Abstract
Antibody-cytokine fusion proteins (“immunocytokines”) represent a promising class of armed antibody products, which allow the selective delivery of potent pro-inflammatory payloads at the tumor site. The antibody-based selective delivery of interleukin-2 (IL2) is particularly attractive for the treatment of metastatic melanoma, an indication for which this cytokine received marketing approval from the US Food and drug administration. We used the K1735M2 immunocompetent syngeneic model of murine melanoma to study the therapeutic activity of F8–IL2, an immunocytokine based on the F8 antibody in diabody format, fused to human IL2. F8–IL2 was shown to selectively localize at the tumor site in vivo, following intravenous administration, and to mediate tumor growth retardation, which was potentiated by the combination with paclitaxel or dacarbazine. Combination treatment led to a substantially more effective tumor growth inhibition, compared to the cytotoxic drugs used as single agents, without additional toxicity. Analysis of the immune infiltrate revealed a significant accumulation of CD4+ T cells 24 h after the administration of the combination. The fusion proteins F8–IL2 and L19–IL2, specific to the alternatively spliced extra domain A and extra domain B of fibronectin respectively, were also studied in combination with tumor necrosis factor (TNF)-based immunocytokines. The combination treatment was superior to the action of the individual immunocytokines and was able to eradicate neoplastic lesions after a single intratumoral injection, a procedure that is being clinically used for the treatment of Stage IIIC melanoma. Collectively, these data reinforce the rationale for the use of IL2-based immunocytokines in combination with cytotoxic agents or TNF-based immunotherapy for the treatment of melanoma patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1562-7) contains supplementary material, which is available to authorized users.
Keywords: Immunocytokines, Interleukin-2, Oncofetal fibronectin, Vascular targeting
Introduction
The approval by FDA of interleukin-2 (IL2) for metastatic melanoma patients dates back to 1998. The use of very high doses of IL2 (e.g., 600.000–720.000 IU/kg up to 14 doses in 1 week of treatment) can yield objective responses in approximately 15 % of patients. Notably, complete responses are typically durable, with >10 % of patients alive after 10 years [1]. This treatment regimen is, however, very toxic and is normally reserved to young and physically fit patients only, because of side effects that include fever and chills, together with hypotension, gastrointestinal toxicities and vascular leak syndrome. Increased vascular permeability results in peripheral edema, fluid retention and increased body weight in patients. In extreme cases, vascular leak syndrome can lead to organ failure, especially at pulmonary and cardiovascular sites [2, 3]. Certain premedication strategies, such as the intravenous administration of fluids, the use of antipyretics and antiemetics, facilitate the clinical implementation of IL2-based therapies [4].
One avenue to increase the selectivity of IL2 therapy consists in the targeted delivery of this cytokine at the tumor site by means of suitable antibody “vehicles” [5, 6]. Two decades of research in this field have now shown that such pharmacodelivery strategies may rely either on the use of antibodies in IgG format (carrying the IL2 moiety at the C-terminal extremity of the heavy chains; [7]), or on fusion proteins featuring antibody fragments such as scFv’s [8] or diabodies as delivery vehicles [9]. Six immunocytokines based on human IL2 are currently being investigated in clinical trials for the therapy of cancer: DI-Leu 16-IL2, EMD 273066, EMD 521873, EMD 273063, L19-IL2 and F16-IL2 [10–15].
Our group has focused on the development of IL2-based immunocytokines, using non-covalent homodimeric scFv fragments as preferred delivery vehicles, because they clear rapidly from circulation and do not display Fc-associated activities. We have mainly concentrated on antibodies that recognize the sub-endothelial extracellular matrix at tumor neovascular sites, as delivery vehicles with the potential to achieve an efficient and stable localization on neoplastic lesions. The F8 and L19 human antibodies recognize the alternatively spliced extra domain A (EDA) and extra domain B (EDB) of fibronectin with identical affinity in mouse and man [16, 17]. F8–IL2 and L19–IL2 have shown activity in various mouse models of cancer. Combination with chemotherapy [18, 19] or with other biologics [20, 21] was shown to potentiate the therapeutic action of IL2-based immunocytokines in mice. In collaboration with the Giavazzi group, we have recently reported that F8–IL2 potently synergizes with paclitaxel for the therapy of mouse models of human melanoma and ovarian cancer, with a strong dependence on the schedule used [18]. In that study, the administration of PTX was able to increase tumor perfusion and vessel permeability ultimately improving the selective accumulation of the immunocytokine at the tumor site. Thus, F8–IL2 plus PTX led to cures in the majority of treated animals if PTX was administered before or together with F8–IL2, while a reversal of the schedule did not lead even to an additive effect [18]. Surprisingly, in the WM1552/5 model of melanoma, the use of dacarbazine (DTIC) plus F8–IL2 did not exhibit a substantial therapeutic effect. In addition, we have fused the L19 and F8 antibodies to murine Tumor necrosis factor (TNF). The resulting immunocytokines have exhibited a potent therapeutic activity in mouse models of cancer [20, 22].
The EDA and EDB domains of fibronectin display a restricted pattern of expression in healthy tissue, mainly confined to placenta and to the endometrium in the proliferative phase [23, 24]. However, these domains are typically included in “oncofetal” fibronectin, which is abundantly found in most cancer lesions, at vascular and/or stromal sites. L19–IL2 (also known as Darleukin) has been studied as monotherapy in a Phase I/II clinical trial in patients with various types of malignancies, mediating a disease stabilization of 51 % of treated patients [25]. When used in combination with DTIC in patients with metastatic melanoma, Darleukin induced objective responses in 8 of the 29 advanced melanoma patients enrolled in a Phase II a trial [26]. Similarly, the L19 antibody fused to human TNF (also known as Fibromun) has been investigated in a monotherapy dose escalation study in patients with cancer [27], as well as in an isolated limb-perfusion procedure in melanoma patients in combination with melphalan [28].
In addition to the systemic administration of immunocytokines for the treatment of metastatic melanoma, there is a potential to use these products for the intralesional treatment of patients with Stage IIIC melanoma, since intratumoral administration of IL2 has been shown to be active in this indication [29, 30]. In collaboration with clinical centers, we have recently reported a first clinical experience on the intralesional use of L19–IL2 in patients with Stage IIIC melanoma [31]. Furthermore, we have observed that the combined use of L19–IL2 with L19–TNF was more potent than either immunocytokine used alone, in a mouse model of teratocarcinoma [20]. In this same study, we could also observe a synergistic effect of L19–IL2 in combination with antibodies directed against murine CTLA-4 (ipilimumab analogs) [20].
In this study, we have investigated the therapeutic action of F8–IL2 in combination with PTX or DTIC in the K1735M2 immunocompetent syngeneic model of murine melanoma. This melanoma had previously been obtained by UV irradiation and croton oil treatment in the C3H/HeN immunocompetent mouse strain [32]. The M2 variant, which had been derived from a spontaneous metastasis, displays an aggressive phenotype [33]. This murine model allowed the study of F8–IL2 in a fully immunocompetent setting, while previous studies in nude mice with human melanoma relied solely on the mitogenic activity of IL2 on NK cells. In the K1735M2 model, F8–IL2 led to a potent tumor growth inhibition when used in combination with PTX or DTIC. In addition, the therapeutic activity of L19–IL2 and F8–IL2 was found to be potentiated by the combination with TNF-based immunocytokines in an intralesional administration protocol, which mimics the one used for the treatment of Stage IIIC melanoma patients.
The experiments described in this article provide support for the use of IL2-based therapeutics in combination with chemotherapy or TNF-based immunotherapy for the treatment of patients with advanced melanoma. Clinical trials with L19–IL2 in combination with DTIC (Stage IV melanoma), or with L19–IL2 in combination with L19–TNF (Stage IIIC melanoma) are currently ongoing.
Methods
Cell lines and mice
The murine melanoma cell line K1735M2 was a kind gift of Prof. Silvio Hemmi (University of Zürich) and was maintained in culture in Dulbecco Modified Eagle Medium (DMEM, Lonza Vervier, Switzerland) supplemented with 10 % fetal calf serum (FCS, Invitrogen, Switzerland). The murine sarcoma cell line Wehi-164 was purchased from Cell Line Service (CLS, Eppelheim, Germany) and maintained in RPMI medium supplemented with 10 % FCS. Female C3H/HeN mice (age 10–12 weeks) and female Balb/c mice (age 7–9 weeks) were obtained from Elevage Janvier, France. Experiments were performed under a project license granted by the Veterinäramt des Kantons Zürich, Switzerland (42/2012).
Drugs and dosages for therapy studies
F8–IL2 was produced from a stably transfected cell line as previously described [34]. Briefly, the clone was maintained in Power CHO-2CD protein-free medium (Lonza Vervier, Switzerland) and the protein was purified from the supernatant by protein A affinity chromatography. The quality of the protein was analyzed by SDS-PAGE and fast protein liquid chromatography gel filtration using a Superdex™ 200 10/300 GL size exclusion column (GE Healthcare, Little Chalfont, UK). Binding to the antigen was confirmed by surface plasmon resonance (Biacore, GE Healthcare) on an antigen-coated chip.
L19–IL2 and KSF–IL2 were produced using similar procedures, as previously described [14, 34].
KSF–IL2 was included in in vivo studies as negative control. The immunocytokine KSF–IL2 comprises of the KSF antibody portion, specific to hen egg lysozyme, and the IL2 cytokine portion [34].
Paclitaxel was purchased in the commercially available form of 30 mg/5 ml solution for infusion (Bristol-Myers Squibb, Switzerland), DTIC as lyophilized powder for infusion Dacin® (Lipomed, Switzerland), dexamethasone (DEXA) as 4 mg/ml solution for infusion (Mepha, Switzerland), metamizol (NOVA) as 1 mg/2 ml solution for infusion (Sanofi-Aventis, France) and paracetamol (PARA) as 10 mg/ml solution for infusion (Fresenius Kabi, Germany).
F8–IL2 was administered i.v. at the dose of 30 μg/mouse, (equivalent to ~1.5 mg/kg assuming a mouse body weight of 20 g, first systemic experiment and intralesional experiment) or 50 μg/mouse (equivalent to ~2.5 mg/kg assuming a mouse body weight of 20 g, following experiments). L19–IL2 was administered i.t. at the dose of 30 μg/mouse. PTX was administered i.v. at 20 mg/kg and DTIC i.p. either at 100 mg/kg (first experiment) or 200 mg/kg (following experiments). Antipyretics were administered i.v. with the following dosages: DEXA at 2 mg/kg, NOVA at 200 mg/kg and PARA at 100 mg/kg.
F8–TNF and L19–TNF were produced using similar procedures [22, 35] and were administered i.t. at the dose of 7 μg/mouse (equivalent to ~0.35 mg/kg assuming a mouse body weight of 20 g). Proleukin (human recombinant IL2, Novartis) was purchased in the commercially available form of 18 Mio IU solution for infusion and administered i.t. at the dose of 10 μg/mouse, (equivalent to ~ 0.5 mg/kg assuming a mouse body weight of 20 g). Mouse recombinant TNFα was purchased from eBioscience and administered i.t. at the dose of 2.5 μg/mouse, (equivalent to ~0.13 mg/kg assuming a mouse body weight of 20 g).
Syngeneic mouse models
K1735M2 or Wehi-164 exponentially growing cells were harvested, repeatedly washed and re-suspended in serum-free medium prior to injection. Tumor cells were injected subcutaneously (1×106-first therapy experiment- or 5×106-subsequent experiments- for the K1735M2 model, 5×106 for the Wehi-164 model) in the right flank of C3H/HeN or Balb/c mice. Tumor growth was measured at least twice a week with the aid of a digital caliper. Tumor weights (mg = mm3) calculated as follows: (length [mm] × width2 [mm2])/2. Treatment started when tumors reached approximately 100 mg. Toxicity was monitored recording mice body weight with the aid of a digital scale. Body weight was plotted as percentage (%) over body weight on the first day of therapy.
Treatment schedules
In the first chemo-immunotherapy experiment, mice were treated every third day for three cycles. In the combination groups, chemotherapy (PTX and DTIC) was administered first, immunotherapy 24 h later.
In the second therapy experiment, treatments followed the clinical schedule with the immunocytokine administered every second day for three cycles. When administered in combination, PTX was administered first, on the same day of the immunocytokine. When administered in combination, DTIC was administered after the immunocytokine and only on the first cycle. When antipyretics were administered, they were administered first, 1 h prior to the immunocytokine.
For the intratumoral therapy experiments, proteins were resuspended in a final volume of 90 μl and injected on the first day of treatment within each tumor lesion. A second injection was performed 7 days later, unless impeded by the presence of necrotic tissue following TNF treatment.
Biodistribution studies with radioiodinated protein preparations
The in vivo tumor-targeting ability of F8–IL2, L19–IL2 and of KSF–IL2 was evaluated as previously described [36]. Briefly, 20 μg of each radiolabeled protein were injected into the lateral tail vein of C3H/HeN mice bearing K1735M2 tumors subcutaneously. Twenty-four hours later, mice were killed, organs excised and radioactivity measured using a Packard Cobra gamma counter. Values are shown as percentage of the injected dose (ID) per gram of tissue (%ID/g) and variability shown as standard error (SE).
Immunofluorescence on frozen tumor samples
Immunofluorescence staining on 10 μm cryostat section of optimal cutting temperature compound (OCT) embedded K1735M2 tumors was performed as previously described [37]. Briefly, acetone-fixed sections were stained for EDA- and EDB-containing fibronectin using the corresponding F8 and L19 antibodies in biotinylated small immunoprotein (SIP) format, using rat anti-CD31 antibodies as counterstain (endothelial cells; BD Biosciences). For the detection of the biotinylated SIPs, Streptavidin Alexa Fluor 488 (Invitrogen, Basel, Switzerland) was used, and anti-rat IgG-AlexaFluor594 was used to detect endothelial cells. As negative control, the stainings were repeated in the absence of primary antibodies.
The immune infiltrate was analyzed on tumors harvested 24 h after one dose of F8–IL2, DTIC or the combination of the two treatments. As primary antibodies rat anti-CD4 (CD4+ T cells, GK1.5; BioXcell), rat anti-CD8 (CD8+ T cells, 2.43; Bio × cell), rat anti-FOXP3 (Foxp3+ cells, eBioscience), rat anti-CD45 (leukocytes; BD Biosciences) and rabbit anti-Asialo/GM1 (NK cells; Wako Pure Chemical Industries) were used and anti-rat IgG-AlexaFluor488 or anti-rabbit IgG-AlexaFluor594 was used as secondary reagent for microscopic detection.
For the evaluation of IL2 residence time in the tumor tissue after intratumoral administration, mice (N = 4 per group) were treated with Proleukin (10 μg/mouse), F8–IL2 (30 μg/mouse) or saline. Mice were killed 3 or 5 days after the injection and tumor embedded in optimal cutting compound and frozen in liquid nitrogen. Detection of IL2 was performed with anti-human IL2 antibody (eBioscience), followed by anti-rat IgG-AlexaFluor488 (Invitrogen) as secondary reagent.
All slides were mounted with fluorescent mounting medium (Dako) and analyzed with an Axioskop2 mot plus microscope (Zeiss).
For semiquantitative evaluation of infiltrating cells and IL2 residence time, pictures of 3–5 randomly selected tumor areas were taken using a 20× objective (0.14 mm2). The percentage of the stained area to the total image area was assessed by computer aided image analysis (Image J software).
Statistical analysis
Statistical analyses were performed with the aid of Prism software. Therapy efficacy was evaluated with the two-way ANOVA followed by Bonferroni as post-test. Differences in the stained area of frozen sections were evaluated with the one-way ANOVA followed by Dunn’s post-test. P values lower than 0.05 were considered significant.
Results
Comparison of L19 and F8 in the K1735M2 model of melanoma
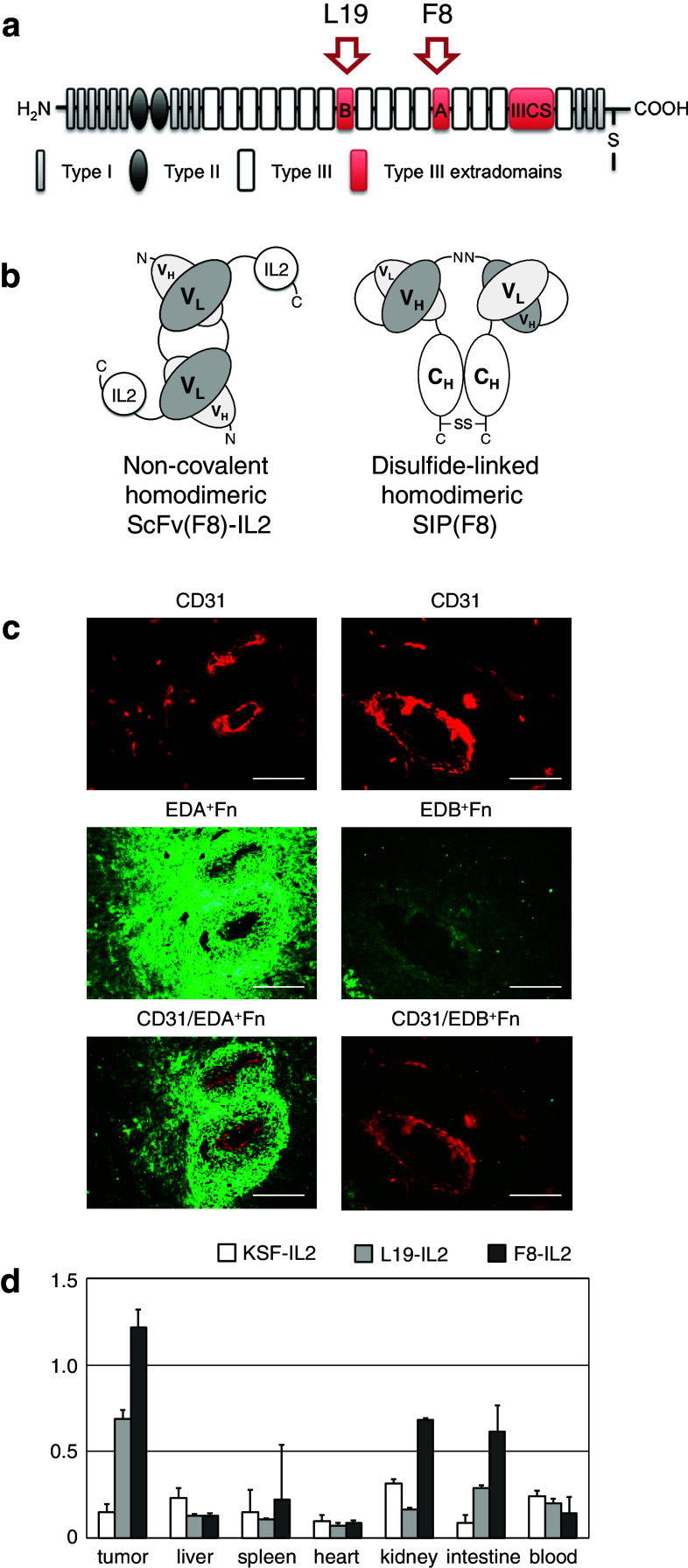
In order to test the ability of L19 and F8 to stain freshly frozen sections of K1735M2, we performed an immunofluorescence experiment with the two antibodies in SIP format, which revealed a stronger staining when the F8 reagent was used (Fig. 1a–c). The two immunocytokines F8–IL2 and L19–IL2 were then labeled with iodine-125 and studied in a biodistribution analysis, following intravenous administration to K1735M2-bearing mice. The results were in keeping with the immunohistochemical data, revealing a higher tumor uptake for F8–IL2, as compared to L19–IL2 (Fig. 1d). The %ID/g values in the tumor observed for F8–IL2 were not as high as for other tumor models recently reported by us [34], but the fusion protein was clearly superior to L19–IL2 and KSF–IL2 in terms of tumor/blood ratios. KSF–IL2 was used as control untargeted immunocytokine, the KSF antibody being specific for the hen egg lysozyme, an antigen of irrelevant specificity in the mouse [34]. For these reasons, F8–IL2 was chosen as our reference immunocytokine for all subsequent studies in the K1735M2 model.
Fig. 1.
Oncofetal fibronectin targeting in K1735M2 syngeneic melanoma model. a Schematic representation of fibronectin structure. Alternatively spliced domains are highlighted in red. b Schematic representation of F8–IL2 immunocytokine, used in the therapy studies, and of the SIP format of F8, used in the immunofluorescence analysis. The N- and C-termini are indicated. c Immunofluorescence analysis of EDA+ and EDB+ fibronectin. Alternatively spliced domains of fibronectin are shown in green, CD31 staining of blood vessel in red. Scale bars 200 µm. d Biodistribution study of radioiodinated KSF–IL2, L19–IL2 and F8–IL2. Immunocompetent C3H/HeN mice-bearing syngenic s.c. K1735M2 melanomas were injected with 20 µg radiolabeled 125I–KSF–IL2 (open square, n = 5), with 20 µg radiolabeled 125I–L19–IL2 (gray square, n = 5) or with 20 µg radiolabeled 125I–F8–IL2 (black square, n = 5). Mice were killed after 24 h. Organs were excised and radioactivity counted, expressing results as percent of ID per gram of tissue %ID/g ± SE
Combination therapy studies with chemotherapy
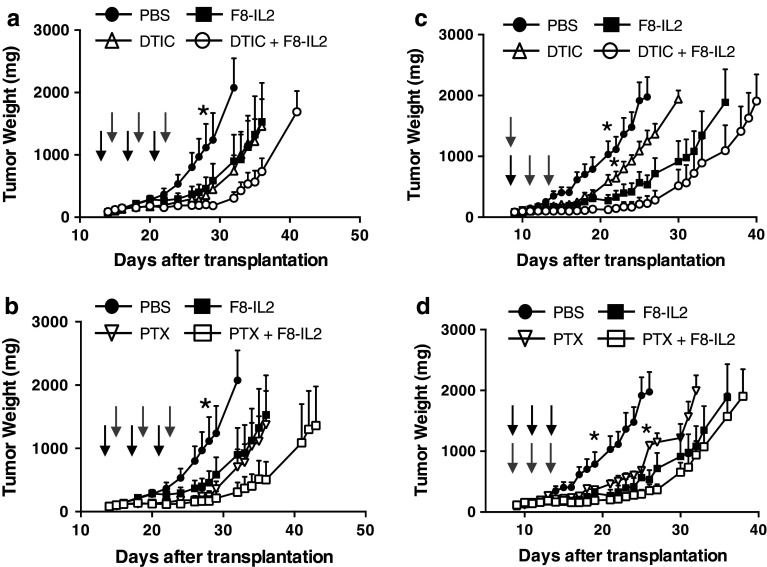
In a first set of therapy experiments, we aimed at assessing the therapeutic activity of F8–IL2 in combination with PTX or with DTIC, in comparison to the activity of the single agents. Based on the experience gained in our previous combination studies, we administered the cytotoxic agent 24 h before the intravenous injection of the immunocytokine (30 µg/mouse, three injections). Figure 2a, b reveals that the combination of F8–IL2 with DTIC or with PTX was always superior to the use of F8–IL2 or the cytotoxic drugs as single agents (T/C = 25 % for F8–IL2 and PTX, 29 % for DTIC, 11 % for both combination therapies). However, in contrast to other mouse models of melanoma, we did not observe complete cures in the K1735M2 model, but only tumor growth retardations compared to saline phosphate buffered saline (PBS), which lasted ~15 days following the last injection. The therapeutic performance of F8–IL2 + DTIC or of F8–IL2 + PTX appeared to be comparable. No significant weight loss was observed in any of the treatment groups (Supplementary Figure 1), which motivated us to investigate higher therapeutic doses. Figure 2c, d shows the results of therapy experiments performed with F8–IL2 at a 50 µg/dose (3 injections), in a more aggressive melanoma setting, established by the injection of a higher number of tumor cells (5×106 per mouse). A statistically significant superiority of F8–IL2 + DTIC over DTIC alone could be observed. The combined treatment gave the best therapeutic results both in the DTIC and PTX combination groups (P < 0.05 from day 18 vs. PBS, P < 0.05 from day 22 or 26 vs. DTIC or PTX, respectively). In spite of the higher doses used, no cures could be observed. A microscopic analysis of the leukocyte infiltration in tumors 24 h after treatment with F8–IL2 + DTIC revealed a statistically significant increase in CD4 (+) T cell infiltration in the neoplastic mass as compared to the control groups, whereas no substantial differences could be observed when staining for CD8, Foxp3, asialo GM1 and CD45 (Supplementary Figure 2).
Fig. 2.
Therapeutic activity of F8–IL2 in combination with dacarbazine or paclitaxel chemotherapy. K1735M2 melanoma-bearing mice were treated with F8–IL2 (30 μg/mouse, a and b; 50 μg/mouse, c and d), dacarbazine (100 mg/kg) or paclitaxel (20 mg/kg) starting when tumors reached ± 100 mg. a, b chemotherapy preceded the immunocytokine by 24 h. Therapies were administered for a total of three cycles, every third day. c Immunotherapy with F8–IL2 preceded three cycles of chemotherapy with dacarbazine on the first day of treatment. d Immunotherapy followed paclitaxel chemotherapy by 1 h during every cycle of therapy, a total of three cycles, every second day was administered. Results are plotted as tumor mean weight over time ±SE. *=P < 0.05 (two-way ANOVA test followed by Bonferroni post-test). Gray down arrow = F8–IL2 treatment, black down arrow = DTIC or PTX treatment, N = 4–5 mice per group
In the ongoing Phase II clinical studies with L19–IL2 in combination with DTIC [26], patients are frequently treated with DEXA, NOVA and PARA following immunocytokine infusion, to counteract flue-like symptoms such as the temperature increase associated with high IL2 values in blood. We performed a therapy experiment, administering DEXA (2 mg/kg), NOVA (200 mg/kg), PARA (100 mg/kg) in combination with F8–IL2 + DTIC, in order to mimic clinical procedures and learn about a possible inhibition of the anticancer effect. Supplementary, Figure 3 shows that no statistically significant reduction of therapeutic effect could be seen with any of the three anti-inflammatory drugs.
Combination therapy studies with TNF-based immunocytokines
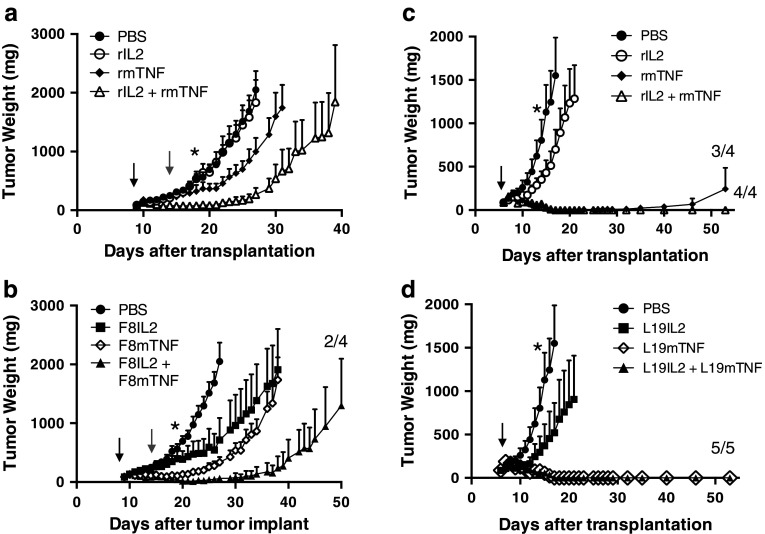
In order to mimic the intralesional treatment of patients with Stage IIIC melanoma, we compared the therapeutic activity of IL2 and TNF, used alone, in combination or fused to the L19 and F8 antibodies, by performing intratumoral injections. F8-based immunocytokines were tested in the K1735M2 melanoma model (Fig. 3a, b), while L19 products were tested in the Wehi-164 sarcoma model, which is strongly positive for the EDB domain of fibronectin [38] (Fig. 3c, d). In the melanoma model, the targeted immunocytokines F8–IL2 and F8–TNF used as single agents resulted to be more effective than an equimolar dose of the corresponding nontargeted cytokines (Fig. 3a, b). The combination of F8–IL2 with F8–TNF yielded the best therapeutic results, curing 2/4 mice with a single intratumoral injection (Fig. 3b).
Fig. 3.
Therapeutic efficacy of the combination of IL2- and TNF-based immunocytokines. Mice-bearing K1735M2 melanoma (a, b) or Wehi-164 sarcoma tumors (c, d) were treated with recombinant human IL2 (10 μg/mouse), murine recombinant TNFα (2.5 μg/mouse) or a combination of the two (a, c). Further therapy groups were treated with the corresponding immunocytokines F8–IL2 (30 μg/mouse), F8–TNF (7 μg/mouse) or a combination of the two (b), L19–IL2 (30 μg/mouse), L19–TNF (7 μg/mouse) or a combination of the two (d). Treatment consisted of a single injection when tumor reached an approximate volume of 100 mg (black arrow). Gray arrows represent a second injection, performed only in therapy groups not receiving TNF. Therapy results are plotted as tumor mean weight over time ±SE. *=P < 0.05 (two-way ANOVA test followed by Bonferroni post-test, N = 4–5 mice per group
In the sarcoma model, recombinant murine TNF exhibited a potent antitumoral activity, which was clearly superior to the one of intralesional IL2 (Fig. 3c), in keeping with the fact that soft tissue sarcomas are the tumors with the highest sensitivity to TNF action [39]. The combination of L19–IL2 with L19–TNF could cure 5/5 mice with a single intratumoral injection (Fig. 3d). Upon rechallenge with a subcutaneous injection of Wehi-164 cancer cells, all cured mice did not develop tumor lesions, indicating that they had acquired a protective immunity. The immunocytokine combination therapies were well tolerated, as evidenced by the profiles of body weight during the course of therapy (Supplementary Figure 4).
In both tumor models, the administration of targeted TNF induced the formation of a necrotic mass, which converted into a black scar prior to the complete disappearance of the neoplastic lesion (Supplementary Figure 5).
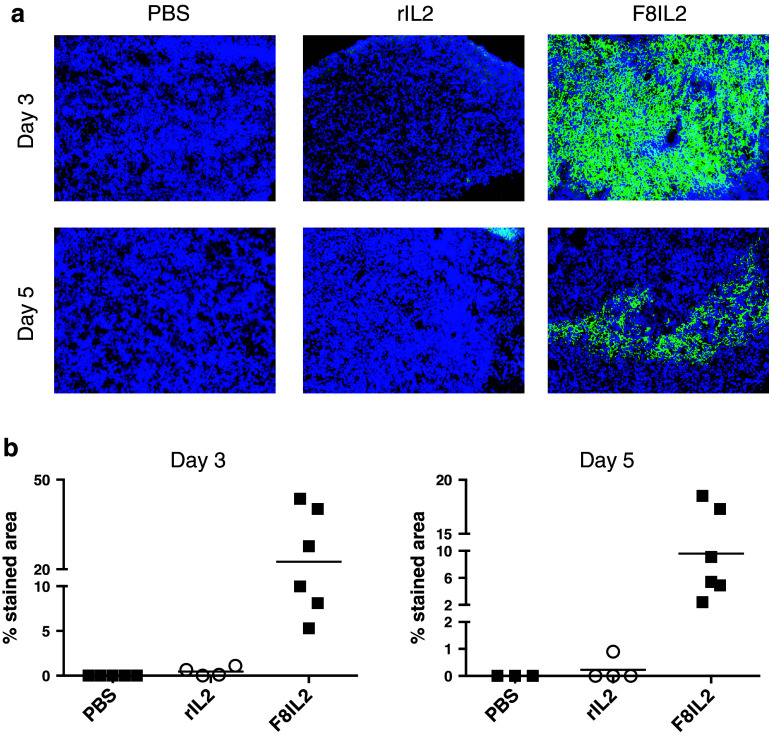
To gain more insights about the superior performance of targeted IL2, compared with recombinant IL2, an immunofluorescence analysis of tumor sections was performed. In the K1735M2 melanoma model, F8-IL2 exhibited a persistent localization at the tumor site following intralesional administration, whereas recombinant IL2 injected at equimolar doses could not be detected after 3 and 5 days (Fig. 4a, b). A similar analysis could not be performed for TNF-based immunocytokines, because of the scar formation induced by these agents.
Fig. 4.
IL2 residence time and distribution in the tumor tissue. K1735M2 melanoma-bearing mice were intralesionally treated once with F8-IL2 (30 μg/mouse), rIL2 (10 μg/mouse) or saline. Two mice per group were killed 3 or 5 days after the injection. 10 μm cryostat sections of OCT embedded tumors were stained for human IL2 (green) and counterstained with DAPI (blue). Representative pictures (a) and the relative quantification (b) are shown
Discussion
Dacarbazine and PTX are frequently used for the treatment of patients with metastatic melanoma, but these agents rarely induce objective responses in patients [40]. In this study, we have shown that the combination of F8–IL2 with either of the two drugs led to improved tumor growth retardation, compared to the drugs used as single agent, without additional loss of body weight or any other sign of toxicity. In contrast to our previous study [18], DTIC did not appear to be inferior to PTX as chemotherapeutic agent for immunocytokine combination in the K1735M2 syngeneic immunocompetent model of melanoma. This observation is of interest, since L19–IL2 has been investigated in the clinic in combination with DTIC in patients with metastatic melanoma and a Phase IIb clinical trial is currently ongoing [26].
Cures were not observed in the systemic therapy study with the K1735M2 model, but the fully human F8–IL2 fusion protein could be administered only three times, as it is immunogenic in the mouse. In the mouse setting, tumors did not grow as long as F8–IL2 was administered. In the clinic, the fully human L19–IL2 and F16–IL2 are routinely given to patients with cancer for over 6 months, without evidence of immunogenicity for the majority of the treated patients [26].
The biodistribution results obtained with F8–IL2 in the murine K1735M2 melanoma model indicate tumor/blood ratios of 8.6 twenty-four hours after intravenous administration, but only 1.2 %ID/g in the tumor. This value is lower compared to the ones observed with F8–IL2 and other F8-based products in other models [34, 36, 41]. Additional parameters, such as tumor blood vessel permeability and interstitial pressure, may influence the antibody uptake in the tumor, which cannot be completely predicted even if the antigen is strongly expressed, as evidenced by immunohistochemical analysis. It is important to compare biodistribution studies in mice with dosimetries obtained in patients. A variability, from lesion to lesion and from patient to patient, has been reported in imaging studies performed with cancer patients using the L19 and F16 antibodies, developed by our laboratory [42, 43]. The tumor-targeting performance of the L19 antibody has been studied in over 200 patients with cancer using Nuclear Medicine Techniques, including >10 melanoma patients [42, 44, 45]. Nuclear Medicine studies for F8 are still missing, but immunofluorescence studies suggest that F8 may be superior to L19 for the staining of certain types of melanoma lesions [46].
The judicious use of antipyretic drugs and the hydration of patients promises to reduce side effects associated with IL2 serum peak concentrations in immunocytokine treatment, thus potentially allowing the administration of higher doses. In the case of pro-inflammatory immunocytokines, there is a clear dose dependence for the therapeutic efficacy, as evidenced not only by dose escalation studies in mice [14, 47], but also by the effect of locoregional treatment modalities [28, 30]. It may be possible to increase therapeutic doses by slowing down infusions, thus reducing cytokine peak concentrations and, consequently, side effects. Importantly, it appears that the tumor/organ ratios (i.e., the targeting selectivity) achieved in vivo with antibodies specific to oncofetal fibronectin do not depend on the absolute amount of injected antibodies [17], thus providing a strong basis for slowing down infusions.
Microscopic analysis of the leukocyte infiltrate in tumors after F8–IL2 + DTIC treatment seems to point to an increased CD4 (+) T cell population in the neoplastic mass. In a number of previous depletion studies featuring use of immunocytokines which lead to cancer cures, CD8+ T cells and NK cells were found to play a crucial role, whereas the contribution of CD4+ T cells was always negligible [18, 36, 48, 49]. These observed differences deserve further investigation (we limited our analysis to the 24-h time point) but, if confirmed, seem to imply that differences in tumor type, animal strain and cytokine might lead to the activation of different mechanisms of tumor rejection.
While the systemic administration of drugs appears to be the method of choice for the treatment of disseminated disease, the control of melanoma with inoperable skin-only manifestations could potentially be achieved using locoregional procedures. A number of interventional strategies, including immune-, electrochemo- and radiotherapy, have been considered [50]. The group of Claus Garbe has pioneered the intralesional administration of recombinant IL2 for the treatment of Stage IIIB and Stage IIIC patients. While the majority of injected lesions respond to treatment [30, 51], a time-to-Stage IV and a survival benefit has so far only been shown for patients with Stage IIIB melanoma disease [52] suggesting a beneficial systemic effect induced by the local treatment in the earlier stages of the disease. For this reason, there is a need for a more efficacious treatment of Stage IIIC melanoma patients. Indeed, the use of a targeted form of IL-2 (L19–IL2) has recently been shown to prompt a sustained systemic immune response and to delay the progression rate to distant metastases for Stage IIIB/IIIC patients [31].
In the pre-clinical setup, we observed that the combined use of L19–IL2 with L19–TNF in F9 teratocarcinoma was more potent than either immunocytokine used alone [20]. In this same study, we could also observe a synergistic effect of L19–IL2 in combination with antibodies directed against murine CTLA-4 (ipilimumab analogs) [20]. These data and the data presented in this study provide a strong rationale for the combination of IL2- and TNF-based immunocytokines, which can persist at the tumor site for a longer period of time compared to the nontargeted cytokines and may thus be efficacious with a limited number of intralesional injections. A Phase II trial featuring the intralesional administration of L19–IL2 plus L19–TNF in patients with Stage IIIC melanoma (EudraCT 2012-001991-13) is currently ongoing.
In summary, we have shown that the therapeutic activity of IL2-based immunocytokines can be potentiated by combination with chemotherapeutic agents and with TNF-based immunocytokines. These data provide support to clinical trials, featuring the use of IL2-based immunocytokines specific to alternatively spliced extra domain of fibronectin. This marker of tumor angiogenesis is conserved from mouse to man, which greatly facilitates translational clinical activities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Kathrin Schwager for her help with biodistribution and immunofluorescence experiments. The study was supported by Swiss National Science Foundation, the Swiss Federal Institute of Technology (ETH), the European Union (FP7 ADAMANT and PRIAT Project) and the Kommission für Technologie und Innovation (KTI MedTech Award to Philochem).
Conflict of interest
Dario Neri is founder and shareholder of Philogen, a biotech company that owns the F8 antibody. Francesca Pretto and Giuliano Elia are Philochem employees. Nadia Castioni declares no conflict of interest.
Abbreviations
- CTLA-4
Cytotoxic T-lymphocyte antigen 4
- DEXA
Dexamethasone
- DTIC
Dacarbazine
- EDA
Extra domain A of fibronectin
- EDB
Extra domain B of fibronectin
- FDA
Food and drug administration
- ID
Injected dose
- IL2
Interleukin-2
- NK
Natural killer cells
- NOVA
Metamizole
- OCT
Optimal cutting temperature compound
- PARA
Paracetamol
- PBS
Phosphate buffered saline
- PTX
Paclitaxel
- SE
Standard error
- SIP
Small immunoprotein
- T/C
Tumor growth inhibition ratio
- TNF
Tumor necrosis factor
References
- 1.Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14(17):5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponce R. Adverse consequences of immunostimulation. J Immunotoxicol. 2008;5(1):33–41. doi: 10.1080/15476910801897920. [DOI] [PubMed] [Google Scholar]
- 3.Baluna R, Sausville EA, Stone MJ, Stetler-Stevenson MA, Uhr JW, Vitetta ES. Decreases in levels of serum fibronectin predict the severity of vascular leak syndrome in patients treated with ricin a chain-containing immunotoxins. Clin Cancer Res. 1996;2(10):1705–1712. [PubMed] [Google Scholar]
- 4.Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001;24(4):287–293. doi: 10.1097/00002371-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kontermann RE. Antibody-cytokine fusion proteins. Arch Biochem Biophys. 2012;526(2):194–205. doi: 10.1016/j.abb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17(11–12):583–590. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Davis CB, Gillies SD. Immunocytokines: amplification of anti-cancer immunity. Cancer Immunol Immunother. 2003;52(5):297–308. doi: 10.1007/s00262-002-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huston JS, McCartney J, Tai MS, et al. Medical applications of single-chain antibodies. Int Rev Immunol. 1993;10(2–3):195–217. doi: 10.3109/08830189309061696. [DOI] [PubMed] [Google Scholar]
- 9.Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90(14):6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies SD, Lan Y, Williams S, et al. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood. 2005;105(10):3972–3978. doi: 10.1182/blood-2004-09-3533. [DOI] [PubMed] [Google Scholar]
- 11.Ko YJ, Bubley GJ, Weber R, et al. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27(3):232–239. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Gillessen S, Gnad-Vogt US, Gallerani E, et al. A phase I dose-escalation study of the immunocytokine EMD 521873 (Selectikine) in patients with advanced solid tumours. Eur J Cancer. 2013;49(1):35–44. doi: 10.1016/j.ejca.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 13.King DM, Albertini MR, Schalch H, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22(22):4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnemolla B, Borsi L, Balza E, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–1665. doi: 10.1182/blood.V99.5.1659. [DOI] [PubMed] [Google Scholar]
- 15.Marlind J, Kaspar M, Trachsel E, et al. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res. 2008;14(20):6515–6524. doi: 10.1158/1078-0432.CCR-07-5041. [DOI] [PubMed] [Google Scholar]
- 16.Villa A, Trachsel E, Kaspar M, et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122(11):2405–2413. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 17.Tarli L, Balza E, Viti F, et al. A high-affinity human antibody that targets tumoral blood vessels. Blood. 1999;94(1):192–198. [PubMed] [Google Scholar]
- 18.Moschetta M, Pretto F, Berndt A, et al. Paclitaxel enhances therapeutic efficacy of the F8–IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012;72(7):1814–1824. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- 19.Pedretti M, Verpelli C, Marlind J, et al. Combination of temozolomide with immunocytokine F16–IL2 for the treatment of glioblastoma. Br J Cancer. 2010;103(6):827–836. doi: 10.1038/sj.bjc.6605832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19–IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19–TNF. J Invest Dermatol. 2013;133(3):751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 21.Schliemann C, Palumbo A, Zuberbuhler K, et al. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19–IL2. Blood. 2009;113(10):2275–2283. doi: 10.1182/blood-2008-05-160747. [DOI] [PubMed] [Google Scholar]
- 22.Halin C, Gafner V, Villani ME, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63(12):3202–3210. [PubMed] [Google Scholar]
- 23.Schwager K, Kaspar M, Bootz F, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther. 2009;11(5):R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwager K, Villa A, Rosli C, Neri D, Rosli-Khabas M, Moser G. A comparative immunofluorescence analysis of three clinical-stage antibodies in head and neck cancer. Head Neck Oncol. 2011;3:25. doi: 10.1186/1758-3284-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johannsen M, Spitaleri G, Curigliano G, et al. The tumour-targeting human L19–IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Eigentler TK, Weide B, de Braud F, et al. A dose-escalation and signal-generating study of the immunocytokine L19–IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 27.Spitaleri G, Berardi R, Pierantoni C, et al. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19–TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol. 2013;139(3):447–455. doi: 10.1007/s00432-012-1327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadia F, Basso V, Patuzzo R, et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19–TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol. 2013;107(2):173–179. doi: 10.1002/jso.23168. [DOI] [PubMed] [Google Scholar]
- 29.Weide B, Eigentler TK, Pflugfelder A, et al. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol Immunother. 2011;60(4):487–493. doi: 10.1007/s00262-010-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weide B, Derhovanessian E, Pflugfelder A, et al. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116(17):4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]
- 31.Weide B, Eigentler TK, Pflugfelder A, et al. Intralesional treatment of stage III metastatic melanoma patients with L19–IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 32.Kripke ML. Speculations on the role of ultraviolet radiation in the development of malignant melanoma. J Natl Cancer Inst. 1979;63(3):541–548. doi: 10.1093/jnci/63.3.541. [DOI] [PubMed] [Google Scholar]
- 33.Talmadge JE, Fidler IJ. Enhanced metastatic potential of tumor cells harvested from spontaneous metastases of heterogeneous murine tumors. J Natl Cancer Inst. 1982;69(4):975–980. [PubMed] [Google Scholar]
- 34.Frey K, Schliemann C, Schwager K, Giavazzi R, Johannsen M, Neri D. The immunocytokine F8–IL2 improves the therapeutic performance of sunitinib in a mouse model of renal cell carcinoma. J Urol. 2010;184(6):2540–2548. doi: 10.1016/j.juro.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Hemmerle T, Probst P, Giovannoni L, Green AJ, Meyer T, Neri D. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109(5):1206–1213. doi: 10.1038/bjc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasche N, Wulhfard S, Pretto F, Carugati E, Neri D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin Cancer Res. 2012;18(15):4092–4103. doi: 10.1158/1078-0432.CCR-12-0282. [DOI] [PubMed] [Google Scholar]
- 37.Schwager K, Bootz F, Imesch P, Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum Reprod. 2011;26(9):2344–2352. doi: 10.1093/humrep/der195. [DOI] [PubMed] [Google Scholar]
- 38.Borsi L, Balza E, Carnemolla B, et al. Selective targeted delivery of TNF alpha to tumor blood vessels. Blood. 2003;102(13):4384–4392. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- 39.Starnes CO. Coley’s toxins. Nature. 1992;360(6399):23. doi: 10.1038/360023b0. [DOI] [PubMed] [Google Scholar]
- 40.Pretto F, Neri D. Pharmacotherapy of metastatic melanoma: emerging trends and opportunities for a cure. Pharmacol Ther. 2013;139(3):405–411. doi: 10.1016/j.pharmthera.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Gutbrodt KL, Schliemann C, Giovannoni L et al. (2013) Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med 5 (201):201ra118. doi 10.1126/scitranslmed.3006221 [DOI] [PubMed]
- 42.Erba PA, Sollini M, Orciuolo E, et al. Radioimmunotherapy with radretumab in patients with relapsed hematologic malignancies. J Nucl Med. 2012;53(6):922–927. doi: 10.2967/jnumed.111.101006. [DOI] [PubMed] [Google Scholar]
- 43.Heuveling DA, de Bree R, Vugts DJ, et al. Phase 0 microdosing PET study using the human mini antibody F16SIP in head and neck cancer patients. J Nucl Med. 2013;54(3):397–401. doi: 10.2967/jnumed.112.111310. [DOI] [PubMed] [Google Scholar]
- 44.Santimaria M, Moscatelli G, Viale GL, et al. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clin Cancer Res. 2003;9(2):571–579. [PubMed] [Google Scholar]
- 45.Sauer S, Erba PA, Petrini M, et al. Expression of the oncofetal ED-B-containing fibronectin isoform in hematologic tumors enables ED-B-targeted 131I-L19SIP radioimmunotherapy in hodgkin lymphoma patients. Blood. 2009;113(10):2265–2274. doi: 10.1182/blood-2008-06-160416. [DOI] [PubMed] [Google Scholar]
- 46.Frey K, Fiechter M, Schwager K, et al. Different patterns of fibronectin and tenascin-C splice variants expression in primary and metastatic melanoma lesions. Exp Dermatol. 2011;20(8):685–688. doi: 10.1111/j.1600-0625.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 47.Halin C, Rondini S, Nilsson F, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20(3):264–269. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 48.Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67(10):4940–4948. doi: 10.1158/0008-5472.CAN-07-0283. [DOI] [PubMed] [Google Scholar]
- 49.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134(2):467–477. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- 50.Testori A, Faries MB, Thompson JF, et al. Local and intralesional therapy of in-transit melanoma metastases. J Surg Oncol. 2011;104(4):391–396. doi: 10.1002/jso.22029. [DOI] [PubMed] [Google Scholar]
- 51.Radny P, Caroli UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89(9):1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weide B, Zelba H, Derhovanessian E, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol. 2012;30(15):1835–1841. doi: 10.1200/JCO.2011.40.2271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.