Abstract
Growing evidence suggests that concurrent loco-regional and systemic treatment modalities may lead to synergistic anti-tumor effects in advanced melanoma. In this retrospective multicenter study, we evaluate the use of electrochemotherapy (ECT) combined with ipilimumab or PD-1 inhibition. We investigated patients with unresectable or metastatic melanoma who received the combination of ECT and immune checkpoint blockade for distant or cutaneous metastases within 4 weeks. Clinical and laboratory data were collected and analyzed with respect to safety and efficacy. A total of 33 patients from 13 centers were identified with a median follow-up time of 9 months. Twenty-eight patients received ipilimumab, while five patients were treated with a PD-1 inhibitor (pembrolizumab n = 3, nivolumab n = 2). The local overall response rate (ORR) was 66.7 %. The systemic ORR was 19.2 and 40.0 % in the ipilimumab and PD-1 cohort, respectively. The median duration of response was not reached in either group. The median time to disease progression was 2.5 months for the entire population with 2 months for ipilimumab and 5 months for PD-1 blockade. The median overall survival was not reached in patients with ipilimumab and 15 months in the PD-1 group. Severe systemic adverse events were detected in 25.0 % in the ipilimumab group. No treatment-related deaths were observed. This is the first reported evaluation of ECT and simultaneous PD-1 inhibition and the largest published dataset on ECT with concurrent ipilimumab. The local response was lower than reported for ECT only. Ipilimumab combined with ECT was feasible, tolerable and showed a high systemic response rate.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1856-z) contains supplementary material, which is available to authorized users.
Keywords: Electrochemotherapy, Ipilimumab, Pembrolizumab, Nivolumab, Melanoma, Immune checkpoint blockade
Introduction
Melanoma is one of the most aggressive and treatment-resistant types of cancer with increasing incidence and a strong tendency to metastasize. The most commonly affected sites by distant metastases are lungs, liver, and the central nervous system. Additionally, 5–10 % of patients develop skin and soft tissues metastases. Immune checkpoint blockade has recently emerged as one mainstay of the treatment of advanced stage melanoma. Ipilimumab was the first substance to achieve survival benefits in metastatic disease, enhancing the anti-tumor immune response by blocking cytotoxic T lymphocyte-associated protein 4 (CTLA-4), a negative regulator of T cell activation [1–3]. Nivolumab and pembrolizumab act by inhibition of the cell surface receptor programmed cell death protein 1 (PD-1), another immune checkpoint preventing T cell activation in melanoma. Both agents have been shown to lead to significantly longer survival, higher response rates, and less immune-related toxicity compared to ipilimumab [4–8]. However, primary non-response and disease progression remain a major challenge in the management of the disease, underlining the need for novel treatment regimens.
A growing body of evidence suggests enhanced efficacy and potential synergy when systemic and local treatment modalities are combined [9, 10]. Whenever cutaneous, subcutaneous, or soft tissue metastases are present, the combination of electrochemotherapy (ECT) with systemic therapy may have unattended therapeutic potential. ECT is a method of local tumor ablation that combines the administration of otherwise poorly permeable chemotherapeutics such as bleomycin with short high-intensity electroporation pulses, resulting in a massively enhanced local drug uptake. It is a well-tolerated, safe, and effective therapeutic strategy with high local tumor control rates [11]. One case study reported complete regression of melanoma skin metastases after treatment with ECT and sequential application of ipilimumab [12]. Mechanistically, inflammatory responses in the surroundings of treated lesions and a release of tumor antigens are thought to synergize with T cell activation resulting from checkpoint inhibition and thereby augment the systemic anti-tumor immune response [13, 14]. A recently published retrospective analysis on 15 patients treated with ipilimumab in an early access program who also received ECT revealed high overall local and systemic response rates without notable toxicity [15]. However, the number of patients was limited, the included population was highly selected among patients who were actually treated, and the combination of ECT with PD-1 inhibition was not assessed.
Here, we present a multicenter analysis of 33 patients with metastatic melanoma who received ECT simultaneously with either ipilimumab or a PD-1 inhibitor (nivolumab or pembrolizumab). The combination of immune therapy and ECT was evaluated with respect to safety and efficacy.
Patients and methods
Study design
The study included patients with histologically confirmed unresectable or metastatic melanoma undergoing concurrent ECT and immune checkpoint blockade with ipilimumab or PD-1 inhibition for distant or cutaneous metastases. Concurrent therapy was defined as application of intralesional ECT in combination with at least one cycle of ipilimumab, pembrolizumab, or nivolumab within 4 weeks. Patients were recruited from 13 major centers for dermatologic oncology within Germany. Clinical and treatment data were extracted from preexisting patient files and merged to a central database prior to analysis. The study was approved by the Institutional Review Board of the Medical Faculty of the Munich University Hospital (UE-No. 718-15).
Treatment protocols
All agents were administered according to standard protocols. Ipilimumab was given at a dosage of 3 mg/kg body weight once every 3 weeks for a maximum of four doses; pembrolizumab was applied at 2 mg/kg every 3 weeks and nivolumab at 3 mg/kg every 2 weeks until disease progression or development of unacceptable toxicity. In all cases, ECT was performed according to the European Standard Operating Procedures for Electrochemotherapy (ESOPE) guidelines, specifically following the operating procedures “local anesthesia—intratumoral chemotherapy” or “general sedation—intravenous chemotherapy” [16]. Bleomycin was used for ECT in all cases at doses of 15,000 IU/m2 body surface area if given intravenously and at 250–500 IU/cm3 of tumor tissue if applied intralesionally. High-voltage pulses (730–1000 V) were applied to cutaneous target lesions with a Cliniporator™ (IGEA, Carpi, Italy) or Sennex® (BIONMED Technologies, Saarbrücken, Germany) pulse generator 8–28 min after infusion of bleomycin (if given intravenously). Needle electrodes with a linear configuration and a length of 10–30 mm (type II electrodes) were used for rather small tumor nodules. Needles with a hexagonal configuration and a length of 10–30 mm (type III electrodes) were used for bigger nodules (>1 cm diameter). If small and large lesions were present in the same patient, adjustable needle electrodes with a linear or hexagonal configuration and a variable length of 5–30 mm were used. The application of plate electrodes (type I electrodes) was not reported in any patient. In accordance with recently published guidelines for improving the quality of reporting clinical ECT studies, more detailed information on the settings and key electrical parameters of the ECT procedures can be found in supplementary Table 1 [16, 17].
Table 1.
Patient characteristics
| Ipilimumab (n = 28) | PD-1 (n = 5)a | Total (n = 33) | |
|---|---|---|---|
| Median age (range) | 73 (39–83) | 60 (46–72) | 72 (39–83) |
| Gender, n (%) | |||
| Male | 15 (53.6) | 4 (80.0) | 19 (57.6) |
| Female | 13 (46.4) | 1 (20.0) | 14 (42.4) |
| Genotype, n (%) | |||
| BRAF-mut. V600 | 6 (21.4) | 1 (20.0) | 7 (21.2) |
| NRAS-mut. Q61 | 6 (21.4) | 0 (0.0) | 6 (18.2) |
| KIT (Exon 18, 829A > p) | 1 (3.6) | 0 (0.0) | 1 (3.0) |
| WT | 15 (53.6) | 4 (80.0) | 19 (57.6) |
| Disease stage, n (%) | |||
| IIIc | 14 (50.0) | 0 (0.0) | 14 (42.4) |
| IV | 14 (50.0) | 5 (100.0) | 19 (57.6) |
| Brain metastases, n (%) | |||
| Yes | 2 (7.1) | 0 (0.0) | 2 (6.1) |
| No | 26 (92.9) | 5 (100.0) | 31 (93.9) |
| Site of cutaneous metastases, n (%) | |||
| Legs | 15 (53.6) | 2 (40.0) | 17 (51.5) |
| Arms | 2 (7.1) | 0 (0.0) | 2 (6.1) |
| Head/neck | 1 (3.6) | 1 (20.0) | 2 (6.1) |
| Trunk | 10 (35.7) | 2 (40.0) | 12 (36.4) |
| Baseline LDH, n (%) | |||
| Normal | 16 (57.1) | 2 (40.0) | 18 (54.5) |
| Elevated | 8 (28.6) | 3 (60.0) | 11 (33.3) |
| Unknown | 4 (14.3) | 0 (0.0) | 4 (12.1) |
| Number of previous local therapies, n (%) | |||
| 0 | 6 (21.4) | 0 (0.0) | 6 (18.2) |
| 1 | 13 (46.4) | 3 (60.0) | 16 (48.5) |
| 2 | 9 (32.1) | 2 (40.0) | 11 (33.3) |
| Number of previous systemic therapies, n (%) | |||
| 0 | 12 (42.9) | 1 (20.0) | 13 (39.4) |
| 1 | 13 (46.4) | 2 (40.0) | 15 (45.5) |
| 2 | 3 (10.7) | 2 (40.0) | 5 (15.2) |
| Type of previous systemic therapy, n (%) | |||
| Chemotherapy | 8 (28.6) | 1 (20.0) | 9 (27.3) |
| Targeted therapy | 6 (21.4) | 1 (20.0) | 7 (21.1) |
| Ipilimumab | 0 (0.0) | 4 (80.0) | 4 (12.1) |
| None | 12 (42.9) | 1 (20.0) | 13 (39.4) |
| Unknown | 2 (7.1) | 0 (0.0) | 2 (6.1) |
aPembrolizumab n = 3; nivolumab n = 2
Data collection
Collected data comprised patient demographics, basic laboratory values with lactate dehydrogenase (LDH) and S100β, mutational status of BRAF, NRAS, and KIT, previous local and systemic therapies, disease stage according to the 7th edition of the American Joint Committee on Cancer (AJCC) melanoma staging system from 2009 [18], and presence of brain metastases before onset of the combination therapy. The sites of cutaneous melanoma metastases were categorized as follows: head and neck including ears and nose; upper extremities including hands and palms; trunk including shoulder blades, groins, and buttocks; and lower extremities with feet and soles. Local and systemic responses to therapy were graded by the site investigators according to RECIST criteria version 1.1 [19] and indicated as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR and PR were summarized to indicate the overall response rate (ORR). Adverse events (AE) were graded according to the Common Terminology Criteria for Adverse Events v4.03 published by the National Institutes of Health in 2010.
Statistical analyses
Overall survival (OS) and progression-free survival (PFS) were calculated as time from onset of the combination therapy until melanoma- or treatment-related death and disease progression, respectively. If no such event occurred or if patients were lost to follow-up, the date of the last documented contact was registered and used as censored observation. Survival curves and progression fractions were analyzed with the product limit (Kaplan–Meier) method for censored failure time data assuming proportional hazards. Univariate comparisons of Kaplan–Meier estimators were performed with the log-rank test. The median follow-up time was calculated as published previously [20]. Comparisons among groups with categorical variables were assessed with the Fisher’s exact and Chi-square test. Two-tailed p values were calculated and considered significant with values p < 0.05. All analyses were performed with the GraphPad Prism software (version 5.01).
Results
Patient population and treatment characteristics
The database was open from June through December 2015. In total, 33 patients from 13 centers were identified who matched the inclusion criteria. Twenty-eight patients received ECT with ipilimumab; five patients were treated with ECT and PD-1 inhibition (n = 3 for pembrolizumab and n = 2 for nivolumab). The detailed patient characteristics are shown in Table 1. The most common sites of cutaneous metastases treated with ECT were legs and feet (51.5 %), followed by trunk (36.4 %). The median number of locally treated lesions was 4 (range 1–100). The median size of the largest lesion of one treatment session was 2 cm (range 1–20 cm). One patient (3.0 %) received bleomycin intratumorally with local anesthesia only. All other patients (n = 32, 97.0 %) underwent ECT in general anesthesia or sedation and received bleomycin intravenously. Safety margins of 1 cm around the tumor lesions were applied in one case and otherwise not consistently documented. The electric pulses covered the entire surface area of the metastatic lesions in all cases. Brain metastases were present in two patients (6.1 %) in the ipilimumab group at baseline. All four cycles of ipilimumab were given to 19 patients (67.9 %), while nine patients (32.1 %) received three cycles or less. Three patients were treated with pembrolizumab with 3, 5, and 9 applications, respectively. The two patients with nivolumab received four and seven cycles each. ECT was performed once in 20 patients (60.6 %). Thirteen patients (39.4 %) received ECT multiple times during the course of the disease. It was applied prior to the first cycle of ipilimumab in nine cases and after the first cycle in 19 cases. One patient received ECT prior to the first cycle of PD-1 inhibition, while four patients had ECT after the first cycle of PD-1 inhibition.
Previous therapies
The cutaneous metastases of most patients had been treated prior to the combination therapy with other local treatments such as surgical excision (n = 20), radiation (n = 10), one or more cycles of ECT (n = 8), isolated limb perfusion (n = 3), intralesional interleukin-2 (n = 2), local interferon α (n = 1), and topical application of 2,4-dinitrochlorobenzene (n = 1). Six patients had received no local treatment, 16 patients had one, and 11 patients had two local treatment regimens previously.
Thirteen individuals (39.4 %) were naïve for any systemic therapy and received the combination as primary intervention. Chemotherapy was given as previous therapy in nine patients (27.3 %). Targeted therapy with kinase inhibitors was reported in seven patients (vemurafenib n = 3, dabrafenib n = 2, imatinib n = 1, dabrafenib plus trametinib n = 1). Four patients in the PD-1 group received ipilimumab prior to PD-1 blockade (Table 1).
Safety and tolerability
The most common local AE included ulceration/necrosis (45.5 %), erythema (42.4 %), and infection of treated areas (30.3 %). Pain was reported as local AE by eight patients (24.2 %). Severe local AE (≥grade 3) were found in five patients (15.2 %) and comprised ulceration, infection, pain, and bleeding. No significant difference was observed between the ipilimumab- and PD-1 inhibitor-treated cohort with respect to incidence and seriousness (p = 0.82).
Systemic AE occurred in 15 cases (45.5 %). Most commonly, patients suffered from pain as systemic AE (15.2 %), followed by nausea (9.1 %), and colitis with diarrhea (9.1 %). Severe systemic AE occurred in seven patients all of whom were treated with ECT and ipilimumab, revealing a rate of 21.2 and 25.0 % for the entire population and the ipilimumab group, respectively. In contrast, no severe systemic AE were observed in the PD-1 inhibitor-treated cohort (p = 0.20). Grade 3 events were observed in four individuals (12.1 %) comprising endocrine abnormalities with hypophysitis or hyperthyroidism (n = 2) and autoimmune-related colitis (n = 2). Three patients (9.1 %) experienced grade 4 systemic AE including peritonitis/ascites (n = 1) and acute kidney injury (n = 2). Systemic treatment was discontinued in two cases due to severe colitis and in one case due to hypophysitis. No treatment-related deaths were reported in either group. All AE are listed in detail in Table 2.
Table 2.
Adverse events
| Ipilimumab (n = 28) | PD-1 (n = 5) | Total (n = 33) | |
|---|---|---|---|
| Local events, n (%) | |||
| None | 3 (10.7) | 0 (0.0) | 3 (9.1) |
| Erythema | 12 (42.9) | 2 (40.0) | 14 (42.4) |
| Necrosis/ulcerationa | 12 (42.9) | 3 (60.0) | 15 (45.5) |
| Infectiona | 7 (25.0) | 3 (60.0) | 10 (30.3) |
| Paina | 6 (21.4) | 2 (40.0) | 8 (24.2) |
| Others | |||
| Rash | 4 (14.3) | 1 (20.0) | 5 (15.2) |
| Bleedinga | 2 (7.1) | 2 (40.0) | 4 (12.1) |
| Edema/swelling | 1 (3.6) | 1 (20.0) | 2 (6.1) |
| Pruritus | 4 (14.3) | 1 (20.0) | 5 (15.2) |
| Systemic events, n (%) | |||
| None | 16 (57.1) | 2 (40.0) | 18 (54.5) |
| Colitis/diarrheaa, b | 3 (10.7) | 0 (0.0) | 3 (9.1) |
| Nausea | 2 (7.1) | 1 (20.0) | 3 (9.1) |
| Endocrine abnormalities | |||
| Hyperthyroidisma, b | 1 (3.6) | 0 (0.0) | 1 (3.0) |
| Hypophysitisa, b | 2 (7.1) | 0 (0.0) | 2 (6.1) |
| Pain | 2 (7.1) | 0 (0.0) | 2 (6.1) |
| Fatigue | 2 (7.1) | 3 (60.0) | 5 (15.2) |
| Laboratory abnormalities | |||
| Leukocytosis | 0 (0.0) | 1 (20.0) | 1 (3.0) |
| Increase of liver enzymes | 0 (0.0) | 1 (20.0) | 1 (3.0) |
| Hyperkalemia | 1 (3.6) | 0 (0.0) | 1 (3.0) |
| Others | |||
| Acute kidney failurea | 2 (7.1) | 0 (0.0) | 2 (6.1) |
| Sepsis/peritonitisa | 2 (7.1) | 0 (0.0) | 2 (6.1) |
| Loss of appetite | 1 (3.6) | 0 (0.0) | 1 (3.0) |
| Fever | 1 (3.6) | 0 (0.0) | 1 (3.0) |
| Insomnia | 1 (3.6) | 0 (0.0) | 1 (3.0) |
| Dyspnea | 0 (0.0) | 1 (20.0) | 1 (3.0) |
aSevere adverse events (≥grade 3)
bAdverse events leading to discontinuation of the combination treatment
Efficacy
The median follow-up time of the entire cohort was 9 months. The median time to assessment of the local response was 3 months (range 1–8 months). The local overall response rate (ORR) was 66.7 % with complete responses (CR) in 15.2 % and partial responses (PR) in 51.5 % of cases (Fig. 1). Stable disease (SD) was achieved with the combination treatment in 9.1 % resulting in 75.8 % local disease control. Two patients were not assessable for systemic response. The systemic ORR amounted to 19.2 and 40.0 % for the ipilimumab and PD-1 group, respectively, revealing an overall systemic ORR of 22.6 %. The median duration of the response was not reached. Two more patients (6.5 %) had stable disease; thus, systemic disease was controlled in 29.1 % (Table 3).
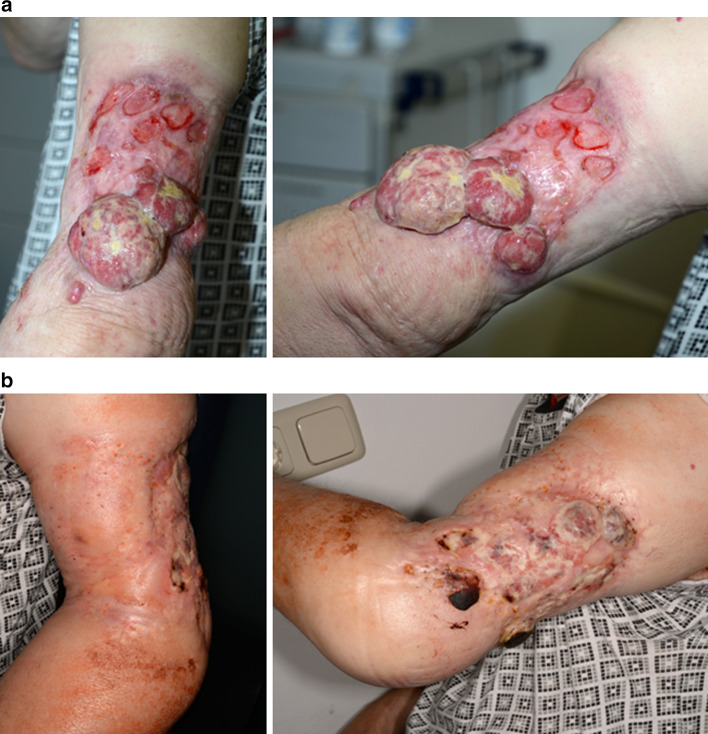
Fig. 1.
Complete local response before (a) and 3 months after (b) the combination therapy on the left upper arm. The patient received three cycles of ipilimumab along with ECT and experienced grade 3 autoimmune-related colitis
Table 3.
Response rates
| Ipilimumaba (n = 28) | PD-1 (n = 5) | Total (n = 33) | p value | |
|---|---|---|---|---|
| Local response, n (%) | ||||
| Complete response (CR) | 5 (17.9) | 0 (0.0) | 5 (15.2) | p = 0.65 |
| Partial response (OR) | 14 (50.0) | 3 (60.0) | 17 (51.5) | |
| Stable disease (SD) | 3 (10.7) | 0 (0.0) | 3 (9.1) | |
| Progressive disease (PD) | 6 (21.4) | 2 (40.0) | 8 (24.2) | |
| Systemic responsea, n (%) | ||||
| Complete response (CR) | 2 (7.7) | 0 (0.0) | 2 (6.5) | p = 0.61 |
| Partial response (PR) | 3 (11.5) | 2 (40.0) | 5 (16.1) | |
| Stable disease (SD) | 2 (7.7) | 0 (0.0) | 2 (6.5) | |
| Progressive disease (PD) | 19 (73.1) | 3 (60.0) | 22 (71.0) | |
aThe systemic response was not assessable in two patients in the ipilimumab group
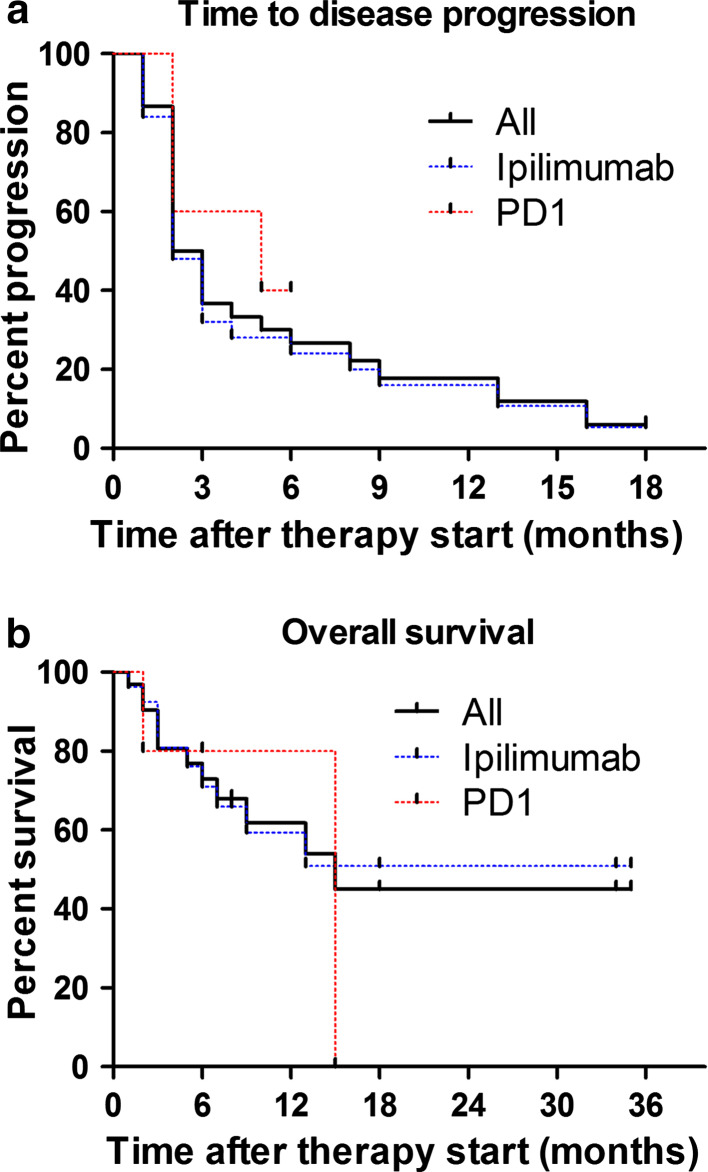
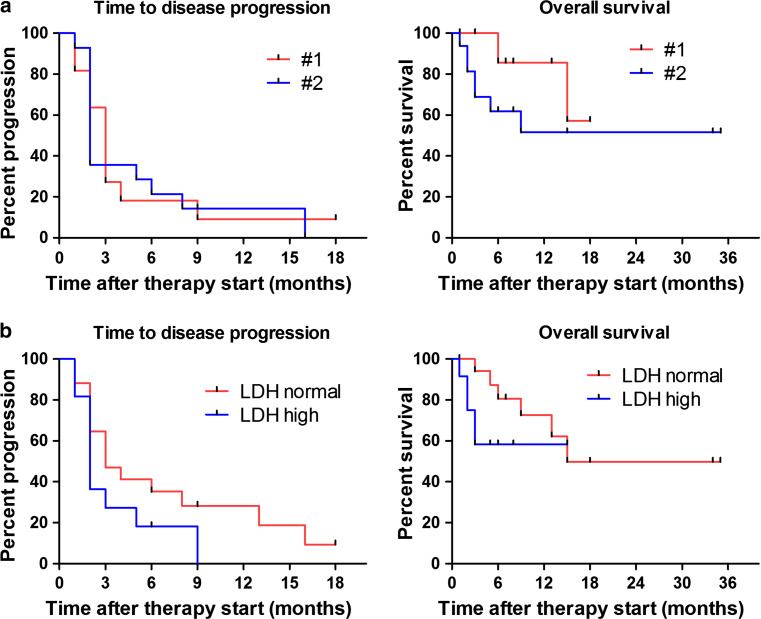
Four patients (12.1 %) did not show any disease progression during the observation period. The median time to disease progression (PFS) for the entire cohort was 2.5 with 2 months for the ipilimumab and 5 months for the PD-1 group (p = 0.77). No significant difference regarding PFS was observed between treatment line (p = 0.95) nor elevation of LDH at baseline (p = 0.14).
Thirteen melanoma-related deaths were reported (39.4 %). The median overall survival (OS) was not reached in patients treated with ipilimumab and 15 months in the PD-1 blockade-treated cohort (p = 0.98). OS did not significantly differ according to treatment line (p = 0.21) nor elevated serum LDH at baseline (p = 0.21) (Figs. 2, 3).
Fig. 2.
Kaplan–Meier estimates for progression-free (a) and overall survival (b) of the study population. The entire cohort comprised 33 patients receiving the combination therapy with n = 28 for ipilimumab and n = 5 for PD-1 inhibition with either pembrolizumab (n = 3) or nivolumab (n = 2). The median time to disease progression was 2.5 months (2 months for ipilimumab, 5 months for PD-1; p = 0.77). The median OS was 15 months (not reached for ipilimumab, 15 months for PD-1; p = 0.98)
Fig. 3.
Kaplan–Meier estimates of the study population according to therapy line (a) and elevation of serum LDH at baseline (b). Time to disease progression and overall survival are shown and did not show significant differences between therapy lines 1 and 2 (p = 0.95 for PFS; p = 0.21 for OS) nor LDH elevation (p = 0.14 for PFS; p = 0.21 for OS)
Discussion
Considerable progress has been achieved with immune checkpoint blockade in the treatment of melanoma. Recent studies suggest that the anti-tumor response can be further augmented by simultaneously adding a loco-regional treatment regimen. In this study, we evaluated the combination of ECT and ipilimumab or PD-1 inhibition. ECT-treated areas showed a local ORR of 66.7 % for all cases. The systemic ORR was 19.2 % for ipilimumab and 40.0 % for PD-1 inhibition, revealing an ORR of 22.6 % for the entire study population. Severe systemic AE were observed in 25.0 % of patients treated with ECT and ipilimumab, while no severe events were reported in the PD-1 cohort. No treatment-related deaths occurred, and treatment was discontinued in three patients due to severe AE.
The majority of patients in our analysis received the combination of ECT plus ipilimumab with a best ORR of 19.2 % and disease control in 26.9 %. Even though cross-trial comparisons only allow limited conclusions, the response rates achieved with ipilimumab as monotherapy in previous studies were slightly lower, ranging from 10.9 % in previously treated to 15.2 % in treatment-naïve patients [1, 2]. An ORR of 11 % was observed for patients with asymptomatic brain metastases who were initially excluded from major phase III trials [21]. Most patients in our population did not have brain metastases before the combination was started and might hence represent a cohort with a more favorable prognosis. In a recent study involving 142 patients who had not received any treatment before, ipilimumab and nivolumab were compared to ipilimumab alone. The ipilimumab monotherapy group showed an objective response of 11 %. Drug-related adverse events of grade 3 or 4 occurred in 24 % [22]. The investigator-assessed objective response was 19.0 % for patients who received ipilimumab monotherapy in the phase III trial CheckMate 067 with treatment-related adverse events of grade 3 or 4 occurring in 27.3 % [23]. The phase III trial KEYNOTE-006 compared pembrolizumab at different dosages to ipilimumab and revealed a response rate of 11.9 % in patients with ipilimumab only. Severe adverse events developed in 19.9 % of cases in the ipilimumab treatment arm [5]. Our study demonstrated a best overall systemic response rate of 19.2 % in the 28 patients who received ipilimumab and ECT. This rate is higher than in the ipilimumab monotherapy arms of the pertinent trials, suggesting increased efficacy of the combination with ECT. A recent study on 15 patients receiving ECT and ipilimumab in an expanded access program found a systemic response rate of 60.0 % which is by far higher than in our population and under monotherapy with ipilimumab [15]. However, the sample size was smaller and the study was performed as monocentric analysis, raising the possibility of overestimating the true treatment effect and the risk of a strong selection bias.
The median OS was not reached in the ipilimumab cohort in our study. In contrast, it was constantly indicated as 10–11 months throughout the pertinent trials investigating ipilimumab alone [1, 2], further underlining that the concurrent application of ECT as local treatment modality may indeed add to the systemic efficacy in advanced melanoma. Such synergistic relationships were also reported for the combination of ipilimumab with radiotherapy and the oncolytic agent talimogene laherparepvec (T-VEC) [9, 10]. These studies suggest that the local destruction of tumor tissue may result in a release of antigens and induce a local inflammatory response that further augments the efficacy of systemic immune therapy.
Five patients received ECT plus PD-1 inhibition with partial responses observed in two cases treated with pembrolizumab, resulting in a formal response rate of 40.0 % in this group. To the best of our knowledge, this is the first report on this combination. Since the PD-1 cohort is small, it does not allow reliable conclusions or comparisons to the treatment responses that were previously achieved with nivolumab or pembrolizumab monotherapies [4, 5, 22, 24].
Notably, the local ORR of 66.7 % was almost identical to the study performed by Mozzillo and colleagues who found a response rate of 67 % in ECT-treated cutaneous lesions. Both values are distinctly lower than previously published for bleomycin-based ECT alone in a large meta-analysis [11]. This might be due to the fact that the assessment of the local response after ECT follows little standardization and that many patients in our study had previous local treatments. The local response was independent of the tumor size. Thus, we conclude that the lower response rate may be due to patient selection and pre-treatment. Twenty-seven patients (81.8 %) had undergone other local therapy regimens before and 11 patients (33.3 %) even received two previous local treatments.
The most common local adverse events in our study comprised erythema, necrosis/ulceration, and pain. Except for necrosis/ulceration, the local AEs were comparable regarding frequency and seriousness to ECT alone and there was no evidence of additional local toxicity induced by simultaneous immune checkpoint blockade [25]. ECT-induced ulceration was observed both in small and in large lesions, indicating that it was independent of the tumor size. It is conceivable that bleomycin reaches higher lesional concentrations when it is combined with ipilimumab or PD-1 inhibitors. It is distributed with a mean volume of 17.5 L/m2 after an intravenous bolus with almost no binding to plasma proteins. The drug is inactivated by the enzyme bleomycin hydrolase which is expressed in most tissues except for skin and lungs, both known target organs of bleomycin toxicity. Ipilimumab, pembrolizumab, and nivolumab are catabolized through non-specific pathways. Although pharmacologic interaction studies of immune checkpoint blockade and bleomycin have not been published yet, immune therapy agents may interfere with the enzymatic inactivation of bleomycin, resulting in higher local toxicity with an increased risk of ulceration and necrosis.
Regarding safety, severe drug-related systemic adverse events (≥grade 3) were observed in 25.0 % among patients receiving the combination of ECT and ipilimumab. Given that this rate is lower than the 27.3 % detected in the CheckMate 067 study and that no treatment-related deaths were observed, we conclude that the combination was clinically feasible and tolerable [23]. However, the systemic treatment was discontinued due to ipilimumab-induced autoimmune-related colitis and hypophysitis in three cases, but no treatment-related deaths were registered. Hence, these data may indicate that the combination while more efficient may also bear more toxicity than ipilimumab alone, warranting close monitoring and adequate management of patients receiving this combination.
The major limitations of our report are the retrospective design and the limited sample size. In particular, the cohort receiving ECT and PD-1 inhibition was small. The main objective of this study was to generate exploratory data for future prospective trials which are adequately powered and have well-defined endpoints. Nevertheless, our data are the largest set on the combination of ECT with ipilimumab and the first report evaluating ECT with PD-1 inhibition. The best timing of such combination therapy is currently unknown. To account for true synergistic effects, we chose a relatively close time frame of 4 weeks in which at least one cycle of immune therapy and ECT had to be administered, regardless of their application sequence. Albeit recruited from several major cancer centers with ECT, the small sample size of our study reveals that these strict inclusion criteria were rarely applicable.
Taken together, we show that the simultaneous application of ECT and immune checkpoint blockade is feasible and tolerable. The local response rate was lower than previously reported for ECT alone, presumably because many patients had received one or more previous local treatments. Ipilimumab and ECT showed a high response rate and potent anti-tumor activity which may be higher than with immune therapy alone. A significant rate of severe adverse events requires a close and vigilant clinical monitoring of patients receiving ECT and concurrent ipilimumab.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Carla Lingner and Diana Lingk for their support with Fig. 1. Bastian Schilling receives research grants from Bristol-Myers Squibb and MSD Sharp and Dohme.
Abbreviations
- AE
Adverse event(s)
- AJCC
American Joint Committee on Cancer
- CR
Complete response
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- ECT
Electrochemotherapy
- ESOPE
European Standard Operating Procedures for Electrochemotherapy
- LDH
Lactate dehydrogenase
- ORR
Overall response rate
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PFS
Progression-free survival
- PR
Partial response
- SD
Stable disease
- T-VEC
Talimogene laherparepvec
Compliance with ethical standards
Conflict of interest
Beatrice Schell, Edgar Dippel, Fanny Matheis, Markus V. Heppt, Susanne G. Schäd, and Thilo Gambichler declare no conflict of interest. Bastian Schilling: advisory for Roche, M.S.D. Sharp and Dohme, and Bristol-Myers Squibb, travel support from Roche, Bristol-Myers Squibb and Amgen, honoraria from Roche, M.S.D. Sharp and Dohme and Bristol-Myers Squibb. Carola Berking: advisory for Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche, speaker’s honoraria by Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche. Carmen Loquai: advisory for Roche, Amgen, Novartis, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Ribological, speaker’s honoraria from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, travel reimbursement from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis. Daniela Göppner: advisory for Roche, Amgen, Bristol-Myers Squibb and M.S.D. Sharp and Dohme, speaker’s honoraria from Roche and Bristol-Myers Squibb, travel reimbursement from Roche, Amgen and Novartis. Erwin S. Schultz: advisory for Bristol-Myers Squibb and Novartis, speaker’s honoraria for Novartis. Julia K. Tietze: speaker’s honoraria from Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, Roche. Jens Ulrich: advisory for Roche, speaker’s honoraria from Bristol-Myers Squibb, M.S.D. Sharp and Dohme, GlaxoSmithKline, IGEA, Novartis, Roche, travel reimbursement from Bristol-Myers Squibb, IGEA, Medac, Roche. Katharina C. Kähler: advisory for Roche, Amgen, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, speaker’s honoraria from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, travel reimbursement from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis. Rudolf A. Herbst: advisory for Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Roche, speaker’s honoraria from Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche. Thomas K. Eigentler: advisory for AMGEN, Roche, Bristol-Myers Squibb, travel support from Bristol-Myers Squibb and Novartis, speaker’s honoraria from Roche.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, Wolchok JD. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and braf wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433–440. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 8.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 9.Thallinger C, Prager G, Ringl H, Zielinski C. Abscopal effect in the treatment of malignant melanoma. Hautarzt. 2015;66(7):545–548. doi: 10.1007/s00105-014-3567-8. [DOI] [PubMed] [Google Scholar]
- 10.Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39(1):4–16. doi: 10.1016/j.ejso.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Brizio M, Fava P, Astrua C, Cavaliere G, Savoia P. Complete regression of melanoma skin metastases after electrochemotherapy plus ipilimumab treatment: an unusual clinical presentation. Eur J Dermatol. 2015;25(3):271–272. doi: 10.1684/ejd.2015.2522. [DOI] [PubMed] [Google Scholar]
- 13.Sersa G, Teissie J, Cemazar M, Signori E, Kamensek U, Marshall G, Miklavcic D. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol Immunother. 2015;64(10):1315–1327. doi: 10.1007/s00262-015-1724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Queirolo P, Marincola F, Spagnolo F. Electrochemotherapy for the management of melanoma skin metastasis: a review of the literature and possible combinations with immunotherapy. Arch Dermatol Res. 2014;306(6):521–526. doi: 10.1007/s00403-014-1462-x. [DOI] [PubMed] [Google Scholar]
- 15.Mozzillo N, Simeone E, Benedetto L, et al. Assessing a novel immuno-oncology-based combination therapy: ipilimumab plus electrochemotherapy. Oncoimmunology. 2015;4(6):e1008842. doi: 10.1080/2162402X.2015.1008842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006;4(11):3–13. doi: 10.1016/j.ejcsup.2006.08.002. [DOI] [Google Scholar]
- 17.Campana LG, Clover AJ, Valpione S, et al. Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol Oncol. 2016;50(1):1–13. doi: 10.1515/raon-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 21.Queirolo P, Spagnolo F, Ascierto PA, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol. 2014;118(1):109–116. doi: 10.1007/s11060-014-1400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 25.Testori A, Rossi CR, Tosti G. Utility of electrochemotherapy in melanoma treatment. Curr Opin Oncol. 2012;24(2):155–161. doi: 10.1097/CCO.0b013e32834fcaa8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.