ABSTRACT
Background:
The management of recurrent lumbar disc herniation (rLDH) lacks a consensus. Consequently, the choice between repeat microdiscectomy (MD) without fusion, discectomy with fusion, or endoscopic discectomy without fusion typically hinges on the surgeon’s expertise. This study conducts a comparative analysis of postoperative outcomes among these three techniques and proposes a straightforward classification system for rLDH aimed at optimizing management.
Patients and Methods:
We examined the patients treated for rLDH at our institution. Based on the presence of facet resection, Modic-2 changes, and segmental instability, they patients were categorized into three groups: Types I, II, and III rLDH managed by repeat MD without fusion, MD with transforaminal lumbar interbody fusion (TLIF) (MD + TLIF), and transforaminal endoscopic discectomy (TFED), respectively.
Results:
A total of 127 patients were included: 52 underwent MD + TLIF, 50 underwent MD alone, and 25 underwent TFED. Recurrence rates were 20%, 12%, and 0% for MD alone, TFED, and MD + TLIF, respectively. A facetectomy exceeding 75% correlated with an 84.6% recurrence risk, while segmental instability correlated with a 100% recurrence rate. Modic-2 changes were identified in 86.7% and 100% of patients experiencing recurrence following MD and TFED, respectively. TFED exhibited the lowest risk of durotomy (4%), the shortest operative time (70.80 ± 16.5), the least blood loss (33.60 ± 8.1), and the most favorable Visual Analog Scale score, and Oswestry Disability Index quality of life assessment at 2 years. No statistically significant differences were observed in these parameters between MD alone and MD + TLIF. Based on this analysis, a novel classification system for recurrent disc herniation was proposed.
Conclusion:
In young patients without segmental instability, prior facetectomy, and Modic-2 changes, TFED was available should take precedence over repeat MD alone. However, for patients with segmental instability, MD + TLIF is recommended. The suggested classification system has the potential to enhance patient selection and overall outcomes.
Keywords: Endoscopic discectomy, facetectomy, microdiscectomy, Modic changes, segmental instability, transforaminal lumbar interbody fusion
INTRODUCTION
Recurrent lumbar disc herniation (rLDH) represents a complex and challenging clinical entity within the realm of spinal pathology. Characterized by the reemergence of disc material through a previously operated site,[1] rLDH poses significant therapeutic dilemmas due to its elusive etiology, unpredictable presentation, and lack of universally accepted management strategies.[2,3] In the absence of a standardized classification system, the diversity of clinical manifestations and surgical outcomes further complicates decision-making for both clinicians and patients.
Despite the advancements in surgical techniques and imaging modalities, the optimal approach to managing rLDH remains controversial. As a result, clinicians are often confronted with the difficult task of selecting from an array of options that include conservative treatment, minimally invasive procedures, and revision surgeries.[1,3,4,5] This conundrum underscores the pressing need for comprehensive research aimed at elucidating the nuances of these interventions and their impact on patient outcomes.
This research paper seeks to address the existing void in the literature by conducting a comparative analysis of three distinct surgical approaches: Transforaminal endoscopic discectomy (TFED), repeat microdiscectomy (MD) alone, and MD with transforaminal lumbar interbody fusion (TLIF). By evaluating the efficacy, safety, and long-term outcomes of these interventions, this study aims to provide valuable insights into the management of rLDH. The primary goal is to facilitate evidence-based decision-making for clinicians while affording patients a clearer understanding of the potential benefits and risks associated with each approach.
PATIENTS AND METHODS
This retrospective study involves 127 patients managed at our institution for rLDH between 2019 and 2022. These patients had initially been treated for disc herniation at other institutions.
Recurrent intervertebral disc herniation was defined according to the criteria set forth by Yao et al.[5] Specifically, the patient must have previously undergone a successful discectomy without fusion surgery. Furthermore, the patient should have experienced a pain-free period of no <1 month following the initial surgery. In addition, the patient’s symptoms upon recurrence should be consistent with the affected level, substantiated by magnetic resonance imaging (MRI) confirmation of disc herniation recurrence at the same level as the prior discectomy surgery. Patients presenting with recurrent pain or the presence of a disc herniation within 1 month of their most recent surgery were excluded, as such instances are regarded as surgical failures rather than true recurrences. Furthermore, patients with herniation at a level different from the site of prior surgical intervention were also excluded.
Patients without segmental instability on flexion/extension X-rays or Modic-2 changes on preoperative MRI were typically managed with TFED when available. In the presence of Modic-2 changes without segmental instability, these patients underwent either MD alone. MD with TLIF (MD + TLIF) was typical performed in patients with segmental instability and bony stenosis. The three procedures were compared based on intraoperative blood loss, duration of surgery (defined as the time from skin incision to the placement of the final stitch), dura and nerve root injury, and postoperative hospitalization.
The patients were followed up for an average of 2 years. Early postoperative pain was evaluated using the Visual Analog Scale (VAS), with assessments conducted every 4 weeks for 3 months, allowing for a comparison of the three procedures.
Postoperative quality of life was gauged using the Oswestry Disability Index (ODI), administered every 6 months over 24 months.
Patients experiencing recurrence during the follow-up period were subject to analysis through computed tomography (CT) scans to assess the extent of facet resection during the prior MD. This evaluation was conducted in tandem with MRI confirmation of recurrence at the same level, and functional X-rays were utilized to examine sagittal instability.
RESULTS
There were 127 patients included in this study, divided into three groups based on the surgical procedures performed: MD alone (50 patients), MD + TLIF (52 patients), and TFED (25 patients). The distribution of sex is presented and analyzed in Table 1.
Table 1.
Analysis of sex in each study groups
| Sex | Total | ||
|---|---|---|---|
|
| |||
| Female | Male | ||
| MD only | 27 | 23 | 50 |
| MD + TLIF | 22 | 30 | 52 |
| TFED | 12 | 13 | 25 |
| Total | 61 | 66 | 127 |
MD - Microdiscectomy; MD + TLIF - MD + transforaminal lumbar interbody fusion; TFED - Transforaminal endoscopic discectomy
Comparison of intraoperative parameters and postoperative duration of hospital stay
The recorded intraoperative parameters encompassed intraoperative blood loss and the duration of surgery, as outlined in Table 2.
Table 2.
Analysis of the intraoperative parameters and postoperative duration of hospitalization
| Type of operation | Blood loss | Duration of operation | Hospitalization duration |
|---|---|---|---|
| MD only | 85.40±27 | 104.60±29.8 | 4.06±1.5 |
| MD + TLIF | 110.77±41 | 103.85±32.7 | 3.12±1.1 |
| TFED | 33.60±8.1 | 70.80±16.5 | 2.24±0.8 |
MD - Microdiscectomy; MD+TLIF - MD + transforaminal lumbar interbody fusion; TFED - Transforaminal endoscopic discectomy
TFED exhibited a statistically significant lower blood loss of 33.6 ± 8.1 mL compared to the other two procedures, yielding a P = 0.002. Although MD + TLIF displayed a higher intraoperative blood loss of 110 ± 41 mL, no statistically significant difference was observed when compared to repeat MD alone with a blood loss of 85.4 ± 27 mL, resulting in a P = 0.057 [Figure 1].
Figure 1.
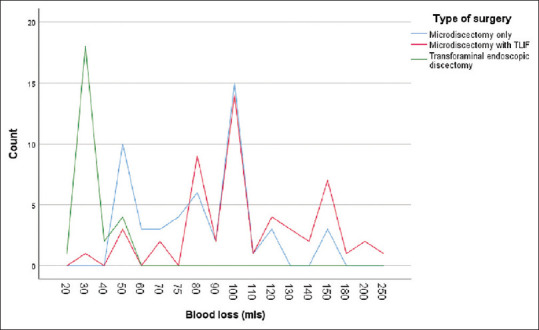
Distribution of intraoperative blood loss in each study group. TLIF: Transforaminal lumbar interbody fusion
Regarding the duration of surgery, no statistically significant difference was discerned between MD alone (104.60 ± 29.8 min) and MD + TLIF (103.85 ± 32.7 min), yielding a P = 0.45. Conversely, the duration of TFED was significantly lower when contrasted with MD alone and MD + TLIF, with values of 70.80* ±16.5 min and a P = 0.00 [Figure 2].
Figure 2.
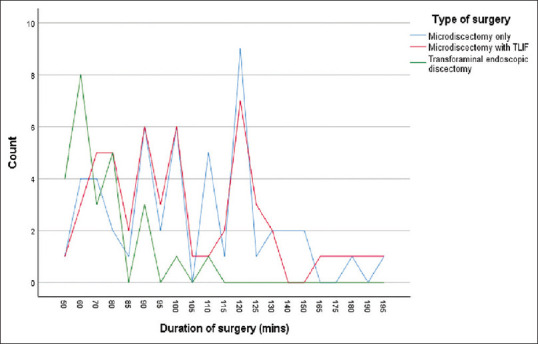
Distribution of duration of surgery in each study group. TLIF: Transforaminal lumbar interbody fusion
Complications
There was no deterioration in the postoperative neurological status observed within the three study groups. The primary complication documented in this study was durotomy [Table 3]. Durotomy occurred in 4% (n = 1), 5.8% (n = 3), and 10% (n = 5) of patients who underwent TFED, MD + TLIF, and MD alone, respectively.
Table 3.
Analysis of durotomy and recurrence in the three study groups
| Durotomy | Recurrence | |||
|---|---|---|---|---|
|
|
|
|||
| Absent | Present | Absent | Present | |
| MD only (n=50) | 90% (n=45) | 10% (n=5) | 80% (n=40) | 20% (n=10) |
| MD + TLIF (n=52) | 94.6% (n=49) | 5.8% (n=3) | 100% (n=52) | 0 |
| TFED (n=25) | 96% (n=24) | 4% (n=1) | 88% (n=22) | 12% (n=3) |
| Total | 118 | 9 | 114 | 13 |
MD - Microdiscectomy; MD+TLIF - MD+transforaminal lumbar interbody fusion; TFED - Transforaminal endoscopic discectomy
Postoperative pain and quality of life
Immediate and early postoperative pain was assessed using the VAS. Discharge VAS scores were recorded as 4, 8, and 8 for TFED, MD, and MD + TLIF, respectively. Subsequent improvement was observed across all three groups, with no significant difference at the 3-month mark [Figure 3]. Quality of life was evaluated using the ODI at discharge, 6 months, 12 months, and 24 months [Figure 4].
Figure 3.
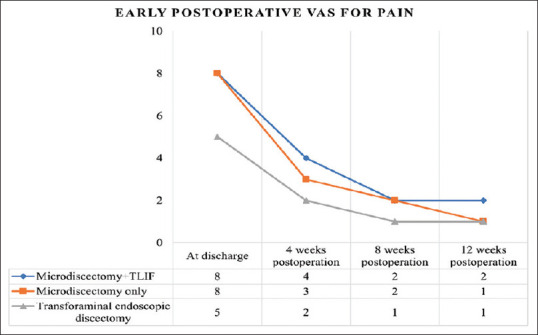
Mean pain Visual Analog Scale at the time of discharge, 4 weeks, 8 weeks and 12 weeks postoperatively. TLIF: Transforaminal lumbar interbody fusion
Figure 4.
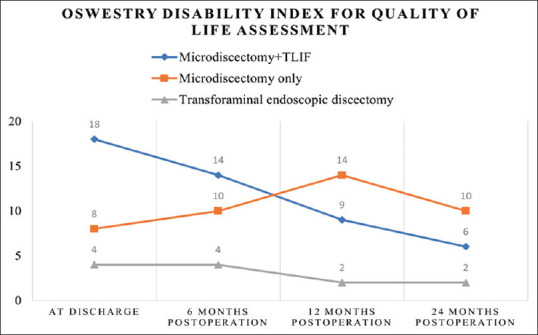
Mean Oswestry Disability Index in each study group at the time of discharge, 6 months, 12 months and 24 months postoperatively. TLIF: Transforaminal lumbar interbody fusion
Recurrence
Throughout the follow-up period, recurrence was identified in 12% (n = 3) of patients who underwent TFED and 20% (n = 10) of those who underwent MD alone. Conversely, no instances of recurrence were reported in the MD + TLIF group [Table 3].
Analysis of facetectomy, Modic-2 changes, segmental instability, and recurrence
Postoperative CT scans were conducted on all patients with recurrence and 13 patients without recurrence during follow-up. Patients subjected to >75% facetectomy exhibited an 88.9% recurrence risk [Table 4].
Table 4.
Analyzing the degree of facetectomy and associated risk of recurrence
| Recurrence | Degree of facetectomy | ||
|---|---|---|---|
|
| |||
| <50% | 51%–75% | 75%–100% | |
| Yes | 10% (n=1) | 33.3% (n=1) | 84.6% (n=11) |
| No | 90% (n=9) | 66.7% (n=2) | 15.4% (n=2) |
| total(n) | 10 | 3 | 13 |
Within the TFED group, Modic-2 changes were detected in 100% of recurrence cases. Among patients with recurrence after MD alone, 86.7% displayed Modic-2 changes. Recurrence without Modic changes was observed in 13.3% and 0% for MD alone and TFED, respectively [Figure 5].
Figure 5.
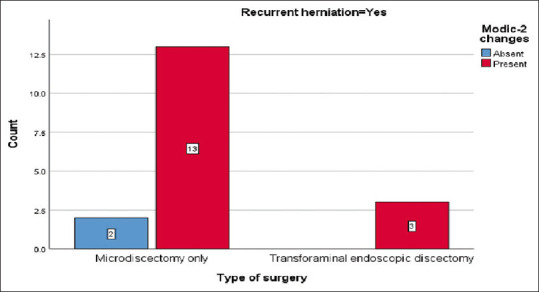
Comparing the risk of recurrence in patients with and without Modic-2 changes
Recurrence was correlated with segmental translation >3 mm and angulation >8° in 100% (n = 3) and 100% (n = 10) of patients who underwent TFED and MD, respectively. However, sagittal segmental instability was associated with recurrence in only 4% and 8% of patients after TFED and MD, respectively [Figures 6 and 7].
Figure 6.
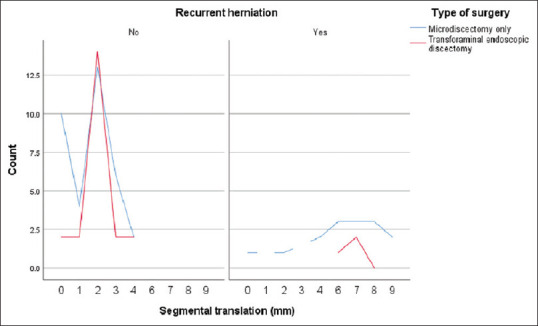
Association between recurrence and segmental translation. No recurrence was report with <3 mm translation
Figure 7.
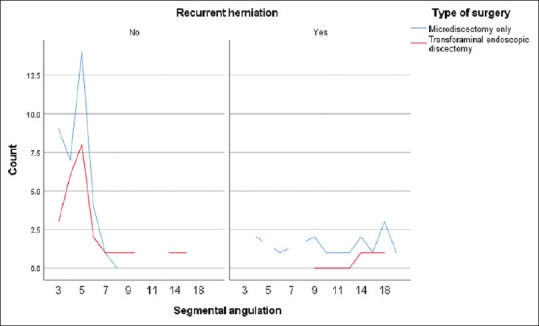
Association between recurrence and segmental angulation. Recurrence was reported with >8° angulation
DISCUSSION
The management of recurrent disc herniation remains a challenge in spinal surgery, with no universally accepted treatment algorithm or classification to guide patient selection. Repeat spine surgery is generally associated with various risk factors, including failed spine syndrome, the risk of spinal nerve and dural injury, as well as exposure to anesthetic agents.[6,7,8,9] This underscores the necessity for a treatment algorithm and classification for rLDH, aiming to standardize and optimize the selection of surgical intervention and enhance the patient outcomes.
Consistent with literature findings, MD + TLIF is linked to higher intraoperative blood loss and surgical duration in comparison to repeat MD alone and TFED.[5,10,11,12] However, the disparity between MD + TLIF and repeat MD alone did not attain statistical significance. Notably, TFED, being the least invasive of the three approaches, demonstrated significantly reduced blood loss and surgical duration.
The presence of adhesions escalates the likelihood of complications across surgical interventions. Specifically, the hazards of durotomy and spinal nerve injury come to the forefront. Even when operating with the aid of a microscope, distinguishing and separating scar tissue from the dura and nerve root remains challenging, thereby heightening the potential for iatrogenic injury.[2,13,14,15] In cases of repeat MD alone, direct traversal through scar tissue elevates the risk of durotomy, aligning with existing literature.[16,17] In contrast, MD + TLIF and TFED, characterized by lateral approaches that circumvent scar tissue, exhibit a reduced risk of durotomy, at 5.8% and 4% respectively. It’s notable that our study did not observe any postoperative deterioration in neurological deficits.
Early postoperative pain control is superior following TFED in comparison to MD alone and MD + TLIF. However, there is no discernible difference in VAS scores at the 3-month postoperative mark. This outcome stems from the fact that MD alone and MD + TLIF, due to their heightened invasiveness and requisite bone resection, exhibit an augmented propensity for early postoperative pain manifestation.[18,19,20] Throughout the duration of the follow-up, TFED consistently manifests as a conduit for good quality of life. It is noteworthy that repeat MD alone was associated with more favorable ODI scores than MD + TLIF during the initial year of follow-up, although this distinction attenuates by the 2-year mark. This trend mirrors observations documented in existing literature.[5,21,22] The interplay of degeneration progression, herniation recurrence, and resultant instability in individuals subjected to repeat MD alone contributes to the diminution in quality of life over the long term.[23,24,25]
In select cases of repeat MD for rLDH, partial or total facetectomy is often pursued as a strategy to circumvent extensive adhesions.[26] While the predication of adhesion severity and the necessity for facetectomy elude precise preoperative anticipation, the act of facetectomy itself has been linked with the induction of instability.[27] Hafez et al. reported a staggering 77.7% incidence of instability progression following facetectomy, although without stratification based on the extent of facet resection.[28] Abumi et al. underscored that partial facetectomy ranging from one-third to half may precipitate notable lumbar spine instability.[26] In contrast, our study embarked on an evaluation of the risk of disc herniation recurrence subsequent to facetectomy, serving as a surrogate for instability assessment. Our findings unveiled that the hazard of recurrence escalates proportionally with 75%–100% facetectomy. In this subset, the incidence of recurrence stood at 84.6%. This starkly elucidates that patients presenting with recurrence alongside at least 75% facet resection necessitate contemplation of fusion surgery, as this signifies a state of significant segmental instability.
The phenomenon of Modic-2 changes has been intrinsically associated with segmental instability and the proclivity for primary disc herniation.[29,30,31] However, few studies have analyzed their significance in recurrent herniation and possible role in management tactic selection. The presence of Modic-2 changes is strongly associated with recurrence of lumbar disc herniation, 88.9%. This is especially so in patients undergoing TFED[32] where all the patients with recurrence had Modic-2 changes in our study. However, some patients had Modic changes without recurrence. Whether the presence of Modic-2 changes alone requires fusion remains controversial but what is clear is that there is an increased risk of recurrence in these patients.[4,29,32] Our analysis underscored instability in 88% of patients featuring Modic changes and undergoing treatment for rLDH. Guided by these observations, our recommendation advocates for a strong consideration of fusion surgery in cases involving rLDH alongside Modic-2 changes.
Segmental instability, whether it serves as a causal factor or an outcome within the ambit of degenerative disc disease, remains a topic marked by contentious discourse. A number of authors including Atul Goel have expounded on the premise that spinal instability constitutes the principal pathological mechanism culminating in disc bulges.[33,34,35,36,37,38,39] Consequently, they advocate for fusion as the sole therapeutic recourse.[24,33,34,35,36,37,38,39] Within our study, dynamic flexion-extension radiographs were executed on patients who exhibited recurrence, with robust correlation outcomes being discerned. Notably, sagittal instability in the form of translation and angulation was universally present among patients experiencing recurrence. While primary disc herniation lies beyond the purview of our study, it is conceivable to infer that segmental instability assumes a pivotal role in the trajectory and genesis of rLDH. For this subset of patients, we advocate for fusion as the therapeutic intervention of choice.[40,41,42,43,44,45,46]
Grounded upon the findings and discourse elucidated above, we posit the ensuing classification for rLDH, poised to foster enhanced precision in both patient selection and surgical technique adoption [Table 5].
Table 5.
Musa’s Classification of recurrent lumbar disc herniation (rLDH)
| Description | Treatment | ||
|---|---|---|---|
| Type I | rLDH without high-risk radiological signs of instability*. | Transforaminal endoscopic discectomy |
|
| Type II | rLDH with Modic-2 changes (orange arrows). | ||
| IIa | < 40 or >60 years old | Transforaminal endoscopic discectomy or repeat microdiscectomy |
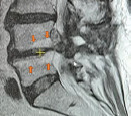
|
| IIb | 40-60 years old | Consider Microdiscectomy with TLIF | |
| Type III | rLDH with: 1. Facetectomy >75% or; 2. Bony spinal canal stenosis or deformity (A). 3. Segmental instability, i.e., translation >4 mm or angulation >9 degrees (B) on flexion-extension radiographs. |
Microdiscectomy with TLIF |
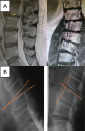
|
*High risk radiological features: Modic-2 changes, facetectomy >75%, segmental instability
The study presented certain limitations that warrant acknowledgment. First, the retrospective nature of the research design may entail inherent biases and uncontrolled confounding variables, potentially affecting the accuracy of the findings. Second, the relatively limited sample size might limit the generalizability of the results to broader populations. Third, the absence of a standardized follow-up protocol and variations in follow-up intervals could influence the consistency of the collected data. Fourth, the study’s focus on a single institution introduces the potential for selection bias and restricts the diversity of patient demographics and surgical practices. Fifth, the lack of a comparative control group receiving conservative management restricts the scope of contrasting outcomes and treatment options. Finally, the absence of long-term outcomes data beyond the 2-year mark may not fully capture the dynamic trajectory of rLDH and its associated complications.
CONCLUSION
In young patients without segmental instability, prior facetectomy, and Modic-2 changes, TFED were available should take precedence over repeat MD alone. However, for patients with segmental instability, MD + TLIF is recommended. The suggested classification system has the potential to enhance patient selection and overall outcomes.
Ethical approval
Ethical approval was waived by the local Ethics Committee of the scientific and technical center, family clinic in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The publication was carried out with the support of the Peoples Friendship University of Russia (RUDN) named after Patrice Lumumba Strategic Academic Leadership Program.
REFERENCES
- 1.Yao Y, Zhang H, Wu J, Liu H, Zhang Z, Tang Y, et al. Comparison of three minimally invasive spine surgery methods for revision surgery for recurrent herniation after percutaneous endoscopic lumbar discectomy. World Neurosurg. 2017;100:641–7.e1. doi: 10.1016/j.wneu.2017.01.089. [DOI] [PubMed] [Google Scholar]
- 2.Dai LY, Zhou Q, Yao WF, Shen L. Recurrent lumbar disc herniation after discectomy: Outcome of repeat discectomy. Surg Neurol. 2005;64:226–31. doi: 10.1016/j.surneu.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhuo X, Hu J, Li B, Sun H, Chen Y, Hu Z. Comparative study of treating recurrent lumbar disc protrusion by three different surgical procedures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:1422–6. [PubMed] [Google Scholar]
- 4.Cao P, Chen Z, Zheng Y, Wang Y, Jiang L, Yang Y, et al. Comparison of simple discectomy and instrumented posterior lumbar interbody fusion for treatment of lumbar disc herniation combined with modic endplate changes. Chin Med J (Engl) 2014;127:2789–94. [PubMed] [Google Scholar]
- 5.Yao Y, Zhang H, Wu J, Liu H, Zhang Z, Tang Y, et al. Minimally invasive transforaminal lumbar interbody fusion versus percutaneous endoscopic lumbar discectomy: Revision surgery for recurrent herniation after microendoscopic discectomy. World Neurosurg. 2017;99:89–95. doi: 10.1016/j.wneu.2016.11.120. [DOI] [PubMed] [Google Scholar]
- 6.Reid PC, Morr S, Kaiser MG. State of the union: A review of lumbar fusion indications and techniques for degenerative Spine disease. J Neurosurg Spine. 2019;31:1–14. doi: 10.3171/2019.4.SPINE18915. [DOI] [PubMed] [Google Scholar]
- 7.Taha A, Youssef M. Surgical outcome of fusion in recurrent lumbar disc herniation. Open J Mod Neurosurg. 2019;10:157. [Google Scholar]
- 8.Tanavalee C, Limthongkul W, Yingsakmongkol W, Luksanapruksa P, Singhatanadgige W. A comparison between repeat discectomy versus fusion for the treatment of recurrent lumbar disc herniation: Systematic review and meta-analysis. J Clin Neurosci. 2019;66:202–8. doi: 10.1016/j.jocn.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-term outcomes of standard discectomy for lumbar disc herniation: A follow-up study of more than 10 years. Spine (Phila Pa 1976) 2001;26:652–7. doi: 10.1097/00007632-200103150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Tang J, Hou S, Ren D, Li L, Lu X, et al. Four-year follow-up results of transforaminal lumbar interbody fusion as revision surgery for recurrent lumbar disc herniation after conventional discectomy. J Clin Neurosci. 2015;22:331–7. doi: 10.1016/j.jocn.2014.06.098. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Zhou Y. Percutaneous endoscopic lumbar diskectomy and minimally invasive transforaminal lumbar interbody fusion for recurrent lumbar disk herniation. World Neurosurg. 2017;98:14–20. doi: 10.1016/j.wneu.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 12.Yan DL, Pei FX, Li J, Soo CL. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17:1311–6. doi: 10.1007/s00586-008-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdu RW, Abdu WA, Pearson AM, Zhao W, Lurie JD, Weinstein JN. Reoperation for recurrent intervertebral disc herniation in the spine patient outcomes research trial: Analysis of rate, risk factors, and outcome. Spine (Phila Pa 1976) 2017;42:1106–14. doi: 10.1097/BRS.0000000000002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed OE, Morad SH, Abdelbar AS. Management of recurrent unilateral lumbar disc herniation in a single level: Unilateral versus bilateral pedicle screws fixation with interbody fusion. Egypt J Neurol Psychiatry Neurosurg. 2020;56:1–10. [Google Scholar]
- 15.Drazin D, Ugiliweneza B, Al-Khouja L, Yang D, Johnson P, Kim T, et al. Treatment of recurrent disc herniation: A systematic review. Cureus. 2016;8:e622. doi: 10.7759/cureus.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musa G, Barrientos RE, Makirov SK, Chmutin GE, Antonov GI, Kim AV, et al. Repeat discectomy for recurrent same level disc herniation: A literature review of the past 5 years. Surg Neurol Int. 2023;14:100. doi: 10.25259/SNI_168_2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kogias E, Franco Jimenez P, Klingler JH, Hubbe U. Minimally invasive redo discectomy for recurrent lumbar disc herniations. J Clin Neurosci. 2015;22:1382–6. doi: 10.1016/j.jocn.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Zhang L, Dong J, Xie P, Liu B, Wang Q, et al. Percutaneous transforaminal endoscopic discectomy versus microendoscopic discectomy for lumbar disc herniation: Two-year results of a randomized controlled trial. Spine (Phila Pa 1976) 2020;45:493–503. doi: 10.1097/BRS.0000000000003314. [DOI] [PubMed] [Google Scholar]
- 19.Fujita M, Inui T, Oshima Y, Iwai H, Inanami H, Koga H. Comparison of the Outcomes of Microendoscopic Discectomy Versus Full-Endoscopic Discectomy for the Treatment of L4/5 Lumbar Disc Herniation. Global Spine Journal. 2022 doi: 10.1177/21925682221127997. doi: 10.1177/21925682221127997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goker B, Aydin S. Endoscopic surgery for recurrent disc herniation after microscopic or endoscopic lumbar discectomy. Turk Neurosurg. 2020;30:112–8. doi: 10.5137/1019-5149.JTN.27360-19.3. [DOI] [PubMed] [Google Scholar]
- 21.Wang A, Yu Z. Comparison of percutaneous endoscopic lumbar discectomy with minimally invasive transforaminal lumbar interbody fusion as a revision surgery for recurrent lumbar disc herniation after percutaneous endoscopic lumbar discectomy. Ther Clin Risk Manag. 2020;16:1185–93. doi: 10.2147/TCRM.S283652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshikane K, Kikuchi K, Izumi T, Okazaki K. Full-endoscopic lumbar discectomy for recurrent lumbar disc herniation: A retrospective study with patient-reported outcome measures. Spine Surg Relat Res. 2021;5:272–7. doi: 10.22603/ssrr.2020-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belykh E, Krutko AV, Baykov ES, Giers MB, Preul MC, Byvaltsev VA. Preoperative estimation of disc herniation recurrence after microdiscectomy: Predictive value of a multivariate model based on radiographic parameters. Spine J. 2017;17:390–400. doi: 10.1016/j.spinee.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Heindel P, Tuchman A, Hsieh PC, Pham MH, D’Oro A, Patel NN, et al. Reoperation rates after single-level lumbar discectomy. Spine (Phila Pa 1976) 2017;42:E496–501. doi: 10.1097/BRS.0000000000001855. [DOI] [PubMed] [Google Scholar]
- 25.Mashhadinezhad H, Sarabi E, Mashhadinezhad S, Ganjeifar B. Clinical outcomes after microdiscectomy for recurrent lumbar disk herniation: A single-center study. Arch Bone Jt Surg. 2018;6:397–401. [PMC free article] [PubMed] [Google Scholar]
- 26.Abumi K, Panjabi MM, Kramer KM, Duranceau J, Oxland T, Crisco JJ. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142–7. doi: 10.1097/00007632-199011010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Fox MW, Onofrio BM, Onofrio BM, Hanssen AD. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: A comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg. 1996;85:793–802. doi: 10.3171/jns.1996.85.5.0793. [DOI] [PubMed] [Google Scholar]
- 28.Hafez AA, Ashry AH, Elsayed A, El Tayeb A, ElShenawy MB. Incidence of iatrogenic lumbar spinal instability after laminectomy, discectomy or facetectomy. Open Access Macedonian Journal of Medical Sciences. 2021;9(B):588–92. [Google Scholar]
- 29.Kjaer P, Korsholm L, Bendix T, Sorensen JS, Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15:1312–9. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahme R, Moussa R. The modic vertebral endplate and marrow changes: Pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am J Neuroradiol. 2008;29:838–42. doi: 10.3174/ajnr.A0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang YH, Zhao CQ, Jiang LS, Chen XD, Dai LY. Modic changes: A systematic review of the literature. Eur Spine J. 2008;17:1289–99. doi: 10.1007/s00586-008-0758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao L, Li S, Liu J, Shan Z, Fan S, Zhao F. Recurrent disc herniation following percutaneous endoscopic lumbar discectomy preferentially occurs when Modic changes are present. J Orthop Surg Res. 2020;15:176. doi: 10.1186/s13018-020-01695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: Review of current trends and controversies. Spine (Phila Pa 1976) 2005;30:S71–81. doi: 10.1097/01.brs.0000155579.88537.8e. [DOI] [PubMed] [Google Scholar]
- 34.Goel A. Indicators of spinal instability in degenerative spinal disease. J Craniovertebr Junction Spine. 2020;11:155–6. doi: 10.4103/jcvjs.JCVJS_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel A. Letter to the editor. Recurrent disc herniation and spinal instability. J Neurosurg Spine. 2021;34:819–20. doi: 10.3171/2020.12.SPINE202101. [DOI] [PubMed] [Google Scholar]
- 36.Goel A, Patil A, Shah A, Rai S, Vutha R, Ranjan S, et al. Lumbar radiculopathy: Outcome analysis following treatment by only fixation – A report of an early experience of 44 cases. J Craniovertebr Junction Spine. 2019;10:203–9. doi: 10.4103/jcvjs.JCVJS_113_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goel A. Is disc herniation “secondary” to spinal instability?. Is it a protective natural response? J Craniovertebr Junction Spine. 2021;12:213–5. doi: 10.4103/jcvjs.jcvjs_111_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel A. Prolapsed, herniated, or extruded intervertebral disc-treatment by only stabilization. J Craniovertebr Junction Spine. 2018;9:133–4. doi: 10.4103/jcvjs.JCVJS_84_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou QS, Sun X, Chen X, Xu L, Qian BP, Zhu Z, et al. Utility of natural sitting lateral radiograph in the diagnosis of segmental instability for patients with degenerative lumbar spondylolisthesis. Clin Orthop Relat Res. 2021;479:817–25. doi: 10.1097/CORR.0000000000001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahsan K, Khan SI, Zaman N, Ahmed N, Montemurro N, Chaurasia B. Fusion versus nonfusion treatment for recurrent lumbar disc herniation. J Craniovertebr Junction Spine. 2021;12:44–53. doi: 10.4103/jcvjs.JCVJS_153_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahsan MK, Hossain MR, Khan MS, Zaman N, Ahmed N, Montemurro N, et al. Lumbar revision microdiscectomy in patients with recurrent lumbar disc herniation: A single-center prospective series. Surg Neurol Int. 2020;11:404. doi: 10.25259/SNI_540_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicoletti GF, Umana GE, Chaurasia B, Ponzo G, Giuffrida M, Vasta G, et al. Navigation-assisted extraforaminal lumbar disc microdiscectomy: Technical note. J Craniovertebr Junction Spine. 2020;11:316–20. doi: 10.4103/jcvjs.JCVJS_146_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmisciano P, Balasubramanian K, Scalia G, Sagoo NS, Haider AS, Bin Alamer O, et al. Posterior epidural intervertebral disc migration and sequestration: A systematic review. J Clin Neurosci. 2022;98:115–26. doi: 10.1016/j.jocn.2022.01.039. [DOI] [PubMed] [Google Scholar]
- 44.Saghebdous S, Zare R, Chaurasia B, Vakilzadeh MM, Yousefi O, Boustani MR. Dynamic rod constructs as the preventive strategy against adjacent segment disease in degenerative lumbar spinal disorders: A retrospective comparative cohort study. Arch Bone Jt Surg. 2023;11:404–13. doi: 10.22038/ABJS.2022.68498.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pahwa B, Tayal A, Chowdhury D, Umana GE, Chaurasia B. Endoscopic versus microscopic discectomy for pathologies of lumbar spine: A nationwide cross-sectional study from a lower-middle-income country. J Craniovertebr Junction Spine. 2023;14:373–80. doi: 10.4103/jcvjs.jcvjs_39_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra S, Garg K, Chaurasia B, Budihal BR, Deora H, Tandon V, et al. An assessment of the variation in the practice of lumbar discectomy and its role in axial back pain. J Craniovertebr Junction Spine. 2023;14:259–67. doi: 10.4103/jcvjs.jcvjs_46_23. [DOI] [PMC free article] [PubMed] [Google Scholar]


