Abstract
Purpose
Suppression of cellular immunity resulting from tumorigenesis and/or therapy might promote cancer cells’ growth, progression and invasion. Here, we explored whether T lymphocyte subtypes from peripheral blood of metastatic breast cancer (MBC) female patients could be used as alternative surrogate markers for cancer progress. Additionally, plasma levels of interleukin (IL)-2, IL-4, IL-6, IL-10, IFN-γ, and transforming growth factor-β1 were quantitated from MBC and healthy volunteers.
Experimental design
This study included 89 female MBC patients during the post-salvage chemotherapy follow-up and 50 age- and sex-matched healthy volunteers as control. The percentages of T lymphocyte subpopulations from peripheral blood and plasma levels of cytokines were measured.
Results
Both CD8+CD28− and CD4+CD25+ were elevated in MBC patients compared to the control cohort (P < 0.05). In contrast, CD3+ and CD8+CD28+cells were significantly lower in MBC patients (P < 0.0001, P = 0.045, respectively). MBC patients had elevated levels of immunosuppressive cytokines IL-6 and IL-10. Patients with elevated CD8+CD28− and CD4+CD25+ cells showed increased levels of IL-6, and only patients with elevated CD8+CD28− had decreased interferon-γ. Univariate analysis indicated increased CD3+CD4+ or CD8+CD28+correlated with prolonged progression-free survival (PFS), while elevated CD8+CD28−associated with shorten PFS. The percent of CD8+CD28− T lymphocytes is an independent predictor for PFS through multivariate analysis.
Conclusions
This study suggests that progressive elevated levels of CD8+CD28− suppressor T lymphocytes represent a novel independent predictor of PFS during post-chemotherapy follow-up.
Keywords: Regulatory T lymphocyte, Peripheral blood, Metastatic breast cancer, Progression-free survival, Cytokine
Introduction
Breast cancer is the most common form of cancer among women in the world. Although patients with metastatic breast cancer (MBC) can be effectively managed with appropriate treatment strategies, most patients eventually die from refractory disease with multiple organ metastases leading to functional organ failure. Alteration of cellular immunity is regarded as an internal accompanying event from the onset of cancer to terminal disease [1–3]. A number of factors, including cancer cell interactions with lymphocytes or anticancer treatment–related chemotherapy and radiotherapy, can have a significant impact on the immune system [4–7]. Cellular immunity plays an important role in eradicating residual tumor cells and maintaining homeostasis, since an active immune system targets mutant pathogenic cells or cancer cells and can induce cancer cell apoptosis. Therefore, the loss of cellular immunity and an imbalance of humoral immunity could trigger the progression of cancer and contribute to disease progression and treatment failure [8].
Most cancer patients do not develop a satisfactory antitumor response due to T cell functional impairment. Previous studies demonstrated that CD8+(CD8+CD28−) and CD4+ (CD4+CD25+Foxp3+) regulatory T cells (Tregs) inhibit T cell activation and proliferation, particularly cytotoxic T cells, through cell–cell interactions and secretion of suppressive cytokines such as interleukin (IL)-6, IL-10, and transforming growth factor (TGF)-β [9–14]. Cytokines also play an important role in evoking immune responses and immune endurance. IL-2 and interferon (IFN)-γ are key cytokines in triggering immune responses, while IL-6, IL-10, and TGF-β1, secreted by lymphocytes and tumor cells, are responsible for immunosuppression. It is thought that complex interactions between the host’s immune system and an existing tumor eventually result in impairment of cellular immunity [15–17]. Previous studies have shown significant changes of peripheral blood lymphocyte cell subsets in patients with different malignant lesions [16, 18–20]. However, it is not known whether certain populations of lymphocytes or types of cytokines could be regarded as prognostic or predictive indicators for MBC [15, 21–25]. Therefore, the present study attempted to expand previous observations to determine whether certain lymphocyte subsets could predict progression-free survival (PFS) during post-chemotherapy follow-up among MBC female patients.
Materials and methods
Patients and healthy volunteers
This study was approved by the both Peking University Cancer Hospital and Institute and Beijing Shijitan Hospital Institutional Review Board (IRB). Written consent was obtained from all patients. Patient population (n = 89 female) was accrued to the study between January 2005 and June 2006. The average patient age was 52.3 years (range 28–70). MBC patients who were eligible for receiving standard salvage chemotherapy had at least one measurable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and a life expectancy >3 months. We excluded patients that did not qualify for chemotherapy as determined by their physician and patients that had the addition of immune modulations following chemotherapy. The control group (n = 50) consisted of age- and sex-matched healthy volunteers with the mean age of 47.5 years old. They were free from active viral infection, Hepatitis A (HAV), Hepatitis B (HBV), HIV, tuberculosis, and autoimmune diseases complications.
Treatment protocol
A taxane-based chemotherapy regimen was undertaken for each individual. Patients received six cycles of the chemotherapy regimen if there was no disease progression. Demographic and clinical data of patients were reported in case report forms designed for data collection for this study. Blood samples were taken at baseline and follow-up intervals of every 2 months. The response to chemotherapy was assessed using the RECIST criteria. The primary endpoint of this study was PFS, defined as time to progressive disease.
Cell isolation and antibodies reagents
Two milliliters of heparinized peripheral blood was obtained from each patient and healthy volunteers. Whole blood (100 μl) was incubated in the dark with primary antibody at 4 °C for 15 min. After hemolysis for 10 min, samples were centrifuged for 10 min at 1,500 rpm at room temperature, and then washed twice in PBS and subjected to flow cytometric analysis (Becton–Dickinson, Franklin Lakes, NJ). Primary antibodies included: anti-CD4-FITC, anti-CD8-PE, anti-CD3-PerCP (Becton–Dickinson), anti-CD4-FITC (Beckman-Coulter), anti-CD25-PE (Beckman-Coulter), anti-CD28-FITC (Beckman-Coulter), anti-CD8-PE (Beckman-Coulter), anti-CD3-FITC, anti-CD16-PE, anti-CD56-PE (Becton–Dickinson), and anti-CD19-PC5 (Beckman-Coulter).
Flow cytometric analysis
Three-color flow cytometric analysis was performed to determine cell phenotypes. Lymphocyte subset levels were reported as percentages of the total population. Flow cytometry was performed using an Epics XL (Beckman-Coulter), and Expo32 ADC software (Beckman-Coulter) was used for analysis. Lymphocytes were gated by forward scatter versus side scatter. T lymphocytes were gated on CD3+ (PerCP) positive cells, B lymphocytes were gated on CD19+ (PerCP) cells, and NK lymphocytes were gated on CD16+CD56+ (PE) cells in the lymphocyte gate. Analysis was set to collect 5,000 gated events. Lymphocyte subsets analyzed included T cells (CD3+, CD3+CD4+, CD3+CD8+, CD4+CD25+, CD8+CD28+, CD8+CD28−), natural killer cells (CD3−CD56+), and B cells (CD19+).
Measurement of plasma cytokines by enzyme-linked immune sorbent assay (ELISA)
Blood samples were processed for plasma within 30 min of each blood draw. Blood was centrifuged at 1,000×g for 30 min. Plasma samples were kept frozen at −80 °C until analysis. The plasma concentrations of cytokines were determined using sandwich enzyme immunoassay kits from Boster Biological Tech Co.(Wuhan, China) for IL-2, IL-4, IL-6, IL-10, IFN-γ, and TGF-β1 (lot numbers EK0397, EK0404, EK0410, Ek0416, EK0373, and EK0513, respectively).
The minimum detectable levels of the cytokine assays were 1 pg/ml for IL-2, 1.5 pg/ml for IL-4, 0.3 pg/ml for IL-6, 0.5 pg/ml for IL-10, 2 pg/ml for IFN-γ, and 1 pg/ml for TGF-β1.
Statistical analysis for lymphocytes variations and PFS
The SAS system for Windows 9.0 statistical software was used for all data analysis. A P value of <0.05 was considered statistically significant. Results of the relative cell numbers were presented as mean values ± standard deviation (SD). Statistical analyses were performed using the Student’s t test to compare lymphocyte subsets between patients and healthy controls. The plasma concentration of cytokines was analyzed using Curve Expert 1.3 software. The levels of plasma cytokines in different groups were analyzed using independent sample t tests.
Cumulative survival time was calculated by the Kaplan–Meier methods. The univariate analyses of the correlation between PFS and experimental data were based on log-rank test. The multivariate analyses were based on the Cox proportional hazards regression model. The dependent variable was PFS, while the independent variables included age, estrogen receptor (ER) status, progesterone (PR) status, HER2 status, metastasis site, menopausal status, prior chemotherapy exposures, and the parameters of eight peripheral blood leukocyte subtypes. Data collection from the laboratory was blinded from the clinicians, and an independent group compared the experimental data with clinical responses.
Results
Patient cohort
All patients were female MBC patients. Patients had a mean age of 52.3 years (range 28–70 years), with 19 (21.3 %) patients being older than 60 years. Patients had previously received 0 (39.3 %), 1–3 (28 %), or 4 or more (32.7 %) lines of regimens of metastatic chemotherapy. Of the tumors present, 27.7 % were HER-2 positive and 21.3 % were triple negative. Sites of metastatic disease were visceral (19.1 %), lymph nodes/soft tissue/bone (44.9 %), or both (34.8 %). The median PFS was 4.0 months (range 1–24 months).
Percentage of lymphocyte subsets in peripheral blood of MBC patients and healthy volunteers
We compared the percentages of CD3+, CD3+CD4+, CD3+CD8+, CD8+CD28+, CD8+CD28−, CD4+CD25+, CD3−CD56+, and CD19+ cells in the peripheral blood of all MBC patients to those in healthy controls and observed significant differences in the percentages of CD3+, CD8+CD28+, CD8+CD28− and CD4+CD25+cells (P < 0.05) (Table 1). A significant increase in the percentages of CD8+CD28− and CD4+CD25+ subsets was observed in the peripheral blood of MBC patients compared to that of healthy controls (21.3 ± 8.0 vs. 17.4 ± 7.5, 9.6 ± 4.6 vs. 5.4 ± 2.7, respectively P < 0.05). In contrast, the percentages of CD3+ and CD8+CD28+cells were significantly lower in MBC patients than controls (59.1 ± 11.3 vs. 69.1 ± 8.0, P ≤ 0.0001; 11.0 ± 4.9 vs. 12.1 ± 3.5, P = 0.045).
Table 1.
Percentages of lymphocyte subsets in the peripheral blood of patients with metastatic breast cancer and healthy controls
| Subset % | Patients cohort (N = 89) | Healthy controls (N = 50) | T value | P value |
|---|---|---|---|---|
| CD3+ | 59.1 ± 11.3 | 69.05 ± 8.0 | −8.354 | <0.0001 |
| CD3+/CD4+ | 32.5 ± 8.2 | 33.7 ± 7.9 | −1.382 | 0.171 |
| CD3+/CD8+ | 23.7 ± 7.7 | 23.85 ± 5.75 | −0.145 | 0.885 |
| CD8+/CD28+ | 11.0 ± 4.9 | 12.05 ± 3.5 | −2.038 | 0.045 |
| CD8+/CD28− | 21.3 ± 8.0 | 17.35 ± 7.45 | 4.660 | <0.0001 |
| CD4+/CD25+ | 9.6 ± 4.6 | 5.4 ± 2.7 | 9.520 | <0.0001 |
| CD3−/CD56+ | 16.9 ± 8.2 | 16.8 ± 8.7 | 0.087 | 0.9310 |
| CD19+ | 13.6 ± 6.1 | 12.7 ± 5.4 | 1.272 | 0.207 |
Values represent the mean ± SD
P values were calculated by Student’s t test
Percentages of lymphocyte subsets in peripheral blood of MBC patients and baseline characteristics
The level of lymphocyte subsets was not associated with ECOG performance status, HER2 receptor status, or pathological classification of tumors. However, the percentages of CD3+ and CD3+CD8+ subsets among premenopausal MBC patients were higher than those of post-menopause patients (P = 0.01 and P = 0.011, respectively). The percentages of lymphocyte subsets in patients who had been off chemotherapy for more than 3 months were similar to those of patients who were “chemo naïve” (Table 2).
Table 2.
The influence of prior chemotherapy regimens on lymphocyte subsets
| Pre-chemotherapy | 0 | 1–3 | ≥4 | P value |
|---|---|---|---|---|
| No. | 35 | 22 | 32 | |
| CD3+ | 58.08 ± 10.21 | 58.78 ± 13.26 | 59.06 ± 11.29 | 0.632 |
| CD3+CD4+ | 32.03 ± 8.83 | 32.31 ± 8.95 | 32.49 ± 8.24 | 0.801 |
| CD3+CD8+ | 23.22 ± 7.28 | 24.35 ± 8.73 | 23.73 ± 7.65 | 0.836 |
| CD8+CD28+ | 10.71 ± 4.16 | 9.83 ± 4.63 | 12.1 ± 4.89 | 0.227 |
| CD8+CD28− | 20.29 ± 6.48 | 21.52 ± 10.15 | 21.3 ± 8.01 | 0.58 |
| CD4+CD25+ | 8.84 ± 4.51 | 9.71 ± 4.79 | 9.64 ± 4.6 | 0.291 |
| CD3−CD16+56+ | 15.38 ± 6.75 | 17.33 ± 9.94 | 16.93 ± 8.23 | 0.297 |
| CD19+ | 14.51 ± 4.81 | 13.02 ± 7.07 | 13.57 ± 6.05 | 0.5 |
Values represent the mean ± SD
P values were calculated by Student’s t test
Correlation of independent variables with PFS
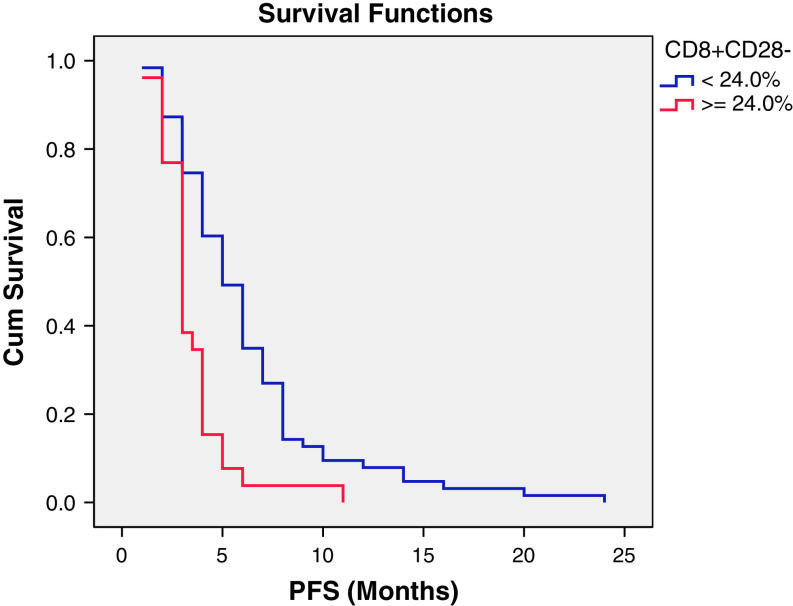
In univariate analyses, the log-rank test was employed to test the relation between PFS and independent variables. The results are shown in Table 3. Four indicators significantly correlated with PFS. The CD3+CD4+ and CD8+CD28+ T lymphocytes and positive ER status positively correlated with PFS, while the CD8+CD28− T lymphocytes negatively correlated with PFS. However, when considering the multivariate analyses using Cox proportional hazards regression model, only the CD8+CD28− subset was independent predictor that correlated with PFS (Table 4). The Kaplan–Meier plots of PFS for patients with different CD8+CD28− subset status are shown in Fig. 1. The median PFS for patients with an elevated CD8+CD28− T cell subset percentage (≥24.0 %) was significantly shorter than that in patients with a normal CD8+CD28− subset percentage (<24.0 %) (3.0 ± 0.3 vs. 5.0 ± 0.5 months P < 0.001).
Table 3.
Correlation between PFS and clinical data by univariate analysis
| Item | PFS (months) | P value |
|---|---|---|
| Age | ||
| <60.0 | 4.0 ± 0.21 | 0.419 |
| ≥60.0 | 5.0 ± 0.62 | |
| ER | ||
| Negative | 4.0 ± 0.55 | 0.025 |
| Positive | 5.0 ± 0.47 | |
| PR | ||
| Negative | 4.0 ± 0.52 | 0.345 |
| Positive | 4.0 ± 0.49 | |
| HER2 | ||
| Negative | 4.0 ± 0.18 | 0.209 |
| Positive | 5.0 ± 0.57 | |
| Metastatic site | ||
| Visceral | 4.0 ± 0.59 | 0.785 |
| Skin/lymph/bone | 5.0 ± 0.78 | |
| Both | 4.0 ± 0.28 | |
| Menopausal Status | ||
| Premenopause | 4.0 ± 0.74 | 0.238 |
| Post-menopause | 4.0 ± 0.31 | |
| Pre-chemotherapy (vs.) | ||
| ≤3 cycles | 4.0 ± 0.31 | 0.788 |
| >3 cycles | 4.0 ± 1.13 | |
| CD8+CD28+ | ||
| <11.0 % | 4.0 ± 0.34 | 0.029 |
| ≥11.0 % | 6.0 ± 0.73 | |
| CD8+CD28− | ||
| <24.0 % | 5.0 ± 0.50 | <0.001 |
| ≥24.0 % | 3.0 ± 0.25 | |
| CD4+CD25+ | ||
| <9.6 % | 4.0 ± 0.22 | 0.236 |
| ≥9.6 % | 5.0 ± 0.68 | |
| CD3+ | ||
| <59.1 % | 4.0 ± 0.68 | 0.717 |
| ≥59.1 % | 4.0 ± 0.38 | |
| CD3+CD4+ | ||
| <32.5 % | 4.0 ± 0.30 | 0.015 |
| ≥32.5 % | 5.0 ± 0.53 | |
| CD3+CD8+ | ||
| <23.7 % | 5.0 ± 0.58 | 0.419 |
| ≥23.7 % | 4.0 ± 0.19 | |
| CD3−CD16+56+ | ||
| <16.9 % | 5.0 ± 0.50 | 0.703 |
| ≥16.9 % | 4.0 ± 0.34 | |
| CD19+ | ||
| <13.6 % | 4.0 ± 0.39 | 0.674 |
| ≥13.6 % | 4.0 ± 0.66 | |
P values were determined by log-rank test
ER estrogen receptor, PR progesterone receptor, PFS progression-free survival
Table 4.
Analysis of relationship between PFS and possible influence factors
| Item | PFS HR (95 % CI) | P value |
|---|---|---|
| CD8+CD28+ (<11.0 vs. ≥11.0 %) | 1.441 (0.9209 ± 2.257) | 0.111 |
| CD8+CD28− (<24.0 vs. ≥24.0 %) | 0.476 (0.2859 ± 0.796) | 0.005 |
| CD3+CD4+ (<32.5 vs. ≥32.5 %) | 1.369 (0.8289 ± 2.262) | 0.221 |
| CD4+CD25+ (<9.6 vs. ≥9.6 %) | 1.046 (0.6269 ± 1.747) | 0.865 |
P values were determined by multiple Cox regression analysis
PFS progression-free survival, CI confidence interval, HR hazard rate
Fig. 1.
Kaplan-–Meier plot of progression-free survival for patients with different CD8+CD28− subset status. The median progression-free survival (PFS) in patients with an increased CD8+CD28− subset percentage (≥24.0 %) was significantly lower than that in patients with a normal CD8+CD28− subset percentage (<24.0 %). (3.0 ± 0.3 vs. 5.0 ± 0.5 months, P < 0.001)
Plasma levels of cytokines
The plasma levels of cytokines are shown in Table 5. In the MBC group, the mean levels of IL-6 and IL-10 were significantly higher than in controls (P = 0.002, P = 0.015, respectively). The plasma levels of IL-2, IFN-γ and IL-4 decreased in MBC patients but without statistical significance (P = 0.169, P = 0.208 and P = 0.842, respectively). The plasma levels of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TGF-β1 in MBC patients with different percentages of CD8+CD28− lymphocytes or CD4+CD25+cells are shown in Table 6. The plasma levels of IL-6 in patients with increased CD8+CD28−cells (≥24.0 %) was significantly higher than that of patients with a normal level of CD8+CD28− cells (<24 %) (P = 0.005). In contrast, the concentration of IFN-γ was significantly lower in patients with increased CD8+CD28−cells than that of patients with a normal level of CD8+CD28− cells (P = 0.008). The plasma levels of IL-10 were higher in patients with increased CD8+CD28− cells than in their normal counterparts, but the difference was not statistically significant (P = 0.516). In patients with increased CD4+CD25+cells, only IL-6 levels were statistically higher than in the healthy group (P = 0.031).
Table 5.
Plasma levels of cytokines in metastatic breast cancer patients and healthy controls
| Cytokines | Groups | N | Plasma concentration (pg/ml) | P value |
|---|---|---|---|---|
| IL-2 | Health | 42 | 11.96 ± 5.32 | |
| Patients | 89 | 4.50 ± 0.34 | 0.169 | |
| IFN-γ | Health | 42 | 50.53 ± 13.07 | |
| Patients | 89 | 32.19 ± 6.04 | 0.208 | |
| IL-10 | Health | 41 | 11.55 ± 2.53 | |
| Patients | 89 | 30.26 ± 7.10 | 0.015 | |
| IL-6 | Health | 42 | 3.20 ± 0.41 | |
| Patients | 89 | 7.07 ± 1.13 | 0.002 | |
| IL-4 | Health | 42 | 3.46 ± 0.32 | |
| Patients | 92 | 3.36 ± 0.30 | 0.842 | |
| TGF-β 1 | Health | 42 | 51.61 ± 8.17 | |
| Patients | 89 | 39.08 ± 11.21 | 0.478 |
Data were presented as mean values ± standard deviation
IL interleukin, TGF transforming growth factor, IFN interferon
Table 6.
Plasma levels of cytokines in metastatic breast cancer patients with different lymphocyte subset status
| Cytokines | Subgroup | CD8+CD28− lymphocyte subset | CD4+CD25+ lymphocyte subset | ||||
|---|---|---|---|---|---|---|---|
| N | pg/ml | P value | N | pg/ml | P value | ||
| IL-2 | Normal | 36 | 27.55 ± 16.42 | 46 | 27.55 ± 16.42 | ||
| Increased | 53 | 12.57 ± 8.60 | 0.425 | 43 | 5.05 ± 0.84 | 0.186 | |
| IFN-γ | Normal | 36 | 71.43 ± 19.26 | 46 | 71.43 ± 19.26 | ||
| Increased | 53 | 14.50 ± 1.85 | 0.008 | 43 | 29.71 ± 12.10 | 0.077 | |
| IL-10 | Normal | 36 | 28.19 ± 7.08 | 46 | 28.19 ± 7.08 | ||
| Increased | 53 | 43.04 ± 18.15 | 0.516 | 43 | 42.87 ± 24.26 | 0.557 | |
| IL-6 | Normal | 36 | 2.12 ± 0.45 | 46 | 2.12 ± 0.45 | ||
| Increased | 53 | 5.95 ± 1.20 | 0.005 | 43 | 11.13 ± 3.88 | 0.031 | |
| IL-4 | Normal | 36 | 5.61 ± 0.82 | 46 | 5.61 ± 0.82 | ||
| Increased | 53 | 12.87 ± 8.30 | 0.464 | 43 | 14.26 ± 11.13 | 0.432 | |
| TGF-β 1 | Normal | 36 | 29.42 ± 14.62 | 46 | 29.42 ± 14.62 | ||
| Increased | 53 | 16.21 ± 4.15 | 0.326 | 43 | 29.27 ± 8.94 | 0.993 | |
Data were presented as mean values ± standard deviation
IL interleukin, TGF transforming growth factor, IFN, interferon
Normal: patients within normal percentage of lymphocyte subsets. Increased: patients with increased percentage of lymphocyte subsets. The group with an increased CD8+CD28− lymphocyte subset contained ≥24.0 % CD8+CD28− cells, whereas the normal group CD8+CD28− lymphocyte subset was set as <24.0 %. The increased group of CD4+CD25+ percentage was ≥10 %, and the normal CD4+CD25+ group was set as <10 %
Discussion
Immune dysfunction is prevalent among most malignancies including MBC [26–28], suggesting that immunosuppression may contribute to the progression and chemotherapy resistance of cancer. There is increasing evidence suggesting tumor-infiltrating immune cells have prognostic value. Recently, Suzuki et al. [29] reported that the relative proportion of stromal FoxP3+ cells to CD3+ cells was a strong predictor of recurrence in 956 patients with stage I adenocarcinoma of lung cancer than the number FoxP3+ cells alone. High expression of tumor IL-12 receptor beta 2 (IL-12 Rβ) was associated with better outcome while high expression of IL-7R resulted in poorer outcome. Tumor biomarkers from different sources, including body fluids and peripheral blood, have been widely used to evaluate clinical responses to therapy as well as disease progression or recurrent status. Currently used biomarkers may only reflect relatively later events of cancer progression and may have little value in revealing latent early changes within cancer tissues. Currently, it is not known whether subsets of lymphocytes or cytokines could be used as prognostic or predictive indicators, especially in MBC [15, 21–24]. Since the evaluation of tumor responses only reflect the effectiveness of anticancer treatments after several cycles, there is no reliable parameter that can be used to predict the benefits of a given treatment. Thus, we have prospectively compared the relative percentages of different lymphocyte subsets in the peripheral blood between patients with MBC and healthy controls to determine whether lymphocytes could be used to predict the status of disease as well as PFS among MBC patients.
In the past decade, researchers have noticed that regulatory CD4+/CD25+ T cells are increased among non-small-cell lung cancer tumor infiltration lymphocytes and ovarian cancer tumor-associated lymphocytes, and most of these cells were capable of secreting TGF-β [9]. In the present study, we also analyzed both the percentage of different lymphocyte subpopulations and the plasma levels of cytokines IL-2, IL-4, IL-6, IL-10, IFN-γ, and TGF-β1 to evaluate their potential roles in disease progression. The results showed the significant decreases in the percentages of total CD3+ T cells and CD8+CD28+ T cells, concurrent with a significant increase in the percentage of CD8+CD28− and CD4+CD25+ T cells in MBC patients. In addition, the plasma levels of IL-6 and IL-10 were significantly elevated. These results indicated that the MBC patients had impaired immunity. We should address that CD4+/CD25+ T cells measured in this study were not representative of CD4+/CD25+FoxP3 regulatory T cells. The purpose of this study was not designed for discrimination of Treg subtypes; therefore, CD4+/CD25+ Treg phenotype indicated here may represent cell surface expression as previously reported. Cutting-edge flow cytometry supports the use of the CD4+/CD25+/CD127 phenotype as a surrogate phenotype for FoxP3+Treg [30].
Although immune cells, especially T cells, play an active role in immune surveillance and control of tumor growth during the early stages of cancer, suppressive CD4+ and CD8+ Treg can develop after chronic stimulation and interactions with tumor cells, thus promoting rather than inhibiting cancer development and progression [31, 32]. Treg plays a crucial role in regulating immune responses to maintain immune homeostasis [31]. Antigen-induced Treg mediate immune suppression either through cell–cell contact or through secretion of soluble suppressive cytokines such as IL-10 and TGF-β, which can also induce Treg differentiation [33–35]. Recent studies have shown an increased proportion of CD4+CD25+Foxp3+Treg in peripheral blood lymphocytes of patients with gastrointestinal malignancies, breast cancer, and lung cancer [19, 36, 37]. The increased prevalence of Treg could induce T cell dysfunction in cancer patients by down-regulating T cell signaling molecules or by the induction of T cell apoptosis, resulting in immunologic tolerance and immunosuppression [9, 10, 38–40]. Moreover, the relative increase of Treg may be associated with immunosuppression and tumor progression in patients with lung cancer, ovarian cancer, and gastrointestinal malignancies [9, 10]. In this study, we observed a significantly higher percentage of CD4+CD25+ T cells in MBC patients than in healthy controls. The plasma levels of IL-10 and IL-6 were also increased among MBC patients. Previous studies showed an expansion of CD8+CD28− lymphocyte subsets in patients with malignancies [20, 41–43] and that increased CD8+CD28−lymphocyte subsets in cancer patients may be associated with advanced stages of disease and poor survival [15, 44, 45]. However, the prognostic value of different lymphocyte subsets in MBC patients has not been previously studied. This study has showed an increase in the percentage of the CD8+CD28− lymphocyte subset from the peripheral blood of MBC patients accompanied by increased IL-6 and IL-10. Based on the results of univariate analysis for PFS, the percentages of CD3+CD4+ and CD8+CD28+lymphocytes positively correlated with PFS, while elevated CD8+CD28− lymphocytes associated with shorten PFS (Table 3). In the multivariate analyses using Cox proportional hazards regression model, CD8+CD28− lymphocytes were shown to be an independent factor to predict a short PFS (Table 4).
One of the interesting findings from this study was that interferon gamma (IFNγ) levels were concurrently lower in patients with elevated CD8+/CD28− cells. However, a direct relationship between CD8+/CD28 and production of IFNγ cannot be assumed since, following chemotherapy, the immune system could be impaired by IL-12, resulting in a reduction in IFNγ secretion [46]. Furthermore, because this is a population-based clinical study, the basic demographic of patients was not uniform since previous anticancer treatments were variable among patients, including cytotoxic regimen, radiotherapy, cycles of treatments, and clinical responses. We should further explore CTL function in vitro to analyze lymphocyte function based on exposure to therapeutic interventions, which might give rise to alterations of different cytokines.
In conclusion, the present study suggests that MBC female patients have impaired immunity, and CD8+CD28− lymphocytes are a significant predicator for PFS. Thus, identification of the mechanism of immunosuppression and the molecules involved in blocking the differentiation and generation of CD8+CD28− lymphocytes may lead to the development of CD8+CD28− lymphocyte subset–targeted immunomodulatory interventions, which may represent an effective treatment strategy for patients with MBC.
Acknowledgments
The authors thank Dr. Wei Sun for excellent technical assistance in flow cytometric analysis, Amy Hobeika Ph.D. from Duke university medical center, Durham, NC, USA for assistance with language editing. Authors received financial support from Natural Science Foundation of China (No.81172534) and Komen-Duke Project in China (3833989) from Susan G. Komen for the Cure Foundation.
Conflict of interest
No potential conflict of interests were disclosed.
Contributor Information
Jun Ren, Phone: +86-10-69326317, FAX: +86-10-63926298, Email: renjun9688@yahoo.com.
Herbert Kim Lyerly, Phone: +919-684-5613, FAX: +919-684-5653, Email: kim.lyerly@duke.edu.
References
- 1.Marr LA, Gilham DE, Campbell JD, Fraser AR. Immunology in the clinic review series; focus on cancer: double trouble for tumours: bi-functional and redirected T cells as effective cancer immunotherapies. Clin Exp Immunol. 2012;167(2):216–225. doi: 10.1111/j.1365-2249.2011.04517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow MT, Möller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. 2012;22(1):23–32. doi: 10.1016/j.semcancer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25(24):2559–2572. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leber MF, Efferth T. Molecular principles of cancer invasion and metastasis. Int J Oncol. 2009;34(4):881–895. doi: 10.3892/ijo_00000214. [DOI] [PubMed] [Google Scholar]
- 5.Miller FR. Immune mechanisms in the sequential steps of metastasis. Crit Rev Oncog. 1993;4(3):293–311. [PubMed] [Google Scholar]
- 6.Solomayer EF, Feuerer M, Bai L, Umansky V, Beckhove P, Meyberg GC, Bastert G, Schirrmacher V, Diel IJ. Influence of adjuvant hormone therapy and chemotherapy on the immune system analyzed in the bone marrow of patients with breast cancer. Clin Cancer Res. 2003;9(1):174–180. [PubMed] [Google Scholar]
- 7.Ceschia T, Beorchia A, Guglielmi R, Mandoliti G, Fongione S, Cereghini M, Tonutti E, Sala PG, Pizzi G. Influence of radiotherapy on lymphocyte subpopulations. Radiol Med. 1991;81(4):532–536. [PubMed] [Google Scholar]
- 8.Zou W. Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 9.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 10.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9(12):4404–4408. [PubMed] [Google Scholar]
- 11.Gilberto F, Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65(2):142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Leong PP, Mohammad R, Ibrahim N, Ithnin H, Abdullah M, Davis WC, et al. Phenotyping of lymphocytes expressing regulatory and effector markers in infiltrating ductal carcinoma of the breast. Immunol Lett. 2006;102(2):229–236. doi: 10.1016/j.imlet.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang RF. CD8 + regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol. 2008;69(11):811–814. doi: 10.1016/j.humimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 14.Aandahl EM, Torgersen KM, Taskén K. CD8+ regulatory T cells-A distinct T-cell lineage or a transient T-cell phenotype? Hum Immunol. 2008;69(11):696–699. doi: 10.1016/j.humimm.2008.08.291. [DOI] [PubMed] [Google Scholar]
- 15.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179(7):4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 16.Melichar B, Tousková M, Solichová D, Králicková P, Kopecký G. CD4 + T-lymphocytopenia and systemic immune activation in patients with primary and secondary liver tumours. Scand J Clin Lab Invest. 2001;61(5):363–370. doi: 10.1080/003655101316911404. [DOI] [PubMed] [Google Scholar]
- 17.Lissoni P, Brivio F, Ferrante R, Vigore L, Vaghi M, Fumagalli E, et al. Circulating immature and mature dendritic cells in relation to lymphocyte subsets in patients with gastrointestinal tract cancer. Int J Biol Markers. 2000;15(1):22–25. doi: 10.1177/172460080001500104. [DOI] [PubMed] [Google Scholar]
- 18.Melichar B, Jandik P, Krejsek J, Solichova D, Drahosova M, Skopec F, et al. Mitogen-induced lymphocyte proliferation and systemic immune activation in cancer patients. Tumori. 1996;82(3):218–220. [PubMed] [Google Scholar]
- 19.Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4 + CD25 + regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep. 2005;14(5):1269–1273. [PubMed] [Google Scholar]
- 20.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, et al. Foxp3 Expressing CD4 + CD25 + and CD8 + CD28 − T Regulatory Cells in the Peripheral Blood of Patients with Lung Cancer and Pleural Mesothelioma. Hum Immunol. 2006;67(1–2):1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 22.Holcombe RF, Jacobson J, Dakhil SR, Stewart RM, Betzing KS, Kannan K, et al. Association of immune parameters with clinical outcome in stage III colon cancer: results of Southwest Oncology Group Protocol 9009. Cancer Immunol Immunother. 1999;48(9):533–539. doi: 10.1007/s002620050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaloudik J, Lauerova L, Janakova L, Talac R, Simickova M, Nekulova M, et al. Significance of pre-treatment immunological parameters in colorectal cancer patients with unresectable metastases to the liver. Hepatogastroenterology. 1999;46(25):220–227. [PubMed] [Google Scholar]
- 24.Vesely P, Tousková M, Melichar B. Phenotype of peripheral blood leukocytes and survival of patients with metastatic colorectal cancer. Int J Biol Markers. 2005;20(2):126–133. [PubMed] [Google Scholar]
- 25.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari M. From tumor immunology to cancer immunotherapy: miles to go. J Cancer Res Ther. 2010;6(4):427–431. doi: 10.4103/0973-1482.77071. [DOI] [PubMed] [Google Scholar]
- 27.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010 doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, KadotaK Sima CS, Nitadori J, Rusch VW, Travis WD, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2(IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31(4):490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saison J, Demaret J, Venet F, Chidiac C, Malcus C, Poitevin-Later F, et al. CD4+CD25+CD127- assessment as a surrogate phenotype for FOXP3+ regulatory T cells in HIV-1 infected viremic and aviremic subjects. Cytometry B Clin Cytom. 2013;84(1):50–54. doi: 10.1002/cyto.b.21047. [DOI] [PubMed] [Google Scholar]
- 31.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19(2):217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe MA, Oda JM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 2010;29(4):569–579. doi: 10.1007/s10555-010-9247-y. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S. Naturally arising Foxp3-expressing CD25 + CD4 + regulatory T cells in immunological tolerance to self and nonself. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 34.Shevach EM. CD4 + CD25 + suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2(6):389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4 + CD25– naive T cells to CD4 + CD25 + regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 37.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4 + CD25 + regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98(5):1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 38.Javia LR, Rosenberg SA. CD4 + CD25 + suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26(1):85–93. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somasundaram R, Jacob L, Swoboda R, Caputo L, Song H, Basak S, et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4 + CD25 + regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor. Cancer Res. 2002;62(18):5267–5272. [PubMed] [Google Scholar]
- 40.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119(2):254–264. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu YR, Yang CL, Chen LB, Wang Q. Analysis of CD8(+) and CD8(+)CD28(−) cell subsets in patients with hepatocellular carcinoma. Di Yi Jun Yi Da Xue Xue Bao. 2002;22(1):72–73. [PubMed] [Google Scholar]
- 42.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52(10):599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strioga M, Pasukoniene V, Characiejus D. CD8 + CD28- and CD8 + CD57 + Tcells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Eng J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 45.Karagöz B, Bilgi O, Gümüs M, Erikçi AA, Sayan O, Türken O, et al. CD8 + CD28- cells and CD4 + CD25 + regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol. 2010;27(1):29–33. doi: 10.1007/s12032-008-9165-9. [DOI] [PubMed] [Google Scholar]
- 46.DeBenedette MA, Calderhead DM, Tcherepanova IY, Nicolette CA, Healey DG. Potency of mature CD40L RNA electroporated dendritic cells correlates with IL-12 secretion by tracking multifunctional CD8(+)/CD28(+) cytotoxic T-cell responses in vitro. J Immunother. 2011;34(1):45–57. doi: 10.1097/CJI.0b013e3181fb651a. [DOI] [PubMed] [Google Scholar]