Abstract
Due to their ability to differentiate into various cell types and to support tissue regeneration, stem cells simultaneously became the holy grail of regenerative medicine and the evil obstacle in cancer therapy. Several studies have investigated niche-related conditions that favor stemness properties and increasingly emphasized their association with an inflammatory environment. Tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) are major orchestrators of cancer-related inflammation, able to dynamically express different polarized inflammatory programs that promote tumor outgrowth, including tumor angiogenesis, immunosuppression, tissue remodeling and metastasis formation. In addition, these myeloid populations support cancer cell stemness, favoring tumor maintenance and progression, as well as resistance to anticancer treatments. Here, we discuss inflammatory circuits and molecules expressed by TAMs and MDSCs as guiding forces of cancer cell stemness.
Keywords: Cancer stem cells, Tumor-associated macrophages, Regulatory myeloid suppressor cells, Myeloid-derived suppressor cells, Inflammation
Introduction
While in homeostatic conditions stem cells localize in specialized niches and control the physiological cell turnover (basal regeneration), during injury inflammatory cytokines promote their proliferation to induce efficient tissue regeneration. Inflammation plays a key role in the dynamic regulation of wound healing, through overlapping phases of acute inflammation, resolving inflammation, proliferation and remodeling [1]. Skewing of macrophage polarization critically orchestrates the onset and resolution phases of the inflammatory response, as well as the different stages of tissue repair. An initial classical activation (M1 polarization) of macrophages supports the inflammatory response after injury and evolves to an alternative activation (M2 polarization), involved in the resolution of inflammation and the regeneration of injured tissues. In support of this paradigm, dynamic changes from M1 to M2 phenotype of recruited monocytes have been observed in models of ischemic heart and kidney diseases [2], and an impaired switch from an M1 to an M2 phenotype prevents resolution of chronic inflammation favoring chronic wounds, as occurring in venous ulcers [3]. A bidirectional interplay between mononuclear phagocytes and progenitor cells, or bona fide stem cells, crucially contributes to tissue repair and remodeling. While mesenchymal stem cells (MSCs) induce an alternative (M2) activation phenotype in macrophages, M2-like macrophages promote growth and motility of MSCs [4]. This cross-talk between mononuclear phagocytes and stem cells can play a critical role in cancer, where the acquisition of stemness properties by cancer cells represents a major hurdle for anticancer therapies. It becomes therefore urgent to elucidate how extrinsic signals, delivered by distinct microenvironmental components, regulate cancer cell stemness.
The tumor microenvironment displays many features of a chronic wound and is characterized by infiltration of leukocytes and other stimuli that support tumor growth, invasion, and angiogenesis. Tumors affect the normal developmental roles of myeloid cells (in particular of macrophages) to promote their own development; moreover, new evidence indicates that alterations of the inflammatory processes that support wound recovery, may confer stemness properties to cancer cells and impair healing [5]. Importantly, cancer growth is supported by a pathological expansion of immature myeloid cells (MDSCs) that establishes tumor-promoting conditions in both the micro- and macro-environments of tumor bearers. These immature myeloid cells cooperate with TAMs to establish a bidirectional interaction with neoplastic cells, sculpting a tumor-promoting niche that determines cancer cell stemness and resistance to anticancer treatments.
Cancer stem cells
Despite Cancer Stem Cells (CSCs) have been recognized as key supporters of tumor outgrowth, maintenance, and progression, their origin is still a matter of debate. While on the one hand cancer cell has the same chance to be transformed into a tumor-initiating cell (TIC), on the other emerging evidence supports the idea that CSCs may originate from a tissue-specific stem cell endowed with unlimited proliferation ability which could, at least in part, explain the accumulation of oncogenic mutations that leads to its neoplastic transformation. CSCs share many features with their normal counterpart, including the extensive proliferative potential, the ability to grow in vitro under specific culture conditions as rounded structures called spheroids, and the ability to differentiate into all cell lineages of the tissue where they home (pluripotency) [6]. However, the most important feature is self-renewal: CSCs, similarly to normal stem cells, can in fact generate two identical daughter stem cells (symmetric division) or one daughter cell with a stemness phenotype and one more differentiated (asymmetric division). This ability permits CSCs to guarantee a reservoir of stem cells within the tissue.
The regulation of asymmetric vs symmetric division (differentiation vs stemness), as well as the self-renewal ability are driven by signals from the microenvironment which control specific pathways such as NOTCH, Sonic Hedgehog and Wnt/β-catenin [7]. Notwithstanding the stemness genes NANOG, SOX2 and OCT4 are overexpressed in tumor cells endowed with a stem phenotype, a CSC universal marker has not been described as yet and, according to the tissue of origin, CSCs can be identified and enriched on the basis of different surface markers. The most common marker is CD44, a type I transmembrane glycoprotein that acts as a cellular adhesion molecule for hyaluronic acid. CD44 exists in several isoform: besides the standard form (CD44 s), the most important is a variant isoform (CD44v) that identifies CSCs alone or in association with other markers such as CD166 or EpCAM in colon cancer [8] and CD117 in epithelial ovarian cancer (EOC) [9].
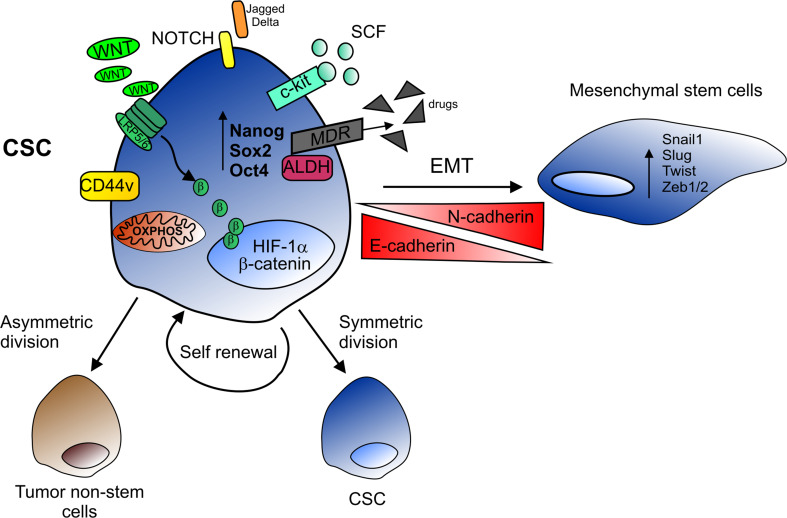
In addition to surface marker expression, many other specific stemness features have been exploited for CSC identification (Fig. 1). In particular, CSCs could be isolated based on their ability to retain membrane labels such as PKH or to exclude lipophilic substrates such as Hoechst 33342 (side population). However, the golden standard for CSC identification remains the evaluation of their tumorigenic potential when injected into immunocompromised mice.
Fig. 1.
Cancer stem cell features. CSCs are characterized by high activity of NOTCH and Wnt/β-catenin pathways, mitochondrial OXPHOS metabolism, and expression of tissue-specific surface markers (CD44v and the SCF receptor c-kit), as well as embryonal stemness genes (NANOG, SOX2 and OCT4). The expression of detoxifying enzyme (ALDH) and MDR pumps, which extrude chemotherapeutic drugs, allows CSCs to survive conventional therapy. CSCs are endowed with self-renewal ability and can generate two identical daughter cells maintaining the stemness features (symmetric division) or give rise to one CSC daughter and one differentiated tumor cell (asymmetric division). By losing E-cadherin and acquiring N-cadherin expression, CSCs can undergo EMT, driven by the expression of regulatory genes (SNAIL, SLUG, TWIST and ZEB1/2), thus acquiring a mesenchymal morphology that contributes to metastasis formation
Epithelial-to-mesenchymal transition
Epithelial-to-mesenchymal transition (EMT) is a multifaceted program normally occurring during embryonic development, which in neoplastic tissues transforms epithelial cells, making them able to invade and disseminate. Recent evidence indicates that only some tumor cells are able to respond to the triggering signals, undergo EMT and generate metastasis. Since metastatic cells are endowed with the ability to recreate the architectural structure and cellular composition of the primary tumor, it follows that only CSCs present all these features, thanks to their self-renewal capacity and unlimited proliferative potential. Indeed, in several neoplastic tissues CSCs are enriched in the expression of transcriptional repressors which orchestrate the transition from the epithelial to the mesenchymal state, such as Zinc finger E-box-binding homeobox 1/2 (ZEB1/2), Snail homolog 1 (SNAIL), Snail homolog 2 (SLUG) and TWIST [9]. Moreover, overexpression of Nanog is associated with a reduction in E-cadherin levels and an increased expression of SLUG, a positive regulator of EMT, resulting in enhanced cell motility and tumor metastasis [10]. Furthermore, EMT can induce de-differentiation and generate CSCs from non-stem precursors.
The CSC niche in the tumor microenvironment
The tumor microenvironment (TME) includes different types of cells: endothelial cells, fibroblasts or stromal cells and infiltrating inflammatory cells, which not only create a physical support for tumor growth but also orchestrate its neoplastic development through stromal cell recruitment, induction of angiogenesis and secretion of specific factors. Akin normal stem cells, CSCs reside in a well-defined space within TME, called “niche”, characterized by fibroblasts, endothelial, perivascular and immune cells which participate in a network of cytokines and growth factors that regulate CSC maintenance. The cross-talk between tumor cells, CSCs and the other components of the niche can activate stemness-associated programs, promote ultimate neoplastic transformation, influence tumor growth and its response to therapies, which could eventually lead to drug resistance.
The CSC niche is characterized by hypoxia, often resulting from chronic inflammation. Hypoxia activates the hypoxia inducible factor-1α (HIF-1α), which in turn stimulates the release of pro-inflammatory cytokines such as Tumor Necrosis Factor-α (TNF-α) and Transforming Growth Factor-β (TGF-β) that regulate the undifferentiated state of CSCs by inhibiting cell proliferation/differentiation and enhancing stemness through the Wnt/β-catenin pathway [11]. HIF-1α accumulation within the microenvironment induces the surface expression of the C-X-C chemokine receptor type 4 (CXCR4), which stimulates cell migration and metastatisation [12]. Moreover, HIF-1α induces nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, increasing CSC aggressiveness through the production of Reactive Oxygen Species (ROS), whose levels stimulate cell proliferation, increase cytokine and growth factor release, and activate the Nuclear Factor-κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3) signaling [13].
Potential mechanisms protecting CSCs
CSCs represent a limit to the common therapeutic treatments. Classical agents such as chemotherapy and radiotherapy reduce tumor burden by eliminating highly proliferative tumor cells; nonetheless, CSCs are largely resistant to these approaches [14]. Thus, by the end of the therapies, the tumor often relapses mainly due to reactivation of CSCs, which start to proliferate again reproducing the entire tumor mass heterogeneity. Indeed, the ability of CSCs to escape both the often hostile conditions of the TME (hypoxia, ROS and toxic metabolite accumulation, and necrosis-associated nutrient shortage) and the activity of most anti-tumor agents renders their radical elimination the key issue of modern oncology.
Proliferative quiescence
Several mechanisms have been described to be involved in CSC resistance, including the ability of CSCs to enter quiescence under appropriate conditions. Indeed, in response to many microenvironmental signals, CSCs can exit the cell cycle and persist in a dormant status controlled by several factors including HIF-1α and NANOG. The latter, modulated by the Wnt/β-catenin pathway, plays a central role in regulation of proliferation and cell cycle progression by binding the Cyclin D1 promoter and inducing G0/G1 cell cycle arrest, eventually increasing drug resistance [15].
Extrusion pump expression
Another important defense mechanism exploited by CSCs is their ability to reduce drug accumulation by extruding toxic compound through the expression of ATP-binding cassette transporters, such as ABCG2 or multidrug resistance protein (MDR1, MDR2) [16]. In lung, ovarian and breast carcinoma it has been demonstrated that chemoresistance is in part mediated by NANOG and OCT4 which, through a STAT3-mediated pathway, can enhance mRNA expression levels of ATP-binding cassette sub-family B member 1 (ABCB1), a membrane-bound transporter involved in the extrusion of toxic agents [17]. In addition, NF-κB activation stimulates the expression of P-glycoprotein in MDR pumps resulting in chemoresistance. Ironically, chemotherapeutic drugs themselves, as well as radiation, are powerful activators of NF-κB.
Autocrine circuits
Autocrine loops could also contribute to preferential survival of these cells. Some authors reported a crucial role of c-kit in EOC and anti-tumor effects of Imatinib mesylate (Gleevec) in experimental models of EOC xenotransplantation. Similar results were also reported in colon cancer [18], where a c-kit/Stem cell factor (SCF) axis seems to be required for the maintenance of the CSC subset. However, most of the above results were obtained in in vitro and not in clinically relevant samples; indeed, phase II trials with Gleevec as a single agent did not provide substantial results [19]. On the other hand, very recently we found that both stem-like and non-stem cells from EOC patients express only the transmembrane form of SCF (unpublished results); it is, therefore, possible that an autocrine/paracrine circuit could be in place within the EOC microenvironment, and contribute to steady CSC activation within the tumor mass. Furthermore, both patient-derived tumor-associated fibroblasts (TAFs) and TAMs express the membrane form of SCF and also are a potent source of the soluble form of this cytokine.
Metabolic adaptation
Tumor cells preferentially use aerobic glycolysis (the so-called Warburg effect) even in the presence of adequate oxygen tensions. It has been demonstrated that CSCs preferentially exploit oxidative phosphorylation (OXPHOS) and present lower levels of ROS, compared to the non-stem counterpart [20], thus indicating a more active machinery of antioxidants such as aldehyde dehydrogenases (ALDH), glutathione (GSH) and the reduced form of NADPH. Therefore, CSCs are equipped with an emergency program to respond to conditions of oxygen and nutrient deficiency, which could allow their preferential survival under stress conditions.
Immune responses
The mechanisms underlying the evasion of malignant cells from immune system recognition are innumerable and may potentially be exploited also by CSCs. They include poor tumor antigen expression, down-regulation of HLA molecules, release of immunosuppressive factors, intrinsic resistance to apoptosis, expression of checkpoint inhibitor molecules and many others, that determine the three phases of tumor growth according to the Schreiber’s model [21]. However, CSCs could be endowed with special additional or enhanced features, which could allow their preferential resistance to immune effectors. Interestingly, a relatively more intact DNA repair machinery in CSCs, compared to bulk tumor cells, could justify a lower incidence of mutations in these cells compared to the differentiated malignant counterpart, thus rendering CSCs stealthier to specific immunity. Further evidence indicates that loss of antigen expression may not imply loss of the stemness status [22], whereas the immunogenicity of CSCs may be reduced by their ability to produce immunosuppressive cytokines.
Interestingly, a cytotoxic activity of a Vγ9Vδ2 T-cell receptor (TCR) subset of γδ T cells has been demonstrated against neuroblastoma and colon carcinoma CSCs treated with zoledronic acid [23]. The recent discovery that the activity of tumor-specific CTL is finely tuned by the network of checkpoint inhibitors places these molecules into play [24]. It is known that in most tumors malignant cells display on their surface Programmed death-ligand 1 (PD-L1), which by interacting with its ligand Programmed cell death protein 1 (PD1) on CTL membrane can block the cytotoxic activity of these latter. It would be important to ascertain whether PD-L1 or other checkpoint inhibitors are equally expressed in CSCs and non-stem populations, since a higher expression by CSCs could be a further element protecting this population against immune attack.
The cross-talk between tumor-associated myeloid cells and CSCs
TAMs and MDSCs are populations of regulatory myeloid cells that contribute to cancer-related immunosuppression. Noteworthy, both populations are key components of the CSC niche and provide signals that protect and promote CSC functions.
Tumor-associated macrophages
TAMs are the major component of tumor-infiltrating leukocytes and the main actors in the link between inflammation and cancer [25]. In established tumors, TAMs generally display an M2-like phenotype, which perpetuates the smoldering inflammation of the tumor microenvironment: they are devoid of cytotoxic activity, produce growth factors for cancer and endothelial cells, and have immunosuppressive functions [26]. However, considerable TAM heterogeneity emerged in recent years with distinct features in different types of tumors and even in different sites of the same microenvironment. A growing body of evidence indicates that TAMs produce several inflammatory cytokines promoting neoplastic transformation and supporting a unique microenvironment that protects and promotes CSC functions, both in primary tumors and in pre-metastatic niches in distant organs [27]. Among these, the interleukin-6 (IL-6)/STAT3 axis plays a key role in wound healing [28, 29], being also crucial for cancer cell stemness [30]. Interestingly, the expression levels of IL-6 in human hepatocellular carcinoma (HCC) samples correlate with markers of stemness, as well as with tumor stage [31]. Additionally, TAMs isolated from HCC patients induced the expansion of CD44+ HCC stem-like cells in a IL-6/STAT3-dependent manner, whereas either IL-6 neutralization or targeted STAT3 disruption inhibited CSC expansion, both in vitro and in vivo [31]. The IL-6/STAT3 pathway was recently reported to be a crucial inducer of CD133, another marker of stemness, whose expression levels are associated with HCC progression [32]. In a hypoxic liver microenvironment, the induction of CD133 is promoted by the transcriptional cooperation of the IL-6-induced STAT3 active form with the p65 NF-κB subunit and HIF-1α, while hampering STAT3 activation, by Sorafenib or nifuroxazide treatment, inhibits HIF-1α and CD133 expression, as well as HCC growth in vivo [32]. IL-6 also cooperates with milk-fat globule-epidermal growth factor-VIII (MFG-E8), mainly derived from CSC-associated macrophages, to activate the STAT3 and Sonic Hedgehog pathways and trigger tumorigenicity and resistance of CSCs to anticancer drugs, in colon and lung cancers [33]. Moreover, whereas TAMs secrete factors that activate STAT3 in pancreatic CSCs, supporting their expansion and resistance to gemcitabine, combining chemotherapy with TAM depletion (e.g., blocking colony-stimulating factor-1 receptor CSF-1R or C–C chemokine receptor type 2 CCR2) resulted in decreased number of pancreatic CSCs, reduced tumor growth and metastasis formation [34]. Additional evidence sustains a key role of STAT3 as a crossroad-pathway supporting cancer cell stemness. IL-6-dependent STAT3 activation is crucial for breast CSC self-renewal capacity [35] and breast cancer cell mammosphere formation is selectively promoted in co-culture with M2-polarized macrophages [36]. M2-polarized macrophages can be the direct source of IL-6 or can educate bone marrow-derived MSCs to produce IL-6, which in turn favors mammosphere formation [37]. In mice co-injected with inflammatory breast cancer (IBC) cells and MSCs, inhibition of TAMs by anti-CSF1 treatment restrained STAT3 activation in tumor cells, thus limiting tumor growth, skin invasion and local recurrence [37].
In addition to IL-6, TAMs can promote CSC maintenance through the secretion of other STAT3 activating molecules. Among them, epidermal growth factor (EGF), one of the first molecule identified in the TAM/cancer cell cross-talk, emerged as a crucial factor for murine breast CSC maintenance [38]. The EGF/epidermal growth factor receptor (EGFR) signaling activates STAT3 that drives the expression of the transcription factor Sox2, a crucial regulator of CSC phenotype [38]. Sox2 promotes the expression of other CSC markers (e.g., Oct-4, Nanog, ABCG2 and Sca-1) and its knock-down inhibits primary tumor growth, lung metastasis formation and chemoresistance [38]. Since aberrant activation of the EGFR signaling pathway is a common feature of several human epithelial cancers, blocking EGFR/STAT3/Sox-2 signaling may represent a valuable therapeutic approach to target CSCs in these types of cancer. Furthermore, hampering the CSF-1/CSF-1R axis might provide an alternative approach to interrupt the paracrine loop established by TAM-derived EGF and tumor cell secretion of CSF-1, that might eventually limit CSCs. Interleukin-10 (IL-10) acts through STAT3 activation as an essential homeostatic mechanism that controls the degree and resolution of inflammation [39, 40]. Of note, TAMs produce copious amount of IL-10 [41], which acts as immunosuppressive cytokine endowed with the ability to enhance cancer cell stemness [42]. Interestingly, comparing the secretion profile of human melanospheres, adherent melanoma cells, and primary melanocytes, it emerged that IL-10 (rather than IL-6) is selectively more expressed by melanoma CSCs and it is involved in the expansion of undifferentiated melanoma stem-like compartment [43]. In agreement, IL-10-dependent activation of the Janus kinase 2 (Jak2)/STAT3 signaling pathway promotes CSC properties in Burkitt lymphoma cells [44].
Over the last years, other molecular players involved in the bidirectional cross-talk between TAMs and CSCs emerged. For example, in pancreatic ductal adenocarcinoma (PDAC) cancer cells produce Interferon β (IFNβ) that induces the expression of IFN-stimulated gene 15 (ISG15) by TAMs. In turns, the secreted ISG15 acts on PDAC CSCs enhancing their inherent “stem-like” properties, including self-renewal and tumorigenicity [45]. More recently, it was shown that the PDAC CSC-secreted TGF-β family members Nodal and Activin A induce human cathelicidin-18 (hCAP-18)/LL-37 expression by TAMs that, by interacting with the formyl peptide receptor 2 (FPR2) and the P2X(7) purinergic receptor (P2X7R) expressed by pancreatic CSCs, enhanced their stemness [46]. In agreement, pharmacological inhibition of the G-protein coupled receptors (FPR2, P2X7R) impaired tumor growth and malignant progression in preclinical models of PDAC [46].
Cancer cells that have passed through an EMT gain mesenchymal traits, acquire motility, invasiveness, and increased resistance to apoptosis. Accordingly, in mouse models of breast and pancreatic cancer, EMT endows metastatic cancer cells with resistance to chemotherapeutic drugs, by inducing genes encoding for drug-metabolizing enzymes and drug-transporter proteins. Similarly to macrophages in wound healing, TAMs support EMT through several mechanisms, involving both release of soluble mediators (e.g., ISG15, LL-37, TGF-β) and direct cell-to-cell interactions [47]. In particular, the immunoregulatory peptide TGF-β1 plays a crucial role in the promotion of keratinocyte EMT during re-epithelialization of cutaneous wounds [48], as well as in the induction of mesenchymal and stem-like features of breast cancer cells [49]. Through the promotion of both EMT and stemness, TGF-β1 is a crucial orchestrator of tumor spread and metastasis formation. While stromal cells are the major source of TGF-β1 in the primary tumor, breast CSCs autonomously produce TGF-β1 to support their seeding in distant organs where they give rise to metastasis [50].
Quantitative proteomic profiling indicated that EMT upregulates the expression of CD90 and Ephrin type-A receptor 4 (EphA4), which mediate the physical interactions of CSCs with TAMs by binding their respective counter-receptors on these cells. This EphA4 ligand-receptor juxtacrine signaling activates the NF-κB p50 subunit, which then enters the nucleus and cooperates with Twist to induce a variety of inflammatory mediators such as IL-8, IL-6 and Granulocyte macrophage colony-stimulating factor (GM-CSF) that sustain the stem cell state [47].
Chemotactic molecules play a key role during wound healing and have been also identified as supporters of cancer cell stemness. In particular, IL-8 is abundantly produced at the site of injury and is involved in the recruitment of inflammatory cells that start the initial phases of tissue repair [51]. CXCR1/2 receptor engagement by IL-8 triggers both the self-renewal and activity of breast CSCs [52] and treatment of various breast cancer cell lines with IL-8 induced the expansion of the CSC pool. This effect was also confirmed in primary samples isolated from either invasive breast tumors or metastatic cells from ascites and pleural effusions [52]. Of note, Ginestier and colleagues demonstrated that CXCR1 promotes breast CSC self-renewal and survival through the activation of the Focal adhesion kinase (FAK)/AKT/Wnt pathway and the inhibition of the transcription factor Forkhead box O3A (FOXO3A) [53]. Intriguingly, although the CXCR1+ cells accounted for less than 1% of the total population, the CXCR1/2 inhibitor Repertaxin reduced the entire breast CSC population, by inducing cell death in CXCR1− cells via a FASL/FAS-dependent bystander effect [53]. Engagement of CXCR1/2 in breast CSCs was also found to induce the EGFR/HER2 transduction pathway leading to the activation of AKT and extracellular signal-regulated kinases 1/2 (ERK1/2) signaling pathways, which are critical regulators of breast CSC activity [52]. Consistently, inhibition of the CXCR1/2 signaling significantly improved the efficacy of HER2 blocking that leads to the abrogation of the IL-8-promoting effect on CSCs in HER2 positive cancers [52]. In human clear cell renal cell carcinoma (ccRCC) distribution of CD44+ cancer stem cells was significantly associated with a high density of CD163+ TAMs and supported through the TNF-α production by macrophages [54]. Depending on the type of tumors, the determinants of TAM/CSC cross-talk may be different, supporting the concept that heterogeneous mechanisms converge to create the TAM/CSC niche. In a spontaneous murine model of melanoma (RETAAD mice), TAMs increased survival and proliferation of CD34− CSCs, through TGF-β and arginase I-dependent polyamine production [55]. Inhibition of TGF-β signaling, or myeloid-specific ablation of Arginase I, resulted in impaired TAM-induced melanospheres in vitro [55].
Chemotherapy remains the standard of care for many types of cancer, but such treatment can select for more aggressive TIC clones. In a model of spontaneous melanoma, during temozolamide-based chemotherapy, arginase-expressing TAMs stimulate sphere formation in CD34− TICs [55]. Interestingly, in tumor cells from post-surgical resection, macrophages stimulate tumor sphere formation, suggesting that depletion of macrophages can influence tumor growth by eliminating the source of polyamines and proposing their elimination as an adjunct strategy to surgical resection [56]. Similarly to wound healing, emerging evidence suggests a bidirectional interaction between CSCs and TAMs. In glioblastoma multiforme, CD133+CD15+ glioma stem cells secrete copious amounts of Periostin (POSTN) that recruit CCR2+CX3CR1− monocytes in αVβ3 integrin-dependent manner [57]. Accordingly, blocking αVβ3 integrin or silencing POSTN in glioma stem cells inhibits TAM accumulation in vivo, reducing tumor growth and increasing survival of mice bearing CD133+CD15+-derived xenografts [57]. CSCs also affect TAM activities. In pancreatic cancer models, STAT3+ PDAC CSCs can skew TAM polarization toward the M2 phenotype [45], which subsequently inhibits anti-tumor CD8+ T-lymphocyte responses [34]. New evidence obtained in human cholangiocarcinoma (CCA) indicates that TAMs are selectively educated by CCA CSCs through IL-13, IL-34 and osteoactivin (OA), [58]. These CCA CSC-secreted molecules induce TAMs to express a peculiar M1/M2 profile, in association with high invasion and adhesion capacity [58]. The CD47 “Don’t Eat Me” signal is exploited by CSCs of different type of cancers, to avoid phagocytosis by TAMs. Interestingly, in vitro neutralization of CD47 allows macrophages to phagocytose CSCs, while in vivo administration of anti-CD47 neutralizing antibodies, alone [59] or in combination with chemotherapeutic drugs [60], significantly inhibited primary tumor growth.
Myeloid-derived suppressor cells
Similarly to TAMs, MDSCs establish a bidirectional interaction with CSCs. As a prototypical example, human pancreatic cancer cells trigger healthy CD14+ peripheral blood monocytes to adopt a monocytic-MDSC (M-MDSC) phenotype characterized by low expression of HLA-DR and acquisition of immunosuppressive activities. Conversely, M-MDSCs promote expansion of CSCs and acquisition of mesenchymal features [61]. This tight MDSC/CSC association is also present in G-CSFR−/− tumor-bearing mice, that show a reduced number of tumor-infiltrating MDSCs, in association with a reduced percentage of ALDH1Bright CSCs and expression of EMT markers [61]. Of relevance, either in vitro generated or tumor isolated M-MDSCs show increased levels of p-STAT3, that is crucial for both immunosuppressive properties [62] and promotion of cancer cell stemness [61]. New evidence in breast cancer patients indicates that the level of tumor-infiltrating CD33+ MDSCs is associated with the number of ALDH+ CSCs and correlates with poor outcome [63]. Along with their immunosuppressive activities, MDSCs isolated from immunodeficient NOD scid gamma (NSG) mice transplanted with a human breast cancer cell line (MCF7), displayed suppressive activity and the capacity to support cancer cell stemness, both in vitro and in vivo [63]. Mechanistically, in breast cancer cells, nitric oxide (NO) produced by MDSCs activates the NOTCH signaling, which supported a long-lasting activation of STAT3 [63]. The cooperation between STAT3 and NOTCH, which affects patient outcome, is required for the promotion of breast cancer stemness and tumor growth, and is halted by combination of anti-IL6 mAb and inducible NO synthetase (iNOS) inhibitors [63]. Although with a different mechanism, MDSCs isolated from human ovarian cancer also affect primary tumor cell phenotype by enhancing the formation of tumor spheres, the expression of stemness-associated gene transcripts (e.g. OCT3/4, SOX2, NANOG, NOTCH2/3) and the expansion of ALDH+ CSC population [64]. Accordingly, when MDSC-conditioned primary ovarian cancer cells were transplanted into NSG mice, they engrafted better and gave rise to more metastatic foci [64]. By studying the molecular mechanisms underlying MDSC-CSC interplay, Cui and co-workers found that MDSCs stimulate ovarian cancer cells to express microRNA101, that in turn promotes cancer stemness by targeting the corepressor C-terminal-binding protein 2 (CtBP2) [64]. By recruiting histone deacetylases, methylases, and demethylases, CtBP2 inhibits the transcription of stem cell core genes [64]. In agreement, a high number of MDSCs, elevated microRNA101 expression levels, and low CtBP2 amounts correlate with worse clinical outcome in human ovarian cancer [64].
MDSCs finely tune tumor senescence by promoting cellular stemness. In genetic models of prostate (specific ablation of phosphatase and tensin homolog, PTEN in prostate epithelium) and lung (Ki-rasG12/V) cancers, infiltration of CD11b+Gr1+ MDSCs protected a fraction of proliferating tumor cells from senescence, thus sustaining tumor growth [65]. By producing Interleukin-1 receptor antagonist (IL-1Ra), MDSCs blocked the Interleukin-1α (IL-1α)-IL-1R axis and antagonized cancer cell senescence driven by loss of the tumor suppressor gene PTEN [65]. Accordingly, either impairment of MDSC tumor infiltration by CXCR2 blocking or adoptive transfer of IL-1Ra−/− myeloid cells, enhanced the senescence of prostate cancer cells and improved the efficacy of chemotherapy [65]. Accordingly, tumor-infiltrating CD33+ myeloid cells inversely correlate with p16INK4A1 senescent cells in human prostate cancer specimens, and high levels of intra-tumor IL- 1Ra predict poor response to chemotherapy and unfavorable patient outcome [65]. In glioblastoma, CSCs play a crucial role in the promotion of an immunosuppressive tumor microenvironment. Glioblastoma stem cells were shown to promote an immunosuppressive tumor microenvironment, by favoring high T regulatory/cytotoxic T-cell ratio [66] and M2-TAM accumulation [57]. Further, new studies highlighted that CSCs isolated from glioblastoma patients also promote MDSC survival and immunosuppressive activities [67]. In comparison to non-stem tumor cells, glioblastoma CSCs produce very high levels of macrophage migration inhibitory factor (MIF) [67], an immunosuppressive factor expressed in several types of cancer. Accordingly, in a syngeneic mouse model of glioma, depletion of MDSCs by low dose 5-flurouracil treatment or inhibition of MIF production by siRNA increased the cytotoxic T-cell response in association with reduced tumor growth [67]. In the brain of glioblastoma-bearing patients, the observed vicinity of CD33+HLA-DR− MDSCs and CD133+ or SOX2+ CSCs is predictive of poor clinical patient outcome, hence underscoring the pathological importance of MIF-mediated CSC-MDSC cross-talk [67].
Concluding remarks
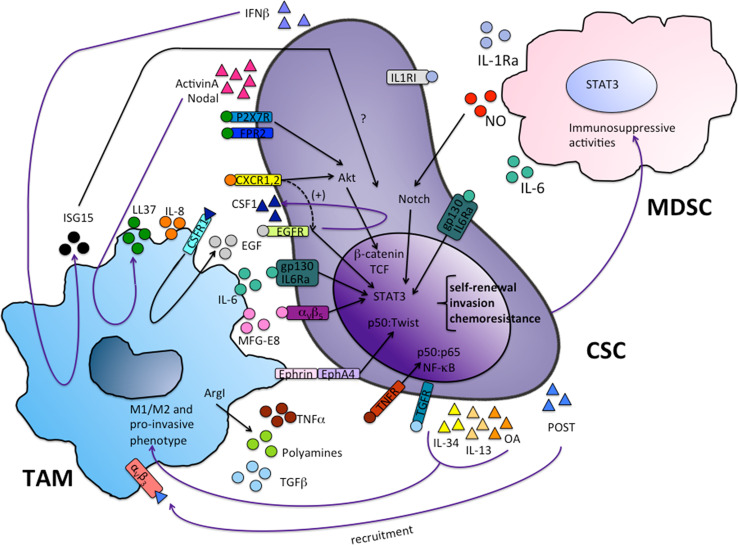
Understanding the cross-talk between the inflammatory microenvironment and CSCs can reveal evolutionary mechanisms that support tumor growth, treatment resistance and disease relapse. M1 and M2 polarized programs, expressed by myeloid cells, control, respectively, the onset and the resolution of the inflammatory response. This myeloid cell plasticity appears as a determinant player in cancer progression and a key functional determinant in their bidirectional interaction with CSCs. As key components of the tumor microenvironment, TAMs and MDSCs play a primary role in orchestrating cancer-associated inflammation and, through this, cancer cell stemness (Fig. 2). Recent studies have clarified ontogeny, differentiation pathways and functions of TAMs and MDSCs and revealed key factors driving their recruitment, expansion and activation during tumor growth [26, 62, 68]. Tumors promote a dynamic expansion of these populations by altering normal differentiation and maturation of hematopoietic precursors [69] and by eliciting microenvironmental signals able to reprogram myeloid cells towards a tumor-promoting mode [25]. This multistep pathway of altered myeloid cell differentiation has been partially dissected and reprogramming of TAM and MDSC functions has been pharmacologically pursued in both preclinical and clinical settings [70]. Inhibition of pro-tumor myeloid (TAM and MDSC) mechanisms has provided significant therapeutic benefits in preclinical models [26, 62, 71] and drugs targeting the TME are currently under investigation in clinical trials [72]. Inherent and acquired resistance to current cytotoxic therapies fail to eradicate CSC clones, thereby resulting in treatment resistance and subsequent relapse in these patients. In a therapeutic perspective, pharmacological reprogramming of myeloid cells may provide a useful unexplored strategy to prevent the two-way interaction between TAM/MDSC and neoplastic cells and to elude cancer cell stemness.
Fig. 2.
Bidirectional interaction between TAM/MDSC and cancer stem cells. TAMs and MDSCs secrete cytokines (IL-6, IL-8, TNF-α, TGF-β, IL1ra), inflammatory mediators (ISG15, LL37, NO), growth factors (MFG-E8, EGF, polyamines) which activate different signaling pathways converging on the transcription factors STAT3, NF-κB, Twist, β-catenin. These transcription factors induce a gene program supporting cancer cell stemness. In turn, CSCs produce factors that promote myeloid cell recruitment (CSF1, POST) and activation (IL-34, IL-13, OA, IFNβ, Activin A, Nodal)
Acknowledgements
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC), project #15585, #14032 Italy; Fondazione Cariplo, Italy; Ministero Università Ricerca (MIUR), Italy, (Grant Numbers: RBAU01PTYW; RBNE01XHB2_002; RBAP11H2R9_005); Ministero della Salute (GR-2011-02349580); Istituto Oncologico Veneto 5x1000 grant.
Abbreviations
- ALDH
Aldehyde dehydrogenases
- CCR2
C–C chemokine receptor type 2
- CSCs
Cancer stem cells
- CtBP2
C-terminal-binding protein 2
- EMT
Epithelial–mesenchymal transition
- EOC
Epithelial ovarian cancer
- HCC
Hepatocellular carcinoma
- HIF-1α
Hypoxia-inducible factor-1α
- IL-1Ra
Interleukin-1 receptor antagonist
- ISG15
Interferon-stimulated gene 15
- MDR1/2
Multidrug resistance protein 1/2
- MDSCs
Myeloid-derived suppressor cells
- MFG-E8
Milk fat globule-epidermal growth factor-VIII
- MSCs
Mesenchymal stem cells
- OXPHOS
Oxidative phosphorylation
- PDAC
Pancreatic ductal adenocarcinoma
- ROS
Reactive oxygen species
- SCF
Stem cell factor
- TAF
Tumor-associated fibroblast
- TAMs
Tumor-associated macrophages
- TIC
Tumor-initiating cell
- TME
Tumor microenvironment
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the conference Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application which was hosted by the Wistar Institute in Philadelphia, PA, USA, 16th–19th June, 2016. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews.
Antonio Sica, Chiara Porta, Alberto Amadori, Anna Pastò have equally contributed to the work.
References
- 1.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton K, Banerjee D, Glod J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS ONE. 2012;7(4):e35036. doi: 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 6.Shiozawa Y, Nie B, Pienta KJ, Morgan TM, Taichman RS. Cancer stem cells and their role in metastasis. Pharmacol Ther. 2013;138(2):285–293. doi: 10.1016/j.pharmthera.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra GK, Zhao X, Band H, Band V. Shared signaling pathways in normal and breast cancer stem cells. J Carcinog. 2011;10:38. doi: 10.4103/1477-3163.91413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasto A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E, Nicoletto MO, Manicone M, Indraccolo S, Amadori A. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5(12):4305–4319. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial–mesenchymal transdifferentiation. Cancer Res. 2010;70(24):10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 11.Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, Cuartas I, Raventos C, Martinez-Ricarte F, Poca MA, Garcia-Dorado D, Lahn MM, Yingling JM, Rodon J, Sahuquillo J, Baselga J, Seoane J. TGF-beta receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18(6):655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9):1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh CH, Shyu WC, Chiang CY, Kuo JW, Shen WC, Liu RS. NADPH oxidase subunit 4-mediated reactive oxygen species contribute to cycling hypoxia-promoted tumor progression in glioblastoma multiforme. PLoS ONE. 2011;6(9):e23945. doi: 10.1371/journal.pone.0023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaylock RL. Cancer microenvironment, inflammation and cancer stem cells: a hypothesis for a paradigm change and new targets in cancer control. Surg Neurol Int. 2015;6:92. doi: 10.4103/2152-7806.157890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial–mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 17.Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283(25):17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatrai S, van Schelven SJ, Ubink I, Govaert KM, Raats D, Koster J, Verheem A, Borel Rinkes IH, Kranenburg O. Maintenance of clonogenic KIT(+) human colon tumor cells requires secretion of stem cell factor by differentiated tumor cells. Gastroenterology. 2015;149(3):692–704. doi: 10.1053/j.gastro.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Schilder RJ, Sill MW, Lee RB, Shaw TJ, Senterman MK, Klein-Szanto AJ, Miner Z, Vanderhyden BC. Phase II evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26(20):3418–3425. doi: 10.1200/JCO.2007.14.3420. [DOI] [PubMed] [Google Scholar]
- 20.Matassa DS, Amoroso MR, Lu H, Avolio R, Arzeni D, Procaccini C, Faicchia D, Maddalena F, Simeon V, Agliarulo I, Zanini E, Mazzoccoli C, Recchi C, Stronach E, Marone G, Gabra H, Matarese G, Landriscina M, Esposito F. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016;23(9):1542–1554. doi: 10.1038/cdd.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 22.Belli C, Piemonti L, D’Incalci M, Zucchetti M, Porcu L, Cappio S, Doglioni C, Allavena P, Ceraulo D, Maggiora P, Dugnani E, Cangi MG, Garassini G, Reni M. Phase II trial of salvage therapy with trabectedin in metastatic pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2016;77(3):477–484. doi: 10.1007/s00280-015-2932-3. [DOI] [PubMed] [Google Scholar]
- 23.Nishio N, Fujita M, Tanaka Y, Maki H, Zhang R, Hirosawa T, Demachi-Okamura A, Uemura Y, Taguchi O, Takahashi Y, Kojima S, Kuzushima K. Zoledronate sensitizes neuroblastoma-derived tumor-initiating cells to cytolysis mediated by human gammadelta T cells. J Immunother. 2012;35(8):598–606. doi: 10.1097/CJI.0b013e31826a745a. [DOI] [PubMed] [Google Scholar]
- 24.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. 2016;35(6):671–682. doi: 10.1038/onc.2015.132. [DOI] [PubMed] [Google Scholar]
- 28.Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14(15):2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 29.Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18(17):4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 31.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Won C, Kim BH, Yi EH, Choi KJ, Kim EK, Jeong JM, Lee JH, Jang JJ, Yoon JH, Jeong WI, Park IC, Kim TW, Bae SS, Factor VM, Ma S, Thorgeirsson SS, Lee YH, Ye SK. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62(4):1160–1173. doi: 10.1002/hep.27968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA. 2011;108(30):12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward R, Sims AH, Lee A, Lo C, Wynne L, Yusuf H, Gregson H, Lisanti MP, Sotgia F, Landberg G, Lamb R. Monocytes and macrophages, implications for breast cancer migration and stem cell-like activity and treatment. Oncotarget. 2015;6(16):14687–14699. doi: 10.18632/oncotarget.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfe AR, Trenton NJ, Debeb BG, Larson R, Ruffell B, Chu K, Hittelman W, Diehl M, Reuben JM, Ueno NT, Woodward WA. Mesenchymal stem cells and macrophages interact through IL-6 to promote inflammatory breast cancer in pre-clinical models. Oncotarget. 2016;7(50):82482–82492. doi: 10.18632/oncotarget.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31(2):248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 39.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10(1):39–49. doi: 10.1016/S1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 40.Lang R. Tuning of macrophage responses by Stat3-inducing cytokines: molecular mechanisms and consequences in infection. Immunobiology. 2005;210(2–4):63–76. doi: 10.1016/j.imbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164(2):762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 42.Kang YJ, Yang SJ, Park G, Cho B, Min CK, Kim TY, Lee JS, Oh IH. A novel function of interleukin-10 promoting self-renewal of hematopoietic stem cells. Stem Cells. 2007;25(7):1814–1822. doi: 10.1634/stemcells.2007-0002. [DOI] [PubMed] [Google Scholar]
- 43.Tuccitto A, Tazzari M, Beretta V, Rini F, Miranda C, Greco A, Santinami M, Patuzzo R, Vergani B, Villa A, Manenti G, Cleris L, Giardiello D, Alison M, Rivoltini L, Castelli C, Perego M. Immunomodulatory factors control the fate of melanoma tumor initiating cells. Stem Cells. 2016;34(10):2449–2460. doi: 10.1002/stem.2413. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Wang X, Wang J, Liu D, Wang Y, Huang Z, Tan H. Hypoxia-induced secretion of IL-10 from adipose-derived mesenchymal stem cell promotes growth and cancer stem cell properties of Burkitt lymphoma. Tumour Biol. 2016;37(6):7835–7842. doi: 10.1007/s13277-015-4664-8. [DOI] [PubMed] [Google Scholar]
- 45.Sainz B, Jr, Martin B, Tatari M, Heeschen C, Guerra S. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Cancer Res. 2014;74(24):7309–7320. doi: 10.1158/0008-5472.CAN-14-1354. [DOI] [PubMed] [Google Scholar]
- 46.Sainz B, Jr, Alcala S, Garcia E, Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo I, Hidalgo M, Gomez-Lopez G, Canamero M, Erkan M, Kleeff J, Garcia-Silva S, Sancho P, Hermann PC, Heeschen C. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64(12):1921–1935. doi: 10.1136/gutjnl-2014-308935. [DOI] [PubMed] [Google Scholar]
- 47.Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, Weinberg RA. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16(11):1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber CE, Li NY, Wai PY, Kuo PC. Epithelial-mesenchymal transition, TGF-beta, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res. 2012;33(3):311–318. doi: 10.1097/BCR.0b013e318240541e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fazilaty H, Gardaneh M, Bahrami T, Salmaninejad A, Behnam B. Crosstalk between breast cancer stem cells and metastatic niche: Emerging molecular metastasis pathway? Tumour Biol. 2013;34(4):2019–2030. doi: 10.1007/s13277-013-0831-y. [DOI] [PubMed] [Google Scholar]
- 51.Rennekampff HO, Hansbrough JF, Kiessig V, Dore C, Sticherling M, Schroder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res. 2000;93(1):41–54. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]
- 52.Singh JK, Farnie G, Bundred NJ, Simoes BM, Shergill A, Landberg G, Howell SJ, Clarke RB. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19(3):643–656. doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120(2):485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma C, Komohara Y, Ohnishi K, Shimoji T, Kuwahara N, Sakumura Y, Matsuishi K, Fujiwara Y, Motoshima T, Takahashi W, Yamada S, Kitada S, Fujimoto N, Nakayama T, Eto M, Takeya M. Infiltration of tumor-associated macrophages is involved in CD44 expression in clear cell renal cell carcinoma. Cancer Sci. 2016;107(5):700–707. doi: 10.1111/cas.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tham M, Tan KW, Keeble J, Wang X, Hubert S, Barron L, Tan NS, Kato M, Prevost-Blondel A, Angeli V, Abastado JP. Melanoma-initiating cells exploit M2 macrophage TGFbeta and arginase pathway for survival and proliferation. Oncotarget. 2014;5(23):12027–12042. doi: 10.18632/oncotarget.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tham M, Khoo K, Yeo KP, Kato M, Prevost-Blondel A, Angeli V, Abastado JP. Macrophage depletion reduces postsurgical tumor recurrence and metastatic growth in a spontaneous murine model of melanoma. Oncotarget. 2015;6(26):22857–22868. doi: 10.18632/oncotarget.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, Chiorino G, Forti E, Glaser S, Alpini G, Destro A, Sozio F, Di Tommaso L, Roncalli M, Banales JM, Coulouarn C, Bujanda L, Torzilli G, Invernizzi P. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66(1):102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, Tong M, Lo J, Ng IO. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60(1):179–191. doi: 10.1002/hep.27070. [DOI] [PubMed] [Google Scholar]
- 60.Cioffi M, Trabulo S, Hidalgo M, Costello E, Greenhalf W, Erkan M, Kleeff J, Sainz B, Jr, Heeschen C. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res. 2015;21(10):2325–2337. doi: 10.1158/1078-0432.CCR-14-1399. [DOI] [PubMed] [Google Scholar]
- 61.Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, Mukherjee P, Wang-Gillam A, Link DC, Denardo DG, Goedegebuure SP, Linehan DC. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63(5):513–528. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, Wan S, Wei S, Wang Y, Liu Y, Staroslawska E, Szubstarski F, Rolinski J, Grywalska E, Stanislawek A, Polkowski W, Kurylcio A, Kleer C, Chang AE, Wicha M, Sabel M, Zou W, Kryczek I. Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 2016;76(11):3156–3165. doi: 10.1158/0008-5472.CAN-15-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, Kotarski J, Tarkowski R, Wicha M, Cho K, Giordano T, Liu R, Zou W. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39(3):611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, D’Antuono R, Montani E, Garcia-Escudero R, Guccini I, Da Silva-Alvarez S, Collado M, Eisenberger M, Zhang Z, Catapano C, Grassi F, Alimonti A. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515(7525):134–137. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 66.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Priebe W, Sawaya R, Lang FF, Heimberger AB. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9(1):67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otvos B, Silver DJ, Mulkearns-Hubert EE, Alvarado AG, Turaga SM, Sorensen MD, Rayman P, Flavahan WA, Hale JS, Stoltz K, Sinyuk M, Wu Q, Jarrar A, Kim SH, Fox PL, Nakano I, Rich JN, Ransohoff RM, Finke J, Kristensen BW, Vogelbaum MA, Lathia JD. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells. 2016;34(8):2026–2039. doi: 10.1002/stem.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strauss L, Sangaletti S, Consonni FM, Szebeni G, Morlacchi S, Totaro MG, Porta C, Anselmo A, Tartari S, Doni A, Zitelli F, Tripodo C, Colombo MP, Sica A. RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell. 2015;28(2):253–269. doi: 10.1016/j.ccell.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212(4):435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buque A, Bloy N, Aranda F, Cremer I, Eggermont A, Fridman WH, Fucikova J, Galon J, Spisek R, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch-small molecules targeting the immunological tumor microenvironment for cancer therapy. Oncoimmunology. 2016;5(6):e1149674. doi: 10.1080/2162402X.2016.1149674. [DOI] [PMC free article] [PubMed] [Google Scholar]