Abstract
DNA vaccine has been suggested to use in cancer therapy, but the efficacy remains to be improved. The immunostimulatory effect of a fungal immunomodulatory protein Ling Zhi-8 (LZ-8) isolated from Ganoderma lucidum has been reported. In this study, we tested the adjuvanticity of LZ-8 for HER-2/neu DNA vaccine against p185neu expressing tumor MBT-2 in mice. We found that recombinant LZ-8 stimulated mouse bone marrow-derived dendritic cells (DCs) via TLR4 and its stimulatory effect was not due to any microbe contaminant. In addition, LZ-8 enhanced the ability of DCs to induce antigen-specific T cell activation in vitro and in a subunit vaccine model in vivo. Surprisingly, LZ-8 cotreatment strongly improved the therapeutic effect of DNA vaccine against MBT-2 tumor in mice. This increase in antitumor activity was attributed to the enhancement of vaccine-induced Th1 and CTL responses. Consistent with the results from DCs, the promoting effect of LZ-8 on DNA vaccine was diminished when the MBT-2 tumor cells were grown in TLR4 mutant mice. Thus, we concluded that LZ-8 may be a promising adjuvant to enhance the efficacy of DNA vaccine by activating DCs via TLR4.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1016-4) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cell, Adjuvant, DNA vaccine, Cancer therapy, TLR4
Introduction
DNA vaccination is a powerful strategy for antigen-specific immunotherapy against various diseases, including cancer [1, 2]. They can be repeatedly administered, high-temperature stability, easily prepared in large scale with high purity, and ability to induce long-lasting immune responses [3]. The preclinical studies reveal that DNA vaccines can generate antigen-specific immunity against established tumors [4]; however, the low efficacy of DNA vaccines in large animals and human has impaired their practical use [5]. Therefore, development of novel approaches to increase the DNA vaccine potency is a primary goal in cancer therapy [6]. Identification of a strong adjuvant is one of the strategy for enhancing the immunogenicity of DNA vaccine [7].
Dendritic cells (DCs) are professional antigen-presenting cells, and play a critical role in initiating immune responses induced by vaccine [8]. Toll-like receptors (TLRs) play an important role in the innate recognition of pathogen-associated molecular patterns (PAMPs) and initiation of immune responses in DCs [9]. Because of the key regulatory role in immune responses, DCs are being developed as potent new vaccines for the treatment of cancer and viral infections [10]. In addition, finding materials that can modulate the DC function is an emerging field that is developing alongside DC immunobiology [11]. Natural or artificial substances that promote DC activation can potentially be adjuvant candidates and applied to immunotherapy and vaccination.
A number of pharmaceutically active compounds have been isolated from Ganoderma lucidum, a well-known medicinal fungus in Asia [12, 13]. A fungal immunomodulatory protein (FIP) Ling Zhi-8 (LZ-8) is isolated from G. lucidum mycelia [14] and forms a FIP family together with FIP-gts (identical to LZ-8 but isolated from G. tsugae), FIP-fve (from Flammulina velutipes), FIP-vvo (from Volvariella volvacea), and FIP-gsi (from G. sinensis) [15–17]. Some studies have shown the immunomodulatory effect of LZ-8 on autoimmunity and transplantation [18], and LZ-8 can also work as a mitogen to activate T cells [19]. Recently, the immunostimulatory effect of LZ-8 on human DCs has been reported [20], implying that LZ-8 may be an adjuvant candidate. However, there is no evidence for applying LZ-8 in vaccination or cancer therapy yet.
In this study, we investigated whether LZ-8 can improve the immunogenicity of DNA vaccine in a preclinical model used HER-2/neu as antigen [21]. Our results showed that recombinant LZ-8 activated mouse DCs as it did in human DCs via TLR4. Significantly, LZ-8 cotreatment enhanced the therapeutic efficacy of HER-2/neu DNA vaccine against HER-2/neu-overexpressing tumor, and this promoting effect was mediated by TLR4 in vivo. Thus, we demonstrated that LZ-8 could be a novel adjuvant and has potential to apply in cancer therapy and vaccination.
Materials and methods
Mice and cell cultures
C57BL/6, C3H/HeN, and C3H/HeJ (TLR4 mutant) mice were purchased from National Laboratory Animal Center (Taipei, Taiwan) or National Cheng-Kung University (Tainan, Taiwan). OT-I and OT-II TCR transgenic mice were provided by Dr. Clifford Lowell (UCSF, San Francisco, CA). All mice were housed in the barrier facility at NHRI (Taiwan) under an Institutional Animal Care and Use Committee-approved protocol. Mouse DCs were generated from bone marrow as previously described [22]. The murine bladder tumor cell line MBT-2 has been described and is known to express high levels of p185neu [21].
Preparation and inactivation of LZ-8
LZ-8 was cloned and expressed in Saccharomyces cerevisiae. Cells expressing LZ-8 (or empty vector as control) were disrupted, centrifuged, and the supernatant was passed through filter and molecular sieves to obtain proteins between 10 and 100 kDa. The filtrate was further purified using FPLC with Superdex 75 columns (GE Healthcare). The purity was determined by FPLC. To inhibit LPS activity, the sample was treated with polymyxin B (5 μg/ml, Sigma–Aldrich) at 37°C for 30 min. To inactivate LZ-8, the protein was treated with proteinase K (2 mg/ml, Promega) at 37°C for 1 h or boiled at 100°C for 50 min.
Detection of TNF-α production
DCs were treated with LZ-8 for 6 h, and the intracellular TNF-α was detected by anti-TNF-α mAb (Biolegend) as described previously [23]. For quantifying the production of TNF-α, supernatants were collected from 1 × 106 DCs/ml after incubation with LZ-8 (5 μg/ml), LPS (20 ng/ml, Sigma–Aldrich), or CpG (500 nM, InvivoGen) for 6 h, and then assayed using ELISA kits (eBioscience).
Assays for T cell activation
Antigen presentation by DCs was determined as described previously [24]. DCs were incubated with 1 μg/ml OVA257–264 (OVAP1) or OVA323–339 (OVAP2) (synthesized by Echo Chemical Co., Taiwan) in the presence or absence of LZ-8 for 3 h, and LZ-8 was washed out to avoid its mitogenic effect on T cells [19], and then OT-I or OT-II T cells were added to the culture. T cell proliferation was determined by [3H]thymidine incorporation after 72 h. For recall assay in vivo, a subunit vaccine model was used. C57BL/6 mice were immunized with OVAP2 (10 μg) mixed with incomplete Freund’s adjuvant (IFA, Sigma–Aldrich) alone or IFA and LZ-8 (10 μg) via footpad injection. Draining lymph node (LN) cells were isolated after 10 days and cultured with OVAP2 for 3 days, and T cell proliferation was determined by [3H]thymidine incorporation. Supernatants from DC–OT-I/OT-II or LN cell cultures were collected after 4 days, and IFN-γ production was measured by ELISA (eBioscience).
Therapeutic efficacy in a mouse model of established MBT-2 tumors
The MBT-2 tumor model was described previously [25]. Briefly, MBT-2 cells (1 × 106) were injected subcutaneously (s.c.) into the flank of C3H/HeN (WT) or C3H/HeJ (TLR4 mutant) mice. Ten days after injection, the mice were immunized with the naked HER-2/neu DNA vaccine or pRc/CMV vector (10 μg) from the shaved abdominal region for three times at weekly intervals using a low pressure-accelerated gene gun (Bioware, Technologies Co. Ltd, Taipei, Taiwan), with or without LZ-8 treated by local intraperitoneal injection. The effects of these treatments on the growth of MBT-2 tumors were then monitored triple a week. Tumor size was calculated using the formula for a rational ellipse: (m1 × m2 × m2 × 0.5236), where m1 represents the longer axis and m2 the shorter axis. Mice were euthanized when tumor size reached >2,500 mm3 in mean diameter.
Quantitative RT–PCR
After the last DNA vaccination for 3 days, inguinal LN cells (2 × 106 cells/well) from immunized C3H/HeN mice were stimulated with recombinant HER-2/neu protein (10 μg/ml, R&D Systems) in 24-well plate with complete RPMI 1640 medium. After 20 h, CD4+ or CD8+ T cells were purified by negative selection (Dynal CD4 and CD8 Negative Isolation Kit, Dynal AS, Oslo, Norway; purity >90%). Then, total RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was generated from denatured total RNA using oligo dT as the primer and MMLV-Reverse Transcriptase (Promega, Madison, Wisconsin, USA) according to the manufacturer’s instructions as described previously [26]. The used primers were: IFN-γ F: 5′-ACTGGCAAAAGGATGGTGAC-3′ and R: 5′-ACCTGTGGGTTGTTGACCTC-3′; and hypoxanthine guanine phosphoribosyl transferase 1 (HPRT), F: 5′-GTTGGATAAGGCCAGACTTTGTTG-3′ and R: 5′-GATTCAACTTGCGCCATCTTAGGC-3′. ABI 7500 Fast Real-Time system with SYBR Green PCR Master Mix (Applied Biosystems) was used for quantitative real-time PCR. The expression was normalized against HPRT. These normalized data were then expressed relative to the vector control group.
Inguinal LN cell-mediated cytotoxicity assay for targeting MBT-2 cells
The protocol was modified from our previous report [27]. In brief, inguinal LN cells from C3/HeN or C3/HeJ mice were cultured in complete RPMI 1640 medium and stimulated with recombinant HER-2/neu protein (10 μg) for 5 days. The antigen-stimulated cells (effector cells) were cocultured with 1 × 104 MBT-2-luciferase cells at various E:T ratios in 96-well round-bottomed plates for 12 h. Cytolysis was determined by quantitatively measuring luciferase activity in the supernatant from each well as reported previously.
Statistical analysis
Statistical analysis in cytokine production, T cell proliferation, and CTL activity assay were evaluated by a Student’s t-test with 2 sample equal variance with a 2-tailed distribution. Data are showed as mean + SD of three samples. The P < 0.05 is considered statistically significant. For the comparison between the survival rates of various vaccinated mice, we used the Kaplan–Meier method and log-rank analysis using GraphPad Prism software package version 4.0 (GraphPad Software; San Diego, CA, USA).
Results
The activation of mouse DCs by recombinant LZ-8 was not due to microbial contaminants
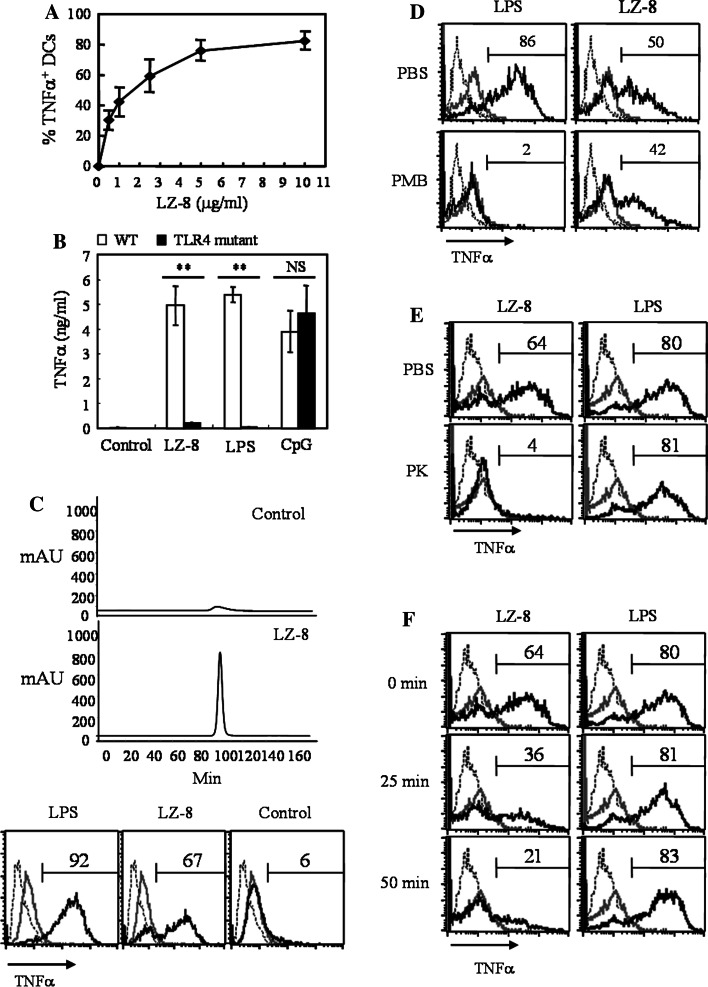
The stimulatory activity of LZ-8 on human DCs has been identified [20]; however, the application of LZ-8 in vaccination and cancer therapy is not studied. Since we used a mouse model to evaluate the adjuvant effect of LZ-8, we first tested whether LZ-8 could activate mouse DCs. As shown in human DCs previously, LZ-8 promoted cytokine and chemokine production and maturation of bone marrow-derived DCs from C57BL/6 mice (Fig. 1a and Supplementary Figs. 1, 2). The saturated dose of our LZ-8 for maximal TNF-α production was at 5 μg/ml. We observed no significant cytotoxicity at the highest dose of LZ-8 as measured by propidium iodide staining (data not shown). Furthermore, the LZ-8-induced TNF-α production was dramatically reduced in TLR4 mutant (C3H/HeJ) DCs when compared with WT (C3H/HeN) cells (Fig. 1b), consistent to the report in human DCs [20]. A very important issue is that we must rule out the effect of microbial contamination because we used a yeast expression system to generate recombinant LZ-8 protein. Therefore, we conducted a series of experiments to address this issue. First, we prepared a control solution from yeasts that did not express LZ-8 but were processed in identical way to those expressing LZ-8, especially used the same column. FPLC analysis showed nothing in control solution except for a few of LZ-8, probably from the LZ-8 residues in column (Fig. 1c upper). This control solution did not activate DCs from C57BL/6 mice (Fig. 1c lower), indicating that our process of LZ-8 preparation did not introduce any microbial contaminant. Next, polymyxin B treatment did not significantly inhibit the activity of LZ-8, suggesting that endotoxin contamination was negligible (Fig. 1d). Furthermore, proteinase K-digested (Fig. 1e) and heat-inactivated (Fig. 1f) LZ-8 lost the ability to stimulate DCs, illustrating that DCs were specifically activated by the LZ-8 protein. Overall, these data strongly argue against the possibility that any microbial contaminant would contribute to the activating effect of LZ-8 on DCs.
Fig. 1.
Stimulatory activity of LZ-8 on DCs was not due to contamination. Mouse bone marrow-derived DCs were incubated with LZ-8 at indicated doses. After 6 h, the percentages of TNF-α-producing CD11c+ cells were determined by intracellular staining and flow cytometry. a Dose response curve of LZ-8 to DCs derived from C57BL/6 mice. b DCs derived from C3H/HeN (WT) and C3H/HeJ (TLR4 mutant) mice were untreated or treated with LZ-8 (5 μg/ml), LPS (20 ng/ml), or CpG (500 μM). Supernatants were collected after 6 h and TNF-α production was measured by ELISA. Error bars indicate ±SD of three samples. NS P > 0.05; **P < 0.01 (Student’s t-test) was comparison between WT and mutant cells. c Upper panels: the FPLC map for control solution (prepared from yeasts expressing empty vector) and LZ-8; Lower panels: DCs from C57BL/6 mice were untreated (gray line) or treated with LPS (20 ng/ml), LZ-8 (5 μg/ml), or control solution (black line) for 6 h, and the percentages of TNF-α-producing CD11c+ cells were showed above the regional marker. DCs were treated as in (c) except that LPS and LZ-8 were treated with polymyxin B (PMB) for 30 min (d), incubated with proteinase K (PK) for 1 h (e), or boiled for 25 and 50 min (f) before being added to DC cultures. Dotted line represents staining with an isotype-matched control antibody. All data are representative of two to four independent experiments
LZ-8 facilitated DC-induced Ag-specific T cell activation in vitro and in vivo
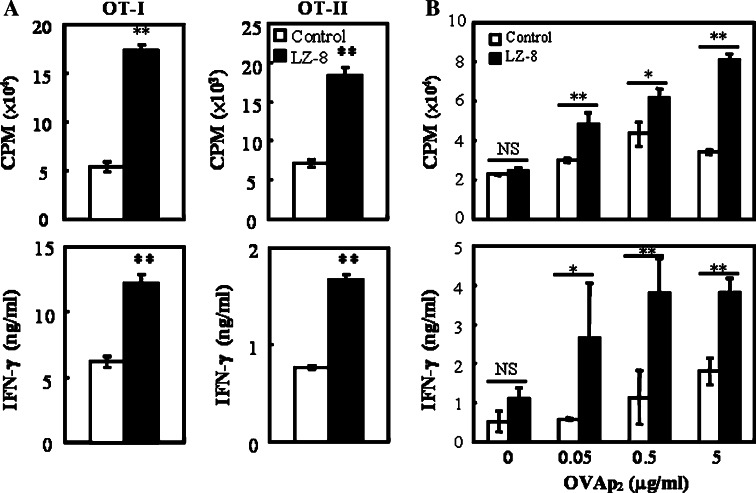
Induction of antigen-specific T cell activation is the primary function of mature DCs, so we next tested whether LZ-8-stimulated DCs are able to activate naïve T cells. OT-I or OT-II T cells were cocultured with LZ-8-treated, OVAP1- or OVAP2-pulsed DCs, and T cell proliferation and IFN-γ production were determined. LZ-8-activated DCs induced more OVA-specific T cell proliferation and IFN-γ production than control cells in vitro (Fig. 2a). Then, we performed a subunit vaccine model to evaluate the adjuvant effect of LZ-8 on T cell priming in vivo. C57BL/6 mice were immunized with OVAP2 mixed with IFA alone or IFA plus LZ-8, and draining LN cells were collected after 10 days. The LN T cells isolated from LZ-8-immunized mice showed more proliferation and IFN-γ production than cells from control mice in response to OVAp2 (Fig. 2b). These data reveal that LZ-8-stimulated DCs can induce Ag-specific T cell activation both in vitro and in vivo. The secretion of IL-12 from DCs (Supplementary Fig. 1a) and the production of IFN-γ from activated T cells indicate that LZ-8-stimulated DCs preferentially promote Th1 differentiation.
Fig. 2.
LZ-8 enhanced the ability of DCs to induce Ag-specific T cell activation. a Untreated (control) or LZ-8 (5 μg/ml)-treated, OVAP1 or OVAP2 (1 μg/ml)-pulsed DCs were cocultured with OT-I or OT-II T cells for 72 h. T cell proliferation was determined by [3H]thymidine incorporation (upper panels), and IFN-γ production was measured by ELISA (lower panels). b C57BL/6 mice were immunized with OVAP2 (10 μg) mixed with IFA alone (control) or IFA + LZ-8 (10 μg). Draining LN cells were collected after 10 days and cultured with indicated concentration of OVAP2 for 3 days. T cell proliferation was determined by [3H]thymidine incorporation (upper), and IFN-γ production was measured by ELISA (lower). Error bars indicate ±SD of three samples. *P < 0.05; **P < 0.01 (Student’s t-test) was comparison between LZ-8-treated and untreated cells or mice. All data are representative of two to four independent experiments
LZ-8 enhanced the antitumor effect of HER-2/neu DNA vaccine
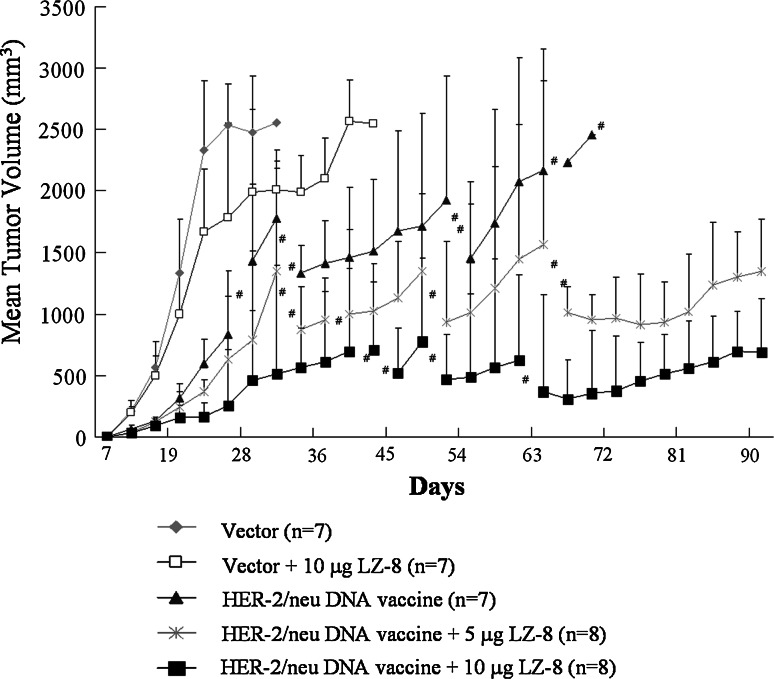
We have demonstrated that gene gun-delivered naked HER-2/neu DNA vaccine has therapeutic effect on established p185neu-expressing MBT-2 tumors in C3H/HeN mice [28]. Based on this model, we further tested whether LZ-8 can increase its efficacy. As shown in Fig. 3, immunization with neither the control vector alone nor LZ-8 alone could reduce tumor growth in mice, and all mice in these two groups had to be sacrificed due to the size of their tumors or their condition; it is expected that these mice would have become moribund by day 41 at the latest. In contrast, vaccination with the HER-2/neu DNA alone or HER-2/neu DNA plus LZ-8 (5 or 10 μg) significantly inhibited tumor growth after tumor implantation for 22 days. However, the HER-2/neu DNA plus LZ-8 was more effective than the HER-2/neu DNA alone in prolonging the survival of the mice. At day 69 after tumor cells implantation, all mice with HER-2/neu DNA vaccine alone had to be sacrificed, but 2/8 mice with HER-2/neu DNA vaccine plus 5 μg LZ-8 and 4/8 mice in HER-2/neu DNA vaccine plus 10 μg LZ-8 survived to day 90, at which time the experiment was terminated. These results clearly indicate that LZ-8 efficiently enhances the therapeutic efficacy of HER-2/neu DNA vaccine against p185neu-expressing MBT-2 tumors in vivo.
Fig. 3.
LZ-8 enhanced the efficacy of HER-2/neu DNA vaccine against MBT-2 tumor in C3H/HeN mice. C3H/HeN mice were implanted with MBT-2 tumor cells and then vaccinated with various formulas as indicated. Line graphs showed the depiction of tumor volumes at the indicated time. The symbol (#) indicates that some mice were sacrificed due to the mean diameter of the tumor reaching 2,500 mm3 or the poor condition of the mice. Subsequently, the mean tumor volume was calculated from the remaining mice. Error bars indicate +SD of remaining mice. Data are representative of three independent experiments
LZ-8 promoted the Th1 and CTL responses induced by HER-2/neu DNA vaccination
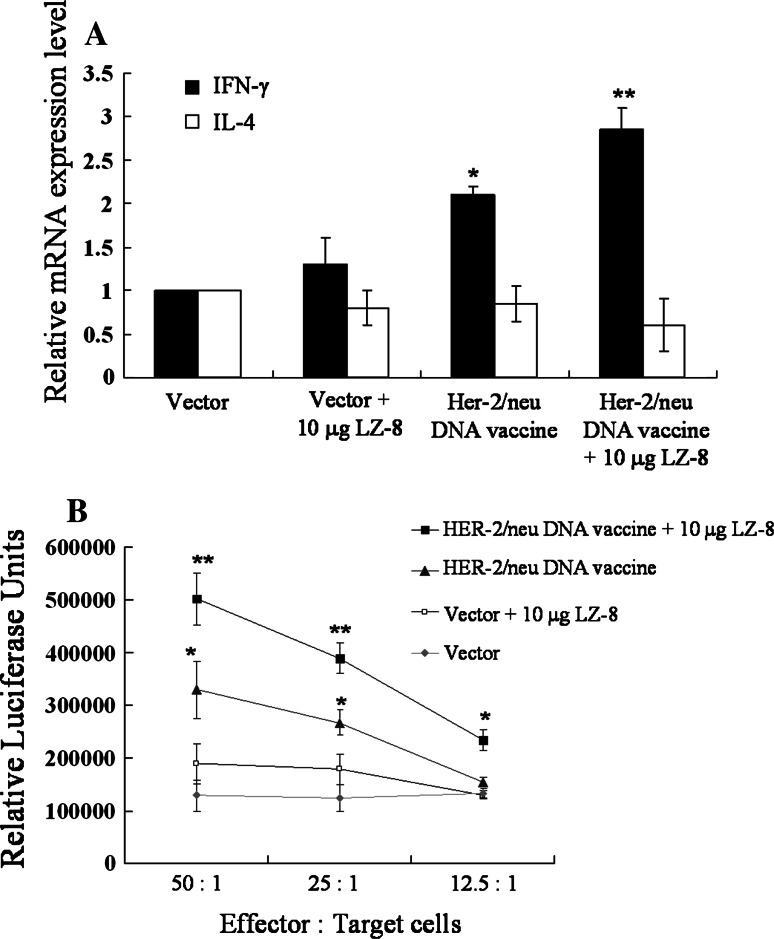
Th1-based immune responses have been identified to play a crucial role in suppressing implanted MBT-2 tumor in mice [27, 28]. In addition, we have showed that LZ-8-stimulated DCs preferentially facilitate Th1 differentiation (Fig 2). Therefore, we examined whether LZ-8 can promote Th1 responses induced by HER-2/neu DNA vaccination. LN cells collected from vaccinated mice were stimulated with recombinant HER-2/neu protein, and the expression of IFN-γ (Th1) and IL-4 (Th2) was determined by quantitative real-time PCR. Mice immunized with HER-2/neu DNA vaccine plus LZ-8 produced significantly higher amount of IFN-γ than that immunized with HER-2/neu DNA vaccine alone (Fig. 4a), whereas no significant difference in IL-4 production. Since Th1 differentiation promotes cellular immune responses such as cytotoxic T lymphocyte (CTL) expansion and activation, we further examined the CTLs responses in vaccinated mice. As shown in Fig. 4b, the LN cells collected from mice immunized with LZ-8 plus HER-2/neu DNA vaccine generated higher level of HER-2/neu-specific CTL response than that from the mice immunized with HER-2/neu DNA vaccine alone. The cells isolated form control vector-immunized mice showed no significant p185neu-induced MBT-2 cytotoxicity. These data illustrate that LZ-8 promotes HER-2/neu-specific Th1 responses induced by HER-2/neu DNA vaccination, which could contribute to the enhancement of antitumor efficacy of HER-2/neu DNA vaccine.
Fig. 4.
LZ-8 promoted Th1 differentiation and CTL response to MBT-2 cells in vaccinated mice. The inguinal LN cells were isolated from vaccinated C3H/HeN mice as indicated formulas and then were stimulated with recombinant HER-2/neu protein (10 μg/ml). a After 3 days, the expression levels of IFN-γ or IL-4 mRNA expression in the stimulated CD4+ T cells were determined using quantitative real-time PCR. The data were normalized to HPRT expression in each sample, and error bars indicate mean ± SD of five mice. b After 5 days, the stimulated LN cells were collected as effector cells. CTL-mediated cytotoxicity assay was performed by incubating the stimulated LN cells with serial dilutions of MBT-2-luciferase cells (target cells), and then detecting luciferase activity in the supernatants. The data are presented as the mean ± SD of three mice. The symbol (**) indicates a statistically significant difference when compared LZ-8-treated to untreated DNA vaccinated mice (P < 0.05). The symbol (*) indicates a statistically significant difference when compared DNA vaccine-treated to control vector-treated mice (P < 0.001). All data are representative of two to three independent experiments
The adjuvanticity of LZ-8 on HER-2/neu DNA vaccine is mediated via TLR4
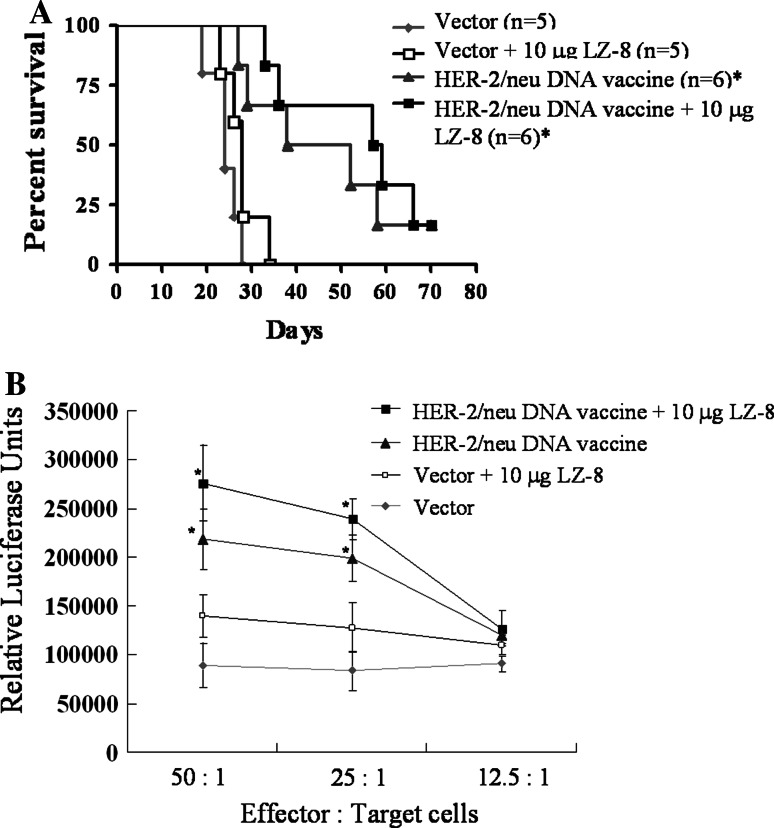
TLR4 has been shown to involve in LZ-8-induced DC activation (Fig. 1b). Thus, we asked whether the enhanced efficacy of LZ-8-cotreated HER-2/neu DNA vaccine was related to TLR4. As shown in Fig. 5a, HER-2/neu DNA vaccine significantly prolonged the survival of tumor-bearing C3H/HeJ (TLR4 mutant) mice compared with control group; however, the cotreatment of LZ-8 (10 μg) had almost no effect on the efficacy of HER-2/neu DNA (P = 0.69). Furthermore, limiting increment of the CTL activity was induced in TLR4 mutant mice immunized with HER-2/neu DNA vaccine plus LZ-8 when compared with the immunization of HER-2/neu DNA vaccine alone (P = 0.17 in DC/T = 50, P = 0.21 in DC/T = 25) (Fig. 5b). These results suggest that TLR4 is required for the adjuvanticity of LZ-8 on HER-2/neu DNA vaccine.
Fig. 5.
TLR4 played a major role in the enhancing effect of LZ-8 on HER-2/neu DNA vaccine. C3H/HeJ (TLR4 mutant) mice were implanted with MBT-2 tumor cells and then were inoculated with HER-2/neu DNA vaccines with various formulas as indicated. a Kaplan–Meier survival curves of surviving mice (%) as a function of time after tumor challenge. The symbol (*) indicates a statistically significant difference when compared DNA vaccine-treated to control vector-treated mice (P < 0.05). There were no significant difference between HER-2/neu DNA vaccine and HER-2/neu DNA vaccine plus 10 μg LZ-8 (P = 0.69). Similar data were obtained from two independent experiments. b As described in Fig. 4b, the activated LN cells from vaccinated C3H/HeJ mice were cocultured with MBT-2-luciferase cells at indicated ratios, and then luciferase activity in the supernatants was determined. The data are presented as the mean ± SD of three mice. The experiments were repeated two times, and similar results were obtained. The symbol (*) indicates a statistically significant difference when compared DNA vaccine-treated to control vector-treated mice (P < 0.001). There were no significant difference between HER-2/neu DNA vaccine and HER-2/neu DNA vaccine plus 10 μg LZ-8 (P = 0.17 in DC/T = 50, P = 0.21 in DC/T = 25)
Discussion
To identify substances that can enhance DC function for vaccination purpose is currently under intense investigations. In this study, we demonstrated that LZ-8 exhibits potent activating activities on mouse DCs and enhances the efficacy of DNA vaccine against cancer. Interestingly, the immunostimulatory function of LZ-8 is dependent on TLR4 in vivo. Thus, our data provide convincing evidences that LZ-8 is a novel adjuvant for DNA vaccine in cancer therapy.
DNA vaccine has been well established in animal models for preventing and treating various diseases; however, low immunogenicity is the major obstacle for the development of DNA vaccines in large animal models and human. We have tried several approaches to overcome this hurdle, including fusion to activating cytokine genes [21], alternation of administration route [29], treatment of Geldanamycin [25]/indoleamine 2,3-dioxygenase siRNA [30]/histone deacetylase inhibitor [27]/a fungal extract [31], delivery by gene gun [28], and DNA formulation [32]. Here, we report an effective immunostimulator LZ-8 to strengthen the immunogenicity. Similar strategy has been shown by using heat shock proteins (HSPs) [33]. It will be valuable to compare the effect of LZ-8- to HSP-DNA vaccine on cancer therapy.
Very interestingly, LZ-8 (Fig. 1b), as well as HSP70 [34], enhance the immune responses via TLR4. Although TLR4 is well-known for its role in sensing LPS, various proteins from pathogens [35] and host-derived damage-associated molecular patterns (DAMPs) have pro-inflammatory activity via TLR4 pathway [36]. However, the stimulatory activity of HSP70 has been argued by microbial contaminants introduced during the purification of proteins [37]. We have used various approaches, as proposed previously [38], to vigorously rule out the possible microbial contamination in our LZ-8 preparation (Fig. 1c–f). The activity of LZ-8 was not completely removed by boiling (Fig. 1f), probably because of the heat-resistant property of LZ-8 [39]. Therefore, we are confident to conclude that the immunostimulatory activity is specifically attributed to LZ-8. Recently, Yeh et al. reported that TLR4 is not required for LZ-8 stimulation in macrophages [40], which is not consistent with our results and previous report [20] in DCs. In contrast to our data (Supplementary Fig. 1a), they detected very low production of TNF-α by LZ-8-treated macrophages, suggesting that their recombinant LZ-8 may have different property from ours.
Why is LZ-8 recognized by TLR4? The overall fold of LZ-8 consists of an N-terminal dimerization domain and a C-terminal Ig-like domain [41]. Dimerization is necessary for the activity of LZ-8 [15], and the Ig-like domain might be the potential active site. Since dimerization is also required for TLR4 activation [42], it will be interesting to investigate whether the Ig-like domain is responsible for TLR4 binding. Another possibility is that the glycosylated moiety of LZ-8 may interact with TLR4 directly [43]. Since FIP-fve has similar structure to LZ-8 [44] and has also been used as an adjuvant for tumor immunotherapy [45], we suggest that all FIPs probably have such biological activities. Recently, an immunonodulatory protein ACA isolated from Antrodia camphorata has been reported to activate macrophages via TLR2 [46]. It is likely that ACA can be included in FIP family and represents another TLR ligand.
An ideal adjuvant for the cancer vaccine should strongly elicit cellular responses, especially anticancer CTL activity. We have shown that LZ-8 uniquely promotes Th1 differentiation (Figs. 2, 4). Thus, LZ-8 could be more suitable than some clinical adjuvants, such as alum which mainly induces humoral responses, in cancer vaccine development. For developing LZ-8 into a new adjuvant, one practical consideration is to ensure a stable supply of LZ-8 with good quality. Some expression systems have been reported to obtain high amount of LZ-8 [47–49]; however, LZ-8 prepared from these systems have various levels of activity. We have generated large scale of LZ-8 with stable activity from S. cerevisiae system (data not shown). It is valuable to compare our LZ-8 to others for immunoregulatory functions, especially the DC activation. For example, although the activating effect of LZ-8 prepared from Pichia pastoris system on human DCs has been reported recently [20], the saturated dose of their LZ-8 for inducing cytokine production is at 50 μg/ml, which is much higher than our LZ-8 (5 μg/ml) (Fig. 1a). In addition, our LZ-8 promoted IL-2 production by mouse DCs (Supplementary Fig. 1a) that were not reported previously [20], and then may potentially facilitate NK cell activation to help killing cancer cells [50]. Overall, we have shown many results to confirm the availability of LZ-8 in vaccinology, especially the preclinical data from mouse model in vivo. It is feasible to further study the effect of LZ-8 on human and then hasten the clinical trial for LZ-8.
In summary, our study reveals that LZ-8 enhances the efficacy of DNA vaccine by activating DCs and promoting innate and adaptive immune responses through TLR4. Although Lin et al. have reported the stimulatory effect of LZ-8 on human DCs [20], we provide the first evidence to apply LZ-8 to a DNA vaccine model for cancer therapy and determine the immunogenicity in vivo. Because DCs have been used as a therapeutic vaccine [51], LZ-8 may also be used to enhance the efficiency of DC-based vaccine in cancer therapy. Furthermore, our LZ-8-HER2/neu DNA vaccine also has application in breast cancer therapy [52] and may be formulated in nanocarriers [53]. Thus, our data provide a new insight for the application of LZ-8 not only in healthy food for enhancing immunity but also in vaccine technology for preventing and treating various cancer and infectious diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Shih-Yang Hsieh (NHRI, Taiwan) for helping to initiate this project. We also thank Kuan-Yin Shen, Yu-Chen Wu, Chun-Nan OuYang, Kai-Chieh Chen (NHRI, Taiwan), Hsiu-Ling Chen, Dr. Chien-Heng Liu (Yeastern Biotech Co., Taiwan), and Kuan-Pei Chen (Taipei Medical University, Taiwan) for technical help. This work was supported by SBIR grant IZ970524, IZ1000250, STSP grant EY-13-15-10-99 (to C.L.C. and J.D.C.C), and in part by the Ministry of Education, Taiwan, R.O.C. under the ATU plan (to C.C.L.).
Conflict of interest
A patent for the use of LZ-8 as an adjuvant in vaccination has been filed.
References
- 1.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2005;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 2.Liu MA. Gene-based vaccines: recent developments. Curr Opin Mol Ther. 2010;12:86–93. [PubMed] [Google Scholar]
- 3.Cai Y, Rodriguez S, Hebel H. DNA vaccine manufacture: scale and quality. Expert Rev Vaccines. 2009;8:1277–1291. doi: 10.1586/erv.09.84. [DOI] [PubMed] [Google Scholar]
- 4.Loisel-Meyer S, Foley R, Medin JA. Immuno-gene therapy approaches for cancer: from in vitro studies to clinical trials. Front Biosci. 2008;13:3202–3214. doi: 10.2741/2921. [DOI] [PubMed] [Google Scholar]
- 5.Bodles-Brakhop AM, Draghia-Akli R. DNA vaccination and gene therapy: optimization and delivery for cancer therapy. Expert Rev Vaccines. 2008;7:1085–1101. doi: 10.1586/14760584.7.7.1085. [DOI] [PubMed] [Google Scholar]
- 6.Signori E, Iurescia S, Massi E, Fioretti D, Chiarella P, De Robertis M, Rinaldi M, Tonon G, Fazio VM. DNA vaccination strategies for anti-tumour effective gene therapy protocols. Cancer Immunol Immunother. 2010;59:1583–1591. doi: 10.1007/s00262-010-0853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fioretti D, Iurescia S, Fazio VM, Rinaldi M. DNA vaccines: developing new strategies against cancer. J Biomed Biotechnol. 2010;2010:174378. doi: 10.1155/2010/174378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Klechevsky E, Schmitt N, Morita R, Palucka K, Ueno H. Harnessing human dendritic cell subsets to design novel vaccines. Ann N Y Acad Sci. 2009;1174:24–32. doi: 10.1111/j.1749-6632.2009.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 11.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 12.Boh B, Berovic M, Zhang J, Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- 13.Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 14.Kino K, Yamashita A, Yamaoka K, Watanabe J, Tanaka S, Ko K, Shimizu K, Tsunoo H. Isolation and characterization of a new immunomodulatory protein, ling zhi-8 (LZ-8), from Ganoderma lucidium. J Biol Chem. 1989;264:472–478. [PubMed] [Google Scholar]
- 15.Lin WH, Hung CH, Hsu CI, Lin JY. Dimerization of the N-terminal amphipathic alpha-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (Fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis. J Biol Chem. 1997;272:20044–20048. doi: 10.1074/jbc.272.32.20044. [DOI] [PubMed] [Google Scholar]
- 16.Hsu HC, Hsu CI, Lin RH, Kao CL, Lin JY. Fip-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. Biochem J. 1997;323(Pt 2):557–565. doi: 10.1042/bj3230557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Wang X, Chen Y, Lin J, Zhou X. Cytokines expression induced by Ganoderma sinensis fungal immunomodulatory proteins (FIP-gsi) in mouse spleen cells. Appl Biochem Biotechnol. 2010;162:1403–1413. doi: 10.1007/s12010-010-8916-1. [DOI] [PubMed] [Google Scholar]
- 18.van der Hem LG, van der Vliet JA, Kino K, Hoitsma AJ, Tax WJ. Ling-Zhi-8: a fungal protein with immunomodulatory effects. Transplant Proc. 1996;28:958–959. [PubMed] [Google Scholar]
- 19.Hsu HY, Hua KF, Wu WC, Hsu J, Weng ST, Lin TL, Liu CY, Hseu RS, Huang CT. Reishi immuno-modulation protein induces interleukin-2 expression via protein kinase-dependent signaling pathways within human T cells. J Cell Physiol. 2008;215:15–26. doi: 10.1002/jcp.21144. [DOI] [PubMed] [Google Scholar]
- 20.Lin YL, Liang YC, Tseng YS, Huang HY, Chou SY, Hseu RS, Huang CT, Chiang BL. An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and MAPK pathways. J Leukoc Biol. 2009;86:877–889. doi: 10.1189/jlb.0708441. [DOI] [PubMed] [Google Scholar]
- 21.Lin CC, Chou CW, Shiau AL, Tu CF, Ko TM, Chen YL, Yang BC, Tao MH, Lai MD. Therapeutic HER2/Neu DNA vaccine inhibits mouse tumor naturally overexpressing endogenous neu. Mol Ther. 2004;10:290–301. doi: 10.1016/j.ymthe.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Chu CL, Lowell CA. The Lyn tyrosine kinase differentially regulates dendritic cell generation and maturation. J Immunol. 2005;175:2880–2889. doi: 10.4049/jimmunol.175.5.2880. [DOI] [PubMed] [Google Scholar]
- 23.Chu CL, Yu YL, Shen KY, Lowell CA, Lanier LL, Hamerman JA. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur J Immunol. 2008;38:166–173. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang RY, Yu YL, Cheng WC, Ouyang CN, Fu E, Chu CL. Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol. 2010;184:6815–6821. doi: 10.4049/jimmunol.0903991. [DOI] [PubMed] [Google Scholar]
- 25.Lin CC, Tu CF, Yen MC, Chen MC, Hsieh WJ, Chang WC, Chang WT, Lai MD. Inhibitor of heat-shock protein 90 enhances the antitumor effect of DNA vaccine targeting clients of heat-shock protein. Mol Ther. 2007;15:404–410. doi: 10.1038/sj.mt.6300014. [DOI] [PubMed] [Google Scholar]
- 26.Yu YL, Chen IH, Shen KY, Huang RY, Wang WR, Chou CJ, Chang TT, Chu CL. A triterpenoid methyl antcinate K isolated from Antrodia cinnamomea promotes dendritic cell activation and Th2 differentiation. Eur J Immunol. 2009;39:2482–2491. doi: 10.1002/eji.200839039. [DOI] [PubMed] [Google Scholar]
- 27.Lai MD, Chen CS, Yang CR, Yuan SY, Tsai JJ, Tu CF, Wang CC, Yen MC, Lin CC. An HDAC inhibitor enhances the antitumor activity of a CMV promoter-driven DNA vaccine. Cancer Gene Ther. 2010;17:203–211. doi: 10.1038/cgt.2009.65. [DOI] [PubMed] [Google Scholar]
- 28.Lin CC, Yen MC, Lin CM, Huang SS, Yang HJ, Chow NH, Lai MD. Delivery of noncarrier naked DNA vaccine into the skin by supersonic flow induces a polarized T helper type 1 immune response to cancer. J Gene Med. 2008;10:679–689. doi: 10.1002/jgm.1183. [DOI] [PubMed] [Google Scholar]
- 29.Tu CF, Lin CC, Chen MC, Ko TM, Lin CM, Wang YC, Lai MD. Autologous neu DNA vaccine can be as effective as xenogenic neu DNA vaccine by altering administration route. Vaccine. 2007;25:719–728. doi: 10.1016/j.vaccine.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Yen MC, Lin CC, Chen YL, Huang SS, Yang HJ, Chang CP, Lei HY, Lai MD. A novel cancer therapy by skin delivery of indoleamine 2, 3-dioxygenase siRNA. Clin Cancer Res. 2009;15:641–649. doi: 10.1158/1078-0432.CCR-08-1988. [DOI] [PubMed] [Google Scholar]
- 31.Huang CH, Chang CC, Lin CM, Wang ST, Wu MT, Li EI, Chang HC, Lin CC. Promoting effect of Antrodia camphorata as an immunomodulating adjuvant on the antitumor efficacy of HER-2/neu DNA vaccine. Cancer Immunol Immunother. 2010;59:1259–1272. doi: 10.1007/s00262-010-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai MD, Yen MC, Lin CM, Tu CF, Wang CC, Lin PS, Yang HJ, Lin CC. The effects of DNA formulation and administration route on cancer therapeutic efficacy with xenogenic EGFR DNA vaccine in a lung cancer animal model. Genet Vaccines Ther. 2009;7:2. doi: 10.1186/1479-0556-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev Vaccines. 2008;7:1185–1199. doi: 10.1586/14760584.7.8.1185. [DOI] [PubMed] [Google Scholar]
- 34.Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182:1449–1459. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- 35.Mossman KL, Mian MF, Lauzon NM, Gyles CL, Lichty B, Mackenzie R, Gill N, Ashkar AA. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. J Immunol. 2008;181:6702–6706. doi: 10.4049/jimmunol.181.10.6702. [DOI] [PubMed] [Google Scholar]
- 36.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marincek BC, Kuhnle MC, Srokowski C, Schild H, Hammerling G, Momburg F. Heat shock protein-antigen fusions lose their enhanced immunostimulatory capacity after endotoxin depletion. Mol Immunol. 2008;46:181–191. doi: 10.1016/j.molimm.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 38.Tsan MF, Baochong G. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res. 2007;13:6–14. doi: 10.1177/0968051907078604. [DOI] [PubMed] [Google Scholar]
- 39.Tong MH, Chien PJ, Chang HH, Tsai MJ, Sheu F. High processing tolerances of immunomodulatory proteins in Enoki and Reishi mushrooms. J Agric Food Chem. 2008;56:3160–3166. doi: 10.1021/jf800205g. [DOI] [PubMed] [Google Scholar]
- 40.Yeh CH, Chen HC, Yang JJ, Chuang WI, Sheu F. Polysaccharides PS-G and protein LZ-8 from Reishi (Ganoderma lucidum) exhibit diverse functions in regulating murine macrophages and T lymphocytes. J Agric Food Chem. 2010;58:8535–8544. doi: 10.1021/jf100914m. [DOI] [PubMed] [Google Scholar]
- 41.Huang L, Sun F, Liang C, He YX, Bao R, Liu L, Zhou CZ. Crystal structure of LZ-8 from the medicinal fungus Ganoderma lucidium. Proteins. 2009;75:524–527. doi: 10.1002/prot.22346. [DOI] [PubMed] [Google Scholar]
- 42.Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008;29:182–191. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Hsu TL, Cheng SC, Yang WB, Chin SW, Chen BH, Huang MT, Hsieh SL, Wong CH. Profiling carbohydrate-receptor interaction with recombinant innate immunity receptor-Fc fusion proteins. J Biol Chem. 2009;284:34479–34489. doi: 10.1074/jbc.M109.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paaventhan P, Joseph JS, Seow SV, Vaday S, Robinson H, Chua KY, Kolatkar PR. A 1.7A structure of Fve, a member of the new fungal immunomodulatory protein family. J Mol Biol. 2003;332:461–470. doi: 10.1016/S0022-2836(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 45.Ding Y, Seow SV, Huang CH, Liew LM, Lim YC, Kuo IC, Chua KY. Coadministration of the fungal immunomodulatory protein FIP-Fve and a tumour-associated antigen enhanced antitumour immunity. Immunology. 2009;128:e881–e894. doi: 10.1111/j.1365-2567.2009.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheu F, Chien PJ, Hsieh KY, Chin KL, Huang WT, Tsao CY, Chen YF, Cheng HC, Chang HH. Purification, Cloning, and Functional Characterization of a Novel Immunomodulatory Protein from Antrodia camphorata (Bitter Mushroom) That Exhibits TLR2-Dependent NF-kappaB Activation and M1 Polarization within Murine Macrophages. J Agric Food Chem. 2009;57:4130–4141. doi: 10.1021/jf900469a. [DOI] [PubMed] [Google Scholar]
- 47.Jinn TR, Wu CM, Tu WC, Ko JL, Tzen JT. Functional expression of FIP-gts, a fungal immunomodulatory protein from Ganoderma tsugae in Sf21 insect cells. Biosci Biotechnol Biochem. 2006;70:2627–2634. doi: 10.1271/bbb.60232. [DOI] [PubMed] [Google Scholar]
- 48.Yeh CM, Yeh CK, Hsu XY, Luo QM, Lin MY. Extracellular expression of a functional recombinant Ganoderma lucidium immunomodulatory protein by Bacillus subtilis and Lactococcus lactis. Appl Environ Microbiol. 2008;74:1039–1049. doi: 10.1128/AEM.01547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue Q, Ding Y, Shang C, Jiang C, Zhao M. Functional expression of LZ-8, a fungal immunomodulatory protein from Ganoderma lucidium in Pichia pastoris. J Gen Appl Microbiol. 2008;54:393–398. doi: 10.2323/jgam.54.393. [DOI] [PubMed] [Google Scholar]
- 50.Granucci F, Zanoni I, Pavelka N, Van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi-Castagnoli P. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–295. doi: 10.1084/jem.20040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Ladjemi MZ, Jacot W, Chardes T, Pelegrin A, Navarro-Teulon I. Anti-HER2 vaccines: new prospects for breast cancer therapy. Cancer Immunol Immunother. 2010;59:1295–1312. doi: 10.1007/s00262-010-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheng WY, Huang L. Cancer Immunotherapy and Nanomedicine. Pharm Res. 2011;28:200–214. doi: 10.1007/s11095-010-0258-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.