Abstract
Background
CD8+Foxp3+ T lymphocytes have been detected in tumors. However, the distribution, phenotypic features, and regulation of these cells in gastric cancer remain unknown.
Methods
The levels of CD8+Foxp3+ T lymphocytes in the peripheral blood, tumor-draining lymph nodes, non-tumor tissues, and tumor tissues of patients with gastric cancer were detected by flow cytometry. Foxp3 induction in CD8+Foxp3− T cells was investigated in vitro. The suppressive function of CD8+Foxp3+ T lymphocytes was analyzed by their effect on CD4+ T-cell proliferation and IFN-γ production. The percentages of CD8+Foxp3+ T lymphocytes were evaluated for the association with tumor stage.
Results
The frequency of CD8+Foxp3+ T lymphocytes in tumor tissues was significantly higher than that in non-tumor tissues, and similar results were also observed in tumor-draining lymph nodes compared with peripheral blood. Most intratumoral CD8+Foxp3+ T lymphocytes were activated effector cells (CD45RA−CD27−). TGF-β1 levels were positively correlated with the frequency of CD8+Foxp3+ T lymphocytes in tumor tissues, and in vitro TGF-β1 could induce the generation of CD8+Foxp3+ T lymphocytes in a dose-dependent manner. Furthermore, intratumoral CD8+Foxp3+ T lymphocytes suppressed the proliferation and IFN-γ production of CD4+ T cells. Finally, intratumoral CD8+Foxp3+ T lymphocytes were significantly increased with tumor progression in terms of tumor-node-metastasis (TNM) stage.
Conclusions
Our data have shown that increased intratumoral CD8+Foxp3+ T lymphocytes are associated with tumor stage and potentially influence CD4+ T-cell functions, which may provide insights for developing novel immunotherapy protocols against gastric cancer.
Keywords: Gastric cancer, CD8+Foxp3+ T lymphocytes, Regulatory T cells, Tumor stage
Introduction
Gastric cancer (GC) is one of the most common malignancies accounting for 8% total cases and 10% total deaths from cancer worldwide [1]. At present, surgical resection is considered the best procedure in the treatment of GC patients with early-stage disease. However, the control of GC at advanced-stage disease remains difficult. It is generally accepted that tumor progression is regulated by the cross-talk between tumor and host immune system. Many studies focusing on the role of host immune response in cancer suggest that tumor-infiltrating immune cells especially T lymphocytes play an important role in controlling the development of tumors [2–4]. Thus, screening T lymphocytes subsets and investigating their functions in GC may have benefits on the clinical outcome.
Tumor-infiltrating lymphocytes (TIL) include effector and regulatory subsets that have been found in GC. Earlier studies have shown that the presence of effector TIL is associated with host defense against GC and that the type and density of effector TIL determine a clinical outcome [4, 5], suggesting their protective role in anti-tumor immunity of GC. However, tumor growth and metastasis denote a failure of most effector TIL to kill tumor cells. Regulatory T-cell subsets, especially CD4+Foxp3+ regulatory T cells (CD4+ Tregs), are considered to be one of the major mechanisms implicated in blunting effective tumor clearance for their immunosuppressive capability [6, 7]. Indeed, it is demonstrated that TIL contain a high level of CD4+ Tregs, which can be activated to suppress the proliferation and function of effector T cells at tumor site [8–11]. Therefore, the combination of boosting host anti-tumor immune response by application of effector TIL and blocking immunosuppressive effect by depletion of CD4+ Tregs may afford a promising therapy approach [12, 13].
Although most of Tregs belong to CD4+ T cells, increasing evidence indicates that some CD8+ T-cell subsets have been also identified to display regulatory functions in normal thymus [14], neonatal tolerance [15], autoimmune diseases [16–22], and infectious disease [23, 24]. These CD8+ T cells are characterized to express different cell surface molecules and separately referred to as CD8+CD25+, CD8+CD103+, CD8+CD28−, CD8+CD122+, CD8+latency-associated peptide (LAP)+, and CD8+lymphocyte activation gene-3 (LAG-3)+CCL4+ regulatory T cells. Like CD4+ Treg counterparts, CD8+ regulatory T cells suppress the proliferation and function of naïve and effector T cells through three different mechanisms: direct cell–cell contact with the target cells, the secretion of immunosuppressant molecules, and the induction of anergy in antigen-presenting cells [25]. Recently, CD8+Foxp3+ T lymphocytes, also reported as CD8+ regulatory T cells, accumulated in some types of tumors and shared phenotypic and functional features with CD4+ Tregs [26–28]. Although these studies imply a potential immunosuppressive role of CD8+Foxp3+ T lymphocytes in the tumor progression, the regulation and function of CD8+Foxp3+ T lymphocytes in human GC remain unknown.
In the present study, we showed that CD8+Foxp3+ T lymphocytes were enriched in tumors of GC patients. Tumor-infiltrating CD8+Foxp3+ T lymphocytes displayed a CD45RA−CD27−-activated effector phenotype and suppressed CD4+ T-cell proliferation and Th1 cells expansion. Moreover, TGF-β1 levels were positively correlated with the frequency of CD8+Foxp3+ T lymphocytes in tumors and in vitro TGF-β1 could regulate the generation of CD8+Foxp3+ regulatory T cells. Additionally, increased intratumoral CD8+Foxp3+ T lymphocytes were associated with tumor progression.
Materials and methods
Patients and tissue samples
Fresh peripheral blood, tumor-draining lymph nodes (TDLN), tumor, and normal autologous gastric tissues (non-tumor tissues, at least 5-cm distant from the tumor site) were obtained from patients who underwent surgical resection at the Southwest Hospital of the Third Military Medical University. None of the patients had received radiotherapy or chemotherapy before sampling. Individuals with autoimmune disease, infectious diseases, and multi-primary cancer were excluded. Peripheral blood from 15 healthy donors was used as controls. The clinical stages of tumors were determined according to the TNM classification system of International Union Against Cancer (Edition 6). The study was approved by the Ethics Committee of the Third Military Medical University. Written informed consent was obtained from each subject.
Cell isolation
Peripheral blood mononuclear cells (PBMC) from GC patients and healthy donors were isolated by Ficoll density gradient centrifugation. Fresh tumor and non-tumor tissues were used for the isolation of TIL and non-tumor-infiltrating lymphocytes (NIL). In brief, fresh tumor and non-tumor tissues were washed three times in RPMI 1640 before being cut into small pieces. The specimen were then collected in RPMI 1640 containing 1 mg/ml collagenase IV (Sigma-Aldrich, St. Louis, MO, USA) and 10 mg/ml DNase I (Roche, Basel, Switzerland) and mechanically dissociated by using the gentle MACS Dissociator (Miltenyi Biotec, Auburn, CA, USA). Dissociated cell suspensions were further incubated 1 h at 37 °C under continuous rotation and filtered through 70-μm cell strainers to obtain cell suspensions. Fresh TDLN were gently minced and passed through 70-μm cell strainers to obtain cell suspensions. The cell suspensions were then used for flow cytometry analysis.
Antibodies and flow cytometric analysis
The following antibodies were used to stain single-cell suspensions: CD3-APC-H7, CCR4-PE-Cy7 (BD Pharmingen, San Diego, CA, USA), CD4-FITC, CD8-PerCP-Cy5.5, CD25-PE, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)-PE, glucocorticoid-induced TNF receptor (GITR)-APC, CXCR4-PE-Cy7, HLA-DR-APC, CD127-APC, CD122-PE, Foxp3-Alexa Fluor 488 or PE or APC, CD45RA-PE-Cy7, CD27-FITC (eBioscience, San Diego, CA), CD4-PE, or APC (Tianjin Sungene Biotech Co., Ltd). Cells were incubated with appropriate surface antibodies for 30 min at 4 °C. For intracellular staining of Foxp3 and CTLA-4, the Foxp3-Staining Buffer Set was used according to the manufacturer’s protocol. Isotype-matched antibodies were given to enable correct compensation and confirm antibody specificity. Cells were analyzed by flow cytometry with a FACSCantoII (BD Biosciences). Data were analyzed with Flowjo software (BD Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
Tumor and normal autologous gastric tissues were weighted before protein extraction with tissue protein extraction reagents (Pierce, Rockford, IL, USA) according to the manufacturer’s protocol. In brief, tissues were added in tissue protein extraction reagents and homogenized, and then, the supernatants of samples were collected by centrifugation and stored at −80 °C for determination of cytokines. TGF-β1 levels were determined with ELISA kits in accordance with the manufacturer’s recommendations (R&D Systems, Minneapolis, MN, USA).
In vitro generation of CD8+Foxp3+ T cells
CD8+ T cells were obtained from PBMC of healthy individuals by positive selection using the EasySep human CD8+ T-cell enrichment kit (Stem cell, Vancouver, Canada). Purified CD8+ T cells were resuspended at 2 × 105 cells/well in RPMI 1640 containing 10% fetal calf serum (Gibco, Bionova, Uruguay) and seeded in 96-well round bottom plates supplemented with 20 U/ml recombination human (rh) IL-2 (rhIL-2) to each well and combination of rhTGF-β1 at different concentrations (1, 10, and 100 ng/ml) (Peprotech, Rocky Hill, NJ, USA), in the presence of anti-CD3 antibody (2 μg/ml) and anti-CD28 antibody (1 μg/ml) (Biolegend, San Diego, CA, USA). On day 5, plated cells were stained for intracellular Foxp3 expression according to the manufacturer’s recommendations (eBioscience). Cells were then analyzed by flow cytometry.
Sorting of CD8+CD25+ T cells and suppressive assay
CD8+Foxp3+ T cells from TIL of GC patients or in vitro-induced CD8+Foxp3+ T cells were stained anti-CD8 and anti-CD25 antibodies and were sorted by FACSAriaII (BD Biosciences), and then, 2 × 104 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+ T cells were co-cultured with 2 × 104 purified CD8+CD25+ T cells in 96-well plates coated with anti-CD3 antibody (1 μg/ml), and soluble anti-CD28 antibody (5 μg/ml) was supplemented to each well. On day 5, the proliferation and IFN-γ production of CFSE-labeled CD4+ T cells were detected by flow cytometry.
Statistical analysis
All results are summarized as mean ± standard error of the mean (SEM), and statistical analysis was performed with the Prism 5.0 Software. Differences between groups were evaluated by two-tailed Student’s t test. P < 0.05 was considered statistically significant.
Results
Patients’ characteristics
A total of 45 never-treated patients with GC were enrolled from July 2010 to May 2011. The baseline clinicopathologic characteristics was presented in Table 1. Among them, TDLN from 19 patients were investigated. Tumor stage III was most frequent.
Table 1.
Clinical characteristics of patients with GC
| Variable | All patients | Patients with tissue analysis | Patients with LN available |
|---|---|---|---|
| Number | 45 | 40 | 19 |
| Sex (male/female) | 33/12 | 28/12 | 16/3 |
| Age, years (median, range) | 55 (31–82) | 54 (31–75) | 56 (31–75) |
| H. pylori antibody (positive/negative) | 25/20 | 22/18 | 11/8 |
| Tumor stage (T1/T2/T3/T4) | 2/6/31/6 | 2/5/27/6 | 1/3/12/3 |
| Lymphoid nodal status (N0/N1/N2/N3) | 5/9/27/4 | 5/9/22/4 | 2/6/8/3 |
| Distant metastasis (M) status (M0/M1) | 43/2 | 40/0 | 19/0 |
| TNM stage (I/II/III/IV) | 2/7/31/5 | 2/6/27/5 | 1/4/10/4 |
| Histological grade (good/moderate/poor) | 2/13/30 | 0/13/27 | 0/5/14 |
CD8+Foxp3+ T lymphocytes are increased in tumors of GC patients
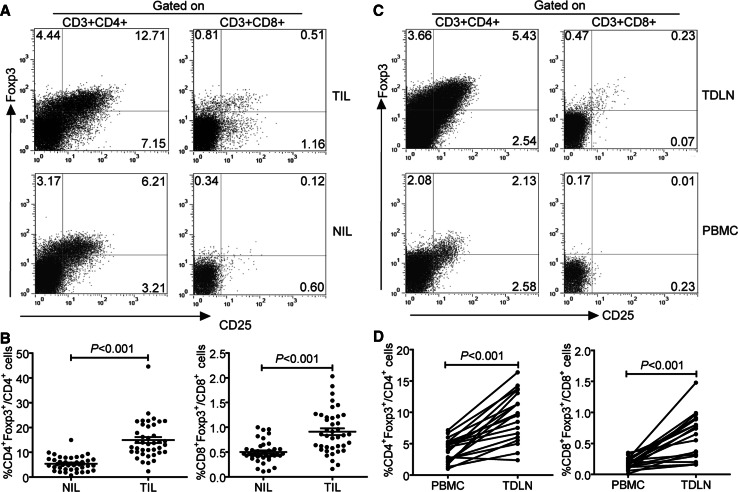
Using flow cytometry, we first analyzed the prevalence of Foxp3+ T lymphocytes in tumor and paired non-tumor tissues of GC patients. We observed that TIL contained not only a substantial amount of CD3+CD4+Foxp3+ T lymphocytes, but also a small fraction of CD3+CD8+Foxp3+ T lymphocytes (Fig. 1a, b). The levels of CD8+Foxp3+ T lymphocytes were significantly higher in TIL than those in NIL (0.85%, 0.16%–2.03% vs. 0.46%, 0.11%–1.00%, P < 0.001). We further analyzed the frequency of CD8+Foxp3+ T lymphocytes in TDLN and peripheral blood of GC patients (Fig. 1c, d). The percentages of these cells in TDLN were also significantly increased compared with those in PBMC (0.65%, 0.30%–1.48% vs. 0.17%, 0.09%–0.35%, P < 0.001). However, CD8+Foxp3+ T lymphocytes were not detected in the peripheral blood of healthy donors (data not shown). Taken together, these data indicate that CD8+Foxp3+ T lymphocytes are present in tumors of GC patients.
Fig. 1.
Distribution of CD3+CD8+Foxp3+ T lymphocytes in TIL, NIL, TDLN, and PBMC of patients with GC. a A representative flow cytometry analysis of lymphocytes in TIL and NIL of the same patients for Foxp3 versus CD25 expression after gating on CD3+CD4+ or CD3+CD8+. b The frequencies of CD3+CD4+Foxp3+ and CD3+CD8+Foxp3+ T cells in TIL and NIL from 40 GC patients (horizontal bars, mean ± SEM; each circle, single donor). c The representative dot plot of CD25+Foxp3+ cells among CD3+CD4+ and CD3+CD8+ T cells in TDLN and PBMC of the same patient. d The frequency of CD3+CD8+Foxp3+ T cells in matched pairs of TDLN and PBL from 19 patients. Statistical analysis in b and d was shown by Student’s paired t test. Data were the mean ± SEM
CD8+Foxp3+ T lymphocytes at tumor site have an activated effector phenotype
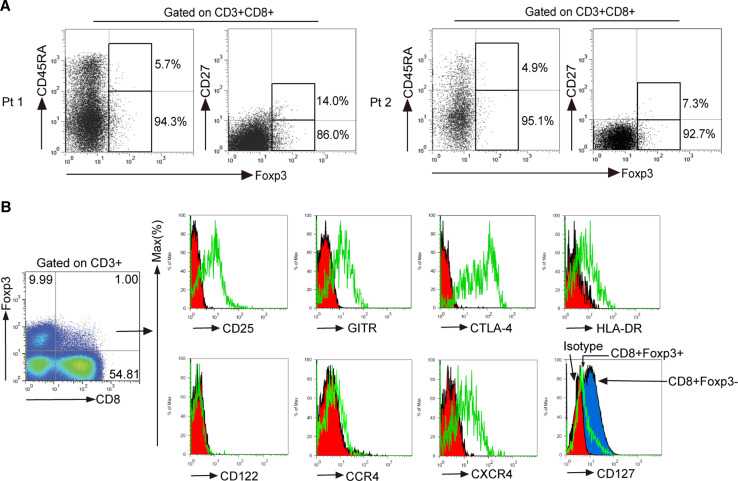
To study phenotypic features of CD8+Foxp3+ T lymphocytes at tumor site, we first examined the surface expression of CD45RA and CD27. Gated on intratumoral CD3+CD8+ T cells, most Foxp3+ cells belonged to the CD45RA− and CD27− subset (Fig. 2a), indicating that most intratumoral CD8+Foxp3+ T lymphocytes were effector-memory cells (CD45RA−CD27−). CD8+Foxp3+ T lymphocytes were found to share a similar phenotype with CD4+ Tregs [25]. Therefore, we next analyzed the regulatory molecules expression profiles of intratumoral CD8+Foxp3+ T lymphocytes. In TIL, CD8+Foxp3+ T lymphocytes expressed CD25, GITR, CTLA-4, HLA-DR, but weakly express CD127 and were negative of CD122 (Fig. 2b). On the basis of our observation, we conclude that tumor-infiltrating CD8+Foxp3+ T lymphocytes have an activated effector phenotype and may perform regulatory functions in GC patients.
Fig. 2.
Phenotypic characteristics of CD8+Foxp3+ T lymphocytes in tumors. a Surface expression of CD27 and CD45RA on CD8+Foxp3+ T lymphocytes from TIL of two patients (Pt). b Freshly isolated TIL from tumor tissues were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-Foxp3, anti-CTLA-4, anti-GITR, anti-HLA-DR, anti-CD122, anti-CCR4, anti-CXCR4, and anti-CD127 antibodies. Cells were gated on CD3+CD8+Foxp3+ T cells and the expressions of CD25, CTLA-4, GITR, HLA-DR, CD122, CCR4, CXCR4, and CD127 were analyzed
TGF-β1 can induce the generation of CD8+Foxp3+ T cells
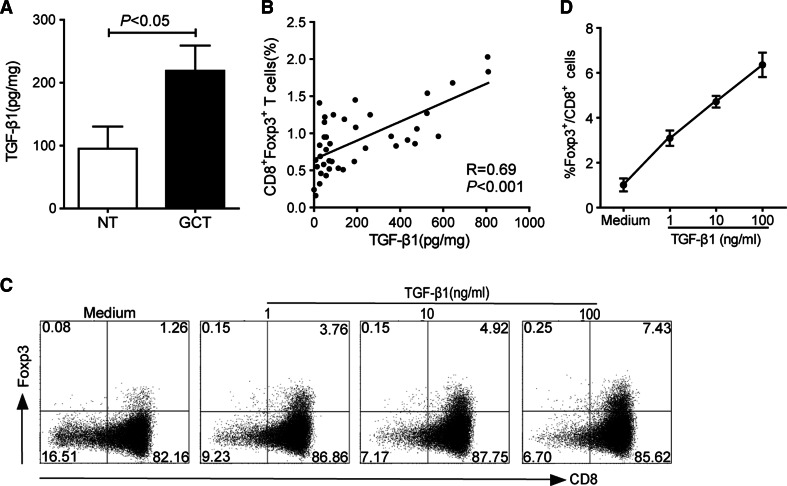
Recent studies suggest that TGF-β1 is a key cytokine for the generation of CD8+Foxp3+ T cells [26, 29]. Given the accumulation of CD8+Foxp3+ T lymphocytes at tumor site, we first investigated whether this cytokine was increased in tumor tissues. Expectedly, the concentration of TGF-β1 was higher in tumor tissues than that in non-tumor tissues (Fig. 3a). Moreover, TGF-β1 levels in tumor tissues were positively correlated with the percentages of CD8+Foxp3+ T lymphocytes (Fig. 3b). We then analyzed whether TGF-β1 could induce the generation of CD8+Foxp3+ T cells from CD8+Foxp3− T cells. Freshly purified CD8+ T cells from peripheral blood of healthy donors were stimulated with rhIL-2 and rhTGF-β1 in the presence of anti-CD3 and anti-CD28 antibodies. As shown in Fig. 3c and d, after culture for 5 days, TGF-β1 significantly induced the generation of CD8+Foxp3+ T cells in a dose-dependent manner. These data imply that tumor environmental TGF-β1 may be involved in facilitating the generation of CD8+Foxp3+ T cells.
Fig. 3.
The differentiation of CD8+Foxp3+ T cells induced by TGF-β1. a TGF-β1 levels in tumor tissues (GCT, black bars) and non-tumor tissues (NT, white bars) of GC patients. Data were the mean ± SEM. b Correlation between the percentages of CD8+Foxp3+ T cells and the concentrations of TGF-β1 in tumor tissues of the patients. c Puried CD8+ T cells were cultured for 5 days with indicated different concentrations of rhTGF-β1 in the presence of rhIL-2, anti-CD3, and anti-CD28 antibodies. A representative dot plot is shown Foxp3 induction in CD8+ cells by flow cytometry. d The mean ± SEM of three independent experiments for the induction of CD8+Foxp3+ T cells
Intratumoral CD8+Foxp3+ T lymphocytes can suppress CD4+ T cells function
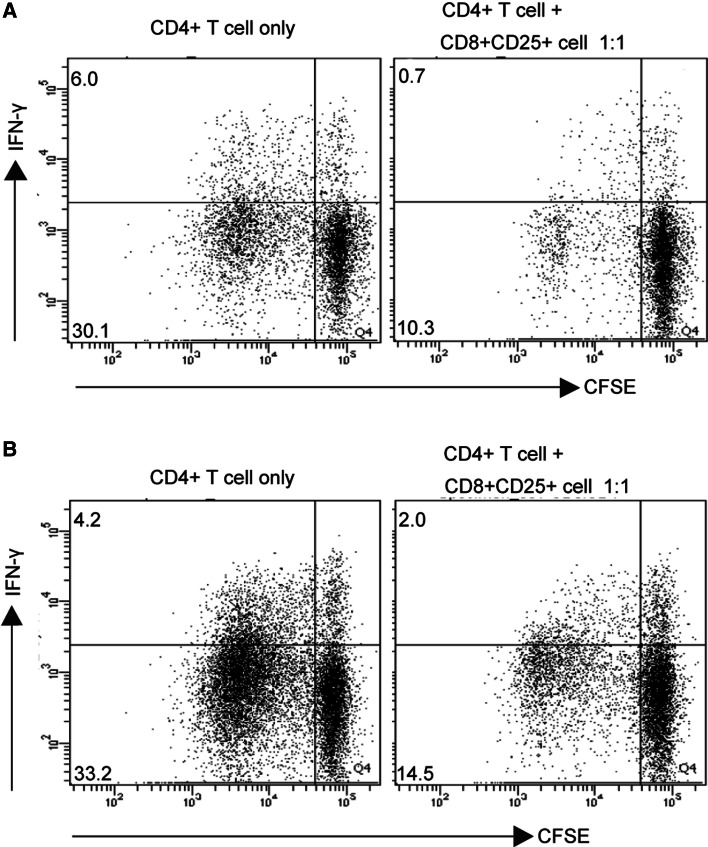
To investigate the suppressive function of CD8+Foxp3+ T lymphocytes, CD8+CD25+ T cells were sorted from TIL of GC patients and then co-cultured with autologous peripheral CD4+ T cells. Interestingly, we found that these sorted cells were able to suppress the proliferation and IFN-γ production of CD4+ T cells (Fig. 4a). Similar results were also obtained when CD4+ T cells were co-cultured with in vitro-induced CD8+Foxp3+ T cells (Fig. 4b). These results suggest that a CD8+ T-cell subset with suppressive function is present in tumors of GC.
Fig. 4.
Suppressive function of CD8+Foxp3+ T cells in vitro. a CFSE-labeled CD4+ T cells were cultured alone or with sorted CD8+CD25+ T cells from TIL of GC patients at 1:1 for 5 days in the presence of anti-CD3 and anti-CD28 antibodies. The suppressive effect of CD8+CD25+ T cells on the proliferation and IFN-γ production of CFSE-labeled CD4+ T cells was detected by flow cytometry. A representative flow cytometry analysis is shown from one of two patients. b CD8+Foxp3+ T cells were induced by TGF-β1 (10 ng/ml) for 5 days and then isolated by sorting CD8+CD25+ T cells. CFSE-labeled CD4+ T cells were cultured alone or with sorted TGF-β1-induced CD8+CD25+ T cells at 1:1 for 5 days in the presence of anti-CD3 and anti-CD28 antibodies. A representative flow cytometry analysis for the proliferation and IFN-γ production of CFSE-labeled CD4+ T cells is shown from one of three independent experiments
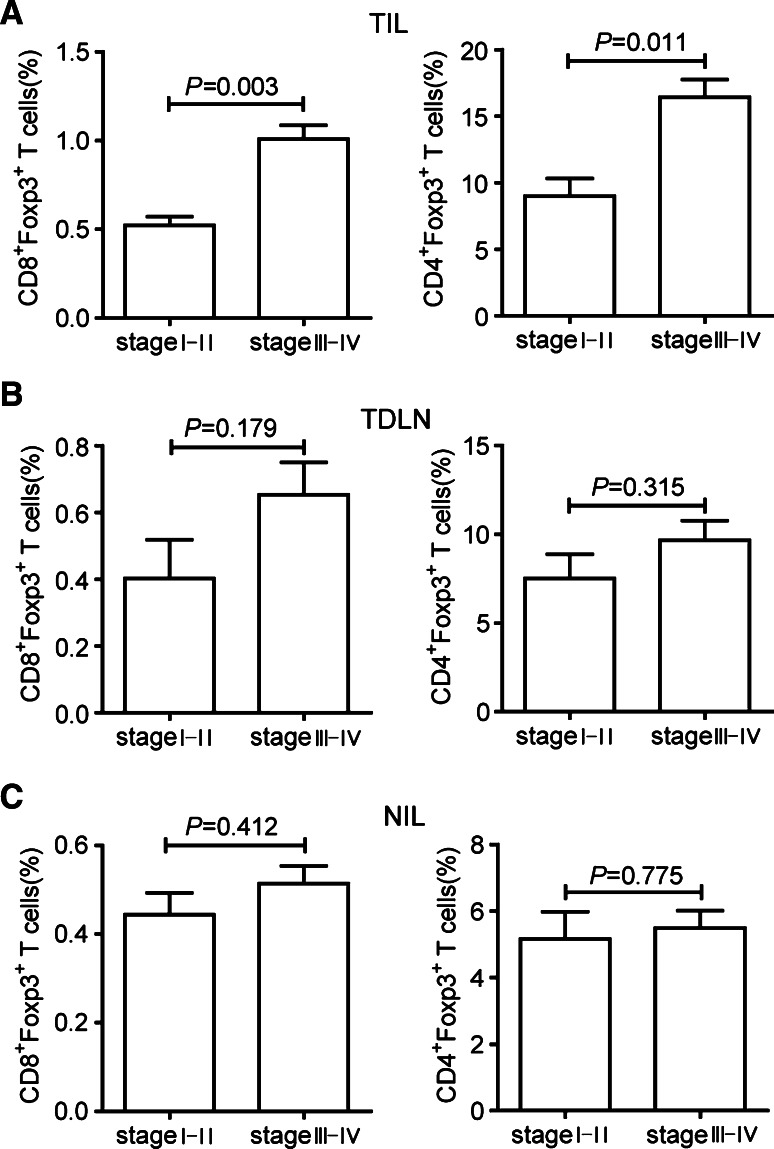
Increased intratumoral CD8+Foxp3+ T lymphocytes correlate with tumor stage
Next, we studied whether increased intratumoral CD8+Foxp3+ T lymphocytes were associated with tumor stage. The comparison was made between stage I–II and stage III–IV GC patients according to the previous studies on GC [30, 31]. As shown in Fig. 5a, there was a significant difference in the percentage of intratumoral CD8+Foxp3+ T lymphocytes between stage I–II and stage III–IV GC patients (stage I–II, 0.52%, 0.16%–0.69% vs. stage III–IV, 1.01%, 0.24%–2.03%, P = 0.003). The increased ratio of CD4+Foxp3+ T lymphocytes was also positively associated with tumor stage (stage I–II, 9.59%, 2.35%–14.40% vs. stage III–IV, 14.46%, 6.02%–44.63%, P = 0.011). However, no significant difference of the percentages of CD8+Foxp3+ and CD4+Foxp3+ T lymphocytes was found between stage I–II and stage III–IV GC patients in TDLN (Fig. 5b) and NIL (Fig. 5c). Furthermore, we analyzed clinical information and correlated data with the levels of CD8+Foxp3+ T lymphocytes in tumors. Here, we did not observe any significant difference of CD8+Foxp3+ T lymphocytes frequency among gender, age, histological grade, and Helicobacter pylori (H. pylori) infection of GC patients (data not shown). Above all, these results indicate that CD8+Foxp3+ and CD4+Foxp3+ T lymphocytes accumulate at tumor site during tumor progression.
Fig. 5.
Association of intratumoral CD8+Foxp3+ and CD4+Foxp3+ T lymphocytes with GC stage. Percentages of CD8+Foxp3+ and CD4+Foxp3+ T lymphocytes in TIL (a), TDLN (b), and NIL (c) between stage I–II and stage III–IV GC patients. P values of < 0.05 are considered significant
Discussion
Although functional effector TIL infiltrate into the tumor microenvironment and mediate anti-tumor immunity by releasing soluble factors and exerting their cytotoxic activity, an immunosuppressive status still occurs at tumor site [32]. This tumor immune tolerance is associated with the immunosuppressive effect of Foxp3+ regulatory T cells on tumor-specific effector T-cell immunity [33]. It has been shown that increased CD4+ Tregs can suppress the proliferation and function of effector T cells and that intratumoral high Foxp3+/CD8+ ratio contributes to tumor progression in GC patients [5, 34].
In our study, we confirmed the reported data that CD4+Foxp3+ T lymphocytes were increased in TIL and TDLN of GC patients. Interestingly, we observed that CD8+Foxp3+ T lymphocytes were also significantly increased in TIL and TDLN of GC patients, and most tumor-infiltrating CD8+Foxp3+ T lymphocytes displayed a CD45RA−CD27− phenotype and co-expressed CD25, GITR, CTLA-4, and HLA-DR but low CD127, showing similar molecule profiles with CD4+ Tregs. These data indicated that intratumoral CD8+Foxp3+ T lymphocytes were likely to exert regulatory functions rather than effector functions in GC tumors. It was reported that tumor-derived CD8+Foxp3+ T lymphocytes expressed CD122 in prostate cancer [27] and that CD122+CD8+ T cells suppressed vaccine-induced anti-tumor immune response in lymphodepleted mice [35]. However, our study showed that intratumoral CD8+Foxp3+ T lymphocytes did not express CD122 molecules, implying that CD122 was not necessary for their functions in human GC. Recently, IL-10-producing CD8+ T cells were involved in the inhibition of T-cell effector functions in human cancer [36, 37], but these CD8+IL-10+ T cells were Foxp3 negative or Foxp3 expression was not confirmed among them. Moreover, Anichini et al. [38] studied that CD8+Foxp3+ T lymphocytes were tumor-reactive CD8+ early-effector T cells with the capability of IFN-γ and perforin production in tumor-invaded lymph nodes of melanoma patients. However, in our study, the expressions of IL-10, IFN-γ, and perforin were not observed in intratumoral CD8+Foxp3+ T lymphocytes (data not shown), indicating that the function of these cells may be different in GC.
It is unknown whether CD8+Foxp3+ T lymphocytes are recruited from peripheral blood or induced to expand in situ by tumor-derived factors. CD4+ Tregs have been shown to express CCR4 molecules and be recruited from peripheral blood by CCR4-CCL17/CCL22 chemotaxis in gastric adenocarcinoma [9, 39]. However, our data showed that tumor-infiltrating CD8+Foxp3+ T lymphocytes expressed an abundance of CXCR4 but not CCR4, suggesting that CXCL12-CXCR4 chemotaxis may be implicated in the migration of CD8+Foxp3+ T lymphocytes in GC microenvironment. Nevertheless, circulating CD8+Foxp3+ T-lymphocyte numbers were rather few in GC patients, implying an almost impossibility pathway for recruiting these cells from peripheral blood. Recent studies indicated that tumor-derived TGF-β could convert CD4+ T cells into CD4+ Tregs [40] and Foxp3 induction in CD8+ T cells required TGF-β stimulation signals [29]. Accordingly, our data showed that the level of tumor microenvironmental TGF-β1 was significantly increased and positively correlated with the frequency of CD8+Foxp3+ T lymphocytes in tumors. Furthermore, TGF-β1 markedly induced the generation of CD8+Foxp3+ cells from peripheral CD8+Foxp3− T cells in a dose-dependent manner. These data suggest that tumor microenvironment may form a cytokine milieu that is suitable for the differentiation and expansion of CD8+Foxp3+ T lymphocytes.
CD8+Foxp3+ T lymphocytes exerted a regulatory role in human prostate and colorectal cancer [26, 27], but the function of this cell population in GC is still unknown. Thus, functional assays were performed to address the suppressive effects of CD8+Foxp3+ T lymphocytes from TIL of GC patients on autologous peripheral CFSE-labeled CD4+ T cells. Interestingly, tumor infiltrating these cells significantly suppressed the proliferation and IFN-γ production of CD4+ T cells, suggesting that CD8+Foxp3+ T lymphocytes in tumors of GC exert an immunosuppressive activity. Similar results were also obtained when CD4+ T cells were co-cultured with in vitro generated CD8+CD25+ T lymphocytes, which is consistent with the studies that human CD8+Foxp3+ regulatory T cells can be generated by in vitro culture [41, 42].
Importantly, our data also demonstrated that increased intratumoral CD8+Foxp3+ and CD4+Foxp3+ T lymphocytes were associated with tumor progression. Within the other sample cohort, the frequency of CD8+Foxp3+ T lymphocytes in TDLN was also elevated compared with that in NIL but not correlated with tumor progression. As for the fact that CD8+Foxp3+ T lymphocytes only account for a small fraction of CD8+ T cells in tumors and TDLN, the origin and clinical relevance of these cells need further investigation.
In conclusion, our data unveil the association of intratumoral CD8+Foxp3+ T lymphocytes with tumor progression and potential implication of these cells in tumor immune escape. Further understanding of their immunological roles will provide insights for developing novel immunotherapy protocols against GC.
Acknowledgments
This work was supported by grants of the National Natural Science Foundation of China (NSFC, No. 81071412) and National Basic Research Program of China (973 program, No. 2009CB522606). We thank Fang Wang (Analysis and Testing Center) for her help in flow cytometry sorting.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Liu-sheng Peng and Yuan Zhuang contributed equally to the work.
Contributor Information
Pei-wu Yu, Phone: +86-023-68752315, FAX: +86-023-68752315, Email: yupeiwu01@vip.sina.com.
Quan-ming Zou, Phone: +86-023-68752315, FAX: +86-023-68752315, Email: qmzou2007@163.com.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104(10):3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99(10):1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136(10):1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3(+) regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 8.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 9.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122(10):2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 10.Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, Xue J, Zhang FM, Ge HL, Xu D. CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. C. Clin Immunol. 2009;131(1):109–118. doi: 10.1016/j.clim.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL, Shen LS, Xu D. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134(3):277–288. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Lim J, Kang JS, Kim HM, Lee HK, Ryu HS, Kim JY, Hong JT, Kim Y, Han SB. Adoptive immunotherapy of human gastric cancer with ex vivo expanded T cells. Arch Pharm Res. 2010;33(11):1789–1795. doi: 10.1007/s12272-010-1111-7. [DOI] [PubMed] [Google Scholar]
- 13.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70(20):7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 14.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S, Annunziato F. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102(12):4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 15.Adams B, Dubois A, Delbauve S, Debock I, Lhommé F, Goldman M, Flamand V. Expansion of regulatory CD8+ CD25+ T cells after neonatal alloimmunization. Clin Exp Immunol. 2011;163(3):354–361. doi: 10.1111/j.1365-2249.2010.04299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005;175(11):7728–7737. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 17.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, Setti M, Puppo F, Indiveri F. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166(10):6452–6457. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183(10):6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, Goronzy JJ, Weyand CM. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005;174(11):7292–7301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 20.Lee YH, Ishida Y, Rifa’i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180(2):825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 21.Chen ML, Yan BS, Kozoriz D, Weiner HL. Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol. 2009;39(12):3423–3435. doi: 10.1002/eji.200939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menager-Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. CD8+CD28- regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131(6):1775–1785. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billerbeck E, Thimme R. CD8+ regulatory T cells in persistent human viral infections. Hum Immunol. 2008;69(11):771–775. doi: 10.1016/j.humimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, de Heer E, Klein MR, Geluk A, Ottenhoff TH. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA. 2007;104(19):8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang RF. CD8+ regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol. 2008;69(11):811–814. doi: 10.1016/j.humimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 26.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58(4):520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 27.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13(23):6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Huang ZF, Xiong G, Mo HY, Qiu F, Mai HQ, Chen QY, He J, Chen SP, Zheng LM, Qian CN, Zeng YX (2011) Distribution, characterization, and induction of CD8+ regulatory T cells and IL-17-producing CD8+ T cells in nasopharyngeal carcinoma. J Transl Med. doi:10.1186/1479-5876-9-189 [DOI] [PMC free article] [PubMed]
- 29.Mayer CT, Floess S, Baru A, M. Lahl K, Huehn J, Sparwasser T. CD8+Foxp3+ T cells share developmental and phenotypic features with classical CD4+Foxp3+ regulatory T cells but lack potent suppressive activity. Eur J Immunol. 2011;41(3):716–725. doi: 10.1002/eji.201040913. [DOI] [PubMed] [Google Scholar]
- 30.Chen YB, Hou JH, Feng XY, Chen S, Zhou ZW, Zhang XS, Cai MY. Decreased expression of Beclin 1 correlates with a metastatic phenotypic feature and adverse prognosis of gastric carcinomas. J Surg Oncol. 2012;105(6):542–547. doi: 10.1002/jso.22151. [DOI] [PubMed] [Google Scholar]
- 31.Xiong H, Du W, Wang JL, Wang YC, Tang JT, Hong J, Fang JY (2012) Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med. doi: 10.1007/s00109-012-0869-0 [DOI] [PubMed]
- 32.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 33.Steer HJ, Lake RA, Nowak AK, Robinson BW. Harnessing the immune response to treat cancer. Oncogene. 2010;29(48):6301–6313. doi: 10.1038/onc.2010.437. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y, Zheng L, Li L (2011) Association of Intra-tumoral Infiltrating Macrophages and Regulatory T Cells Is an Independent Prognostic Factor in Gastric Cancer after Radical Resection. Ann Surg Oncol. doi:10.1245/s10434-011-1609-3 [DOI] [PubMed]
- 35.Wang LX, Li Y, Yang G, Pang PY, Haley D, Walker EB, Urba WJ, Hu HM. CD122+CD8+ Treg suppress vaccine-induced antitumor immune responses in lymphodepleted mice. Eur J Immunol. 2010;40(5):1375–1385. doi: 10.1002/eji.200839210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65(12):5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 37.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179(7):4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 38.Anichini A, Molla A, Vegetti C, Bersani I, Zappasodi R, Arienti F, Ravagnani F, Maurichi A, Patuzzo R, Santinami M, Pircher H, Di Nicola M, Mortarini R. Tumor-reactive CD8+ early effector T cells identified at tumor site in primary and metastatic melanoma. Cancer Res. 2010;70(21):8378–8387. doi: 10.1158/0008-5472.CAN-10-2028. [DOI] [PubMed] [Google Scholar]
- 39.Enarsson K, Lundgren A, Kindlund B, Hermansson M, Roncador G, Banham AH, Lundin BS, Quiding-Järbrink M. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin Immunol. 2006;121(3):358–368. doi: 10.1016/j.clim.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178(5):2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 41.Siegmund K, Rückert B, Ouaked N, Bürgler S, Speiser A, Akdis CA, Schmidt-Weber CB. Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells. J Immunol. 2009;182(4):2124–2130. doi: 10.4049/jimmunol.0802271. [DOI] [PubMed] [Google Scholar]
- 42.Fleissner D, Frede A, Knott M, Knuschke T, Geffers R, Hansen W, Dobos G, Langhorst J, Buer J, Westendorf AM. Generation and function of immunosuppressive human and murine CD8+ T cells by transforming growth factor-β and retinoic acid. Immunology. 2011;134(1):82–92. doi: 10.1111/j.1365-2567.2011.03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]