Abstract
Alterations in the MHC class I surface antigens represent one mechanism of tumor cells to escape from natural or immunotherapy-induced antitumor immune responses. In order to restore MHC class I expression, knowledge about the underlying molecular mechanisms of MHC class I defects in different tumor types is required. In most cases, abnormalities of MHC class I expression are reversible by cytokines suggesting a deregulation rather than structural abnormalities of members of the antigen-processing and presentation machinery (APM). The impaired expression of APM components could be controlled at the epigenetic, transcriptional and/or posttranscriptional level. Furthermore, a direct link between altered transcription factor binding, interferon signal transduction and MHC class I APM component expression has been shown, which might be further associated with cell cycle progression. This information will not only give novel insights into the (patho) physiology of the antigen-processing and presenting pathway, but will help in the future to design effective T cell-based immunotherapies.
Keywords: MHC class I antigens, Antigen processing, Tumors, Gene regulation, Signal transduction
Tumors represent heterogeneous diseases with a variable clinical cause, but a high frequency of recurrences. Although a number of ongoing clinical trials provide promise for the implementation of immunotherapies for the treatment of cancer patients and a number of different immunotherapeutic strategies alone or in combination with irradiation or chemotherapy have been explored, the general efficacies of these therapies are not as potent as expected. This might be due to changes in the tumor host interaction, which is mediated by professional antigen-presenting cells (APC), such as B cells and dendritic cells (DC), effector cells like T, NK and NKT cells, tumor stroma and endothelium as well as immune suppressive cells including regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC). The tumor microenvironment may provide protection of tumors against potent T cell responses by increasing for example the frequency of Treg and MDSC [1, 2].
In general, there exists interplay between immune and tumor cells, which profoundly influences each other. An association between host immune responses and prognosis has been described in a variety of tumor types, which is also influenced by the tumor microenvironment, consisting of a heterogeneous mix of cellular and non-cellular components. The immune cells are able to recognize tumor cells, thereby leading to their apoptosis and tumor rejection. This activity is mainly mediated by T cells, NK cells as well as macrophages. During disease progression, host immune responses have been biased against antitumor immune responses. Tumors could not be recognized by immune cells resulting in tumor cell proliferation and in the induction of an immune suppressive microenvironment, which negatively interferes with the systemic and local adaptive immune responses. Lastly, this could lead to the induction of immune escape variants.
Different immune escape strategies of tumors
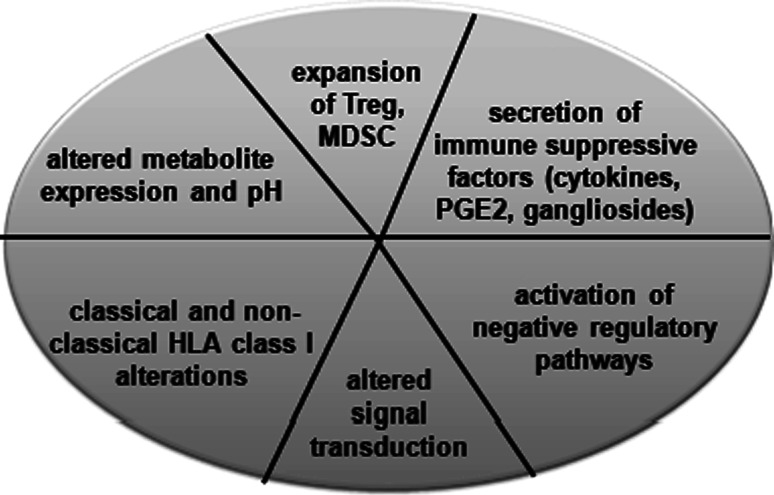
There exist different strategies how tumors evade the immune system. These include defects in the elucidation or maintenance of an effective antitumor responses such as insufficient antigen processing by DC, poor recruitment of or impaired activation of effector cells, the secretion of immune suppressive factors, like cytokines, prostaglandine and growth factors, the expression of molecules of negative regulatory pathways, such as B7-H1, B7-H3 and B7-H4 and the non-classical HLA-G antigen, increased frequency of Treg and MDSC, altered metabolite expression, modulation of the pH as well as downregulation or lack of the expression of classical HLA class I molecules and signal transduction molecules (Fig. 1). Although there exists evidence for improved responses at earlier disease stages, not only activated effector CD8+ T cells, but also tumor antigen (TA)-specific T cells could be induced. For the antitumoral T cell responses, the interaction of the T cell receptors with their specific HLA class I complex is required. Therefore, the HLA class I antigen-processing machinery (APM) plays a crucial role in mediating immune responses by the generation and expression of the trimeric HLA class I, β2-microglobuline (β2-m) and peptide complex. The APM pathway consists of four major steps. The first step involves the peptide generation and peptide trimming, which is mainly mediated by the proteasome and cytosolic peptidases. Endogeneously synthesized proteins are ubiquitinated and then degraded by the multi-catalytic proteasome complex consisting of the constitutive and interferon (IFN)-γ-inducible subunits, the low molecular weight proteins (LMP)2, -7 and -10. The proteasome yields peptides with a correct C-terminus and an extended N-terminus, which is further trimmed by cytosolic peptidases. Then, the peptides are transported in an ATP- and sequence-dependent manner from the cytosol into the endoplasmic reticulum (ER). In the ER the MHC class I molecules are assembled, which is assisted by various chaperones, such as calnexin, calreticulin and in particular tapasin, which facilitates peptide loading onto MHC class I molecules [3]. This trimeric complex is then transported via the trans-Golgi to the cell surface and there exposed to CD8+ cytotoxic T lymphocytes (CTL).
Fig. 1.
Different immune escape mechanisms of tumors. Tumors exert different strategies to escape immune cell surveillance. In addition, the frequency and function of immune cells are altered by the tumor microenvironment, which also results in an impaired anti tumor immune response
Alterations in MHC class I antigens and APM components in tumors
It has been shown by many different groups including ours that MHC class I abnormalities could occur at a relatively high frequency in many solid and hematopoietic tumors [4–6]. A total MHC class I antigen loss, MHC class I downregulation, a selective loss or downregulation of MHC class I allospecificities have been described, which are further associated with disease progression and reduced patients’ survival (Table 1) [7–9]. This is often accompanied by an impaired expression of a single or various APM component(s) [10–12]. For example, an altered expression of LMP2, LMP7, TAP1 and β2-m was found in renal cell carcinoma (RCC), prostate cancers and melanoma when compared to adjacent normal tissues [13–15]. The loss of these APM components was associated with a disease progression and/or an early disease recurrence of patients [16]. In addition, MHC class I APM component expression appears to be tissue- and tumor subtype-specific regulated and differentially expressed during differentiation process and cell cycle progression.
Table 1.
HLA class I abnormalities in human tumors
| Highly variable frequency in many tumors |
| Total HLA class I antigen loss (9–52%) |
| HLA class I downregulation (3–20%) |
| Selective loss or downregulation of HLA class I allospecificities (15–50%) |
| Association with disease progression |
| Tissue and/or tumor subtype specificity |
| Differentiation/cell cycle dependence |
Molecular mechanisms of APM downregulation
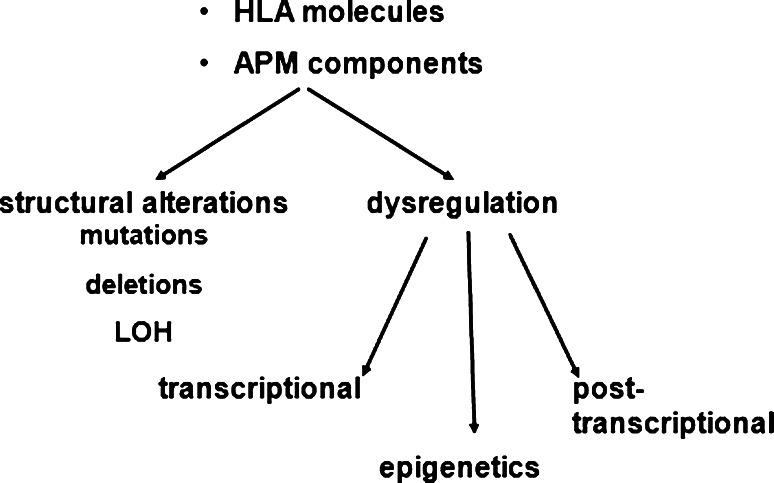
In order to understand the molecular mechanisms of MHC class I loss or downregulation, structural alterations and control mechanisms involved in the deregulation of diverse APM components were determined (Fig. 2) [7–9]. Structural abnormalities of APM components represent a relatively rare event and appear to be tumor specific, for example mutations, deletion and/or loss of heterozygosity of β2-m and the HLA class I heavy chains (HC) have been mainly described in colorectal carcinoma and melanoma, but not in RCC [17–24]. Mutations in TAP have only been found in two melanoma cell lines, in non-small lung carcinoma and leiomyosarcomas, whereas mutations in tapasin and LMPs were detected in neuroblastoma (B. S. unpublished data) [13, 25, 26]. The defective APM component expression could be restored by gene transfer resulting in increased MHC class I surface antigen levels, enhanced antitumoral responses and survival [27, 28]. Thus, the major molecular mechanisms of impaired MHC class I APM component expression appear to be caused by their de-regulation as already demonstrated for virus-transformed cells [29–33]. In addition, cytokine-mediated upregulation of MHC class I APM components further strengthened their deregulation [13, 34, 35]. This could occur either at the epigenetic expression, transcriptional and/or posttranscriptional level. Methylation and histone modifications of APM components appear to be a rare event with a frequency comparable to that of mutations and have been only described for TAP1, β2-m and HLA class I HC [36–39]. In contrast, transcriptional and/or posttranscriptional control of all major APM components was detected in tumors of distinct histology. Although in most cases APM-specific mRNA and protein were coordinately downregulated, some tumor cells expressed high levels of TAP, LMP, tapasin and ERAP mRNA, but lacked respective protein expression [13, 14]. Interestingly, a posttranscriptional downregulation of TAP2 in cells with TAP1 loss due to a mutation in TAP1 leading to an earlier stop codon was reported [13]. It was speculated that the mutation-mediated lack of TAP1 expression directly destabilises TAP2 protein. In order to prove this hypothesis, cells were left untreated or treated with different proteasome inhibitors like epoxomycin and MG-132. An increased ubiquitination and degradation of TAP2 in the absence of the proteasome inhibitors were found. These data were further confirmed using shRNA plasmids for TAP1 demonstrating a concordant TAP2 downregulation by TAP1 silencing resulting in reduced TAP function and downregulated MHC class I surface expression.
Fig. 2.
Molecular mechanisms of MHC class I APM downregulation. The loss or downregulated expression of APM components could occur at the distinct levels of the MHC class I APM pathway and involves transcriptional, posttranscriptional and epigenetic processes resulting in a deregulated expression, whereas structural alterations like mutations, deletions and LOH are rare events
In order to determine the transcriptional activity of MHC class I APM components, the various APM promoters were cloned into a luciferase (luc) expression vector and the APM promoter activity was determined upon transient transfection of the APM-luc constructs into different human tumor cells. In parallel, the mRNA and protein expression of the distinct components was analyzed. Using this approach, a transcriptional as well as posttranscriptional regulation in particular of the LMP subunits, TAP1/2 and tapasin, was observed in melanoma cells. So far, it is not solved, which transcription factors negatively or positively interfere with the APM component expression in tumors. Detailed analysis of the transcription factor binding sites (TFBS) using APM promoter constructs in which the TFBS were altered by site directed mutagenesis will give information about the factors involved in the tumor-mediated suppression of APM components.
Novel insights into MHC class I APM component regulation
By performing cDNA arrays of TAP1+ transfectants and TAP1− counterparts, an altered expression pattern of genes involved in the IFN signal transduction pathway as well as in the cellular metabolism was detected (B. S. unpublished data). Therefore, a link between the IFN signaling and/or metabolism with proper APM component expression was postulated. So far, there exists no information whether the altered energy metabolism affects APM components, which might be an interesting topic to investigate. In order to test the association of IFN signaling with APM components, human melanoma cell lines were screened for the loss of the IFN-γ inducibility of MHC surface antigens [40]. Indeed, some melanoma cells lack IFN-γ inducibility of MHC molecules. To characterize the mechanisms of IFN resistance, the expression and function of diverse IFN-γ signal transduction pathway components was analyzed. Upon IFN-γ-binding to its specific receptors, a number of proteins were induced including the janus kinase (JAK)1, JAK2 and the signal transducer and activation (STAT)1. A loss of IFN-γ, but not of IFN-α and tumor necrosis factor (TNF)-α inducibility of MHC class I antigens, was found in melanoma cells, suggesting that the lack of IFN-γ inducibility of MHC class I molecules was not due to defects in APM component expression. The IFN-γ resistance in these melanoma cells was caused by a deletion of the JAK2 gene on chromosome 9, whereas upstream and downstream genes were still present and expressed. The loss of JAK2 not only resulted in the lack of JAK2 mRNA and protein expression, but was also directly associated with a downregulation of constitutive APM component expression as determined for TAP1, TAP2, tapasin as well as β2-m [35]. JAK2 overexpression into the JAK2-deficient melanoma cells upregulated MHC class I surface expression as well as basal APM component expression and also restored their IFN-γ inducibility. Vice versa treatment of cells with JAK2 inhibitors as well as with JAK2-specific shRNA resulted in a downregulation or loss of HLA class I antigens as well as APM component expression.
In vitro models of oncogenic transformation as suitable tools for analyzing the mechanisms of deficient APM component expression
In order to characterize the molecular mechanisms of transcriptional regulation of APM components and MHC class I abnormalities, oncogene-transformed cells served as models. Downregulation of MHC class I surface antigens was found upon mos-, myc- and ras- as well as HER-2/neu-mediated transformation of murine and/or human cells, which is accompanied by an impaired APM component expression [41–45]. However, MHC class I surface expression could be restored by IFN-γ treatment in these cases. The mechanisms of this oncogene-mediated downregulation could be due to a dysregulation mediated by specific factors and/or repressors modulating the APM component expression. HER-2/neu belongs to the epidermal growth factor (EGF) receptor family (HER-1, -2, -3, -4), has receptor tyrosine kinase activity and represents the preferred dimer partner of the different HERs. So far, no soluble ligands for HER-2/neu have been identified. HER-2/neu is physiologically expressed in epithelial cells, whereas gene amplification and/or overexpression of HER-2/neu occurs in solid tumors, such as breast, lung and colon carcinoma and is often associated with disease progression, poor patients’ outcome and impaired cell recognition [46]. The HER-2/neu-mediated transformation of murine fibroblasts not only caused an enhanced proliferation, development of foci, anchorage-independent cell growth and tumor formation, but also suppressed MHC class I surface antigens, which is mediated by a transcriptional downregulation of various APM components [43]. Furthermore, an inverse correlation of HER-2/neu and MHC class I APM component expression was found upon analysis of HER-2/neu+ versus HER-2/neu− mammary carcinoma lesions. These data were confirmed by shRNA-mediated downregulation of HER-2/neu in human tumor cells, resulting in an increased APM component and MHC class I surface expression [47].
So far, transcriptional regulators of the APM have been not well characterized. The role of PML controlling APM expression is controversially discussed [48], although a concordant regulation of PML and HLA class I antigens was described in prostate cancer [49]. Analysis of the activity of wild-type and mutant APM promoters in HER-2/neu− and HER-2/neu+ cells revealed a downregulation of the activity of the various APM promoters in the presence of HER-2/neu. Regarding the tapasin promoter activity, a mutation of the p300 and E2F1 restored the HER-2/neu-mediated suppression of tapasin promoter activity to levels excluding that of HER-2/neu− cells. Thus, both transcription factors appear to be important for the HER-2/neu-mediated tapasin downregulation [50]. These data were further confirmed by a siRNA approach inhibiting E2F1, which was accompanied by an upregulation of tapasin expression and consequently also MHC class I surface antigen expression [50]. E2F1 is a member of the large E2F family of highly redundant transcription factors and is often overexpressed in human tumors of distinct histologies. This is further associated with an aberrant cell cycle progression since E2F1 targets genes involved in cell cycle regulation. Our recent data postulated a link between cell cycle control and MHC class I surface expression. However, in order to support and confirm that an altered cell cycle progression might play an important role in tumor-associated downregulation of MHC class I APM component expression, combined analyses of cell cycle phases with MHC class I APM molecules should be performed.
Acknowledgments
We would like to thank Sylvi Magdeburg and Nicole Ott for excellent secretarial help. This work was supported by grants from the DFG Se581-11-1/11-2 and DFG Se581-9-1/-2.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- APC
Antigen-presenting cells
- APM
Antigen-processing machinery
- β2-m
β2-microglobuline
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cells
- EGF
Epidermal growth factor
- ER
Endoplasmic reticulum
- HC
Heavy chains
- IFN
Interferon
- JAK
Janus kinase
- luc
Luciferase
- MDSC
Myeloid-derived suppressor cells
- RCC
Renal cell carcinoma
- STAT
Signal transducers and activators of transcription
- TA
Tumor antigen
- TAP
Transporter associated with antigen processing
- TCR
T cell receptor
- TFBS
Transcription factor binding sites
- TNF
Tumor necrosis factor
- Treg
Regulatory T cells
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Second International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2011), held in Budapest, Hungary, 2nd–5th May 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16(4):348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 2.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D (2011) Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. doi:10.1172/JCI45862 [DOI] [PMC free article] [PubMed]
- 3.Scholz C, Tampe R. The peptide-loading complex—antigen translocation and MHC class I loading. Biol Chem. 2009;390(8):783–794. doi: 10.1515/BC.2009.069. [DOI] [PubMed] [Google Scholar]
- 4.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16(10):487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 5.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27(45):5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aptsiauri N, Carretero R, Garcia-Lora A, Real LM, Cabrera T, Garrido F. Regressing and progressing metastatic lesions: resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol Immunother. 2008;57(11):1727–1733. doi: 10.1007/s00262-008-0532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. 2008;57(11):1719–1726. doi: 10.1007/s00262-008-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrido F, Algarra I, Garcia-Lora AM. The escape of cancer from T lymphocytes: immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol Immunother. 2010;59(10):1601–1606. doi: 10.1007/s00262-010-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrido C, Algarra I, Maleno I, Stefanski J, Collado A, Garrido F, Garcia-Lora AM. Alterations of HLA class I expression in human melanoma xenografts in immunodeficient mice occur frequently and are associated with higher tumorigenicity. Cancer Immunol Immunother. 2010;59(1):13–26. doi: 10.1007/s00262-009-0716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seliger B, Schreiber K, Delp K, Meissner M, Hammers S, Reichert T, Pawlischko K, Tampe R, Huber C. Downregulation of the constitutive tapasin expression in human tumor cells of distinct origin and its transcriptional upregulation by cytokines. Tissue Antigens. 2001;57(1):39–45. doi: 10.1034/j.1399-0039.2001.057001039.x. [DOI] [PubMed] [Google Scholar]
- 11.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177(2):265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabrera CM, Lopez-Nevot MA, Jimenez P, Garrido F. Involvement of the chaperone tapasin in HLA-B44 allelic losses in colorectal tumors. Int J Cancer. 2005;113(4):611–618. doi: 10.1002/ijc.20526. [DOI] [PubMed] [Google Scholar]
- 13.Seliger B, Ritz U, Abele R, Bock M, Tampe R, Sutter G, Drexler I, Huber C, Ferrone S. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61(24):8647–8650. [PubMed] [Google Scholar]
- 14.Kamphausen E, Kellert C, Abbas T, Akkad N, Tenzer S, Pawelec G, Schild H, van Endert P, Seliger B. Distinct molecular mechanisms leading to deficient expression of ER-resident aminopeptidases in melanoma. Cancer Immunol Immunother. 2010;59(8):1273–1284. doi: 10.1007/s00262-010-0856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins D, Breuckmann A, Schmahl GE, Binner P, Ferrone S, Krummenauer F, Storkel S, Seliger B. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer. 2004;109(2):265–273. doi: 10.1002/ijc.11681. [DOI] [PubMed] [Google Scholar]
- 16.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, Seliger B. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11(7):2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, McNally BA, Ferrone S, Liu Y, Zheng P. A single-nucleotide deletion leads to rapid degradation of TAP-1 mRNA in a melanoma cell line. J Biol Chem. 2003;278(17):15291–15296. doi: 10.1074/jbc.M300954200. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Lapinski PE, Zhao H, Zhou Q, Zhang H, Raghavan M, Liu Y, Zheng P. A rare transporter associated with antigen processing polymorphism overpresented in HLAlow colon cancer reveals the functional significance of the signature domain in antigen processing. Clin Cancer Res. 2005;11(10):3614–3623. doi: 10.1158/1078-0432.CCR-04-1804. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen CF, Jordanova ES, ter Haar NT, Kolkman-Uljee SM, de Miranda NF, Ferrone S, Peters AA, Fleuren GJ. Expression and genetic analysis of transporter associated with antigen processing in cervical carcinoma. Gynecol Oncol. 2007;105(3):593–599. doi: 10.1016/j.ygyno.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Maleno I, Lopez-Nevot MA, Cabrera T, Salinero J, Garrido F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol Immunother. 2002;51(7):389–396. doi: 10.1007/s00262-002-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi T, Kobayashi Y, Kohsaka S, Sano K. The mutation in the ATP-binding region of JAK1, identified in human uterine leiomyosarcomas, results in defective interferon-gamma inducibility of TAP1 and LMP2. Oncogene. 2006;25(29):4016–4026. doi: 10.1038/sj.onc.1209434. [DOI] [PubMed] [Google Scholar]
- 22.Chen HL, Gabrilovich D, Tampe R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13(2):210–213. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 23.Chang CC, Ogino T, Mullins DW, Oliver JL, Yamshchikov GV, Bandoh N, Slingluff CL, Jr, Ferrone S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel beta2-microglobulin loss-of-function in melanoma cells. J Biol Chem. 2006;281(27):18763–18773. doi: 10.1074/jbc.M511525200. [DOI] [PubMed] [Google Scholar]
- 24.Cabrera CM, Jimenez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;61(3):211–219. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 25.Seliger B, Bock M, Ritz U, Huber C. High frequency of a non-functional TAP1/LMP2 promoter polymorphism in human tumors. Int J Oncol. 2002;20(2):349–353. [PubMed] [Google Scholar]
- 26.Hodson I, Bock M, Ritz U, Brenner W, Huber C, Seliger B. Analysis of the structural integrity of the TAP2 gene in renal cell carcinoma. Int J Oncol. 2003;23(4):991–999. [PubMed] [Google Scholar]
- 27.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63(14):4095–4100. [PubMed] [Google Scholar]
- 28.Kallfelz M, Jung D, Hilmes C, Knuth A, Jaeger E, Huber C, Seliger B. Induction of immunogenicity of a human renal-cell carcinoma cell line by TAP1-gene transfer. Int J Cancer. 1999;81(1):125–133. doi: 10.1002/(SICI)1097-0215(19990331)81:1<125::AID-IJC21>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Verweij MC, Koppers-Lalic D, Loch S, Klauschies F, Salle H, Quinten E, Lehner PJ, Mulder A, Knittler MR, Tampe R, Koch J, Ressing ME, Wiertz EJ. The varicellovirus UL49.5 protein blocks the transporter associated with antigen processing (TAP) by inhibiting essential conformational transitions in the 6 + 6 transmembrane TAP core complex. J Immunol. 2008;181(7):4894–4907. doi: 10.4049/jimmunol.181.7.4894. [DOI] [PubMed] [Google Scholar]
- 30.Oosten LE, Koppers-Lalic D, Blokland E, Mulder A, Ressing ME, Mutis T, Halteren AG, Wiertz EJ, Goulmy E. TAP-inhibiting proteins US6, ICP47 and UL49.5 differentially affect minor and major histocompatibility antigen-specific recognition by cytotoxic T lymphocytes. Int Immunol. 2007;19(9):1115–1122. doi: 10.1093/intimm/dxm082. [DOI] [PubMed] [Google Scholar]
- 31.Koppers-Lalic D, Verweij MC, Lipinska AD, Wang Y, Quinten E, Reits EA, Koch J, Loch S, Marcondes Rezende M, Daus F, Bienkowska-Szewczyk K, Osterrieder N, Mettenleiter TC, Heemskerk MH, Tampe R, Neefjes JJ, Chowdhury SI, Ressing ME, Rijsewijk FA, Wiertz EJ. Varicellovirus UL 49.5 proteins differentially affect the function of the transporter associated with antigen processing, TAP. PLoS Pathog. 2008;4(5):e1000080. doi: 10.1371/journal.ppat.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin BD, Verweij MC, Wiertz EJ. Herpesviruses and immunity: the art of evasion. Vet Microbiol. 2010;143(1):89–100. doi: 10.1016/j.vetmic.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Croft NP, Shannon-Lowe C, Bell AI, Horst D, Kremmer E, Ressing ME, Wiertz EJ, Middeldorp JM, Rowe M, Rickinson AB, Hislop AD (2009) Stage-specific inhibition of MHC class I presentation by the Epstein-Barr virus BNLF2a protein during virus lytic cycle. PLoS Pathog 5(6):e1000490. doi:10.1371/journal.ppat.1000490 [DOI] [PMC free article] [PubMed]
- 34.Rodriguez T, Mendez R, Del Campo A, Jimenez P, Aptsiauri N, Garrido F, Ruiz-Cabello F. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer. 2007;7:34. doi: 10.1186/1471-2407-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Respa A, Bukur J, Ferrone S, Pawelec G, Zhao Y, Wang E, Marincola FM, Seliger B. Association of IFN-gamma signal transduction defects with impaired HLA class I antigen processing in melanoma cell lines. Clin Cancer Res. 2011;17(9):2668–2678. doi: 10.1158/1078-0432.CCR-10-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setiadi AF, Omilusik K, David MD, Seipp RP, Hartikainen J, Gopaul R, Choi KB, Jefferies WA. Epigenetic enhancement of antigen processing and presentation promotes immune recognition of tumors. Cancer Res. 2008;68(23):9601–9607. doi: 10.1158/0008-5472.CAN-07-5270. [DOI] [PubMed] [Google Scholar]
- 37.Setiadi AF, David MD, Seipp RP, Hartikainen JA, Gopaul R, Jefferies WA. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol Cell Biol. 2007;27(22):7886–7894. doi: 10.1128/MCB.01547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sers C, Kuner R, Falk CS, Lund P, Sueltmann H, Braun M, Buness A, Ruschhaupt M, Conrad J, Mang-Fatehi S, Stelniec I, Krapfenbauer U, Poustka A, Schafer R. Down-regulation of HLA Class I and NKG2D ligands through a concerted action of MAPK and DNA methyltransferases in colorectal cancer cells. Int J Cancer. 2009;125(7):1626–1639. doi: 10.1002/ijc.24557. [DOI] [PubMed] [Google Scholar]
- 39.Khan AN, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother. 2008;57(5):647–654. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Epperson DE, Arnold D, Spies T, Cresswell P, Pober JS, Johnson DR. Cytokines increase transporter in antigen processing-1 expression more rapidly than HLA class I expression in endothelial cells. J Immunol. 1992;149(10):3297–3301. [PubMed] [Google Scholar]
- 41.Vertegaal AC, Kuiperij HB, Houweling A, Verlaan M, van der Eb AJ, Zantema A. Differential expression of tapasin and immunoproteasome subunits in adenovirus type 5- versus type 12-transformed cells. J Biol Chem. 2003;278(1):139–146. doi: 10.1074/jbc.M206267200. [DOI] [PubMed] [Google Scholar]
- 42.Seliger B, Harders C, Lohmann S, Momburg F, Urlinger S, Tampe R, Huber C. Down-regulation of the MHC class I antigen-processing machinery after oncogenic transformation of murine fibroblasts. Eur J Immunol. 1998;28(1):122–133. doi: 10.1002/(SICI)1521-4141(199801)28:01<122::AID-IMMU122>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 2004;64(1):215–220. doi: 10.1158/0008-5472.CAN-2522-2. [DOI] [PubMed] [Google Scholar]
- 44.Griffioen M, Peltenburg LT, van Oorschot DA, Schrier PI. C-myc represses transiently transfected HLA class I promoter sequences not locus-specifically. Immunobiology. 1995;193(2–4):238–247. doi: 10.1016/S0171-2985(11)80549-8. [DOI] [PubMed] [Google Scholar]
- 45.Ashrafi GH, Tsirimonaki E, Marchetti B, O’Brien PM, Sibbet GJ, Andrew L, Campo MS. Down-regulation of MHC class I by bovine papillomavirus E5 oncoproteins. Oncogene. 2002;21(2):248–259. doi: 10.1038/sj.onc.1205008. [DOI] [PubMed] [Google Scholar]
- 46.Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, Okita R, Nishimura MI, Handke D, Krug N, Choudhury A, Seliger B, Kiessling R. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer. 2011;128(2):390–401. doi: 10.1002/ijc.25613. [DOI] [PubMed] [Google Scholar]
- 47.Choudhury A, Charo J, Parapuram SK, Hunt RC, Hunt DM, Seliger B, Kiessling R. Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, upregulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer. 2004;108(1):71–77. doi: 10.1002/ijc.11497. [DOI] [PubMed] [Google Scholar]
- 48.Zheng P, Guo Y, Niu Q, Levy DE, Dyck JA, Lu S, Sheiman LA, Liu Y. Proto-oncogene PML controls genes devoted to MHC class I antigen presentation. Nature. 1998;396(6709):373–376. doi: 10.1038/24628. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Melamed J, Wei P, Cox K, Frankel W, Bahnson RR, Robinson N, Pyka R, Liu Y, Zheng P. Concordant down-regulation of proto-oncogene PML and major histocompatibility antigen HLA class I expression in high-grade prostate cancer. Cancer Immun. 2003;3:2. [PubMed] [Google Scholar]
- 50.Bukur J, Herrmann F, Handke D, Recktenwald C, Seliger B. Identification of E2F1 as an important transcription factor for the regulation of tapasin expression. J Biol Chem. 2010;285(40):30419–30426. doi: 10.1074/jbc.M109.094284. [DOI] [PMC free article] [PubMed] [Google Scholar]