Abstract
CD8+ T cells undergoing homeostatic proliferation (HP) in a lymphopenic environment acquire a central memory-like phenotype (CD44+ CD62L+ Ly6c+). Such cells are readily functional in vitro, with a strong capacity to secrete IFNγ and IL-2 and to lyse target cells upon antigen recognition. In vivo, these memory-like T cells display potent anti-tumor reactivity. When addressing whether these remarkable properties were “acquired” or dependent on sustained HP, we observed, for the first time, that memory-like T cells retained full anti-tumor functions even when removed from their lymphopenic environment and retransferred into non-lymphopenic P14/Rag2−/− recipients (where HP is prevented). Moreover, memory-like T cells were superior to in vitro expanded effector T cells. We next sought to determine the conditions required to reproduce such a potent phenotype in vitro, in order to obtain optimal cells for adoptive cell transfer therapy. Assessing ex vivo lymph node cultures, dendritic cells, fibroblastic reticular cells, and HP-associated cytokines, we found that stimulation of naïve T cells with anti-CD3/CD28 beads and IL-15 (IL-7 was dispensable) led to the generation of memory-like T cell with a similar phenotype. Both in vitro and in vivo memory-like T cells retained the capacity to efficiently control tumor growth in non-lymphopenic hosts upon adoptive cell transfer. A similar phenotype could be imparted to human peripheral blood leukocytes with comparable culture conditions. Our data reinforce the idea that in vitro-generated memory-like T cells could benefit adoptive cell transfer therapies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1350-1) contains supplementary material, which is available to authorized users.
Keywords: Homeostatic proliferation, T cell, Memory-like phenotype, Tumor immunotherapy
Introduction
Homeostatic proliferation (HP) is the phenomenon in which T cells proliferate in response to a lymphopenic environment in order to regain homeostasis. Lymphopenia-induced proliferation has been shown to reverse anergy of CD8+ T cells and promote tumor rejection [1–3] as well as to increase anti-pathogen responses and allograft rejection [4]. Upon transfer of naïve T-cell receptor (TCR) transgenic CD8+ T cells in lymphopenic hosts, such as recombination activating gene (RAG)-deficient mice, the T cells undergo lymphopenia-induced HP and adopt a central memory-like phenotype (CD44+ CD62L+ Ly6c+) [5, 6]. Memory-like T cells become resistant to tolerance induction protocols [7] and display phenotypic and functional characteristics of antigen-experienced memory cells [5]. The mechanisms responsible for the switch from naïve to memory-like phenotype are partially elucidated; TCR triggering by self-peptide–MHC and homeostatic signals from IL-7 and IL-15 are considered essential [6, 8]. Such TCR triggering by self-peptide–MHC ligands and memory-phenotype acquisition are associated with subsequent augmented responsiveness toward high-affinity foreign peptide–MHC ligands [6, 9, 10].
Thus, generating T cells with these properties may help optimize tumor rejection in currently used protocols of adoptive cell transfer (ACT) [11, 12]. Increasing the degree of lymphopenia before ACT and thus allowing transferred T cells to undergo HP has, indeed, been shown to improve clinical response rates [11, 13]. The window of induced lymphopenia, however, is short (7–10 days) and the transferred cells must continue to perform their anti-tumor effect in the presence of competing cells following reconstitution of the host. While it has been shown that memory-like HP T cells are capable of controlling Listeria monocytogenes infections similarly to true memory T cells [14], the capacity of memory-like T cells to control tumor growth in non-lymphopenic hosts has not been investigated, to date. To this end, we used the 2C TCR transgenic model, in which CD8+ T cells recognize the allogeneic p2CA antigen presented in the context of H2-Ld by the mastocytoma cell line P1-HTRC [15]. In this model, tumor control must be mediated by direct antigen presentation at the tumor surface as cross-presentation is abrogated for the 2C TCR in H2-Lb hosts (C57BL/6 background). As a result, in vivo tumor control is CD4 help- and dendritic cell (DC) cross-presentation-independent and thus harder to obtain as compared to tumors with high levels of cross-presentable antigen [16]. We show here, for the first time, that HP-induced memory-like CD8+ T cells themselves possess intrinsic potent anti-tumor function upon retransfer into non-lymphopenic P14/Rag2−/− hosts where further lymphopenia-associated proliferation is abrogated due to the presence of a non-tumor-reactive CD8 + TCRtg clone (P14 TCR) [1, 8].
These notable results led us to investigate the key components necessary to generate similar memory-like CD8+ T cells in vitro in order to use such memory-like T cells in future ACT therapies. The study of HP-associated signals and the isolation of lymph node components led us to focus on artificial APCs consisting of anti-CD3/CD28 beads supplemented with HP-associated cytokines. Both in vitro- and in vivo-generated memory-like T cells were equally capable of controlling tumor growth in a model of adoptive cell transfer in non-lymphopenic mice. Moreover, bead stimulation with IL-15 led to the generation of phenotypically and functionally comparable memory-like T cells from human peripheral blood leukocytes (PBL), representing an attractive strategy for clinical translation.
Materials and methods
Mice
C57BL/6 2C TCR transgenic × Rag2−/− (2C/Rag2−/−) mice have been described previously [15, 17, 18]. Briefly, 2C TCRtg mice (obtained from Dr D. Kranz) were backcrossed in house onto Rag2−/− C57BL/6 mice (obtained from Taconic) for more than 12 generations. Rag2−/− recipient mice from these breedings were used to generate HP T cells. The 2C TCR recognizes the p2CA peptide (LSPFPFDL) of mitochondrial alpha-ketoglutarate-dehydrogenase in the context of H-2Ld as well as the synthetic peptide SIYRYYGL in the context of H2-Kb [19]. P14/Rag2−/− mice, obtained from Taconic and bred in house are specific for a H2-Kb-restricted epitope from lymphocytic choriomeningitis virus and is an “irrelevant” TCR in our model. Mice were maintained at the Netherlands Cancer Institute in individually ventilated cages and were used in agreement with our Institutional Animal Care and Use Committee according to the guidelines of the Society for Laboratory Animal Science, the Netherlands.
Tumor cells
Tumor cell lines were cultured in RPMI (Invitrogen) 10 % FCS (Sigma), 100 U/ml penicillin/streptomycin (Roche). The P815.B7.1 mastocytoma cell line expresses the p2CA/H2-Ld recognized by the 2C TCR as well as the costimulatory molecule B7.1 (CD80). P1.HTR.C was derived from P815 and selected for its ability to grow as a solid mass in vivo [20]. The control EL4 T-cell lymphoma line was obtained from ATCC.
Mouse T-cell purification and stimulation
Spleens and lymph nodes of 2C/Rag2−/− were isolated, homogenized, and red blood cells were lysed. CD8+ T cells were negatively selected using the IMag™ Mouse CD8 T Lymphocyte Enrichment Set—DM kit (BD Biosciences). Purity was generally >90 %. To generate memory-like T cells in vivo, 3–5 × 106-purified CD8+ 2C T cells were transferred i.v. into Rag2−/− mice. 14 days later, cells were harvested and CD8+ T cells purified as described above. For bead stimulation, 1 × 106 freshly isolated naïve 2C T cells were co-cultured in 1 ml complete medium with 3 × 106 Dynabeads® Mouse T-Activator CD3/CD28 (Invitrogen) according to the manufacturer’s protocol, in 24-well tissue culture plates. Complete medium consisted of RPMI, 10 % FCS, Glutamax-1 (Invitrogen), MEM NEAA (Invitrogen), Sodium Pyruvate (Invitrogen), 100 U/ml Penicillin/Streptomycin, 5 μg/ml β-mercapto-ethanol (Sigma). Upon culture set-up, 200 ng/ml recombinant mouse IL-15 (Peprotech), 400 ng/ml IL15RFc-Ig (R&D systems), and when indicated IL-7 50 ng/ml (Peprotech) were added once without refreshing during the 14-day culture. Cells were split when cultures reached 100 % confluence. Effector T cells were generated as described before [21]. In short, 1 × 105 naïve 2C T cells were co-cultured with 5 × 105 40 Gy-irradiated P815.B7.1 cells. After 7 days of culture, T cells were harvested, ficolled (Lympholyte®-M, Cedarlane), and restimulated again with freshly irradiated P815.B7.1 cells for an additional 7 days. Expansion was assessed by counting live cells using trypan blue exclusion. A ficoll step was performed at day 14 before continuing with in vitro or in vivo experiments.
FRC and DC-T cell co-culture
Primary fibroblastic reticular cell (FRC) cultures were kindly provided by Prof. Reina Mebius and Dr. Rosalie Molenaar and obtained from peripheral lymph nodes (LNs) of C57BL/6 mice by long-term culturing as previously described [22]. DCs were obtained by culturing 4 × 106/5 ml total bone marrow cells from C57BL/6 mice in a tissue culture 6-well plate (BD Biosciences) with 10 ng/ml mIL-4 (Peprotech) + 25 μg/ml mGM-CSF (Peprotech). On day 2, the non-adherent cells were harvested and further cultured with fresh cytokines for an additional 4 days. Co-cultures were set up in a flat bottom 96-well plate with 2 × 104 CFSE-labeled T cells together with 5 × 104–1 × 105 DC and/or FRCs with either no cytokines, or in the presence of 50 ng/ml IL-7 (Peprotech), 200 ng/ml IL-15 (Peprotech), or a combination of both. FRCs were seeded on day 2 and DCs on day 0 of co-culture. CFSE labeling of T cells was performed according to the manufacturer’s protocol (Vybrant® CFDA SE Cell Tracer Kit, Invitrogen).
Co-cultures on three-dimensional structures were performed using collagen-coated Sponceram® carrier discs (ZellWerk GmbH, ref. 160A1-20-00Collagen) in non-coated tissue culture 24-well plates in IMDM (Invitrogen) 8 % FCS. The discs were placed in 500 μl culture medium and 200 μl medium containing 1 × 105 FRCs was added. After 30-min preincubation at 37 °C, culture medium was adjusted to 2 ml. After 2 days of culture, 1 × 106 naïve 2C T cells were added. Similarly, GM-CSF/IL-4 DCs were coated 1 day before T cell addition.
On day 7, T cells were flushed out of the Sponceram carriers or—in the absence of the structures—harvested from the wells. CFSE dilution and CD62L expression were analyzed by flow cytometry.
Flow cytometric analysis
Fluorochrome-conjugated antibodies directed against the following molecules were purchased from BD Pharmingen or eBioscience: FITC-IgG2a, FITC-CD44, FITC-PD-1, FITC-Ly6c, FITC-Ly6A/E, FITC-CD25, FITC-CD107a, PE-IgG2a, PE-CD62L, PE-CD127, PE-CD103, PE-CD122, PE-NK1.1, PE-PD-1, PE-KLRG1, PE-CTLA4, PE-BTLA, PE-FasL, PE-MHC-II (IA/IE), PerCP-CD8α, APC-CD8α, APC-Ly49D, and APC-CD11. The 1B2 antibody recognizing the antigen-binding site of the 2C TCR [18] was labeled with AlexaFluor 647 or AlexaFluor 488 using a commercially available labeling kit (Invitrogen) and used to stain the 2C TCRtg T cells. For phenotypical analysis of human cells FITC-CD62L, APC-CD45RO, PE-CD45RA, PerCP-CD8, PE-CD8, APC-CD3, and PE-CD107a (BD Biosciences) were used. For CD107a staining, the antibody was added during the 4-h co-culture and again during staining prior to flow cytometry acquisition.
To determine cytokine production, co-cultures were set up with tumor-to-T cells ratio 2:1 for 4 h with exocytosis inhibitor (GolgiPlug; BD Biosciences). The target cell lines used were P1.HTR.C, P815.B7.1, and EL4 (negative control). After co-culture, cells were stained with anti-1B2-Alexa Fluor488 and anti-CD8-PerCP, fixed/permeabilized according to the manufacturer’s protocol (eBiosciences) and stained with anti-IFNγ-APC, and anti-IL-2-PE. FACS acquisition was performed with a Calibur or an LSRII (BD biosciences) and analyzed with FlowJo software (Tree Star).
In vitro functional assays
IFNγ production in response to anti-CD3/CD28 stimulation (3:1 bead: T cell ratio) was measured by ELISA after 18 h (R&D). Lytic capacity of T cells was assessed in a standard 4-h chromium release assay. Target cells were labeled with 51Cr for 1 h at 37 °C and co-cultured with effector cells at the indicated ratios with 2.5 × 103 radiolabeled target cells for 4 h. 51Cr release was determined in the supernatant (LumaPlate; PerkinElmer). Maximal release was determined by the addition of 1 % Triton X-100 in water (Sigma-Aldrich) and spontaneous release was determined in medium alone. Counts were measured in an automatic counter (TopCount; PerkinElmer). The percentage of specific release was calculated as follows: [(cpm experimental release − cpm spontaneous)/(cpm maximal − cpm spontaneous)] × 100.
Tumor challenge and measurement
P14Rag2−/− mice were injected with 1 × 106 P1.HTR.C cells subcutaneously on the flank. Tumor sizes (mm2) were determined with electronic callipers by multiplying the size (in mm) of the greatest tumor diameter and its perpendicular. The following day, mice received 1 × 105 2C T cells intra-venously.
Human T-cell stimulation and functional testing
Human PBL were obtained by ficolling healthy-donor buffy-coats (Sanquin, Amsterdam, the Netherlands). PBL were seeded at 2 × 106/well in 24-well tissue culture plates with 1 μg/ml anti-CD3 (OKT3, Janssen Cilag) + 300 U/ml IL-2 (Proleukin, Novartis) to generate effector T cells. Cells were cultured in 50 % AIMV medium (Invitrogen), 50 % RPMI 1640 supplemented with 10 % human serum (Sanquin), 100 U/ml penicillin–streptomycin (Roche Diagnostics), and 2 mM l-Glutamine (Invitrogen) for 14 days and split when necessary in medium containing IL-2 (without additional anti-CD3). In vitro-generated memory-like T cells were obtained by culturing 1 × 106 human PBL with 3 × 106 anti-CD3/CD28 beads (Invitrogen) per well in 24-well plates + 200 ng/ml human-IL15 ± 50 ng/ml human IL-7 (Cell Genix, CellGro) and split upon confluency without further cytokine addition until day 14.
T cells were TCR-transduced on day 2 after the start of stimulation in 24-well non-tissue culture-treated plates. Wells were coated with 10 μg/ml Retronectin (R-Fibronectin CH-296, TaKaRa) at 4 °C for 18 h, blocked with RPMI 5 % FCS, and loaded with 1 ml of codon optimized 1D3 encoding retroviral vector supernatant [23] by 45-min centrifugation at 1000 RCF. The 1D3 TCR is specific for MART-126–35 epitope. Viral supernatant was removed, T cells were added, and plates were spun 90 min at 1000 RCF. The cultures were then retransferred into tissue culture plates after 18 h.
Intra-cellular cytokine staining and CFSE dilution were performed as described above.
Statistical analysis
Statistical analysis for in vitro experiments was performed using the Student’s t test (Excel software, Microsoft). Results are expressed as the mean ± SD. Statistical analyses of in vivo tumor growth curves were performed using the Wilcoxon rank sum test. Differences were considered statistically significant at p < 0.05.
Results
T cells that have undergone HP acquire a memory-like phenotype, are readily functional, and control tumor outgrowth in secondary non-lymphopenic hosts
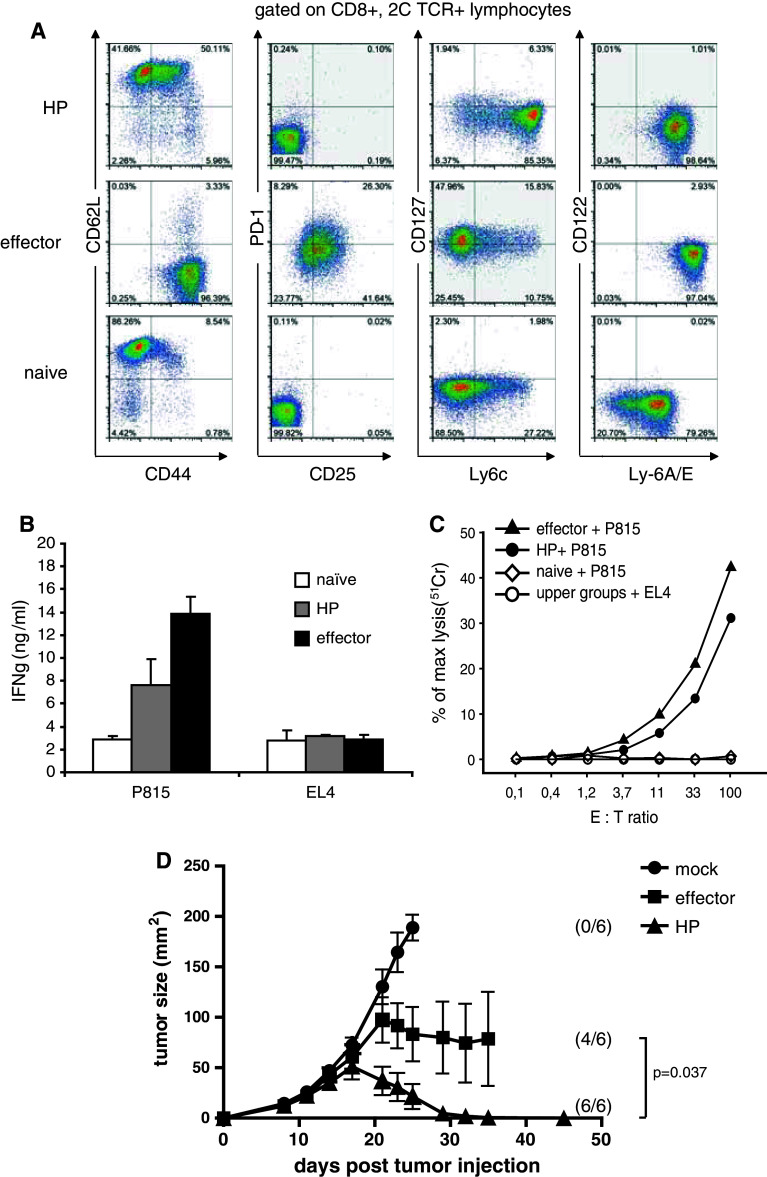
It has been previously shown that 2C/Rag2−/− CD8+ T cells spontaneously proliferate when adoptively transferred in Rag2−/− mice (that have no lymphocytes) but they do not proliferate when transferred into P14/Rag2−/− that have a normally sized but monomorphic repertoire of virus-specific T cells [1]. To obtain T cells having undergone HP, naïve 2C/Rag2−/− CD8+ T cells were isolated and transferred into Rag2−/− lymphopenic hosts. The phenotype and function of the transferred cells were analyzed 14 days later. As shown in Fig. 1a, compared to naïve 2C T cells which are CD62L+, CD44−, Ly6clo, Ly-6A/Elo, CD127lo, PD-1−, 2C T cells that had undergone HP upregulated CD44+, Ly6chi, Ly-6A/Ehi, maintained CD62L expression, but remained PD-1−. Such phenotype is attributed to bona fide central memory T cells; thus, the HP T cells have been termed memory-like T cells [8]. In contrast, 2C Rag2−/− T cells that were primed in vitro by repetitive stimulations with P815.B7.1 displayed a full effector phenotype (CD62L−, CD44+, CD25+, PD-1+, CD127hi) [20, 21]. Interestingly, Ly-6A/E (lymphocyte activation protein-6A/E), also known as Sca-1 (Stem cell antigen 1), a marker of T-cell activation and recently associated with memory stem cells, [24–27] was upregulated on memory-like T cells compared to naïve T cells albeit to a lesser extent than on effector cells, indicating that the memory-like T cells are activated cells. After 14 days of in vivo HP, however, we observed that nearly half the cells (41 %, Fig. 1a) maintained a naïve like CD44 low phenotype. This was the case when cells were recovered at day 14, whereas at later time points (day 14), all cells upregulated CD44 [27]. Considering that virtually all cells underwent HP (as previously shown, after 9 days of HP, all cells display diluted CFSE [1]) and expressed high levels of Ly6A/E, it appears that almost half of the memory-like T cells are phenotypically similar to the recently described CD8+ memory stem cells (Tscm) [24–27] or are in the process of upregulating CD44. In a kinetic analysis, we observed that, given enough time, the majority of HP T cells become CD44+ [28]. Further work is necessary to compare HP T cells with Tscm.
Fig. 1.
Naïve 2C T cells transferred into Rag2−/− mice acquire a memory-like phenotype and become readily functional. Naive 2C T cells (3–5 × 106) were transferred i.v. in Rag2−/− lymphopenic mice to undergo homeostatic proliferation and generate the “HP” T cells. In parallel, effector T cells were generated in vitro by stimulating naïve 2C T cells, on day 0 and day 7, with 40 Gy-irradiated P815.B7.1 mastocytoma tumor cells (at a 1:5 ratio). The phenotype and function of the indicated cell types were analyzed 14 days later from pooled spleens and LNs. a Flow cytometry analysis of naïve, effector, and HP T cell at day 14. b 1 × 105 2C T cells from the indicated groups were stimulated for 18 h with P815 tumor cells at a 1:1 ratio. IFNγ production was measured by ELISA (error bars are SD of triplicates). c Lytic capacity was analyzed in a standard 4-h chromium release assay of 2.5 × 103 radiolabeled target cells and the indicated increasing ratios of T cells. EL4 targets were used as negative controls. d P14/Rag2−/− received 1 × 106 P1.HTR.C tumor cell subcutaneous on day 0. On day 1, the mice received either, no cells (mock), 1 × 105 in vitro-stimulated T cells (effector) or 1 × 105 T cell having undergone 14 days of homeostatic proliferation in Rag2−/− mice (HP). Results are shown for 6 mice/group (mean ± SEM). Differences between tumor curves of effector and HP groups are significant (p = 0.037) as calculated by Wilcoxon rank sum test. Numbers between brackets represent the number of mice alive at completion of the experiment. Data shown are representative of 2 independent experiments
In functional assays, the memory-like T cells resulting from HP, as well as the effector T cells, were capable of producing IFNγ upon overnight stimulation with P815 tumor cells (expressing their cognate targeted antigen), whereas the naïve T cells did not (Fig. 1b). Moreover, both effector and memory-like T cells showed similar lytic activity in a chromium release assay targeting P815 compared to EL4 serving as negative control, while naïve T cells showed no lytic activity as expected (Fig. 1c). These results are consistent with previous findings that HP can induce changes in antigen-specific T cells that render them more readily functional with less stringent requirements for activation than naïve cells [5, 8].
However, it remains unclear whether these memory-like T cells maintain their potent anti-tumor function in vivo in a non-lymphopenic environment. To address this question, naïve 2C/Rag2−/− T cells were transferred to Rag2−/− primary hosts to undergo HP or stimulated in vitro by to obtain effector T cells. On day 14, in vivo-generated memory-like 2C T cells and in vitro-induced effector T cell were transferred into P14/Rag2−/− secondary hosts challenged with P1.HTR.C tumor cells s.c. the previous day (according to standard protocols in this model, which does not allow to use established tumors due to the absence of cross-presentation [17]. P14/Rag2−/− mice were used as non-lymphopenic hosts [8] since the presence of an additional (non-tumor-reactive) CD8+ TCRtg clone prevents HP of transferred CD8+ T cells as measured by the absence of CFSE dilution and absence of tumor control [1]. We observed that the memory-like CD8+ T cells were significantly superior to the effector T cells in controlling tumor growth (p = 0.037) (Fig. 1d).
These data indicate that memory-like T cells generated by HP are highly functional in vivo and could be of benefit for ACT therapy protocols.
Resuming HP in vitro requires dendritic cells and IL-15, but three-dimensional structure, fibroblastic reticular cells, and IL-7 are dispensable
As in vivo HP-induced memory-like T cells cannot be obtained for use in clinical settings, we analyzed the signals that are essential to reproduce such T cells in vitro.
HP of T cells is known to take place in secondary lymphoid organs [28–30]. We thus hypothesized that in order to undergo HP, naïve T cells may depend on signals from DC and/or FRC within a three-dimensional architecture as the one found in LNs, and HP-associated cytokines like IL-7 and IL-15 [30, 31]. We studied the in vitro generation of CD8+ memory-like T cells in a reductive manner starting with the study of whole LN. In a first attempt to understand whether the structure of a LN was necessary, LN from Rag2−/− mice was removed 15 h after injection of CFSE-labeled naïve 2C Rag2−/− CD8+ T cells and placed in culture medium containing IL-7. The 15-h in vivo allowed homing of the T cells to the LN without initiation of proliferation, as confirmed by the absence of CFSE dilution (Supplementary Figure 1, available online). Four days later, LN was homogenized and analyzed for CFSE dilution and CD62L expression of the T cells (Fig. 2a). We observed that in intact LN, 53.9 ± 11.6 % (mean ± SD, n = 4) of the T cells proliferated (CFSE low) and maintained CD62L, whereas only 21.2 ± 6.1 % (mean ± SD, n = 4) of T cells proliferated when the LN was homogenized into a single cell suspension immediately ex vivo and cultured for 4 days. This significant difference in proliferation upon homogenization (p = 0.002, student’s t test) was also accompanied by a loss of CD62L expression (Fig. 2b). Quantification of the T cell numbers recovered was hampered by the low yield and the need to use all T cells recovered from each LN for FACS analysis. This suggested that the three-dimensional organization of a LN played a role in HP. Characterization of the DC content (CD11c+ MHCII+ DCs, Fig. 2a, b) of whole versus homogenized LN at day 4 showed a consistent reduction of DC percentage in the latter. Thus, not only the absence of three-dimensional structure, but also the reduction in APC numbers after homogenization could have been accountable for the reduced in vitro HP. To further address the need for 3D organization, we used a collagen-coated ceramic structure (Sponceram® discs) that has been developed for stem cell cultures (Supplementary Figure 2). Because a major component of lymph nodes are FRCs and DCs [30], we coated these structures with either FRCs or DCs in the presence of IL-7 and IL-15 and analyzed the CFSE dilution and CD62L expression of naïve CFSE-labeled T cells added to the cultures (Fig. 2c). We observed the strongest proliferation of T cells when DCs were coated on the ceramic structure (approximately 70 % CFSE low T cells). In FRC-coated structures, more than 40 % of T cells remained CFSE high. Surprisingly, when the ceramic structures were omitted and T cells were co-cultured with plate-bound DCs, IL-7, and IL-15, ~80 % T cells underwent proliferation and the majority maintained CD62L expression (Fig. 2c).
Fig. 2.
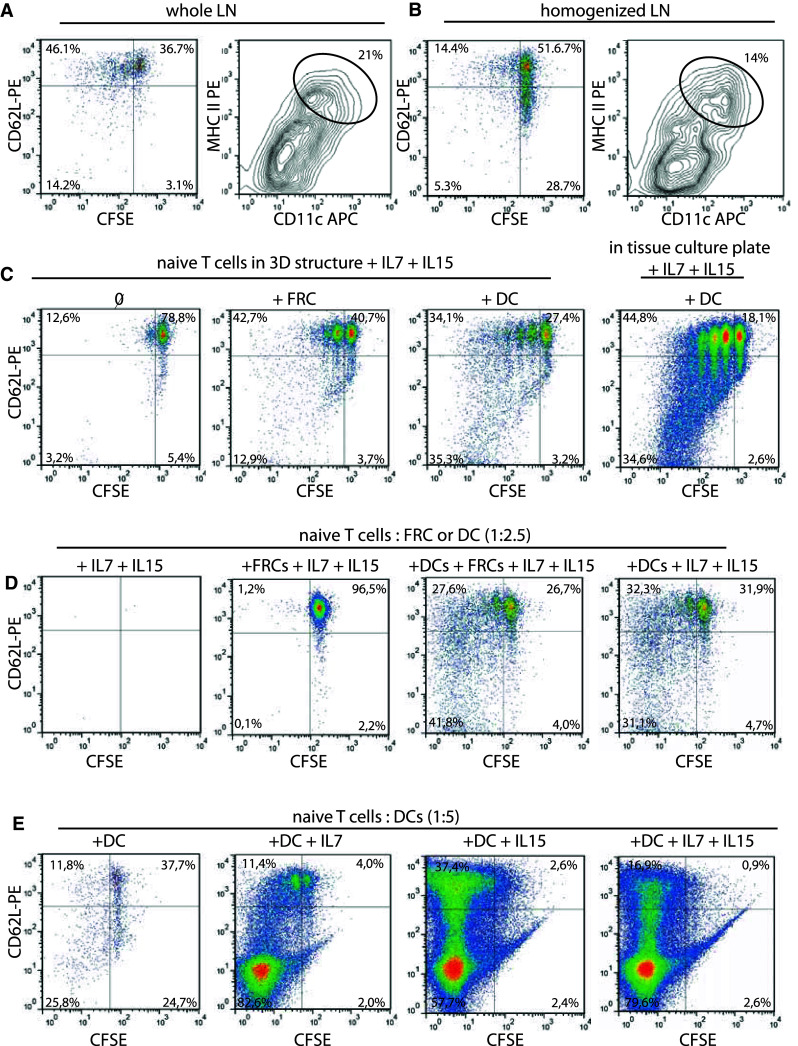
Assessing the factors necessary to reproduce HP-like proliferation in vitro. CFSE-labeled naïve 2C T cells (5 × 106) were transferred into a Rag2−/−. After 15 h, inguinal LNs were removed and either placed a intact in culture or b as single cell suspension after homogenization for an additional 4 days. Cells were harvested and analyzed by flow cytometry for CD62L, CD11c, MHCII, and CFSE expression. Represented are lymphoid-gated CD8+, 1B2+ (anti-2C TCR) cells (left) and total gated CD3−, CD11c+, MHC-II+ dendritic cells (right). c FRCs or DCs were loaded on collagen-coated ceramic structures on day-2 and day-1, respectively. Naïve CFSE-labeled 2C T cells were added on day 0 and cultured in the presence of IL-15 and IL-7. CFSE dilution, and CD62L expression was analyzed 7 days later. Alternatively, DCs and T cells were cultured in flat bottom 24-well tissue culture plates. d, e CFSE-labeled T cells were co-cultured with the indicated ratio of FRC or DC in the presence of the indicated cytokines for 7 days. CD62L and CFSE expression of CD8+, 1B2+ lymphocytes is shown. Each panel is representative of 2–3 individual experiments
Observing that a 3D scaffold was not required for T-cell proliferation in vitro, we addressed whether FRCs were also dispensable. CFSE-labeled naïve 2C T cells were cultured for 7 days in tissue culture plates with either FRCs or DCs alone or both, in the presence of IL-7 and IL-15 (Fig. 2d). The ratio of DC:T or FRC:T was 2.5:1 in these cultures. CFSE dilution did not occur in the cytokine-only condition (no viable cells remaining) or when FRCs were added. However, proliferation occurred when DCs were added to the cultures, independently of the presence of FRCs (Fig. 2d). Thus, DCs in combination with IL-7 and IL-15 were sufficient to induce T-cell proliferation and CD62L maintenance in the majority of proliferating cells. Next, we tested whether the addition of IL-7 and IL-15 to the T/DC cultures was necessary (Fig. 2e). As the ratio of 1:2.5 T:DC led to suboptimal yield of T cells (Fig. 2d), we increased the ratio to 1:5 to gain higher yields for FACS characterization of the T cells. This was based on previous data from Ge et al. [32] showing that a higher T/DC ratio is important.
DCs alone were not able to sustain potent in vitro proliferation of the naïve T cells, while addition of IL-7 led to a strong expansion of the T cells; however, this was accompanied by a marked loss of CD62L expression (Fig. 2e). DCs with IL-15 led also to a strong T-cell proliferation while CD62L expression was maintained in 40 % of the proliferating T cells. The combination of IL-15 and IL-7 resulted, as for IL-7 alone, in a stronger bias toward CD62L-negative cells (Fig. 2e).
In summary, the minimal combination enabling in vitro proliferation of naïve CD8+ T cells with CD62L maintenance was high DC-to-T cell ratio in the presence of IL-15 signalling.
The memory-like T cell phenotype can be generated in vitro using anti-CD3/CD28 beads and IL-15
It is known from animal experiments that DCs can deliver potent self-peptide–MHC-class I/TCR triggering of T cells as well as costimulatory signals that are important to induce HP and the memory-like phenotype [33–35]. The same signals could be provided, in a “cell-free” system, by beads coated with agonist anti-CD3/CD28 antibodies.
To test whether sustained TCR triggering from CD3 and costimulation via CD28 could replace the DC signals, we used anti-CD3/CD28-coated beads in the presence of IL-15. Naïve 2C/Rag2−/− CD8+ T cells were co-cultured 14 days with an excess of anti-CD3/CD28 beads (3:1 bead: T cell) and IL-15 ± IL-7. For optimal function, IL-15 must be presented in trans by APCs [36, 37]. Therefore, we supplemented the culture with IL-15RFc chimeric molecules as described by Rubinstein et al. [38]. IL-7 was previously shown to be essential for T-cell survival, so its contribution was once more analyzed in this setup, despite the fact that we showed it was dispensable in our previous experiments (Fig. 2e).
In the presence of IL-15/IL-15RFc, bead-stimulated T cells showed a strong CD44 upregulation within 2 days of culture and CD62L expression was maintained in the majority of T cells until 4 days of culture after which a population of CD44+, CD62L− T cells appeared (Supplementary Figure 3). At day 14, the ratio of memory-like (CD62L+ CD44+) and effector memory (CD62L− CD44+) T cells varied per culture. On average, beads + IL-15 induced 56 ± 17.2 % (mean ± SD, n = 4 experiments) memory-like T cells, with comparable CD127+, PD-1−, CD122+, Ly-6c+, Ly49D− expression as in vivo-generated memory-like T cells (Fig. 3). However, bead stimulation led to non-homogenous cultures containing also a fraction of effector T cells that were CD62L− and CD127−. As previously observed in co-cultures with DCs, addition of IL-7 led to a consistent reduction in the average generation of CD62L+ CD44+ memory-like T cells (42.3 ± 6.5 %, n = 3 experiments) (Fig. 3). A clear difference in CD103 expression was observed between in vivo-derived memory-like T cells and bead-stimulated T cells. Its significance is yet unknown. Additionally, bead-stimulated T cells in the presence of IL-15 ± IL-7 and in vivo HP-induced T cells showed a similar phenotype: KLRG1−, CTLA4−, BTLA−, and FasL− (data not shown). Thus, murine CD8+ 2C TCRtg T cells stimulated in vitro with anti-CD3/CD28 beads in the presence of IL-15/IL-15RFc displayed a phenotype with comparable memory-like characteristics as T cells having undergone HP in vivo.
Fig. 3.
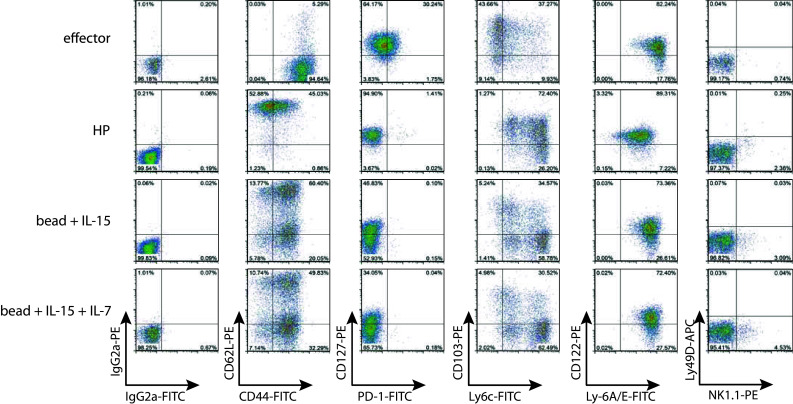
Anti-CD3/CD28 bead stimulation with IL-15 in vitro leads to memory-like phenotype acquisition. The phenotype of 2C T cells at day 14 after in vivo HP, or in vitro stimulation is represented. HP and effector cells were generated as described in Fig. 1. Naïve T cells were stimulated with anti-CD3/CD28 beads at a ratio of 1:3 in the presence of 200 ng/ml IL-15 + 400 ng/ml IL15RFc-Ig and when indicated 50 ng/ml IL-7. Depicted surface stains are gated on CD8+ 1B2+ lymphoid cells. Data are representative of 3 individual experiments using pooled T cells from 3 donor mice each
Functional memory-like T cells can be generated in vitro using anti-CD3/CD28 beads and IL-15
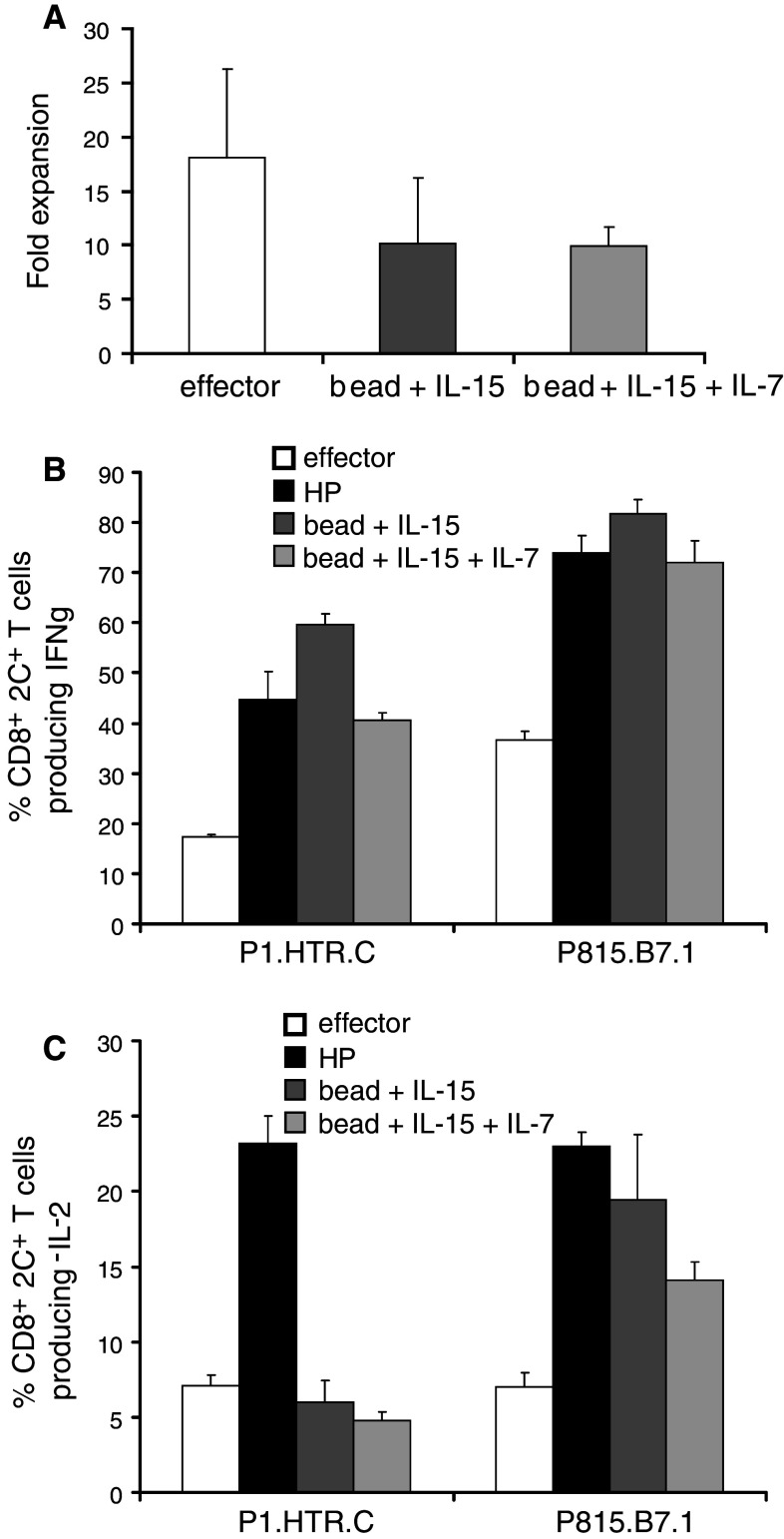
During the 14 days of culture, bead-stimulated T cells underwent a modest 10.1-fold expansion on average (range 6.3–17.1) and the addition of IL-7 did not improve expansion (average 10.0-fold, range 8.2–11.6). Effector cells expanded on average 18.1-fold (range 8.7–24), which was not significantly different from the bead conditions (p = 0.43 and p = 0.25) (Fig. 4a).
Fig. 4.
In vitro-generated memory-like T cells are readily functional upon antigen recognition. Naive 2C T cells (3–5 × 106) were transferred i.v. in Rag2−/− lymphopenic mice to undergo homeostatic proliferation and generate the “HP” T cells. In parallel, effector T cells were generated in vitro by stimulating naïve 2C T cells, on day 0 and day 7, with 40 Gy-irradiated P815.B7.1 mastocytoma tumor cells (at a 1:5 ratio). Bead-stimulated T cells were obtained from naive CD8+ 2C T cells stimulated with anti-CD3/CD28 bead (ratio T:bead = 1:3) and the indicated cytokines. a The effector T cells and bead-stimulated T cells were harvested after 14 days of culture and viable T cells were determined by trypan blue exclusion. The average fold expansion ± SD of three independent experiments is represented. b IFNγ and c IL-2 intracellular cytokine production was determined after a 4-h co-culture in vitro with P1.HTR.C or P815.B7.1 tumor cells at an E:T ratio of 1:2. Shown are the mean percentages ± SD of CD8+, 1B2+ lymphoid cells producing cytokines corrected for background (using irrelevant EL4 target cells). Representative of 2 independent experiments
Having previously observed that in vivo HP induced readily functional memory-like T cells, we assessed whether in vitro-cultured T cells could also produce effector cytokines upon TCR stimulation. Restimulation of bead-stimulated T cells, in vivo HP T cells, and effector T cells with P815-derived tumor cells lines (expressing CD80/B7.1 or not) showed that both in vivo HP and bead-stimulated memory-like T cells were superior to effector T cells against both tumor targets (Fig. 4b), although the number of IFNγ-producing cells was lower when the tumor did not express B7.1. On the other hand, IL-2 production upon stimulation with the B7.1-negative tumor could only be observed in HP T cells, whereas the B7.1-positive tumor induced comparable IL-2 in HP and bead-stimulated T cells (Fig. 4c). This observed difference in IL-2 production upon P1.HTR.C recognition could suggest that the bead stimulation may have either biased T-cell functions or pushed in vitro-generated memory-like T cells to a further state of maturation which may result in a compromised potential to control tumor growth in vivo.
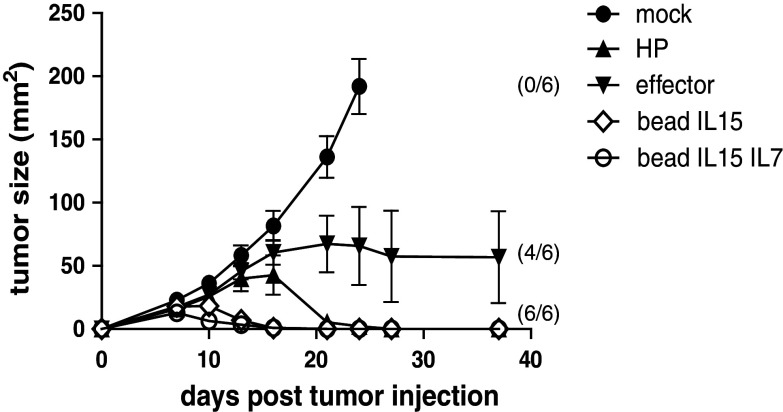
To test their in vivo capacity, in vitro-generated memory-like T cells, effector T cells, and in vivo-generated HP T cells were injected into P14/Rag2−/− mice one day after tumor challenge with P1.HTR.C. As expected, HP-derived T cells controlled very efficiently the tumor growth, whereas effector 2C T cells were inferior in controlling the tumors. Bead-stimulated T cells (with IL15 or IL15 + IL7) displayed a very potent anti-tumor control with a more rapid onset of response than the HP T cells (Fig. 5). As predicted from our in vitro experiments, the addition of IL-7 during culture provided no additional in vivo benefit. Similar to previous data testing in vivo-induced HP T cells targeting B16.SIY melanoma in C57BL/6 host mice [3], we found that additional Treg depletion (in our experiments mediated by CD4 T-cell depletion) was required to achieve melanoma control from in vitro-generated HP T cells (Supplementary Figure 4).
Fig. 5.
Beads + IL-15 stimulation generates T cells with potent anti-tumor activity in non-lymphopenic hosts. P14/Rag2−/− received 1 × 106 P1.HTR.C tumor cell subcutaneous on day 0. On day 1, the mice received either no cells (mock), 1 × 105 in vitro-stimulated T cells (effector or bead-stimulated), or 1 × 105 T cell having undergone 14 days of homeostatic proliferation in Rag2−/− mice. Results are the mean of 6 mice per group (±SEM). Numbers between brackets represent the number of mice alive at completion of the experiment. Data shown are representative of 2 independent experiments
Thus, the in vitro-generated tumor-specific memory-like CD8+ T cells showed comparable phenotype and function to in vivo-generated HP T cells and both were superior to conventional effector cells in controlling tumor growth.
Memory-like human CD8+ T cells can be generated in vitro
In order to determine whether comparable culture conditions could be used to generate similar memory-like T cells from human PBL, we assessed the phenotype and function of in vitro bead-stimulated human PBL. The most conventional way of stimulating open-repertoire T cells, currently used for TIL protocols, is the combination of the agonist anti-CD3 antibody (OKT3) and recombinant IL-2 [39]. Therefore, we included this condition as a reference in our experiments. We stimulated PBL from 5 healthy donors with beads + IL-15 ± IL-7 and compared phenotype and function to the anti-CD3 + IL-2-stimulated T cells after 14 days of culture. In order to test their functionality (against a specific antigen), the T cells were TCR gene-modified by retroviral transduction—using a vector coding the MART-126–35-specific 1D3 TCR—2 days after stimulation. As observed in the murine setting, human PBL stimulated with beads + IL-15 acquired a memory-like phenotype, whereas anti-CD3-stimulated T cells developed toward effector memory T cells (Fig. 6a). Bead-stimulated cells had a tendency to expand better than anti-CD3-stimulated T cells (Fig. 6b) and yielded in average a 50-fold expansion which could be brought to 100-fold expansion when using culture bags instead of 24-well plates (data not shown). Moreover, bead-stimulated T cells were highly functional in expressing IFNγ and IL-2 upon target recognition (Fig. 6c, d). Lytic as well as proliferative capacities were similar between conditions, as determined by CD107a expression and CFSE dilution assays, respectively (Fig. 6e, f).
Fig. 6.
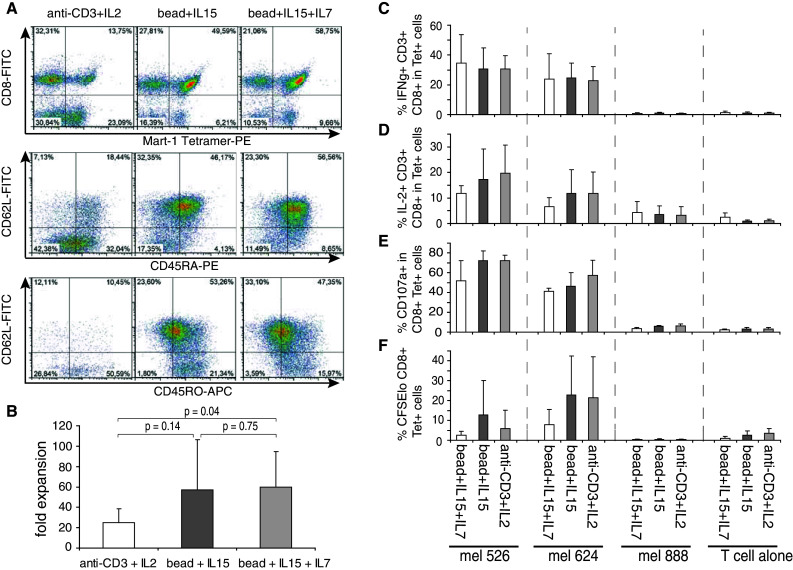
Beads + IL-15 stimulation of human PBL generates memory-like T cells that are readily functional. PBL were stimulated with soluble anti-CD3 antibody and IL-2 or anti-CD3/CD28 beads + IL-15 ± IL-7 at a bead to PBL ratio of 3:1. After 2 days of stimulation, the T cells were retrovirally transduced to express the 1D3 TCR specific for MART-126–35. a Surface expression of CD62L, CD45RA, and CD45RO among CD3+, CD8+, lymphocytes was determined after 14 days of culture. b Mean fold expansion of T cells from 5 different healthy donors after 14 days of culture. c intracellular IFNg d IL-2 (on fixed cells) and e CD107a surface expression (on live cells), was measured after a 4-h in vitro co-culture with the melanoma cell lines mel 526, mel 624 (both HLA-A2+, MART-1+), and mel 888 (HLA-A2−, negative control). T cells alone are shown as control. The graphs represent the mean percentage of CD8+ CD3+ lymphocytes normalized for MART-1-tetramer+ T cells. f Depicts the fraction of CD8+ MART-1-Tetramer+ lymphocytes that have undergone proliferation as measured by CFSE dilution after 4 days of co-culture. Data represent mean ± SD. For a–d and f, 5 different healthy donors were analyzed. Data in e are determined in two healthy donors
In summary, we have shown that bead-stimulated cells in the presence of IL-15 can develop into memory-like T cells in the murine setting, resulting in potent anti-tumor function in vivo. In the human setting, the phenotype and function of bead + IL-15-stimulated PBL were comparable to the murine memory-like T cells.
Discussion
T cells transferred into a lymphopenic host undergo homeostatic proliferation and display superior tumor growth control [1]. A commonly used animal model of HP is to transfer naïve CD8+ TCRtg T cells (e.g., from 2C TCR mice) into RAG−/− mice. Transfer into irradiated C57BL/6 mice has also been used as experimental setup, but requires additional regulatory T-cell depletion to allow tumor control [3]. Therefore, P14/Rag2−/− mice are often used as non-lymphopenic controls [8] since the presence of an additional CD8+ TCRtg clone prevents HP of transferred CD8+ T cells as measured by the absence of CFSE dilution and absence of tumor control [1].
Here, we confirm that naive CD8+ 2C TCRtg T cells undergoing HP acquire a memory-like phenotype and are readily functional.
In addition, we show for the first time, that such T cells are capable of mediating potent anti-tumor responses upon retransfer into secondary, undisturbed, non-lymphopenic hosts (without the need for additional exogenous adjuvants, that is, antigen stimulation or cytokine support). In these experiments, P14/Rag2−/− mice were challenged with the P1.HTR.C mastocytoma, a tumor cell line whose antigens cannot be cross-presented by the host due to the H2-Ld restriction of the 2C TCR. This type of tumor is known to be more difficult to reject compared to tumors expressing high levels of cross-presentable antigen [16]. Since the endogenous CD8+ P14/Rag2−/− T cells are not tumor-reactive, rejection is exclusively dependent on the transferred 2C T cells that do not receive CD4+ T cell help. This model was therefore chosen to investigate the HP dependence and intrinsic anti-tumor potential of memory-like T cells.
The superior anti-tumor efficacy of in vivo-generated HP T cells observed upon transfer into non-lymphopenic hosts led us to address whether the memory-like T-cell phenotype could also be generated in vitro in a manner suitable for clinical approaches like ACT therapy. Unlike conventional memory T cells, which are induced in response to foreign peptide–MHC complexes and inflammatory cues, HP-induced memory-like T cells are known to be induced in response to engagement with self-peptide–MHC complexes and the homeostatic cytokines IL-7 and IL-15 [6, 40]. Similarly to Ge et al. [32], we found in our reductive approach that DCs and IL-15 were sufficient to induce an HP-like proliferation in vitro with a strong maintenance of CD62L expression on CD8+ T cells.
What are DCs providing in this system? It is known that antigen-independent TCR triggering via MHC-I molecules at the surface of DCs is essential for HP induction [33, 34]. Moreover, DCs are professional APCs with high surface expression of costimulatory molecules and CD28 signalling has been shown to play an essential role in the HP response of CD8+ T cells [35]. It has also been reported that naïve T cells generate more memory-like cells after exposure to pathogen-activated DCs, which may reflect more self-recognition due to enhanced costimulation [41]. Consistent with data from Ge et al. [32], we observed that a high DC:T cell ratio induced an increased proliferation of CD62L+ T cells. Thus, it is possible that sustained MHC-class I/TCR interaction and costimulatory signals are the only requirements to induce HP and memory-like phenotype acquisition by T cells. To take this hypothesis one step further, we tested a known cell-free system, taking advantage of agonistic anti-CD3/CD28 beads that may be able to mimic sustained signals. Bead stimulation + IL-15 allowed for the in vitro generation of murine as well as human CD8+ T cells with memory-like phenotype and function. In vitro-generated murine memory-like T cells proved to be as potent as in vivo HP T cells in controlling tumor growth in non-lymphopenic hosts. Moreover, such cells could mediate a potent anti-tumor response in wild-type C57BL/6 mice upon in vivo depletion of CD4 T cells as shown in supplementary Figure 4. Recently, Haluszczak et al. [42] have shown that antigen-driven central memory T cells are Ly49Dhi (also known as α4-integrin, a component of the integrin VLA-4 and LPAM), while naturally occurring memory-like T cells that arise from HP in response to self-ligands are Ly49Dlo. In line with these data, we found that both in vivo HP and in vitro-generated memory-like T cells were Ly49Dlo. Moreover, we observed in kinetic analyses from HP 2C T cells in the blood of Rag2−/− mice that the transferred T cells undergoing HP maintained CD62L expression instead of temporarily shedding and re-expressing it upon time (Supplementary Figure 3). This may be a hallmark to distinguish memory-like T cells from bona fide memory T cells which temporarily downregulate/shed expression of l-selectin [5, 43]. Sustained CD3 and CD28 signalling driven by the beads + IL-15 was also able to maintain a high CD62L expression in 44–77 % T cells, indicative of the memory-like T cell phenotype.
Comparable to the murine T cell cultures, we observed that bead + IL-15 stimulation of human PBL consistently led to the generation of memory-like T cells which were readily functional in vitro and quickly proliferated, secreted cytokines (IFNγ, IL-2) and showed lytic degranulation (CD107a+) upon antigen encounter. Similar results were obtained by Kaneko et al. [44] who showed that bead + IL-15 + IL-7-cultured human PBL were highly alloreactive in a humanized mouse model of skin xenografts. However, they did not address whether beads + IL-15 alone was sufficient to obtain the highly functional T cells, as we found IL-7 to be dispensable in all the conditions tested (both human and murine).
It should be noted, though, that the TCR signalling induced by the bead-coated agonistic anti-CD3 is considered to be closer to a cognate peptide–MHC-class I signal than the self-peptide–MHC-class I/TCR triggering delivered by a DC. Alternatively, artificial APCs loaded with irrelevant peptide–MHC complexes and anti-CD28 could be assessed for their potential to induce memory-like cells [45].
Although the phenotype and function of the in vivo HP and in vitro bead-induced memory-like T cells were comparable, this could potentially be the result of a different gene programming. Analyzing the gene signatures of in vitro memory-like T cells should allow a better understanding of their gene programming and may uncover novel paths to explore the generation and use of memory-like T cells in ACT therapies of cancer.
In conclusion, during our efforts to generate the HP cell type in vitro, we observed that the memory-like T cell phenotype can be reproduced in vitro using sustained anti-CD3/CD28 + IL-15 stimulation and that this approach yields cells that retain their anti-tumor function even in the absence of lymphopenia. Overall, our data further support the use of bead stimulation + IL15 for the generation of TCR gene-modified T cells in clinical trials of ACT therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Wilhelm Sander-Stiftung, grant 2005.020.01 and the Dutch Cancer Society (KWF), grant NKI 2008-3988 to C.B. We thank Prof. Reina Mebius and Dr. Rosalie Molenaar for providing us with early cultures of fibroblastic reticular cells. We thank Prof. Jannie Borst, Dr. Bianca Heemskerk, Anna Hooijkaas, and Leila Périé for critical reviewing of this manuscript. We thank Prof. Ton Schumacher for providing us with the 1D3 encoding retrovirus. The 1D3 TCR sequence was kindly provided by Dr Pedro Romero.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbrevations
- Tg
Transgenic
- HP
Homeostatic proliferation
- TIL
Tumor-infiltrating lymphocytes
- RAG
Recombination activating gene
- TCR
T-cell receptor
- ACT
Adoptive cell transfer
- DC
Dendritic cell
- FRC
Fibroblastic reticular cell
- LN
Lymph node
- PBL
Peripheral blood leukocytes
- ML
Memory-like
- APC
Antigen-presenting cells
References
- 1.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8 + T cell anergy and promotes tumor rejection. J Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 2.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, Zhang L, Gajewski TF. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 4.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, Sacks SH, Wong W. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol. 2008;180:3910–3918. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 5.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Hochweller K, Wabnitz GH, Samstag Y, Suffner J, Hammerling GJ, Garbi N. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc Natl Acad Sci U S A. 2010;107:5931–5936. doi: 10.1073/pnas.0911877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8 + T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8 + T cells during homeostatic proliferation requires CD4 + T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 15.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 16.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/S1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 17.Gajewski TF, Fallarino F, Fields PE, Rivas F, Alegre ML. Absence of CTLA-4 lowers the activation threshold of primed CD8 + TCR-transgenic T cells: lack of correlation with Src homology domain 2-containing protein tyrosine phosphatase. J Immunol. 2001;166:3900–3907. doi: 10.4049/jimmunol.166.6.3900. [DOI] [PubMed] [Google Scholar]
- 18.Kranz DM, Tonegawa S, Eisen HN. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia KC, Tallquist MD, Pease LR, et al. Alphabeta T cell receptor interactions with syngeneic and allogeneic ligands: affinity measurements and crystallization. Proc Natl Acad Sci USA. 1997;94:13838–13843. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajewski TF. B7-1 but not B7-2 efficiently costimulates CD8 + T lymphocytes in the P815 tumor system in vitro. J Immunol. 1996;156:465–472. [PubMed] [Google Scholar]
- 21.Gajewski TF, Markiewicz MA, Uyttenhove C (2001) The p815 mastocytoma tumor model. Current protocols in immunology. Chapter 20: unit 20 4 [DOI] [PubMed]
- 22.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorritsma A, Gomez-Eerland R, Dokter M, et al. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood. 2007;110:3564–3572. doi: 10.1182/blood-2007-02-075010. [DOI] [PubMed] [Google Scholar]
- 24.Kamath AT, Sheasby CE, Tough DF. Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN-alpha beta and IFN-gamma. J Immunol. 2005;174:767–776. doi: 10.4049/jimmunol.174.2.767. [DOI] [PubMed] [Google Scholar]
- 25.Malek TR, Ortega G, Chan C, Kroczek RA, Shevach EM. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986;164:709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8 + memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster K, Gadiot J, Andreesen R, Mackensen A, Gajewski TF, Blank C. Homeostatic proliferation of naive CD8 + T cells depends on CD62L/L-selectin-mediated homing to peripheral LN. Eur J Immunol. 2009;39:2981–2990. doi: 10.1002/eji.200939330. [DOI] [PubMed] [Google Scholar]
- 29.Dummer W, Ernst B, LeRoy E, Lee D, Surh C. Autologous regulation of naive T cell homeostasis within the T cell compartment. J Immunol. 2001;166:2460–2468. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 30.Mueller SN, Ahmed R. Lymphoid stroma in the initiation and control of immune responses. Immunol Rev. 2008;224:284–294. doi: 10.1111/j.1600-065X.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 31.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 32.Ge Q, Palliser D, Eisen HN, Chen J. Homeostatic T cell proliferation in a T cell-dendritic cell coculture system. Proc Natl Acad Sci USA. 2002;99:2983–2988. doi: 10.1073/pnas.052714199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 34.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Ogawa S, Tanabe K, Tahara H, Abe R, Kishimoto H. Induction of antitumor immune response by homeostatic proliferation and CD28 signaling. J Immunol. 2008;180:4596–4605. doi: 10.4049/jimmunol.180.7.4596. [DOI] [PubMed] [Google Scholar]
- 36.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/S1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 37.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 41.Maroof A, Beattie L, Kirby A, Coles M, Kaye PM. Dendritic cells matured by inflammation induce CD86-dependent priming of naive CD8 + T cells in the absence of their cognate peptide antigen. J Immunol. 2009;183:7095–7103. doi: 10.4049/jimmunol.0901330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8 + T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko S, Mastaglio S, Bondanza A, et al. IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood. 2009;113:1006–1015. doi: 10.1182/blood-2008-05-156059. [DOI] [PubMed] [Google Scholar]
- 45.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.