Abstract
Expression levels of VEGF and Her-2, levels of T-regulatory (Treg) cells, levels of CD3+ cells, and ratios of Th (CD4+ T cells)/Tr (Treg) cells were compared between stage I, II, III, and IV breast cancer patients (n = 120) prior to chemotherapy and healthy women (n = 30). Cells from peripheral blood were counted by flow cytometry, Her-2 and VEGF expression was detected by pathological examination, and Her-2 was detected by FISH. Breast cancer patients had more Treg cells and a lower ratio of Th/Tr cells than the healthy women. Stage IV breast cancer patients had more Treg cells and a lower ratio of Th/Tr cells than stage I, II, or III breast cancer patients. Patients positive for VEGF had a lower ratio of Th/Tr cells compared with patients negative for VEGF, and those positive for both VEGF and Her-2 also had a lower ratio of Th/Tr cells compared with patients not positive for both VEGF and Her-2. The decreased Th/Tr cells ratio indicates impaired immune function, suggesting that the stage IV breast cancer and the Her-2/VEGF-positive breast cancer patients have lower immune function.
Keywords: Breast cancer, Her-2, VEGF, Treg cells, Th/Tr cells ratio
Introduction
Immunity plays a dual role in the interactions between tumors and the host, so the role of the immune system in cancer is now described by the concept of “cancer immunoediting,” which involves three Es: elimination, equilibrium, and escape [1]. The occurrence, development, recurrence, and metastasis of tumors are processes of tumor escape from immune surveillance, which is strongly related to host immune function [2]. Assessing the status of immune function remains elusive, since no ideal indicators have been developed. Lymphocyte numbers are used to assess immune function, but this parameter does not apply to all cancer patients. Detecting subsets of lymphocytes may be beneficial to understand immune function; assist in the clinical diagnosis of diseases; provide evidence for the pathogenesis, disease course, and prognosis of disease; and determine clinical treatment [3].
Poor immune responses in breast cancer patients have been associated with increased T regulatory (Treg) cell activity [4]. Treg cells maintain immune tolerance and protect the body against hyper-response-induced injury, yet they also can suppress the immune activity of tumor-specific T cells, allowing tumor escape from immune surveillance, and facilitating the growth and progression of tumors by negatively regulating the immune response. A study of Treg knock-out mice found that in the absence of Treg cells, the host immune system could reject inoculated tumor cells. Also, blocking Treg cell function with CD25 antibody and CTLA-4 antibody reduces the suppression of cytotoxic T cells and natural killer cells by Treg cells, enabling an effective tumor-specific immune response [5]. Non-small cell lung cancer patients with cancer infiltration in the peripheral blood and lymphoid tissues also have been found to have a significantly increased number of Treg cells [6]. In breast cancer patients, Treg cells are valuable in assessing disease prognosis [4].
A poor prognosis for breast cancer patients is predicted by overexpressed human epidermal growth factor receptor 2 (Her-2) and vascular endothelial growth factor (VEGF) proteins [7, 8]. Her-2 is a proto-oncogene and plays important roles in the growth, infiltration, and metastasis of breast cancer. Overexpressed Her-2 is strongly associated with angiogenesis and increased VEGF expression [9, 10]. The protein VEGF is a key factor that can promote angiogenesis by facilitating proliferation of endothelial cells and increasing the permeability of blood vessels to promote the migration of endothelial cells [11]. About 25% of breast cancer patients overexpress Her-2, and about 88% of those patients have up-regulated VEGF expression [12]. Our previous study found a positive correlation between Her-2 and VEGF in primary and recurrent breast cancers, which indicates that Her-2 and VEGF may synergistically affect breast cancer occurrence and development [13].
Here, we have examined the expression of Her-2 and VEGF, the proportions of Treg and CD3+ cells, and the ratios of T helper (Th)/Tr (Treg) cells and of CD4+/CD8+ in peripheral blood from breast cancer patients and healthy women. We aimed to explore correlations among these parameters to understand whether they could be useful as indicators of immune function.
Subjects and methods
Clinical information
The Ethics Committee of Chinese PLA General Hospital in Beijing approved this study, and informed consent was obtained from the patients and volunteers. We recruited 120 breast cancer patients in our department from January 2008 to June 2010, and their mean age was 46.38 ± 8.93 years (range 24–73 years). The inclusion criteria were as follows: invasive ductal carcinoma, women, 18–75 years old, normal liver and kidney functions, Karnofsky performance status ≥60, no radiotherapy and chemotherapy before hospitalization, no immune system-related diseases or other tumors, and no treatment with steroids or immunosuppressants. Cancer was staged according to the criteria of the Sixth Edition of the American Joint Committee on Cancer (AJCC) [14], with 18 participating patients having stage I cancer, 24 having stage II cancer, 23 having stage III cancer, and 55 having stage IV cancer. An additional 30 healthy female volunteers served as controls and had a mean age of 31.56 ± 11.23 years (range 21–64 years).
Methods
Detection of lymphocyte subsets
Fasting patients allowed us to collect 2 ml of blood from their elbow vein, which was stored in EDTA anti-coagulate tubes. Detection was performed within 4 h by mixing 100 μl of peripheral blood in 500 μl of OptiLyse C (Beckman Coulter,Los Angeles, California, USA), incubating the mixture for 10 min, and then adding 500 μl of PBS to discontinue hemolysis. Next, 20 μl of PC5-conjugated mouse anti-human CD antibody, PE-conjugated mouse anti-human CD25, or CD3 antibodies conjugated to FITC were added, along with the isotype antibody that served as the negative control. The antibodies were all from Beckman Coulter (Los Angeles, California, USA). The samples were then incubated at room temperature for 30 min in the dark and then were analyzed with a flow cytometer (FC500 MPL, Beckman Coulter) for regulatory T cells (Treg or Tr), defined as CD4+ CD25+; T helper cells (Th), defined as CD4+; CD3+ T cells, and CD8+ T cells. The data were analyzed with the corresponding CXP software (Beckman Coulter). The proportion of positive cells was calculated and normalized by non-specific staining. The percentage or level of T-cell subsets was based on total leukocytes after hemolysis.
Tissues prepared by standard immunohistochemistry techniques were incubated at 4°C overnight with either Her-2 monoclonal antibody at 1:200 or VEGF monoclonal antibody at 1:200 (both antibodies from Dako, Copenhagen, Denmark). The known positive sections served as positive controls, and the primary antibody that was replaced with PBS served as negative controls.
Pathological evaluation
The slides were pathologically evaluated by randomly selecting five fields at high magnification and counting more than 500 cells in each field. The slides were scored based on the proportion of positive cells and the intensity of the staining. Cells considered positive were scored as: 0 (0% positive), 1 (1–30% positive), 2 (31–70% positive), or 3 (71–100% positive) [15–17]. Staining intensity scores were as follows: 1 (light brown), 2 (yellowish brown), or 3 (dark brown). The final scores were the product of both scores, and they ranged as follows: 0 (negative), 1–2 (+), 3–4 (++), and 5–6 (+++). The cells positive for Her-2 or VEGF had granules in the cytoplasm and the cell membrane. Slides stained for VEGF with scores higher than “+” were considered positive. Slides stained for Her-2 with scores of “+++” or higher were considered positive, and also those with scores of “+ to ++” that had Her-2 gene amplification by FISH were considered positive. Sections that stained positive (either + or ++) for Her-2 were analyzed by FISH [18] using the PathVysion Her-2 DNA probe kit (Vysis, West Chester, Pennsylvania USA).
Statistic analysis
Data are expressed as means and standard deviations (SDs). Means between two groups were compared by the student’s t test. The differences in cell counts among three groups (healthy women, breast cancer patients of stage I–III, and breast cancer patients of stage IV) were compared by one-way analysis of variance (ANOVA). Multiple comparisons were performed by the Bonferroni procedure with type-I error adjustment when significance was found. The level of significance was set at 0.05. Statistics were analyzed with SAS 9.1 software (SAS Institute Inc., Cary, NC).
Results
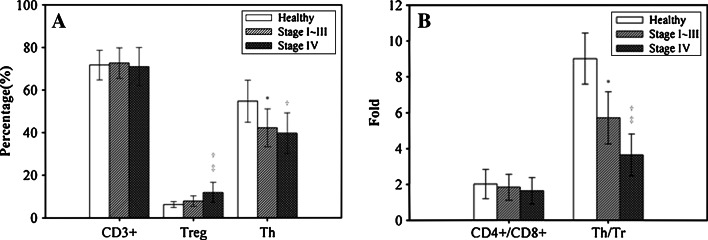
This study enrolled 120 women with breast cancer (mean age 46.38 ± 8.93 years) and 30 healthy women (mean age 31.57 ± 11.23 years). The breast cancer patients with stage IV disease had a higher proportion of Treg cells than patients with stage I, II, or III (11.86 ± 4.75% vs. 7.83 ± 2.47%, P < 0.05) and healthy women (6.20 ± 1.42%, P < 0.05). But, the levels of Treg cells did not significantly differ between healthy women and patients of stage I–III. Regarding Th cells, both patients with stage IV disease (40.62 ± 8.20%) and those with stage I, II, or III (41.19 ± 10.76%) had a significant lower proportion than healthy women (54.77 ± 9.88%), but the difference in Th cells between patients of stage I–III and stage IV was not significant. However, in terms of ratio of Th/Tr cells, the lowest ratio was observed among patients of stage IV (3.64 ± 1.17 fold), the next lowest was among patients of stage I, II, and III (5.71 ± 1.45 fold), and the highest was among healthy women (9.02 ± 1.42 fold). Each pairwise comparison (healthy vs. stage I–III, healthy vs. stage IV, and stage I–III vs. stage IV) had significant differences in the ratio of Th/Tr cells. All three groups had similar proportions of CD3+ T cells and similar ratios of CD4+/CD8+ T cells (Fig. 1).
Fig. 1.
The percentages of CD3+, Treg, and Th cells (a) and the ratios of CD4+/CD8+ cells and Th/Tr cells (b) were compared among healthy adult women (n = 30), breast cancer patients of stage I–III (n = 65), and breast cancer patients at stage IV (n = 55). Data are expressed as means with the standard deviations indicated by the bar. For comparisons where P < 0.05, the * represents a significant difference between stage I–III and the healthy women, the † represents a significant difference between stage IV and the healthy women, and ‡ represents a significant difference between stage I–III and stage IV
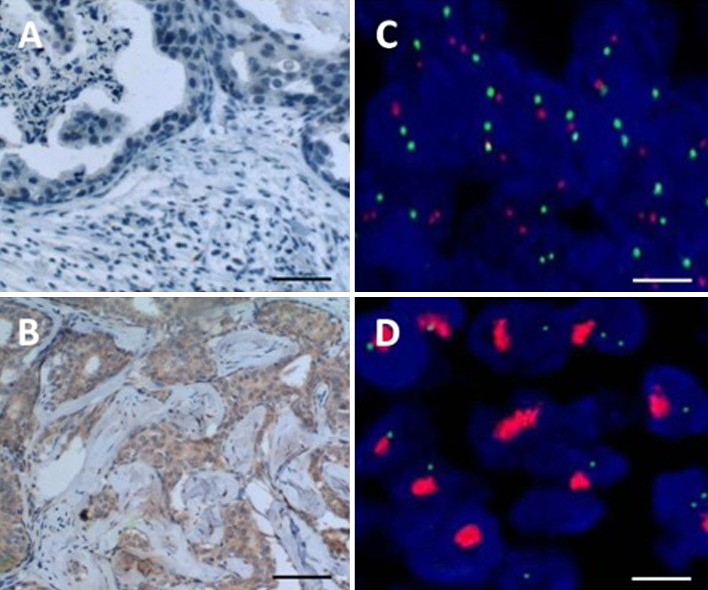
To evaluate whether there is association between the VEGF expression level and another prognostic factor, Her-2, with the relative level of Treg and Th/Treg ratio, the VEGF and Her-2 expression levels in each breast tumor samples were determined by either immunohistochemistry for VEGF or FISH for Her-2 overexpression. The representative result of the immunohistochemistry for low VEGF expression (Fig. 2a), high VEGF (Fig. 2b) expression, normal HER-2 expression (Fig. 2c), and HER-2 overexpression (Fig. 2d) by FISH were shown. The breast cancer patients who were negative for VEGF expression had a higher ratio of Th/Tr cells than those who were positive for VEGF expression (4.99 ± 1.74 vs. 4.22 ± 1.42, P = 0.022) (Table 1). Breast cancer patients who were positive for both Her-2 and VEGF expression had a lower ratio of Th/Tr cells than those who were not positive for both (4.88 ± 1.70 vs. 3.99 ± 1.34, P = 0.047). The proportions of CD3+, Treg, Th, or CD4+/CD8+ cells did not differ significantly based on Her-2 or VEGF expression status.
Fig. 2.
The immunohistochemistry results evaluated the overexpression of VEGF and Her-2. To evaluate the expression of VEGF, breast tissue sections from healthy women (a) and cancer patients (b) were immunostained with anti-VEGF antibody and visualized by DAB. The detection of Her-2 gene expression status was evaluated by FISH. The results from a normal Her-2 expressing (c) or overexpressing (d) tissue were shown. The scale bar = 50 μm in (a) and (b). The scale bar = 2 μm in (c) and (d)
Table 1.
Breast cancer patient Her-2 and VEGF status compared with CD3+ and Treg cell percentages and ratios of CD4/CD8+ and Th/Tr cells
| HER-2 | P value | VEGF | P value | HER-2 and VEGF | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 23) | Negative (n = 97) | Positive (n = 35) | Negative (n = 85) | Both positive (n = 16) | Not both positive (n = 104) | ||||
| CD3+ (%) | 73.03a ± 5.47 | 71.66 ± 8.57 | 0.341 | 72.51 ± 7.21 | 71.68 ± 8.42 | 0.607 | 72.83 ± 5.71 | 71.78 ± 8.38 | 0.632 |
| Treg (%) | 11.37 ± 5.90 | 9.28 ± 3.61 | 0.115 | 11.04 ± 5.18 | 9.11 ± 3.61 | 0.050 | 12.43 ± 6.39 | 9.25 ± 3.61 | 0.069 |
| Th (%) | 41.85 ± 10.00 | 40.90 ± 9.10 | 0.659 | 41.42 ± 9.51 | 40.94 ± 9.19 | 0.798 | 43.77 ± 10.60 | 40.67 ± 9.01 | 0.213 |
| CD4+/CD8+ (ratio) | 1.86 ± 0.92 | 1.72 ± 0.69 | 0.413 | 1.79 ± 0.81 | 1.73 ± 0.71 | 0.689 | 2.04 ± 0.91 | 1.70 ± 0.70 | 0.088 |
| Th/Tr (ratio) | 4.29 ± 1.73 | 4.88 ± 1.66 | 0.130 | 4.22 ± 1.41 | 4.99 ± 1.74 | 0.022* | 3.99 ± 1.34 | 4.88 ± 1.70 | 0.047* |
aData are expressed as mean ± standard deviation
* Indicates P < 0.05, which is a significant difference
Discussion
The breast cancer patients in our study had a higher proportion of Treg (CD4+ CD25+) cells than the healthy control women, in agreement with other research [19]. The stage IV breast cancer patients had significantly more Treg cells than those with stage I, II, or III cancer, indicating the close relationship between the number of Treg cells and the occurrence and development of cancer. This agrees with research indicating that metastatic spread of cancer cells requires Treg cells [20] and with others suggesting that higher numbers of Tregs are associated with a poor prognosis [21, 22]. Ovarian cancer patients having more Treg cells in the cancer and malignant ascites had a poorer prognosis and lower overall survival [23]. Our results suggest that the number of Treg cells in the peripheral blood is closely associated with the occurrence and stage of breast cancer, in agreement with a study finding that Treg cells may be an effective predictor for the value of immunotherapy for advanced stage breast cancer patients [24]. However, another study suggested that Treg cell frequency did not correlate directly with clinical stage of breast cancer, though their study only looked at stage II and IV breast cancer patients [25].
We also found a trend toward higher levels of Treg in Her-2-positive individuals compared with Her-2-negative individuals, though it did not reach significance. This agrees with another study, which found that Her-2-positive individuals had higher levels of Treg than healthy individuals, though they observed similar proportions of Treg cells between breast cancer patients who were negative for Her-2 and healthy individuals [26]. Our study defined Treg cells as those which were CD4+ CD25+, while they defined Treg cells as CD4+ FoxP3+. They explained that reductions in non-regulatory CD4+ T cells may explain the apparent increase in Treg cell numbers that they observed in breast cancer patients.
The breast cancer patients in our study had a lower ratio of Th/Tr cells than in the healthy control women, suggesting that the patients were immunosuppressed and had significantly compromised immune function. However, the association between Th/Tr cell ration and cancer stage is still controversial [26]. Other studies have found that the ratio of Th/Tr cells is an appropriate indicator to evaluate the anti-tumor state [27, 28] and have suggested the use of peripheral blood leukocytes to predict immunotherapy responses [29]. We suggest that the lower Th/Tr cells ratio that we observed in stage IV breast cancer patients compared to stage I, II, or III cancer patients indicates that the peripheral Th/Tr cells ratio decreases as cancer develops. As the disease progressed, immune function progressively declined. Cancer development leads to more imbalanced T-cell subsets, with the compromised immune function resulting in cancer progression [30].
Patients who were positive for VEGF had a lower Th/Tr cells ratio than those negative for VEGF, which suggested the close relationship of angiogenesis and the Th/Tr cells ratio. Patients positive for both Her-2 and VEGF proteins had a lower ratio of Th/Tr cells compared with patients who were not positive for both proteins, indicating that highly malignant tumors have the ability of evading immune surveillance. In those patients with advanced stage breast cancer, we suggest that Treg cell proliferation is promoted, and, in turn, the tumor-specific immune response is suppressed. These conditions benefit the growth and metastasis of cancer, while they are detrimental for immunotherapy and are involved in the escape of tumors from immune surveillance.
Our study is limited by the small sample size and by the age difference between the breast cancer patients and healthy volunteer women. Our data do suggest that the ratio of Th/Tr cells is associated with Her-2 and VEGF expression.
Our results reveal that higher counts of Treg cells are related to the occurrence of breast cancer and to the development of stage IV breast cancer. We suggest that the ratio of Th/Tr cells may be used to evaluate the immune function of patients, as this ratio was lower in stage IV patients than in stage I, II, or III patients. A decreased Th/Tr cells ratio implies impaired immune function and susceptibility to metastasis. This information may provide a link between poor immune function and late stage of breast cancer and also between the expression of both Her-2 and VEGF.
Acknowledgments
This work was supported by the Innovation Fund from the Chinese PLA General Hospital (No. 06LN14).
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Croci DO, Zacarías Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother. 2007;56(11):1687–1700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krupnick AS, Kreisel D, Szeto WY, Popma SH, Amin KM, Moore JS, Rosengard BR. Multiparameter flow cytometric approach for simultaneous evaluation of T lymphocyte endothelial cell interactions. Cytometry. 2001;46(5):271–280. doi: 10.1002/cyto.1168. [DOI] [PubMed] [Google Scholar]
- 4.Abo-Elenein A, Elgohary S, Hashish A, El-Halaby E. Significance of immunoregulatory T cells in different stages of breast cancer patients. Egyptian J Immunol/Egyptian Ass Immunologists. 2008;15(2):145. [PubMed] [Google Scholar]
- 5.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Chao QG, Ping LZ, Xue C, Xia ZY, Qian D, Shi-ang H. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm. 2009;24(3):357–367. doi: 10.1089/cbr.2008.0612. [DOI] [PubMed] [Google Scholar]
- 7.Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Fry EA, Inoue K. Classical and novel prognostic markers for breast cancer and their clinical significance. Cli Med Insights Oncol. 2010;4:15–34. doi: 10.4137/cmo.s4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group IBCS. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: international breast cancer study group trial 13–93. J Clin Oncol. 2006;24(9):1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 9.Erdem O, Dursun A, Coskun U, Gunel N. The prognostic value of p53 and c-erbB-2 expression, proliferative activity and angiogenesis in node-negative breast carcinoma. Tumori. 2005;91(1):46–52. doi: 10.1177/030089160509100109. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell KL, Dewhirst MW, Liotcheva V, Snyder S, Broadwater G, Bentley R, Lal A, Riggins G, Anderson S, Vredenburgh J. HER-2 gene amplification correlates with higher levels of angiogenesis and lower levels of hypoxia in primary breast tumors. Clin Cancer Res. 2004;10(12):4083. doi: 10.1158/1078-0432.CCR-03-0695. [DOI] [PubMed] [Google Scholar]
- 11.Clere N, Bermont L, Fauconnet S, Lascombe I, Saunier M, Vettoretti L, Plissonnier ML, Mougin C. The human papillomavirus type 18 E6 oncoprotein induces Vascular Endothelial Growth Factor 121 (VEGF121) transcription from the promoter through a p53-independent mechanism. Exp Cell Res. 2007;313(15):3239–3250. doi: 10.1016/j.yexcr.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Tai W, Qin B, Cheng K. Inhibition of breast cancer cell growth and invasiveness by dual silencing of HER-2 and VEGF. Mol Pharm. 2010;7(2):543–556. doi: 10.1021/mp9002514. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Meng H, Han C, et al. Expression of human epidermal growth factor 2 and vascular endothelial growth factor in primary or recurrent metastatic breast cancer. Zhong Guo Yi Xue Ke Xue Yuan Xue Bao. 2010;32(4):403–406. doi: 10.3881/j.issn.1000-503X.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Greene F, Page D, Fleming I, Fritz A, Balch C, Haller D, Morrow M. AJCC cancer staging man. NY: Springer; 2002. [Google Scholar]
- 15.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 16.Al-Dissi AN, Haines DM, Singh B, Kidney BA. Immunohistochemical expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 in canine simple mammary gland adenocarcinomas. Can Vet J. 2010;51(10):1109. [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Yang W. Standardization of immunohistochemistry result. China Oncology. 1996;6(4):229–231. [Google Scholar]
- 18.Lan C, Liu JM, Liu TW, Hsu DH, Liang S, Chen JR, Peng JW. erb-b2 amplification by fluorescence in situ hybridization in breast cancer specimens read as 2+ in immunohistochemical analysis. Am J Clin Pathol. 2005;124(1):97. doi: 10.1309/R2X4KK22QCL7PLME. [DOI] [PubMed] [Google Scholar]
- 19.Horlock C, Stott B, Dyson P, Morishita M, Coombes R, Savage P, Stebbing J. The effects of trastuzumab on the CD4+ CD25+ FoxP3+ and CD4+ IL17A+ T-cell axis in patients with breast cancer. Br J Cancer. 2009;100(7):1061. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 22.Wolf AM, Rumpold H, Wolf D, Gastl G, Reimer D, Jenewein N, Marth C, Zeimet AG. Role of forkhead box protein 3 expression in invasive breast cancer. J Clin Oncol. 2007;25(28):4499. doi: 10.1200/JCO.2007.13.2092. [DOI] [PubMed] [Google Scholar]
- 23.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 24.Wada J, Yamasaki A, Nagai S, Yanai K, Fuchino K, Kameda C, Tanaka H, Koga K, Nakashima H, Nakamura M, Tanaka M, Katano M, Morisaki T. Regulatory T-cells are possible effect prediction markers of immunotherapy for cancer patients. Anticancer Res. 2008;28(4C):2401–2408. [PubMed] [Google Scholar]
- 25.Perez SA, Karamouzis MV, Skarlos DV, Ardavanis A, Sotiriadou NN, Iliopoulou EG, Salagianni ML, Orphanos G, Baxevanis CN, Rigatos G. CD4+ CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res. 2007;13(9):2714. doi: 10.1158/1078-0432.CCR-06-2347. [DOI] [PubMed] [Google Scholar]
- 26.Rech AJ, Mick R, Kaplan DE, Chang KM, Domchek SM, Vonderheide RH. Homeostasis of peripheral FoxP3+ CD4+ regulatory T cells in patients with early and late stage breast cancer. Cancer Immunol Immunother. 2010;59(4):599–607. doi: 10.1007/s00262-009-0780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brivio F, Fumagalli L, Parolini D, Messina G, Rovelli F, Rescaldani R, Vigore L, Vezzo R, Vaghi M, Di Bella S. T-helper/T-regulator lymphocyte ratio as a new immunobiological index to quantify the anticancer immune status in cancer patients. In Vivo. 2008;22(5):647. [PubMed] [Google Scholar]
- 28.Lissoni P, Brivio F, Fumagalli L, Messina G, Meregalli S, Porro G, Rovelli F, Vigore L, Tisi E, D’amico G. Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory T lymphocytes in cancer patients. Anticancer Res. 2009;29(5):1847. [PubMed] [Google Scholar]
- 29.Characiejus D, Jacobs JJL, Pasukoniene V, Kazlauskaite N, Danileviciute V, Mauricas M, Den Otter W. Prediction of response in cancer immunotherapy. Anticancer Res. 2011;31(2):639–647. [PubMed] [Google Scholar]
- 30.Ohwada S, Iino Y, Nakamura S, Takeyoshi I, Tanahashi Y, Izumi M, Kawashima Y, Arai M, Kobayashi I, Sato Y. Peripheral blood T cell subsets as a prognostic factor in gastric cancer. Jpn J Clin Oncol. 1994;24(1):7. [PubMed] [Google Scholar]