Abstract
Wilms’ tumor gene (WT1), which is expressed in human pancreatic cancer (PC), is a unique tumor antigen recognized by T-cell-mediated antitumor immune response. Gemcitabine (GEM), a standard therapeutic drug for PC, was examined for the regulation of WT1 expression and the sensitizing effect on PC cells with WT1-specific antitumor immune response. Expression of WT1 was examined by quantitative PCR, immunoblot analysis, and confocal microscopy. Antigenic peptide of WT1 presented on HLA class I molecules was detected by mass spectrometry. WT1-specific T-cell receptor gene–transduced human T cells were used as effecter T cells for the analysis of cytotoxic activity. GEM treatment of human MIAPaCa2 PC cells enhanced WT1 mRNA levels, and this increase is associated with nuclear factor kappa B activation. Tumor tissue from GEM-treated MIAPaCa2-bearing SCID mice also showed an increase in WT1 mRNA. Some human PC cell lines other than MIAPaCa2 showed up-regulation of WT1 mRNA levels following GEM treatment. GEM treatment shifted WT1 protein from the nucleus to the cytoplasm, which may promote proteasomal processing of WT1 protein and generation of antigenic peptide. In fact, presentation of HLA-A*2402-restricted antigenic peptide of WT1 (CMTWNQMNL) increased in GEM-treated MIAPaCa2 cells relative to untreated cells. WT1-specific cytotoxic T cells killed MIAPaCa2 cells treated with an optimal dose of GEM more efficiently than untreated MIAPaCa2 cells. GEM enhanced WT1 expression in human PC cells and sensitized PC cells with WT1-specific T-cell-mediated antitumor immune response.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1033-3) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic cancer, WT1, Gemcitabine, NF kappa B, T-cell response
Introduction
Pancreatic cancer (PC) is a devastating disease with a 5% overall 5-year survival rate [1, 2]. This high mortality rate is due to a combination of factors that include a high incidence of metastatic disease at initial diagnosis, an aggressive clinical course, and the failure of systemic therapies used for treatment. Despite the fact that advanced loco-regional disease is found in 40% of patients [3], only 5–25% of patients with pancreatic cancer are treated surgically [4]. Even in cases where pancreatic cancer is discovered at a resectable stage, only 10–20% of patients are expected to survive for more than 5 years after curative resection [5].
Gemcitabine (GEM) is currently the most commonly used therapeutic drug prescribed in cases of advanced PC [6, 7]. Numerous phase III trials testing gemcitabine in combination with other cytotoxic drugs have failed to reveal any additional benefit compared with gemcitabine alone [8]. Erlotinib, a small molecule inhibitor of the epidermal growth factor receptor tyrosine kinase, is a notable exception in that it is the only drug reported to confer a significant improvement in survival over gemcitabine alone [9]. Recently, Folfirinox was reported to be a more efficient, but more toxic, regimen for pancreatic cancer and might be promising for the patients with good performance status [10]. Ultimately, improved treatment of advanced PC will likely require additional selected and targeted agents that provide the benefit of prolonged survival with minimum risk.
The Wilms’ tumor gene WT1 encodes a zinc finger transcription factor. Although the WT1 gene was originally defined as a tumor suppressor gene [11–13], additional reports demonstrate that it is highly expressed in leukemia and various types of malignant tumors [14] and can confer oncogenic functions [15]. WT1-specific cytotoxic T lymphocytes (CTLs) and WT1 antibodies have both been shown to be induced spontaneously in tumor-bearing leukemia patients [16]. These results indicate that WT1 protein is highly immunogenic and establish it as a promising tumor antigen for recognition by specific CTLs [17]. The safety and clinical efficacy of major histocompatibility complex (MHC) class I-restricted WT1 epitope peptides against various malignancies have been confirmed in clinical immunotherapy trials [14, 15].
Reports indicate that WT1 is frequently overexpressed in human pancreatic cancer cells [18]. Recent clinical reports on treatments combining GEM drug therapy with peptide vaccine immunotherapy have demonstrated safe and promising results in cases of advanced PC [19, 20]. In our recent phase I clinical trial that tested a combination of WT1 peptide vaccine and GEM in treatment of advanced PC, several cases showed marked tumor regression (manuscript in preparation). These results suggest that the actions of WT1-targeted antitumor immunity and GEM can function synergistically against PC cells. In the present study, we demonstrate that GEM treatment up-regulates WT1 expression in PC cell lines, and that antitumor immune activity against PC cells via a WT1-specific T-cell response is augmented by GEM treatment.
Materials and methods
Cell lines, antibodies, and mice
Human pancreatic cancer cell lines (MIAPaCa2, PANC-1, AsPC-1, BxPC-3, Capan-1 and Capan-2) were obtained from the American Type Culture Collection (Manassas, VA, USA) [21]. A rabbit polyclonal antibody against WT1 (C-19) and a goat polyclonal antibody against Lamin B (C-20) were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Eight- to ten-week-old SCID mice were supplied by Nihon SCL Co., Ltd. (Hamamatsu, Japan) and were maintained in our specific pathogen-free facilities. Mice received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institute of Health (NIH publication 86-23 revised 1985).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Tissue or cell samples were lysed directly in Buffer RLT Plus (Qiagen, Hilden, Germany) and homogenized. Reverse transcription (RT) was performed using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). TaqMan primers and non-fluorescent quencher probes complementary to WT1 (Assay ID:Hs00240913_m1) and 18S ribosomal RNA (rRNA, Assay ID:Hs99999901_s1) genes were purchased from Applied Biosystems. qRT-PCR was performed using the 7300 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). WT1 expression levels were normalized relative to those of 18S rRNA.
Inhibition of nuclear factor kappa B (NF-kB)
Inhibition of NF-kB activity in human PC cells was achieved using an NF-kB p65 (Ser276) inhibitory peptide kit (IMGENEX, San Diego, CA, USA). Briefly, MIAPaCa2 cells (6 × 104/well) were seeded in 24-well culture plates and incubated for 24 h. Growth medium was then changed to medium containing GEM (0 or 30 ng/ml) with NF-kB blocking peptide (50 μM) or control peptide (50 μM). After 24-h incubation, cellular expression of NK-kB was determined using qRT-PCR.
Immunoblot analysis
The nuclear fraction of MIAPaCa2 cells used for the detection of WT1 protein was isolated using an Active Motif extraction kit (Carlsbad, CA, USA). Protein samples (30 μg/well) separated by electrophoresis in 15% sodium dodecyl sulfate-polyacrylamide gels were then transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). After blocking in 5% nonfat milk for 1 h, membranes were exposed to antibodies specific to WT1 (1:100) and beta-actin (1:10,000; Sigma–Aldrich, St. Louis, MO, USA) and then to horseradish peroxidase-conjugated secondary antibodies. The ECL-PLUS Detection System (GE Healthcare, Buckinghamshire, UK) was used for chemiluminescent detection of secondary antibodies.
Confocal microscopy
MIAPaCa2 cells cultured on glass coverslips were incubated with or without GEM (30 ng/ml) for 24 h. Cells were then washed and fixed in 4% paraformaldehyde. Immunofluorescent visualization of cells expressing WT-1 was achieved by incubating slides in rabbit anti-WT1 antibody (1/200), followed by Amaxa488-conjugated donkey anti-rabbit IgG antibody (Molecular probes, Eugene, OR, USA). Cell nuclei were stained with TO-PRO-3 iodide (Molecular Probes), and a laser scanning confocal microscope (LSM510, CarlZeiss, Thornwood, NY, USA) was used to obtain fluorescence images.
Positive ion ESI LC–MS/MS analysis of MHC class I binding peptides from MIAPaCa2 cells
MIAPaCa2-bearing mice were injected intraperitoneally with PBS or GEM (3.75 mg/mouse). After 48 h, tumors were resected and digested using collagenase to obtain single cells. MHC class I binding peptides were isolated from 108 cells using the method described by Storkus et al. [22]. Isolated peptides were dissolved in 50% methanol and analyzed via electrospray ionization (ESI) liquid chromatography (LC)-tandem mass spectrometry (MS/MS) using a triple quadrupole mass spectrometer (Q TRAP) (Applied Biosystems, Foster City, CA, USA). The mass spectrometer interfaced with an Agilent 1100 liquid chromatography (Agilent Technologies, Wilmington, DE, USA) was employed. The WT1 antigenic peptide (aa 235–243 CMTWNQMNL; MW = 1,139.5 Da) in 50% methanol was easily produced m/z 1171.5 as a methanol adduct ion (M + MeOH)+. The multiple reaction monitoring (MRM) transition monitored for the detection of this peptide was m/z 1,171.5/1,154.5. This peptide was eluted at a flow rate 0.2 mL/min from an Intersil C8-3 column [50 × 2.1 mm, 3 μm particle size] (GL Science Inc., Tokyo Japan) using a linear gradient of 9.5% min−1 of 5–100% acetonitrile containing 1% formic acid. To estimate cellular peptide concentrations, a standard curve was prepared by increasing concentrations (0–1,000 pmol) with chemically synthesized WT-1 antigenic peptide. The response was considered to be linear if the correlation coefficient (r 2) was greater than 0.99, calculated by least-squares linear regression analysis.
Cytotoxicity assay
WT1-specific cytotoxic effector cells were generated as described below. Full-length WT1-specific T-cell receptor (TCR) a/b genes (Va20/J33/Ca for TCR-a and Vb5.1/J2.1/Cb2 for TCR-b, respectively) isolated from the HLA-A*2402-restricted WT1235–243-specific CD8+ CTL clone TAK-1 [23] were cloned into a pMEI-5 retroviral vector (Takara Bio, Shiga, Japan). WT1-specific TCR genes were then transduced into normal CD8+ lymphocytes as described previously [24]. Cytotoxicity assays were performed using a standard 4-h culture 51chromium (Cr) release assay described elsewhere [25].
Statistical analysis
The significance of differences between groups was analyzed using Student’s t test for two independent groups and with Tukey’s test for multiple-group comparisons. Values that did not fit a Gaussian distribution were analyzed with the Bonferroni method for multiple-group comparisons.
Results
Up-regulation of WT1 mRNA in human PC cells by in vitro treatment with GEM
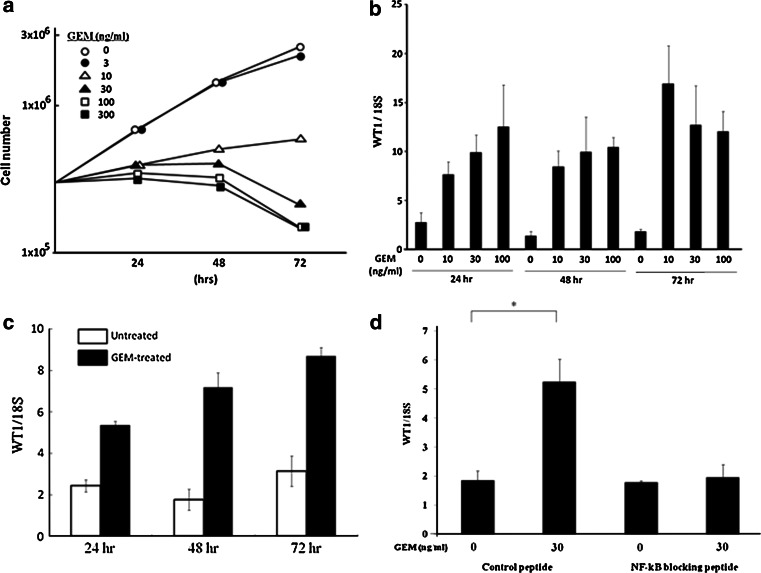
Proliferation of MIAPaCa2 cells was inhibited for 48 h with stable numbers of viable cells following treatment with 30 and 100 ng/ml of GEM (Fig. 1a). Growth of MIAPaCa2 cells was also impaired by treatment with 10 ng/ml of GEM for 72 h. Levels of WT1 mRNA were enhanced significantly by treatment of MIAPaCa2 cells with 10, 30, and 100 ng/ml of GEM for 24, 48 and, 72 h, respectively (Fig. 1b). Enhancement of WT1 mRNA was also observed after 2-h treatment with GEM (100 ng/ml) in following 72 h (Fig. 1c). This GEM-mediated enhancement was suppressed by the addition of NF-kB blocking peptide in the culture (Fig. 1d).
Fig. 1.
a Proliferation of MIAPaCa2 cells in medium containing various concentrations of GEM. MIAPaCa2 cells (3 × 105/well) were seeded in 6-well culture plates in regular culture medium, which was then exchanged for GEM-containing medium after 24 h. At 24-h intervals, cells were detached using trypsin, and cell numbers were counted using a hemocytometer (n = 3). b Up-regulation of WT1 mRNA in MIAPaCa2 cells by GEM treatment. Twenty-four hours after plating, culture medium was exchanged to media containing GEM at indicated concentrations (0, 10, 30 and 100 ng/ml). MIAPaCa2 cells were harvested at 24-h intervals, and WT1 mRNA in cell homogenates was analyzed using qRT-PCR. WT1mRNA levels were normalized relative to those of 18S ribosomal RNA (18S). c Up-regulation of WT1 mRNA in MIAPaCa2 cells after short treatment with GEM. Twenty-four hours after plating, MIAPaCa2 cells were untreated or treated with 100 ng/ml of GEM for 2 h. MIAPaCa2 cells did not proliferate but kept alive for following 72 h by this treatment with GEM. After GEM treatment, cells were washed well, cultured in regular culture medium, and harvested at 24-h intervals. WT1 mRNA in cell homogenates was analyzed using qRT-PCR, and WT1mRNA levels were normalized relative to those of 18S ribosomal RNA (18S). d NF-kB suppresses GEM-induced up-regulation of WT1 mRNA. MIAPaCa2 cells (6 × 104/well) were seeded in 24-well culture plates. After 24 h, medium was exchanged for media containing GEM (0 or 30 ng/ml) and/or NF-kB blocking peptide (50 μM) or control peptide (50 μM). WT1 mRNA levels were quantified after 24-h incubation using qRT-PCR. *P < 0.01
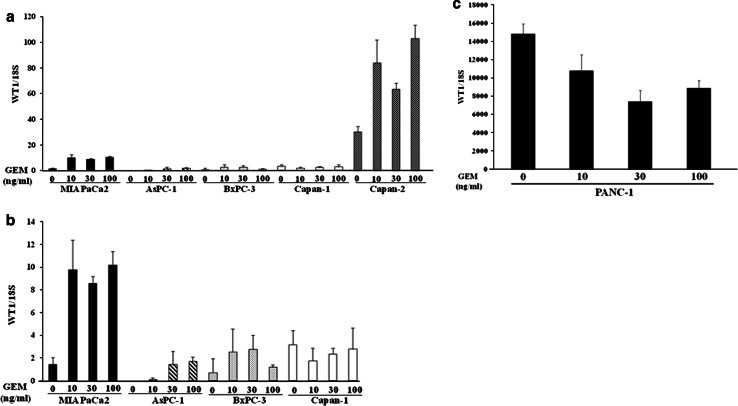
GEM-mediated up-regulation of WT1 mRNA expression was examined in various human pancreatic cancer cell lines. GEM-treated Capan-2 cells showed a significant enhancement of WT1 mRNA expression (Fig. 2a). Low steady-state levels of WT1 mRNA expression in AsPC-1 and BxPC-3 cells were also enhanced by GEM treatment (Fig. 2b). In contrast, expression of WT1 mRNA in Capan-1 and PANC-1 cells was not up-regulated by GEM treatment (Fig. 2b, c).
Fig. 2.
a Up-regulation of WT1 mRNA levels in various human PC cell lines following GEM treatment. Human PC cells (1 × 106 MIAPaCa2, AsPC-1, BxPC-3, Capan-1 or Capan-2) were seeded in 10-cm culture plates. After 24-h incubation, medium was changed to media containing GEM (10, 30 or 100 ng/ml). After 48 h, we used qRT-PCR to quantify the relative ratio of WT1 to 18S mRNA levels in each cell line (n = 3). b GEM-induced up-regulation of WT1 mRNA in human PC cells with low basal levels of WT1 mRNA (MIAPaCa2, AsPC-1, BxPC-3 and Capan-1). To illustrate these results, we replotted data from (a) to represent a considerably narrower range of mRNA level ratios (0–14) on the y-axis. (c) Expression of WT1 mRNA in human PC cells with high basal levels of WT1 mRNA (PANC-1). To illustrate the results, we plotted data to represent a considerably wider range of mRNA level ratios (0–18,000) on the y-axis
Changes in WT1 mRNA expression levels were also examined in MIAPaCa2 cells following in vitro treatment with various other chemotherapeutic agents. Oxaliplatin, Doxorubicin, and five-fluorouracil showed significant enhancement of WT1 mRNA expression, but cisplatin and irinotecan did not (Suppl. 1). Because GEM is the standard drug used to treat human PC, its effect on human PC cells was studied thereafter.
In vivo up-regulation of WT1 mRNA in tumor tissue by treatment of MIAPaCa2-bearing SCID mice with GEM
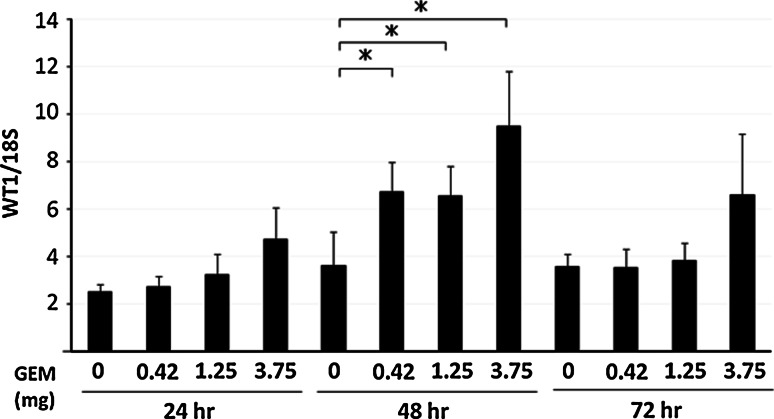
In order to clarify whether in vivo treatment of tumor cells with GEM induces an enhancement of WT1 mRNA expression, SCID mice implanted subcutaneously with MIAPaCa2 cells were treated with a clinical dosage of GEM. We observed a significant increase in the levels of WT1 mRNA 48 h after injection of GEM (Fig. 3).
Fig. 3.
Tumors in PC-bearing SCID mice treated with GEM show increased WT1 mRNA levels. Ten days after subcutaneous inoculation of SCID mice with 5 × 106 MIAPaCa2 cells (formation of approximately 1-cm diameter tumors), mice were injected intraperitoneally with GEM (0, 0.42, 1.25 and 3.75 mg/mouse). Tumors were resected every 24 h thereafter, and relative levels of WT1 mRNA were quantified using qRT-PCR (n = 3). Duplicate trials of the same protocol showed similar results. *P < 0.01
GEM treatment shifts localization of WT1 from the nucleus to the cytoplasm
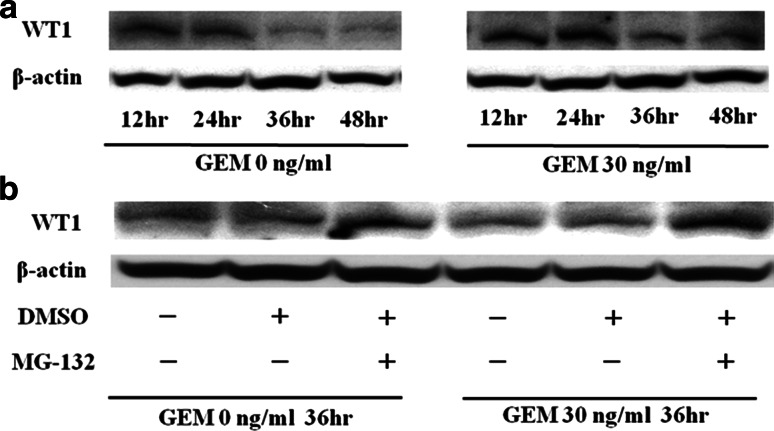
We used immunoblot analysis to examine the levels of WT1 protein in MIAPaCa2 cells cultured in the absence or presence of GEM. Relative to untreated cells, WT1 protein levels in GEM-treated MIAPaCa2 cells were augmented; however, after 36 h of cell culture, levels of WT1 protein diminished in both untreated and GEM-treated cells (Fig. 4a). This decline in WT1 protein levels was rescued by treatment with the proteasome inhibitor MG-132, indicating that WT1 protein is susceptible to proteasomal degradation (Fig. 4b).
Fig. 4.
a WT1 protein is degraded by proteasomal enzymes. Twenty-four hours after 3 × 105 MIAPaCa2 cells/well were seeded in 6-well culture plates, medium was exchanged from untreated to media containing GEM (0 or 30 ng/ml). Expression of WT1 protein in the cells was analyzed every 12 h thereafter from immunoblots described in Sect. “Materials and methods”. b Protease inhibitors block WT1 degradation. Twenty-four hours after incubating MIAPaCa2 cells with GEM (0 or 30 ng/ml), MG-132 in DMSO or DMSO alone was added to each well at a concentration of 5 μM and 0.05%, respectively. Treated and control cells (in 0.05% DMSO alone) were incubated for 12 h before harvesting cells for immunoblot analysis of WT1 and beta-actin proteins
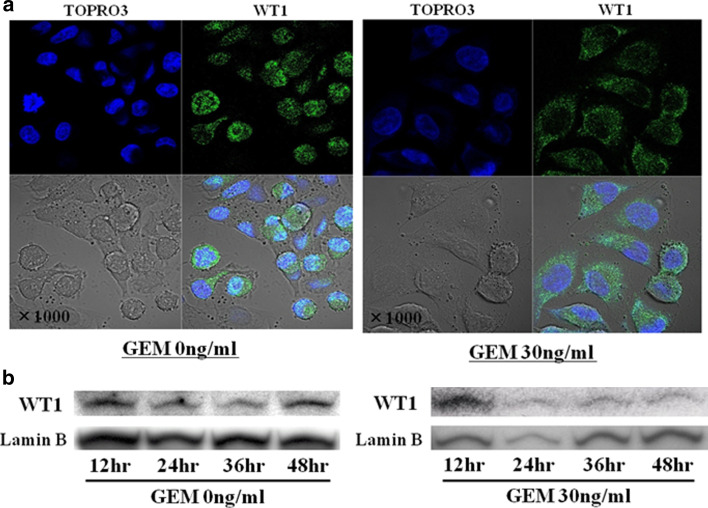
Confocal microscopy images demonstrate that WT1 protein is primarily located in nuclei of untreated cells (Fig. 5a). However, in MIAPaCA2 cells treated with GEM, localization of WT1 protein shifted to the cytoplasm and the intensity of WT1 immunofluorescence in the nucleus decreased (Fig. 5a). Decline in WT1 protein levels following GEM treatment was also observed in immunoblot analyses of the nuclear fraction of treated MIAPaCa2 cells (Fig. 5b).
Fig. 5.
a GEM treatment shifts WT1 protein localization from nucleus to cytoplasm. Twenty-four hours after seeding 3 × 105 MIAPaCa2 cells/well in 6-well culture plates, untreated medium was exchanged for fresh medium with or without GEM (0 or 30 ng/ml). After 24-h incubation, cells were fixed with paraformaldehyde, followed by nuclear staining with TO-PRO-3 iodide (blue color) and detection of WT1 with rabbit anti-WT1 polyclonal antibody and anti-rabbit IgG conjugated with fluorescein isothiocyanate (green color). Stained cells were observed using confocal microscopy (original magnification ×1,000). b GEM treatment diminishes nuclear localization of WT1 protein. Twenty-four hours after seeding 3 × 105 MIAPaCa2 cells/well in 6-well culture plates, medium was exchanged for fresh medium with or without GEM (0 or 30 ng/ml). At 12-hour intervals thereafter, nuclei were isolated and WT1 protein levels of nuclear extracts were analyzed on immunoblots as described in Sect. “Materials and methods”
Enhanced presentation of HLA-A*2402-restricted WT1 antigenic peptide following GEM treatment
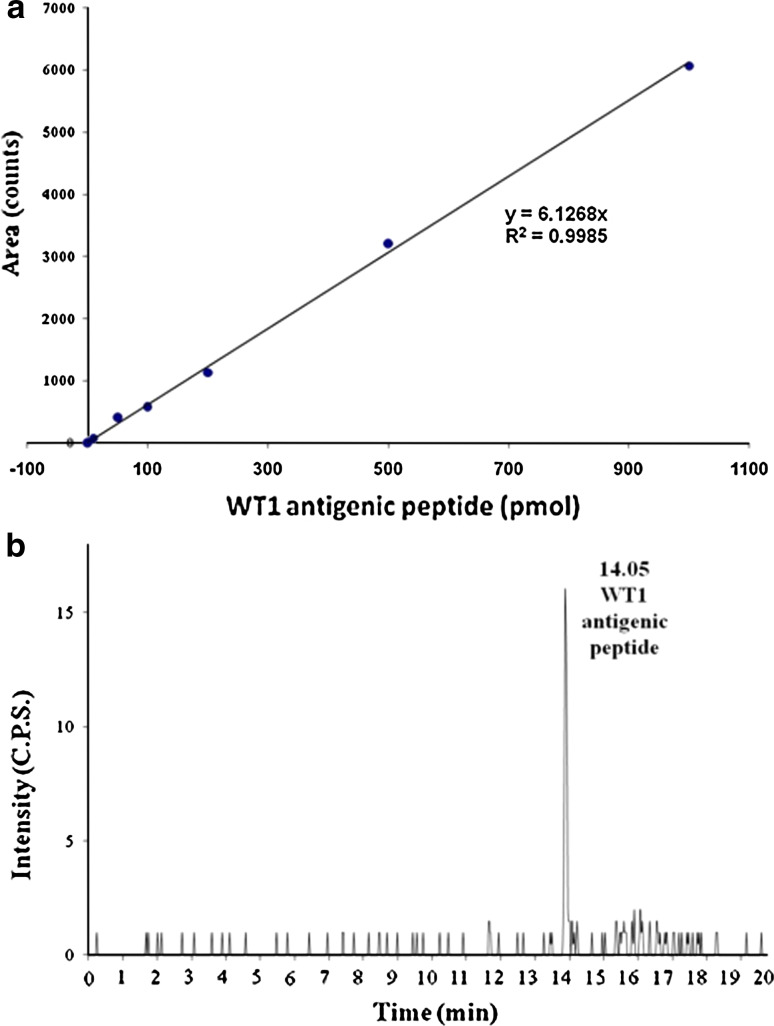
Figure 6a shows typical standard curve obtained with increasing quantities of WT1 antigenic peptide. The data indicate a linear relation over a wide range (0–1,000 pmol) of analyte amount with correlation coefficients greater than 0.99. The data in the Fig. 6b demonstrate the sensitivity as well as the noise background of the LC–MS/MS. The noise background is less than 1 cps. The signal from injection of 10 pmol of this peptide spiked to MIAPaCa2 cells is approximately 16 cps, giving an S/N ratio of approximately 16. The low noise background and signal of 10 pmol of this peptide indicated the extrapolated limit of detection is less than 0.8 pmol on column under S/N = 2.
Fig. 6.
a Standard curve for HLA-A*2402 restricted WT1 antigenic peptide. b Trace of MRM signal during LC–MS/MS analysis of spiked HLA-A*2402-restricted WT-1 antigenic standard peptide (10 pmol) in MIAPaCa2 cells
The level of the WT1 antigenic peptide was estimated among MHC class I binding peptides from MIAPaCa2 cells treated with either PBS or GEM to 6.49 pmol/108cell or 8.78 pmol/108cell, respectively. GEM treatment increased the presentation of HLA-A*2402-restricted WT1 antigenic peptide on MIAPaCa2 cells.
GEM-treated PC cells are killed efficiently by effector cells transduced with genes encoding a WT1-specific T-cell receptor
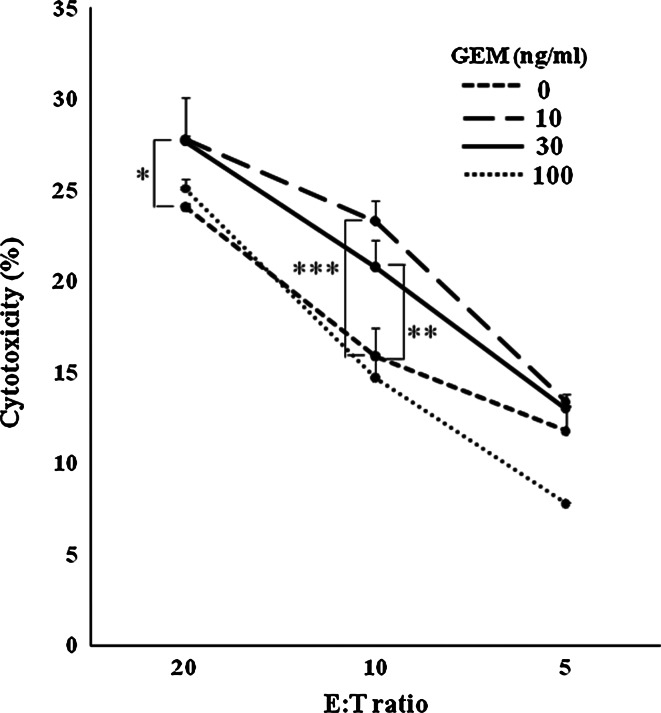
The susceptibilities of untreated and GEM-treated MIAPaCa2 cells to WT1-specific cytotoxic effector T cells were compared. The cytotoxic effect of WT1-specific effector cells on MIAPaCa2 cells was enhanced significantly when PC cells were treated with either 10 or 30 ng/ml of GEM for 48 h (Fig. 7). Notably, effector cell cytotoxicity was not enhanced by treatment of PC cells with 100 ng/ml of GEM, although this high dose of GEM was more toxic to PC cells than 10 or 30 ng/ml. Up-regulation of MHC class I in MIAPaCa2 cells by GEM treatment that possibly provides the similar results was not observed (data not shown).
Fig. 7.
WT1-specific CTLs kill GEM-treated MIAPaCa2 cells efficiently. MIAPaCa2 cells pretreated with 0, 10, 30, or 100 ng/ml GEM for 48 h were labeled with 51Cr. 51Cr release assays were used to measure the cytotoxic activity of WT1-specific effector cells against untreated or GEM-pretreated MIAPaCa2 cells. *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
In the present study, we demonstrate that expression of WT1 mRNA in human PC cells is enhanced by treatment with GEM. MIAPaCa2 cells demonstrating GEM-mediated enhancement of WT1 mRNA levels did not proliferate but maintained stable numbers of viable cells with impaired growth by continuous treatment with low-dose GEM as well as short treatment with high-dose GEM. WT1 is a transcription factor with oncogenic potential, in that it can induce malignant cellular phenotypes, suppress apoptosis, and promote cell proliferation [15]. We hypothesize that up-regulation of WT1 levels in PC cells aids cell survival by conferring chemoresistance against GEM’s toxic effects.
Based on the fact that GEM-mediated augmentation of WT1 mRNA expression was attenuated by addition of an NF-kB blocking peptide in the culture, activation of NF-kB also appears to play a significant role in WT1 enhancement. NF-kB is known to be active in many malignant tumors and has been implicated in cellular resistance to cytotoxic agents and escape from apoptosis [26]. Previous reports demonstrate that GEM activates NF-kB [27] and that the ensuing regulatory cascade activates the WT1 gene downstream [28]. Human PC cell lines with high NF-kB activity are resistant to GEM [27], and that silencing or suppression of NF-kB increases the sensitivity of PC cells to GEM and induces apoptosis [29–31].
It is of note and interest that some chemotherapeutic agents other than GEM showed capability on up-regulation of WT1mRNA expression. Especially, treatment with oxaliplatin (L-OHP) induced marked enhancement of WT1mRNA expression. Folfirinox including L-OHP was recently reported to be a more efficient regimen for metastatic pancreatic cancer (10). However, combined treatment with Folfirinox and WT1 targeting immunotherapy might be unsuccessful because of severe leukopenia by Folfirinox. GEM has relatively low hematologic toxicity and thus seems to be preferable for combination therapy with WT1 targeting immunotherapy.
We also observed up-regulation of WT1 mRNA by GEM treatment in vivo. Within 48 h of treating MIAPaCa2-bearing SCID mice with a clinical dose of GEM, steady-state levels of WT1 mRNA in the tumor increased. Despite its rapid disappearance after intraperitoneal injection, the enhancement of WT1 mRNA expression in tumor tissue was significant. Enhancement of WT1 mRNA expression was also observed after in vitro short treatment with GEM. These results suggest strongly that GEM treatment of human PC in a clinical setting might induce up-regulation of WT1 in PC cells.
In the present study, we found that the localization of WT1 protein shifted from nucleus to cytoplasm following GEM treatment. WT1 protein has been shown to undergo nucleocytoplasmic shuttling [32], and the function of WT1 has been suggested to correlate with its cellular location: Siberstein et al. [33] described that WT1 was localized to the cytoplasm and not to nuclei in some human breast cancers and suggested that such localization may be regulated by alternative splicing of WT1 mRNA. On the other hand, immunohistochemical studies of Nakatsuka et al. [34] demonstrate a majority of WT1-positive tumors with diffuse or granular staining in the cytoplasm. Ye et al. [35] report that phosphorylation of WT1 protein resulted in cytoplasmic retention of WT1, thereby inhibiting DNA binding and altering transcriptional activity. Through the activation of NF-kB, GEM treatment may mediate a similar phosphorylation and translocation of WT1 protein from nucleus to cytoplasm.
In order for MHC class I-restricted antigen to be presented and recognized by antigen-specific CTLs, tumor antigen must be degraded by proteasomal enzymes located in the cytoplasm [36]. Retention of an intra-nuclear tumor antigen such as WT1 in the cytoplasm should favor tumor antigen processing, and in fact, we observed enhanced presentation of HLA-A*2402-restricted WT1 antigenic peptide using ESI LC–MS/MS analyses. GEM-treated MIAPaCa2 cells showed greater susceptibility than untreated cells to the cytotoxic effects of WT1-specific CTLs generated by transduction of a gene encoding a WT1-specific T-cell receptor. Importantly, treatment with 10–30 ng/ml of GEM enhanced the susceptibility of MIAPaCa2 cells to CTL, but treatment with 100 ng/ml did not. This phenomenon indicates that the enhanced susceptibility of GEM-treated MIAPaCa2 cells to CTLs is not due to GEM toxicity, but to augmented expression of the WT1 target antigen.
GEM is a nucleoside analog with clinical relevance to the treatment of several solid tumors, including PC; nonetheless, its antitumor effect is limited. We observed significant clinical response in a phase I clinical study of combined treatment against advanced PC using a WT1 peptide vaccine and GEM (manuscript in preparation). The presumed actions of GEM up-regulating WT1 expression in vivo and WT1-specific CTLs killing GEM-treated tumor cells efficiently may prove valuable for the treatment of human PC. It has been reported that GEM may suppress the activity of myeloid-derived suppressor cells that inhibit antitumor immunity [37]. In addition, GEM has been shown to increase the number of dendritic cells in blood without affecting T-cell activity in patients with PC [38]. We propose that combining GEM’s proven role as an immunopotentiator with its ability to up-regulate target WT1 expression of PC cells will enhance the susceptibility of PC cells to WT1-specific CTLs. Furthermore, PC cells already acquired GEM resistance by the activation of NF-kB might be injured by WT1-specific CTLs. Assessment of the clinical response to combined therapy with WT1 peptide vaccine and GEM is presently underway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work has been supported by Foundation for Promotion of Cancer Research, Mitsui Life Social Welfare Foundation, Grants-in-Aid for Scientific Research (B) and (C) from the Ministry of Education, Cultures, Sports, Science and Technology of Japan, Grantin-Aid of the Japan Medical Association, Takeda Science Foundation, and Pancreas Research Foundation of Japan.
Conflict of interest
There are no financial disclosures of any of the authors to declare.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Heinemann V. Gemcitabine in the treatment of advanced pancreatic cancer: a comparative analysis of randomized trials. Semin Oncol. 2002;29(6 Suppl20):9–16. doi: 10.1053/sonc.2002.37372. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, Strasberg S, Hanna S, Taylor B, Langer B, Gallinger S. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/S1091-255X(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 6.Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA, 3rd, Green MR, Tarassoff PG, Brown TD, Casper ES, Storniolo AM, Von Hoff DD. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–353. doi: 10.1093/oxfordjournals.annonc.a010600. [DOI] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Merl MY, Chabot J, Saif MW. Updates of adjuvant therapy in pancreatic cancer: where are we and where are we going? Highlights from the “2010 ASCO annual meeting”. Chicago, IL, USA. J Pancreas. 2010;11:4–8. [PubMed] [Google Scholar]
- 9.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 11.Coppes MJ, Campbell CE, Williams BR. The role of WT1 in Wilms tumorigenesis. FASEB J. 1993;7:886–895. doi: 10.1096/fasebj.7.10.8393819. [DOI] [PubMed] [Google Scholar]
- 12.Rauscher FJ., 3rd The WT1 Wilms tumor gene product: a developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J. 1993;7:896–903. [PubMed] [Google Scholar]
- 13.Haber DA, Park S, Maheswaran S, Englert C, Re GG, Hazen-Martin DJ, Sens DA, Garvin AJ. WT1-mediated growth suppression of Wilms tumor cells expressing a WT1 splicing variant. Science. 1993;262(5142):2057–2059. doi: 10.1126/science.8266105. [DOI] [PubMed] [Google Scholar]
- 14.Hutchings Y, Osada T, Woo CY, Clay TM, Lyerly HK, Morse MA. Immunotherapeutic targeting of Wilms’ tumor protein. Curr Opin Mol Ther. 2007;9:62–69. [PubMed] [Google Scholar]
- 15.Sugiyama H. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. Expert Rev Vaccines. 2005;4:503–512. doi: 10.1586/14760584.4.4.503. [DOI] [PubMed] [Google Scholar]
- 16.Gaiger A, Carter L, Greinix H, Carter D, McNeill PD, Houghton RL, Cornellison CD, Vedvick TS, Skeiky YA, Cheever MA. WT1-specific serum antibodies in patients with leukemia. Clin Cancer Res. 2001;7(3 Suppl):761s–765s. [PubMed] [Google Scholar]
- 17.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oji Y, Nakamori S, Fujikawa M, Nakatsuka S, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, Yamamoto H, Sakon M, Nezu R, Kawano K, Nishida S, Ikegame K, Kawakami M, Tsuboi A, Oka Y, Yoshikawa K, Aozasa K, Monden M, Sugiyama H. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci. 2004;95:583–587. doi: 10.1111/j.1349-7006.2004.tb02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, Nakamura Y, Yamaue H. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010;101:433–439. doi: 10.1111/j.1349-7006.2009.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagimoto H, Mine T, Yamamoto K, Satoi S, Terakawa N, Takahashi K, Nakahara K, Honma S, Tanaka M, Mizoguchi J, Yamada A, Oka M, Kamiyama Y, Itoh K, Takai S. Immunological evaluation of personalized peptide vaccination with gemcitabine for pancreatic cancer. Cancer Sci. 2007;98:605–611. doi: 10.1111/j.1349-7006.2007.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sipos B, Möser S, Kalthoff H, Török V, Löhr M, Klöppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442:444–452. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- 22.Storkus WJ, Zeh HJ, 3rd, Maeurer MJ, Salter RD, Lotze MT. Identification of human melanoma peptides recognized by class I restricted tumor infiltrating T lymphocytes. J Immunol. 1993;151:3719–3727. [PubMed] [Google Scholar]
- 23.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286–293. [PubMed] [Google Scholar]
- 24.Okamoto S, Mineno J, Ikeda H, Fujiwara H, Yasukawa M, Shiku H, Kato I. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 2009;69:9003–9011. doi: 10.1158/0008-5472.CAN-09-1450. [DOI] [PubMed] [Google Scholar]
- 25.Yasukawa M, Ohminami H, Arai J, Kasahara Y, Ishida Y, Fujita S. Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans. Blood. 2000;95:2352–2355. [PubMed] [Google Scholar]
- 26.Bottero V, Busuttil V, Loubat A, Magné N, Fischel JL, Milano G, Peyron JF. Activation of nuclear factor kappaB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in HeLa human carcinoma cells. Cancer Res. 2001;61:7785–7791. [PubMed] [Google Scholar]
- 27.Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, Schäfer H. Role of NF-kappaB and Akt/PI3 K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 28.Dehbi M, Hiscott J, Pelletier J. Activation of the wt1 Wilms’ tumor suppressor gene by NF-kappaB. Oncogene. 1998;16:2033–2039. doi: 10.1038/sj.onc.1201747. [DOI] [PubMed] [Google Scholar]
- 29.Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, Lopez-Berestein G, Vivas-Mejia PE, Sood AK, McConkey DJ, Logsdon CD. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14:8143–8151. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee S, Wang Z, Kong D, Sarkar FH. 3, 3’-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res. 2009;69:5592–5600. doi: 10.1158/0008-5472.CAN-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Vajjhala PR, Macmillan E, Gonda T, Little M. The Wilms’ tumour suppressor protein, WT1, undergoes CRM1-independent nucleocytoplasmic shuttling. FEBS Lett. 2003;554(1–2):143–148. doi: 10.1016/S0014-5793(03)01144-X. [DOI] [PubMed] [Google Scholar]
- 33.Silberstein GB, Van Horn K, Strickland P, Roberts CT, Jr, Daniel CW. Altered expression of the WT1 wilms tumor suppressor gene in human breast cancer. Proc Natl Acad Sci USA. 1997;94:8132–8137. doi: 10.1073/pnas.94.15.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakatsuka S, Oji Y, Horiuchi T, Kanda T, Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M, Kitamura Y, Oka Y, Kawase I, Sugiyama H, Aozasa K. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol. 2006;19:804–814. doi: 10.1038/modpathol.3800588. [DOI] [PubMed] [Google Scholar]
- 35.Ye Y, Raychaudhuri B, Gurney A, Campbell CE, Williams BR. Regulation of WT1 by phosphorylation: inhibition of DNA binding, alteration of transcriptional activity and cellular translocation. EMBO J. 1996;15:5606–5615. [PMC free article] [PubMed] [Google Scholar]
- 36.Sijts A, Zaiss D, Kloetzel PM. The role of the ubiquitin-proteasome pathway in MHC class I antigen processing: implications for vaccine design. Curr Mol Med. 2001;1:665–676. doi: 10.2174/1566524013363230. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1 +/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 38.Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915–925. doi: 10.1007/s00262-004-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.