirect evidence for the association of Tregs with disease progression which in most cases is derived from estimating the numbers or the function of Tregs at various anatomical sites at different stages of the disease [32, 33]. The most relevant study in this respect could be the phase II PROSTVAC trial [34] in which patients with metastatic castrate-resistant disease who responded to the vaccine had circulating Tregs with decreased suppressor function as opposed to those who did not benefit from vaccination. However, the number of patients analyzed in our study was too small to draw solid conclusions for association of vaccine-induced clinical responses with a concomitant decrease in Treg suppression function. Furthermore, the data obtained with peripheral Tregs may not be representative of what occurs in the tumor microenvironment. Derhovanessian et al. [27] reported no significant correlation between frequency of Tregs and time to progression in late-stage prostate cancer patients undergoing active immunotherapy. In addition, a direct role of Tregs in the induction of tumor-specific tolerance has been questioned in TRAMP mice where their accumulation within prostate tumors and lymph nodes was associated with ongoing antitumor immune responses and not with progression of disease [35].
The effects of TGF-β on the immune response have mostly been described as inhibitory [36]. However, TGF-β has been recently identified as a critical regulator of IL-9 production by memory CD4+ T cells [37]. There are several observations to indicate that IL-9 is a pleiotropic cytokine and functions as both a positive and negative regulator of immune responses. Nonetheless, in a most recent report, IL-9 was demonstrated to protect mice from tumor development by promoting strong CD8+ CTL activation [38]. TGF-β has also been shown to have a positive role in the development of Th1 responses [39] and to counteract suppressor activity of CD8+ suppressor T cells [40]. Moreover, TGF-β signaling has been demonstrated to correlate with increased relapse-free survival in breast cancer patients [41]. In our study, TGF-β levels followed a similar pattern throughout the assessment period as Tregs. Although it is plausible that the increased TGF-β levels could have an impact to the enhanced frequencies of Tregs in the periphery, we cannot make any assumptions whether, and to which extent, these may have influenced Treg functionality. This issue may be clarified after testing patients’ Treg functionality from frozen samples, which is under progress in our laboratory.
Our study also shows accelerated IDO activity in the majority of boosted patients compared with pre-vaccination, as evidenced by increased Kyn/Trp ratios. Higher Kyn/Trp ratios have been associated with immune activation [15, 42], and our study results are in line with this concept, as we observed a high number of patients with increased immunity in parallel with the increased IDO activity 3 years after booster. Considering, however, the decrease in immunity levels on an individual basis at LTI, we may speculate a timely dependent attenuation of AE37 immunity with a concomitant prevalence of immune suppressor circuits. If IDO activity is a major player in this scenario (e.g., by inducing increased frequencies of Tregs), this is presently unknown, and surely, the small size population in our study does not allow any clear conclusions.
The attenuation of immune responses during the 3-year period between LTB and LTI clearly suggests the need for including additional boosters in between. While it is reasonable to assume that AE37 boosting would also reduce Tregs and TGF-β, as observed after the initial series of immunizations, other tumor-suppressor circuits may not be suppressed. It is of interest to note that the combination of two immune checkpoint inhibitors, targeting PD-1 and CTLA4, led to improved treatment outcomes in patients with melanoma [43]. We may propose that, in addition to increasing booster injections, the elimination of suppressor pathways by targeted therapies could be beneficial for increasing the efficiency of AE37 immunotherapy.
In our opinion, the results presented herein warrant further studies with a homogeneous group of patients for evaluating the clinical efficacy of the optimized AE37 booster schedule, either alone or in combination with immunomodulatory antibodies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are indebted to our patients for their voluntary participation in this study. We thank Dr Eric von Hofe for critical reading of the manuscript. We also thank Joanne Kalogeropoulou and Efi Pappou for excellent technical assistance, Dr. Stratos Bissias for helping in patients’ follow-up and C. Zeyher for expert assistance with Trp/Kyn measurements. This study was supported by Antigen Express, Inc. Worcester, Massachusetts, and a grant from OPAP SA to Michael Papamichail.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxevanis CN, Voutsas IF, Gritzapis AD, Perez SA, Papamichail M. HER-2/neu as a target for cancer vaccines. Immunotherapy. 2010;2:213–226. doi: 10.2217/imt.09.89. [DOI] [PubMed] [Google Scholar]
- 3.Carles J, Lloreta J, Salido M, Font A, Suarez M, Baena V, Nogue M, Domenech M, Fabregat X. Her-2/neu expression in prostate cancer: a dynamic process? Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:4742–4745. doi: 10.1158/1078-0432.CCR-04-0115. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Brands FH, Chatterjee S, Feng AC, Groshen S, Schewe J, Lieskovsky G, Cote RJ. Her-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001;166:1514–1519. doi: 10.1016/S0022-5347(05)65822-3. [DOI] [PubMed] [Google Scholar]
- 5.Signoretti S, Montironi R, Manola J, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 6.Agus DB, Sweeney CJ, Morris MJ, et al. Efficacy and safety of single-agent pertuzumab (rhuMAb 2C4), a human epidermal growth factor receptor dimerization inhibitor, in castration-resistant prostate cancer after progression from taxane-based therapy. J Clin Oncolo Off J Am Soc Clin Oncol. 2007;25:675–681. doi: 10.1200/JCO.2006.07.0649. [DOI] [PubMed] [Google Scholar]
- 7.de Bono JS, Bellmunt J, Attard G, et al. Open-label phase II study evaluating the efficacy and safety of two doses of pertuzumab in castrate chemotherapy-naive patients with hormone-refractory prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:257–262. doi: 10.1200/JCO.2006.07.0888. [DOI] [PubMed] [Google Scholar]
- 8.Salazar LG, Fikes J, Southwood S, Ishioka G, Knutson KL, Gooley TA, Schiffman K, Disis ML. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res Off J Am Assoc Cancer Res. 2003;9:5559–5565. [PubMed] [Google Scholar]
- 9.Sotiriadou R, Perez SA, Gritzapis AD, et al. Peptide HER2(776-788) represents a naturally processed broad MHC class II-restricted T cell epitope. Br J Cancer. 2001;85:1527–1534. doi: 10.1054/bjoc.2001.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillogly ME, Kallinteris NL, Xu M, Gulfo JV, Humphreys RE, Murray JL. Ii-Key/HER-2/neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer immunol immunotherapy CII. 2004;53:490–496. doi: 10.1007/s00262-003-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez SA, von Hofe E, Kallinteris NL, Gritzapis AD, Peoples GE, Papamichail M, Baxevanis CN A new era in anticancer peptide vaccines. Cancer. 2010;116:2071–2080. doi: 10.1002/cncr.24988. [DOI] [PubMed] [Google Scholar]
- 12.Sotiriadou NN, Kallinteris NL, Gritzapis AD, et al. Ii-Key/HER-2/neu(776-790) hybrid peptides induce more effective immunological responses over the native peptide in lymphocyte cultures from patients with HER-2/neu + tumors. Cancer Immunol Immunother CII. 2007;56:601–613. doi: 10.1007/s00262-006-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voutsas IF, Gritzapis AD, Mahaira LG, Salagianni M, von Hofe E, Kallinteris NL, Baxevanis CN. Induction of potent CD4 + T cell-mediated antitumor responses by a helper HER-2/neu peptide linked to the Ii-Key moiety of the invariant chain. Int J Cancer. 2007;121:2031–2041. doi: 10.1002/ijc.22936. [DOI] [PubMed] [Google Scholar]
- 14.Perez SA, Kallinteris NL, Bisias S, et al. Results from a phase I clinical study of the novel Ii-Key/HER-2/neu(776-790) hybrid peptide vaccine in patients with prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16:3495–3506. doi: 10.1158/1078-0432.CCR-10-0085. [DOI] [PubMed] [Google Scholar]
- 15.Sperner-Unterweger B, Neurauter G, Klieber M, Kurz K, Meraner V, Zeimet A, Fuchs D. Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiology. 2011;216:296–301. doi: 10.1016/j.imbio.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 17.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA J Am Med Assoc. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 18.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 19.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, Levine EG, Blumenstein BA, Vogelzang NJ. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 20.Sartor O. Combination therapy: abiraterone prolongs survival in metastatic prostate cancer. Nat Rev Clin Oncol. 2011;8:515–516. doi: 10.1038/nrclinonc.2011.111. [DOI] [PubMed] [Google Scholar]
- 21.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother CII. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 23.Ebert LM, MacRaild SE, Zanker D, Davis ID, Cebon J, Chen W. A cancer vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PLoS ONE. 2012;7:e48424. doi: 10.1371/journal.pone.0048424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francois V, Ottaviani S, Renkvist N, et al. The CD4(+) T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res. 2009;69:4335–4345. doi: 10.1158/0008-5472.CAN-08-3726. [DOI] [PubMed] [Google Scholar]
- 25.Welters MJ, Kenter GG, Piersma SJ, et al. Induction of tumor-specific CD4 + and CD8 + T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 26.Ayyoub M, Dojcinovic D, Pignon P, Raimbaud I, Schmidt J, Luescher I, Valmori D. Monitoring of NY-ESO-1 specific CD4 + T cells using molecularly defined MHC class II/His-tag-peptide tetramers. Proc Natl Acad Sci USA. 2010;107:7437–7442. doi: 10.1073/pnas.1001322107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derhovanessian E, Adams V, Hahnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17 + CD4 + T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 28.Jandus C, Bioley G, Dojcinovic D, et al. Tumor antigen-specific FOXP3 + CD4 T cells identified in human metastatic melanoma: peptide vaccination results in selective expansion of Th1-like counterparts. Cancer Res. 2009;69:8085–8093. doi: 10.1158/0008-5472.CAN-09-2226. [DOI] [PubMed] [Google Scholar]
- 29.Gates JD, Clifton GT, Benavides LC, et al. Circulating regulatory T cells (CD4+ CD25+ FOXP3+) decrease in breast cancer patients after vaccination with a modified MHC class II HER2/neu (AE37) peptide. Vaccine. 2010;28:7476–7482. doi: 10.1016/j.vaccine.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 32.Flammiger A, Weisbach L, Huland H, et al. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur J Cancer. 2013;49:1273–1279. doi: 10.1016/j.ejca.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Huen NY, Pang AL, Tucker JA, et al. Up-regulation of proliferative and migratory genes in regulatory T cells from patients with metastatic castration-resistant prostate cancer. Int J Cancer. 2013 doi: 10.1002/ijc.28026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother CII. 2010;59:663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degl’Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, Hess-Michelini R, Doglioni C, Bellone M. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+ CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 36.Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beriou G, Bradshaw EM, Lozano E, et al. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010;185:46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Hong S, Li H, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122:4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smeltz RB, Chen J, Shevach EM. Transforming growth factor-beta1 enhances the interferon-gamma-dependent, interleukin-12-independent pathway of T helper 1 cell differentiation. Immunology. 2005;114:484–492. doi: 10.1111/j.1365-2567.2005.02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabinowitz KM, Wang Y, Chen EY, et al. Transforming growth factor beta signaling controls activities of human intestinal CD8(+)T suppressor cells. Gastroenterology. 2013;144(601–12):e1. doi: 10.1053/j.gastro.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bierie B, Chung CH, Parker JS, Stover DG, Cheng N, Chytil A, Aakre M, Shyr Y, Moses HL. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119:1571–1582. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godin-Ethier J, Pelletier S, Hanafi LA, et al. Human activated T lymphocytes modulate IDO expression in tumors through Th1/Th2 balance. J Immunol. 2009;183:7752–7760. doi: 10.4049/jimmunol.0901004. [DOI] [PubMed] [Google Scholar]
- 43.Riley JL. Combination checkpoint blockade: taking melanoma immunotherapy to the next level. The New England journal of medicine. 2013 doi: 10.1056/NEJMe1305484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.