Abstract
The development of cancer immunotherapy has long been a challenge. Here, we report that prophylactic vaccination with a highly attenuated Trypanosoma cruzi strain expressing NY-ESO-1 (CL-14-NY-ESO-1) induces both effector memory and effector CD8+ T lymphocytes that efficiently prevent tumor development. However, the therapeutic effect of such a vaccine is limited. We also demonstrate that blockade of Cytotoxic T Lymphocyte Antigen 4 (CTLA-4) during vaccination enhances the frequency of NY-ESO-1-specific effector CD8+ T cells producing IFN-γ and promotes lymphocyte migration to the tumor infiltrate. As a result, therapy with CL-14-NY-ESO-1 together with anti-CTLA-4 is highly effective in controlling the development of an established melanoma.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1634-8) contains supplementary material, which is available to authorized users.
Keywords: Cancer, Immunotherapy, NY-ESO-1, Anti-CTLA-4, Trypanosoma cruzi
Introduction
Cancer incidence rates have increased in the past 30 years [1]. Nevertheless, significant improvement in cancer therapy has been achieved over the years, and immunotherapy has emerged as an important alternative to the classic methods. Although many clinical trials of immunotherapy protocols have resulted in the development of measurable immune responses, only a minority of treated patients experienced tumor regression [2]. Thus, the field of cancer immunotherapy continues to face major challenges, such as the selection of appropriate target antigens and effective antigen delivery systems to stimulate robust and durable immune responses [3].
To achieve positive clinical results, cancer vaccines should combine immunogenic tumor-specific proteins with effective delivery systems and immunological adjuvants [4]. Currently, various vaccine formulations against experimental tumors have been employed to induce strong T cell-mediated immunity in particular CD8+ T cells specific for tumor antigens [5]. Due to restricted expression in normal tissues, cancer testis antigens (CTA) are of particular interest as candidates for cancer vaccines [6]. NY-ESO-1 is a CTA expressed in a variety of human cancer cells, including melanoma, breast, lung, and prostate tumors [7, 8]. This antigen induces both cellular and humoral immunity in different vaccination protocols. Additionally, a number of strategies aiming at improving both the magnitude and the quality of anti-NY-ESO-1 responses have been proposed [9, 10].
Recently, we have used the highly attenuated CL-14 clone of Trypanosoma cruzi, engineered to express NY-ESO-1 (CL-14-NY-ESO-1), as a cancer antigen delivery vector [11]. The use of T. cruzi as a live vaccine delivery system is based on the parasites capacity to elicit strong immunity mediated by CD8+ T cells, which are the main arm of the immune system involved in tumor elimination [12, 13]. This transgenic parasite has been shown to be effective in prophylactic vaccines, inducing complete protection against NY-ESO-1-expressing cancer cells. However, this vector proved to be inefficient in therapeutic protocols [11].
The cytotoxic T lymphocyte antigen 4 (CTLA-4) has been widely used in preclinical and clinical trials for solid tumors. Blockade of CTLA-4 with an antibody, used as a means to potentiate T cell activation and initiate responses to target tumor cells, provided a step forward in the development of various treatments against cancer [14]. Here, we report the highly significant therapeutic effect of CL-14-NY-ESO-1 in combination with anti-CTLA-4. The immunological mechanism involved in the inhibition of tumor growth was associated with a high frequency of both effector and effector memory CD8+ T cells. The tumor-specific effector CD8+ T cells were cytolytic and produced high levels of IFN-γ and IL-2 upon stimulation with recombinant NY-ESO-1 protein (rNY-ESO-1). Furthermore, we observed a substantial increase in the frequency of tumor-infiltrating effector CD8+ T cells in animals treated with transgenic parasites plus anti-CTLA-4. The multiple effects resulting from this combined strategy undoubtedly represent a novel tool for a therapeutic anticancer vaccine.
Materials and methods
Mice
Female (8–12 weeks old) C57BL/6 mice were obtained from CEBIO (Federal University of Minas Gerais, Brazil), and β2-microglobulin−/− mice were obtained from the René Rachou Research Institute’s (CPqRR) animal facility center (Fiocruz, Belo Horizonte, Brazil). GzmBCreERT2/ROSA26EYFP mice were provided by Dr. Douglas T. Fearon (Cambridge, UK). GzmBCreERT2/ROSA26EYFP mice received 1 mg tamoxifen daily in 10 % EtOH/90 % sunflower seed oil (Sigma-Aldrich) via intraperitoneal injection (i.p.) for five consecutive days 1 week before the spleens were harvested. Experiments for this study were approved by the Ethical Commission on Animals’ Use (CETEA) at Federal University of Minas Gerais and performed following the Institutional Guide for the Care and Use of Laboratory Animals.
Cells and parasites
B16-NY-ESO-1 melanoma cells were grown at 37 °C under 5 % CO2 in complete RPMI 1640 (Sigma-Aldrich) with 100 U/ml penicillin and 100 μg/ml streptomycin and supplemented with 10 % fetal bovine serum (FBS; GIBCO). The selection was performed with G418 (250 μg/ml), as recommended [15]. To establish subcutaneous tumors, 5 × 104 B16-NY-ESO-1 cells in 100 μl PBS were subcutaneously injected into the right flank of the mice. The monoclonal anti-CTLA-4 antibody (clone 9D9) was produced from a hybridoma and purified on protein G columns. Trypomastigotes from the T. cruzi CL-14 strain were maintained as previously described [11, 16]. Immunizations were performed by inoculating the mice with 107 trypomastigotes i.p. once, or with two doses given 30 days apart.
Determination of cytokine levels
Spleen cells of immunized mice were treated with ACK buffer for erythrocytes lysis and washed twice in RPMI containing 5 % FBS before being diluted in RPMI 1640 cell culture medium (pH 7.4) supplemented with 10 mM HEPES, 0.2 % sodium bicarbonate, 59 mg of penicillin/liter, 133 mg of streptomycin/liter, and 10 % FBS containing recombinant IL-2 (RD402). Spleen cells number were adjusted to 5 × 106 cells per well in cell culture medium and were cultured with or without 10 μg/mL of rNY-ESO-1 (LICR—Cornel University) or the TSKB20 peptide (ANYKFTLV) (Genscript, Piscataway, NJ) for 48 h in 24-well round-bottom plates. Alternatively, 1 × 106 splenocytes from vaccinated mice were cultured with 2 × 104 B16-NY-ESO-1 cells in 96-well round-bottom plates. IL-2 or IFN-γ levels were measured in cell-free supernatants by ELISA (R&D Systems). To determine the IL-6 or IFN-γ levels in sera or peritoneal fluids, we used the BD Cytometric Bead Array Mouse Inflammation Kit, according to the manufacturer’s instructions.
T cell immunophenotyping and intracellular cytokine measurements
A total of 106 freshly isolated cells were analyzed immediately ex vivo for surface marker expression, or after being cultured for 18 h with either medium alone or with rNY-ESO-1 for intracellular cytokine production. During the last 6 h of culture, Brefeldin A (1 μg/mL; GolgiPlug Protein Transport Inhibitor, BD Biosciences) was added for intracellular cytokine accumulation. To evaluate specific immune response, splenocytes were stained for 30 min at room temperature with MHC-tetramers-PE exhibiting the specific epitopes: NY-ESO-1 (87–94) (H-2 Kb LLEFYLAM), TSKB20 (H-2 Kb) or gp100 (25–33) (H-2Db EGSRNQDWL; all from the LICR Tetramer Facility). Additionally, surface markers were labeled with the following mAbs: anti-CD3-APC-Cy7, anti-CD4-AlexaFluor700, anti-CD8-PE, anti-CD8-AlexaFluor700, anti-CD44-PerCP-Cy5.5, anti-CD127-PE-Cy7, anti-CD11b-PerCP-Cy5.5, anti-F4/80-PE-Cy7, anti-CD11c-AlexaFluor700, and anti-MHCII-APC, all from eBioscience; anti-CD4-Pacific Orange from Caltag–Invitrogen; anti-CD62L-APC from BD Pharmingen. Cells were washed, fixed, and permeabilized according the manufacturer’s instructions (Cytofix/Cytoperm, BD Biosciences). The cells were then stained with anti-granzyme B-Alexa647 (eBioscience), anti-IFN-γ-APC, or anti-FoxP3-FITC (BD Biosciences). At least 200,000 gated events were acquired for the analyses using LSR II with Diva (BD Biosciences). FlowJo (v8.8.6) and GraphPad Prism (v5.0b) were used for data analysis and graphic presentation.
B16-NY-ESO-1 melanoma treatment experiments
C57BL/6 and β2-microglobulin−/− mice were challenged on day 0 with B16-NY-ESO-1 cells. Treatment was initiated on day 3 or 11 depending on the adopted protocol (Supplementary Fig. 1). Doses of anti-CTLA-4 (100 μg) were administered every 3 days for a total of four or five doses. The parasites (107 metacyclic forms) were inoculated every 5 days for a total of two or three doses. Tumor growth was monitored for at least 50 days, and the immune response was analyzed 21 and/or 28 days after challenge with tumor cells. An ELISPOT assay was performed essentially as previously described [17]. The splenocytes were stimulated with the peptides CD4-NY-ESO-1 (FYLAMPFATPMEAEL), CD8-NY-ESO-1 (LLEFYLAM), TSKB20, and rNY-ESO-1 for 18 h. The spots were counted on a Series 5 Core ELISPOT Analyzer (CTL). To collect tumor-infiltrating T cells, tumors were minced and treated with 1 mg/mL of collagenase IA (Sigma-Aldrich) in HBSS for 90 min at room temperature [18], followed by passage through a 100 μm filter. The cells were stained for flow cytometry analysis, as previously described.
Migration of parasites
A total of 2 × 107 metacyclic forms of T. cruzi were incubated with 5 µM CFSE for 10 min at 37 °C under 5 % CO2. Labeled parasites were washed three times and inoculated i.p. After 1, 20 h or 3 days, the mice were subjected to intraperitoneal lavage. Additionally, the mesenteric lymph nodes and spleen were harvested. The cells were processed and stained for surface markers as described previously.
Statistical analyses
Statistical significance for the ELISA, ELISPOT, and cytokine staining assays and immunophenotyping were evaluated using a one-way ANOVA and a nonparametric test followed by Bonferroni posttest. Statistical significance for tumor growth was evaluated using a two-way ANOVA with a Bonferroni posttest.
Results
A homologous prime-boost protocol with CL14-NY-ESO-1 is required to protect mice against challenge with the syngeneic B16 melanoma cell line expressing NY-ESO-1
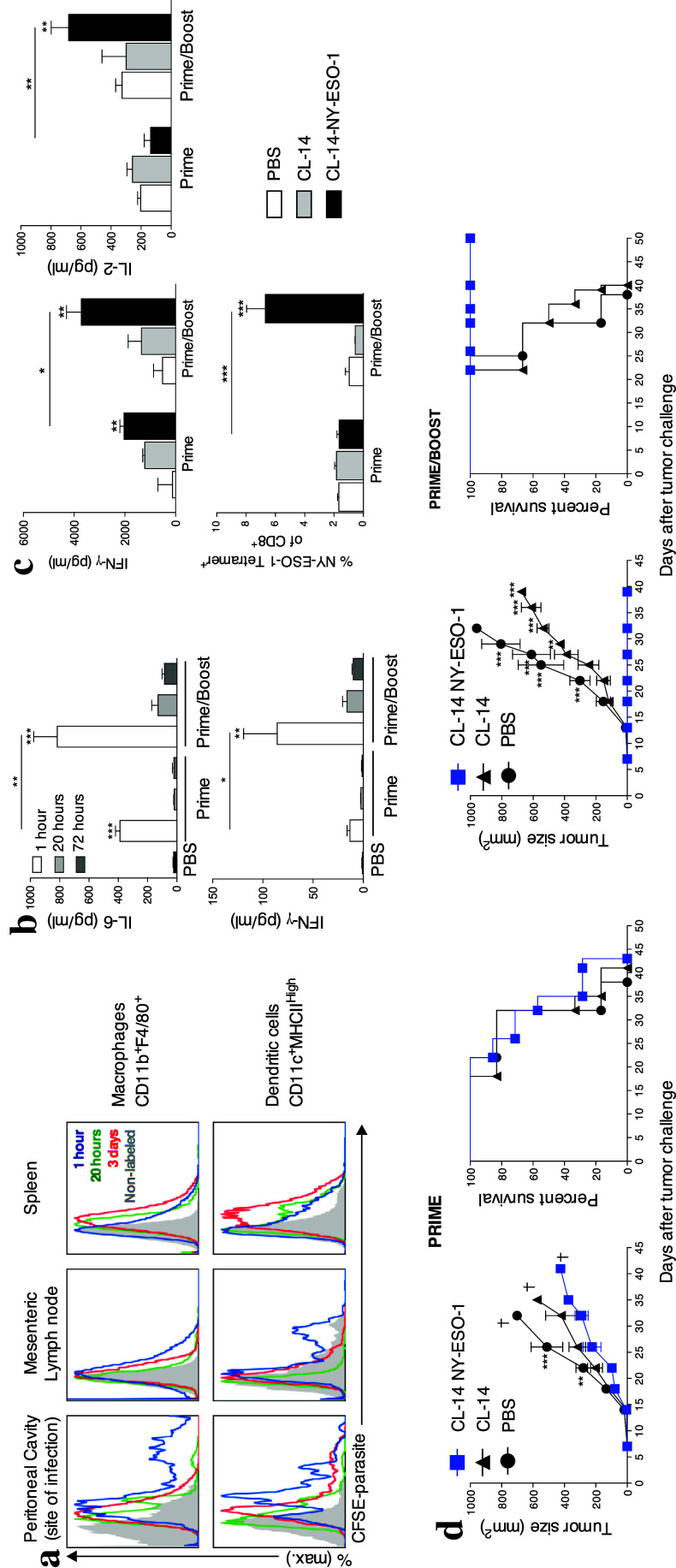
As described previously, the highly attenuated CL-14 strain of T. cruzi is partially impaired in invading host cells [19]. We observed that after internalization of CL-14-NY-ESO-1, dendritic cells, but not macrophages, migrated to the secondary lymphoid tissues to initiate the immune response (Fig. 1a). Thus, we first investigated the number of doses of transgenic T. cruzi required to induce protective immunity in a prophylactic vaccination protocol. Cytokine secretion at the site of infection (Fig. 1b) and activation of T cells in the mesenteric lymph nodes (Supplementary Fig. 2) were increased after the second dose of the parasite (prime/boost) compared with mice that received only one dose (prime). PBS-treated mice were included for all time points (1 h/20 h/72 h), and no significant responses were observed in this group (data not shown). A single dose of CL-14-NY-ESO-1 did not induce high IFN-γ or IL-2 production, nor did it activate NY-ESO-1-specific CD8+ T cells (Fig. 1c). However, after an additional dose of transgenic parasite, mice produced significantly higher levels of IL-2 and IFN-γ, activated large numbers of NY-ESO-1 specific CD8+ T cells, exhibited controlled tumor growth, and survived up to 50 days after challenge (Fig. 1d). Hence, two doses of CL-14-NY-ESO-1 were necessary and sufficient to induce a robust T cell-mediated immune response capable of preventing tumor growth.
Fig. 1.
Two doses of CL-14-NY-ESO-1 were sufficient to ensure antitumor protection. a Ten million metacyclic forms of CL-14-NY-ESO-1 unlabeled (control, gray) or labeled with CFSE were injected intraperitoneally in C57BL/6 mice, and the presence of intracellular parasites was evaluated at 1 h (blue), 20 h (green), and 3 days (red) post-infection. The presence of intracellular parasites in macrophages (CD11b+F4/80+) (upper panels) and dendritic cells (CD11c+MHCIIHigh) (lower panels) was evaluated by flow cytometry. b The peritoneal fluid (site of infection) was collected 1, 20, or 72 h after the infection with one (prime) or two doses (prime/boost) of the transgenic parasite or PBS (control), and the cytokine production was measured by cytometric bead array assay. The statistics at the top of the bar refers to the comparison with group PBS. c To evaluate the level of IFN-γ and IL-2 produced, splenocytes of vaccinated animals were collected and subjected to stimulation with recombinant NY-ESO-1 protein. The profile of NY-ESO-1-specific CD8+ T cells in the spleen was assessed by gating CD3+CD8+ T lymphocytes 21 days post-infection. d Tumor growth and survival rate were evaluated after vaccination and challenge of C57BL/6 mice immunized with one (left panels) or two doses (right panels) of PBS or T. cruzi strain CL-14 expressing or not expressing NY-ESO-1. The challenge with B16-NY-ESO-1 was performed 21 days after the last dose. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-way or one-way ANOVA and Bonferroni posttest. Similar results were observed in three independent experiments with at least four animals in each group
The homologous prime-boost protocol with CL14-NY-ESO-1 induces type 1 cytokine responses by NY-ESO-1-specific T lymphocytes
The population of CD8+ T lymphocytes responsive to the parasite-specific immunodominant peptide TSKB20 expands and contracts during acute infection but is nevertheless maintained during the chronic phase of infection with T. cruzi [12, 20]. We next evaluated the longevity of the specific CD8+ T cell responses. After the prime-boost protocol, we found that both the production of cytokines, assayed after 48 h of stimulation with TSKB20 peptide, and the frequency of TSKB20–specific CD8+ T cells remained high up to 45 days after the boost (Fig. 2a). However, these responses were significantly lower after 85 days. A similar contraction of the CD8+ T cell population was also observed in the specific response to NY-ESO-1 expressed by the transgenic parasite. Upon stimulation with the rNY-ESO-1, we detected the production of high levels of IFN-γ and IL-2 as well as large numbers of NY-ESO-1-specific CD8+ T cells, 21 days after the last immunization (Fig. 2b). In addition, we observed that on day 21, approximately 30 % of the NY-ESO-1-tetramer+ cells were functional and capable of producing IFN-γ following stimulation with rNY-ESO-1 (Fig. 2c, Supplementary Fig. 3a). Although lower, the NY-ESO-1-specific T cell population was maintained on day 45 and became undetectable by day 85 post-immunization. Additionally, splenocytes from vaccinated mice, harvested 21 days after the boost, produced higher levels of IFN-γ when cultured with B16-NY-ESO-1 melanoma cells (Supplementary Fig. 3b).
Fig. 2.
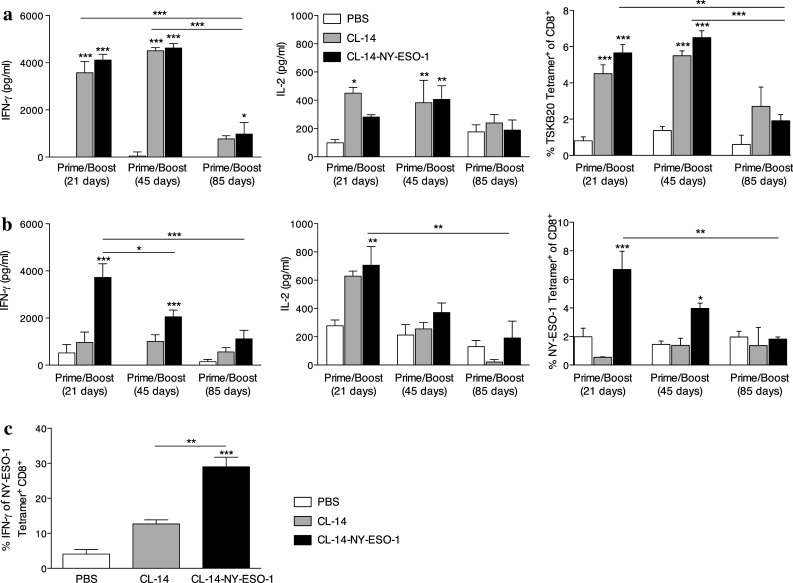
Induction of cytokine production was greater at 21 days after the boost. a, b Activation of antigen-specific immune response was evaluated after a prime/boost protocol at three time points: 21, 45, and 85 days after the boost. The production of the cytokines IFN-γ and IL-2 was measured after stimulating splenocytes for 48 h with either a T. cruzi peptide TSKB20 or b rNY-ESO-1. The culture supernatants were evaluated by ELISA. c NY-ESO-1-specific CD8+ T cells collected 21 days after the boost were re-stimulated with rNY-ESO-1 and evaluated for their ability to produce IFN-γ. The percentage of double-positive cells was summarized in the bar graph. *P < 0.05, **P < 0.01, and ***P < 0.001. Within each time interval, the groups were compared to the control group PBS and this statistic was shown in the top of the bar. Statistical analyses were performed using a one-way ANOVA with a Bonferroni posttest. Data are the mean ± s.e.m. of three to four independent experiments performed in triplicate
Immunization with CL14-NY-ESO-1 induces a high frequency of effector CD8+ T (CD8+TE) cells as well as effector memory cells (CD8+TEM)
The next step was to analyze the phenotypic characteristics of CD8+ T cells induced after immunization with the transgenic parasite. Upon initial priming, naive CD8+ T cells can acquire a variety of effector functions, including cytotoxicity and cytokine production. In mice, short-lived CD8+TE cells express low levels of the IL-7 receptor alpha chain (CD127) and down-regulate L-selectin (CD62L) [21]. Only a few primed cells are maintained as long-term memory cells, a heterogeneous population comprising at least two distinct subtypes: central memory (CD8+TCM) and effector memory (CD8+TEM). The long-living T cells are characterized by their constitutive expression of CD127. CD62L expression is used to further discriminate CD8+TCM (CD62Lhigh) from CD8+TEM cells (CD62Llow) (Supplementary Fig. 4). Prime/boost immunization with the transgenic parasite led to a reduced frequency of naive CD8+ T cells (C44lowCD62high; Fig. 3a) and to a fivefold increase in the proliferation and differentiation of effector CD8+ cells (5.65 ± 0.25 × 106 cells/spleen), compared with the absolute numbers observed in the PBS control group (0.72 ± 0.19 × 106 cells/spleen; Supplementary Fig. 5). Furthermore, when assessed 21 days after the boost, approximately 60 % of activated CD8+ T cells (CD44high) were CD8+TE cells, compared with 36.4 % in the PBS control group (Fig. 3b, c). However, after 45 days, the frequency of CD8+TEM cells increased to 43 % in animals vaccinated with transgenic parasites, while it remained unchanged in the PBS control group (around 30 %). Similarly, for the antigen-specific (tetramer positive) CD8+ T cells, we observed that the majority of TSKB20- and NY-ESO-1-specific cells were CD8+TE 21 days post–boost and became CD8+TEM 45 days after the boost (Fig. 3d, e). Therefore, our findings indicated that transgenic parasites induced a high frequency of effector cells as well as promoting the generation of effector memory cells against the tumor.
Fig. 3.
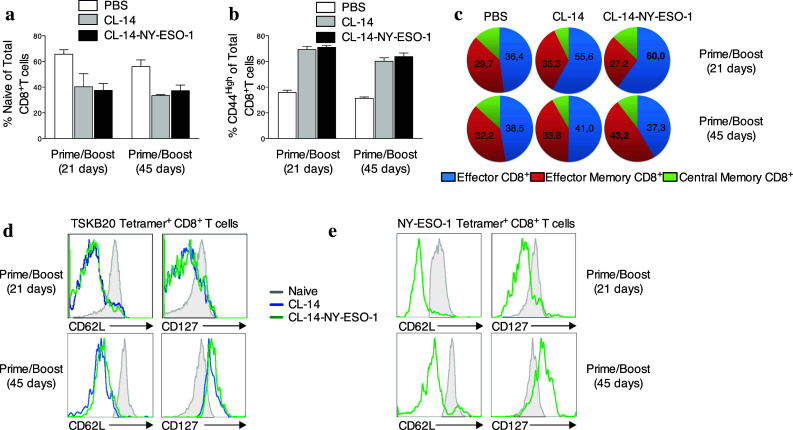
Effector and effector memory CD8+ T cells were generated with immunization. Splenocytes were harvested 21 and 45 days after the boost vaccination. The expression of the surface markers CD44, CD62L and CD127 were used to define subpopulations within the CD3+CD8+ T cells. a The bar graph shows the percentage of naïve cells (CD44lowCD62Lhigh) in the mice vaccinated with parasites at both of time points assessed. b To evaluate the profile of activated cells, CD44high was considered in the gates. c The subpopulations of CD8+ T cells were depicted with different color patterns: effector (CD62LlowCD127low) in blue, effector memory (CD62LlowCD127high) in red, and central memory (CD62LhighCD127high) in green. d, e The profiles of the subpopulation of the cells specific to the parasite and against tumor were evaluated after gating on d TSKB20-tetramers+ and e NY-ESO-1-tetramer+, respectively. The groups that were immunized with CL-14 (blue) or CL-14-NY-ESO-1 (green) are represented in each histogram. The shaded histograms (gray) represent the expression of these molecules on the whole naïve T cells. Similar results were found in three independent experiments with four animals in each group
Immunization with CL14-NY-ESO-1 induces a high frequency of NY-ESO-1-specific CD8+TE cells expressing granzyme B
In functional terms, it has been shown that in addition to effector T cells, effector memory cells also exhibit constitutive levels of lytic activity [22]. The release of cytolytic granules containing protein granzyme B (gzmB) by cytotoxic lymphocytes is an important effector function to exterminate tumor cells. To verify the in vivo expression of this protein in both cell subtypes, we used a transgenic mouse line, gzmBCreERT2/ROSA26EYFP, which allowed the identification of cells transcribing the gzmB gene by the co-expression of EYFP [23]. We first observed that gzmB-positive cells were present in mice immunized with CL-14-NY-ESO-1 (Fig. 4a) both 21 and 45 days after the boost (Fig. 4b). However, these gzmB+ CD8+ T cells changed phenotypically from being predominantly CD8+TE cells at 21 days to almost all being CD8+TEM cells by 45 days after the second dose (Fig. 4c). In agreement with the higher effector function observed on day 21, we observed that more than 30 % of the gzmB+ CD8+ T cells were NY-ESO-1-specific at 21 days after the prime/boost protocol (Fig. 4d). Similar results were observed with respect to gzmB+TSKB20-specific CD8+ T cells (Supplementary Fig. 6a), and the capacity of the vaccine to induce gzmB+ CD8+ T cells was confirmed using an anti-granzyme B monoclonal antibody (Supplementary Fig. 6b). Thus, we conclude that immunization with transgenic parasites promotes the generation of effector CD8+ T cells that are potentially capable of lysing the tumor cells.
Fig. 4.
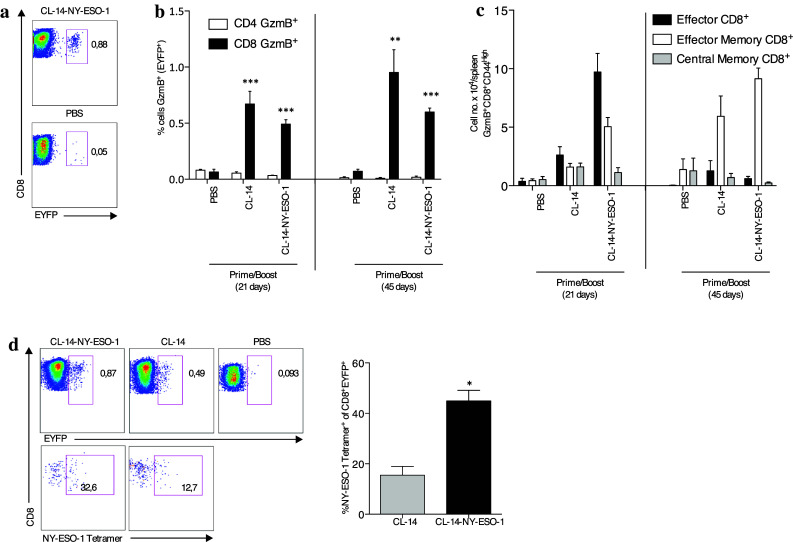
Transgenic parasites were able to induce granzyme B tumor-specific CD8+ T cells. gzmBCreERT2/ROSA26EYFP mice were vaccinated with two doses of PBS or the T. cruzi strain CL-14 expressing or not expressing NY-ESO-1. Treatment with tamoxifen was performed during 5 days as described in the methods section, and the splenocytes were collected 21 and 45 days after the boost vaccination. a The expression of EYFP, which is restricted to cells expressing granzyme B (gzmB), was used for gating. b The frequencies of EYFP+CD3+CD4+ and EYFP+CD3+CD8+ cells were determined. c The different subtypes of CD3+CD8+/gzmB+ lymphocytes were defined from the markers CD44, CD62L, and CD127, and the numbers of cells were plotted. d Representative pseudo-color plots demonstrates the gating of CD8+/gzmB+ cells (upper panels) collected 21 days after the boost and the assessment of the NY-ESO-1-tetramer+ (lower panels). The numbers represent the percentage observed, and the bar graph on the right summarizes the data obtained in three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001. Within each time interval, the groups were compared with the control group PBS. Statistical analyses were performed using one-way ANOVA with Bonferroni posttest. Data are the mean ± s.e.m. of three to four independent experiments performed in triplicate
The high frequency of effector CD8+ T cells correlates with the ability of vaccinated mice to control B16-NY-ESO-1 tumors
After evaluating the immune profile induced by vaccination with CL-14-NY-ESO-1 at different time points, we determined the impact of these responses on the control of tumor growth. We demonstrated that upon an early challenge, 21 days after the boost with the melanoma B16 cell line expressing NY-ESO-1, vaccinated mice could efficiently control tumor growth and exhibited 100 % survival (Fig. 1d). However, upon late challenge at 45 days after boost, we observed a decrease in control of tumor growth and reduced survival (60 %; Fig. 5a). Taken together, the characterization of CD8+ T cell responses and the protection in vaccinated mice suggest that the effector CD8+ T cells induced by the CL-14-NY-ESO-1 are responsible for controlling the tumor growth. However, when used in an immunotherapeutic protocol, the ability of CL14-NY-ESO-1 to control ongoing tumor growth was rather limited [11]. Thus, we were encouraged to use the blockade of CTLA-4 combined with the transgenic parasite to achieve the immune response required to control tumor growth. Therefore, mice were challenged with B16-NY-ESO-1 melanoma and treated with anti-CTLA-4 in the presence or absence of transgenic parasites. Consistent with previous reports in the B16F10 model [24–26], we observed no curative effect of the anti-CTLA-4 treatment alone. However, when combined with transgenic parasite vaccination, CTLA-4 blockade conferred 100 % survival (Fig. 5b). Additionally, tumor growth was reduced fourfold compared with the untreated group and twofold compared with the group treated with the parasite alone. More than 40 % of mice treated with CL-14-NY-ESO-1/anti-CTLA-4 remained tumor-free for at least 12-weeks after challenge (Supplementary Fig. 7). Corroborating our initial hypothesis, we observed that β2-microglobulin−/− mice (deficient in CD8+ T cells) subjected to the same therapeutic protocol were unable to control tumor growth (Fig. 5b, right panel).
Fig. 5.
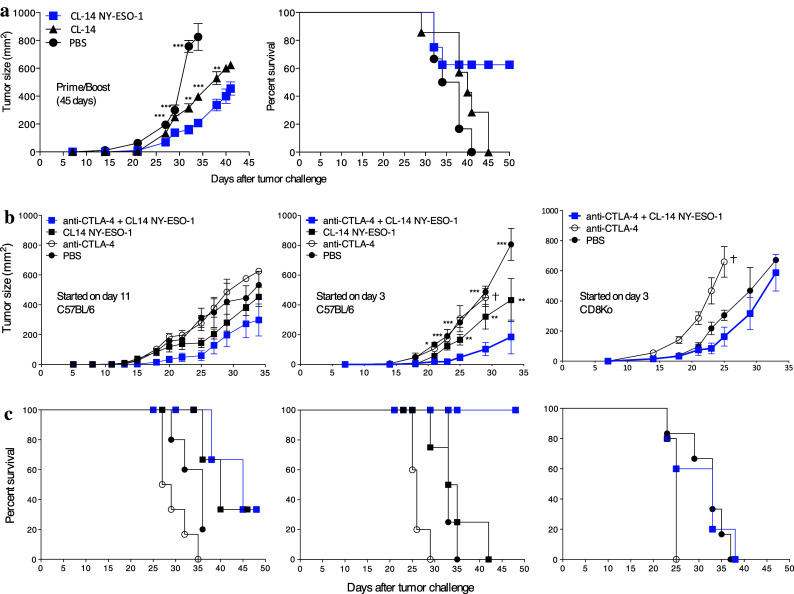
Transgenic parasites control tumor growth. a Prophylactic vaccination efficiency was evaluated in C57BL/6 mice that received a simultaneous prime/boost protocol. The mice were challenged with B16 cells expressing NY-ESO-1 45 days after the last dose. The tumor growth was measured for 45 days, and the rate of survival was observed for 50 days. b For the therapeutic vaccination, the mice were subjected to the treatment starting on day 11 or 3 after challenge and the tumor growth and survival were monitored for 35 and 50 days, respectively. The best result (treatment started on day 3) was applied in the β2-microglobulin−/− mice (c). The tumor growth was compared with the group that received anti-CTLA-4/CL-14NY-ESO-1. *P < 0.05, **P < 0.01, and ***P < 0.001 by a two-way ANOVA and a Bonferroni posttest. Similar results were observed in three independent experiments with five animals in each group
CL14-NY-ESO-1 and anti-CTLA-4 are responsible for the induction and maintenance of NY-ESO-1-specific effector cells
To better understand the immunological mechanism by which treatment with anti-CTLA-4 enhances the efficacy of immunotherapy with CL14-NY-ESO-1, we characterized the phenotypes of CD8+ T cells in mice that received immunotherapy 21 and 28 days after challenge with B16 cells expressing NY-ESO-1. The increased frequency of effector CD8+ T cells in the spleen suggested that the treatment with anti-CTLA-4 enhanced the induction of CD8+TE cells by the transgenic parasites (Fig. 6a). Importantly, we found that blockade of CTLA-4 also significantly enhanced the expansion of NY-ESO-1-tetramer+ T cells (Fig. 6b). The induction of antigen spreading to other melanoma proteins was evaluated using a tetramer specific for the glycoprotein (gp) 100, which is highly expressed in melanocytic cells. We found a significant increase in the frequency of gp100 tetramer+ T cells in treated mice compared with the PBS group (Supplementary Fig. 8a). Next, we evaluated the functional state of CD8+ T cells by measuring the production of IFN-γ, a key cytokine involved in the control of tumor growth. We observed high levels of this cytokine in the mouse sera at 48 h after the last dose of anti-CTLA-4 (Fig. 6c) and a high frequency of IFN-γ producing cells in response to in vitro stimulation with rNY-ESO-1 in mice treated with CL-14-NY-ESO-1/anti-CTLA-4 (Fig. 6d). Interestingly, as previously reported [27, 28], our experiments indicated that the presence of infiltrating CD8+ T cells within tumors was positively correlated with better prognosis of the mice challenged with the B16 melanoma cell lines expressing NY-ESO-1. We observed that approximately 40 % of leukocytes are CD8+ T cells in the tumor infiltrates from mice treated with the CL-14-NY-ESO-1/anti-CTLA-4 and <20 % in animals treated with anti-CTLA-4 (Fig. 6e). Our results indicate that transgenic parasites are responsible for induction of CD8+ T lymphocytes, whereas therapy with CTLA-4 maintains this protective response. Regardless anti-CTLA-4 treatment, no changes in regulatory T cells (Tregs; Fig. 6f; Supplementary Fig. 8b) and ratio of CD8+ T cells and Tregs were observed in the tumor upon CL-14-NY-ESO-1 treatment on day 28 after the challenge (Fig. 6g). It is worth mentioning that anti-CTLA-4 depletes Tregs when this cell subpopulation is assessed 1 or 2 days after treatment [26, 29].
Fig. 6.
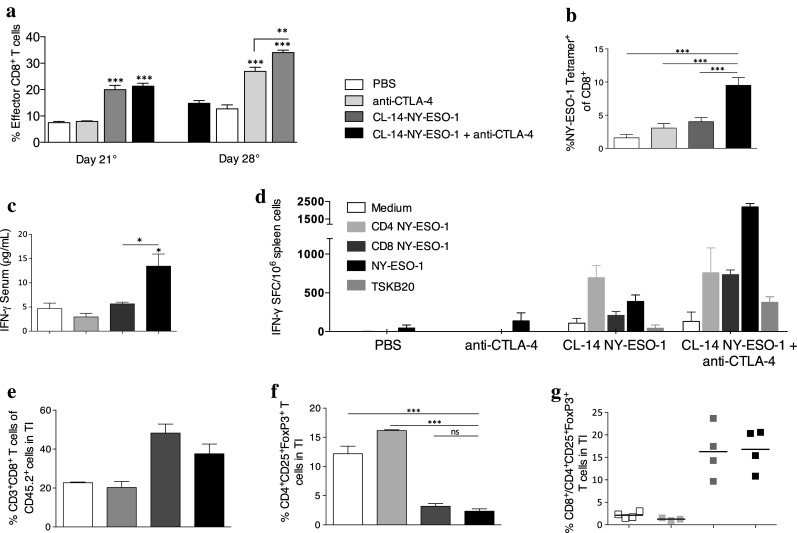
Longevity of specific effector cells protects against tumor development. a C57BL/6 mice were challenged with B16-NY-ESO-1 cells (day 0). After 21 and 28 days, we evaluated the percentage of effector CD8+ T cells in the spleen. The same definition of columns is applied to all figures, except to “panel d”. b The frequency of the tetramer-specific CD8+ T cells was determined by flow cytometry on day 28 for antigen NY-ESO-1. c The production of the cytokine IFN-γ was measured in the serum 48 h after the last dose of anti-CTLA-4 in the treatment. d On day 28 after the challenge, the numbers of IFN-γ-producing spleen cells were estimated by the ELISPOT assay. Cells of individual mice were stimulated in vitro with restricted peptides from NY-ESO-1 (CD4+ T cell epitopes as well as CD8+ T cell epitopes) or NY-ESO-1 recombinant protein or a T. cruzi-specific peptide (TSKB20/ANYDFTLV). e, f At the tumor infiltrate (TI) we observed e CD8+ T cells and f Treg cells (CD4+CD25+FoxP3+). g Ratio of CD8+ T cells to Treg cells was represented. Data are the mean ± s.e.m. of three to five independent experiments performed in triplicate. *P < 0.05, **P < 0.01, and ***P < 0.001 by a one-way ANOVA and a Bonferroni posttest
Discussion
The major challenge in the cancer vaccine field is to induce an effective anti-tumor immune response with an efficient type 1 response and long-term immunological memory. These goals are similar in many infectious diseases, where successful immune protection is ideally induced with live vaccines [4]. Therefore, both virus- and bacteria-based vectors are currently being studied as potential vehicles for antigen and therapeutic gene delivery to immune and tumor cells. Fowlpox virus, attenuated Salmonella strains and Listeria monocytogenes have exhibited great potential as live vectors with broad applications [10, 30, 31]. However, only few clinical trials have been conducted to date, and although they have conclusively shown the safety of some of these systems, the immunogenicity results are less than optimal [31].
The tumoricidal effect of T. cruzi was observed in early experiments where tumor-bearing mice were infected with the parasite. At that time, it was suggested that tumor control might be due to toxins produced by the protozoan [32, 33]. Several clinical studies were conducted later aiming to describe the tumoricidal effect of T. cruzi, but the studies yielded unsatisfactory results, and the tumorigenic action remained unclear [34]. Our group has made valiant efforts in developing an innovative strategy that uses a highly attenuated T. cruzi clone (CL-14) as a vaccine vector for NY-ESO-1 expression, the most immunogenic cancer testis antigen currently described. Using these transgenic parasites as a prophylactic vaccine, we achieved 100 % protection against a syngeneic melanoma cell line [11]. The CL-14 clone was derived from the CL strain, which was isolated in the early 1980s and is consistently avirulent. Both parasitemia and tissue parasitism are absent, even in newborn or immunodeficient mice, which are known to be highly susceptible to T. cruzi infection [35]. We have shown, however, that despite its highly attenuated phenotype, the short-term infection is sufficient to initiate the immune response.
The key to developing an effective antitumor response is the breakdown of immunological tolerance and the activation of antigen-specific T cells with robust functions [36]. There is consensus that an effective cancer vaccine should induce CD8+ T cytotoxic cells and the robust production of cytokines, such as IFN-γ and IL-2, which mediate various effector functions [37]. We believe that at least two attributes make CL-14 a great vaccine vector. To begin with, infection with T. cruzi continuously stimulates the response by the intrinsic expression of TLR agonists, such as glycosylphosphatidylinositol anchors, unmethylated CpG and ssRNA [11, 38, 39]. This results in the polarization of antigen-specific Th1 lymphocytes, which is ideal to control the tumor. Secondly, in its cytoplasmic environment, T. cruzi releases proteins that are processed for presentation by class I MHC molecules. Each intracellular round of replication likely last up to 5 days, thus providing substantial time for the detection of these infected cells by parasite-specific CD8+ T cells [13]. Furthermore, the transgenic CL-14 expressing NY-ESO-1 is able to secrete the CTA into the host cell cytoplasm, leading to direct class I MHC presentation [11].
Here, we demonstrated that two homologous doses of the attenuated parasite CL-14 expressing NY-ESO-1 are able to promote both the expansion of NY-ESO-specific CD8+ T cytotoxic cells expressing granzyme B+ and the production of high levels of IFN-γ and IL-2. A typical CD8+ T cell response, however, consists of three main developmental stages: effector cell expansion and differentiation, effector cell contraction, and stabilization and maintenance of the memory cell population. CD8+ memory cells have been broadly classified into CD8+TCM and CD8+TEM subsets. Similar to other persistent infections, the CD8+TEM cells developed during T. cruzi infection have been shown to be maintained primarily by the continual antigen presentation [13]. The frequency of the CD8+TCM cell subpopulation increases as the infection chronifies [20].
In our model, the attenuated parasite was able to induce CD8+TEM cells; however, most likely due to its elimination, these cells return to basal levels, and only a few CD8+TCM cells were generated. Some studies have also demonstrated that in vitro stimulation of CD8+TCM resulted in the production of IL-2 but little IFN-γ, IL-4 or IL-5 [40]. In contrast, CD8+TEM cells rapidly produced these effector cytokines but produced less IL-2 [41]. Furthermore, only the subpopulation of CD8+TEM cells was found to contain intracellular perforin [41]. Here, we showed that the long-term production of the effector cytokine IFN-γ was correlated with the presence of CD8+TEM cells. The decrease in IL-2 could be related to the stimulation of few CD8+TCM. Moreover, cells expressing the cytotoxic molecule granzyme B, weeks after the boost were CD8+TEM cells. Nevertheless, although the effector functions of effector memory cells are able to be activated, these cells proliferate poorly in response to antigen [42]. In agreement with these descriptions and the need for a robust response to tumor control, we observed that the full protection of the vaccine requires the presence of functional effector cells.
More urgent than the production of an effective vaccine is the establishment of new therapies against cancer. The activation of T cells requires the recognition of specific antigens in concert with co-stimulatory signals from the constitutive CD28 receptor on T cells. Once activated, T cells transiently upregulate the expression of the CTLA-4 receptor. The latter competes with the former for the binding of the same ligands, CD80 and CD86 expressed on the surface of antigen presenting cells. While CD28 engagement promotes T cell activation, CTLA-4 serves as an immune checkpoint, inhibiting cell-cycle progression and IL-2 production. Thus, CTLA-4 signaling provides negative feedback to the activated T cells, thereby dampening the immune response. CTLA-4 deficiency leads to fatal lymphoproliferation and autoimmunity, exemplifying its importance in the physiological negative regulation of T cells [43]. Due to its efficacy, the drug Yervoy® (Ipilimumab, Bristol-Myers Squibb Company), an antibody that binds to CTLA-4, was approved in the U.S. and Europe as an alternative for anti-tumor treatment [44]. Ipilimumab specifically blocks the binding of CTLA-4 to its ligands and thereby augments T cell activation and proliferation and tumor regression [44]. However, ipilimumab works in only 20–30 % of the patients, and severe toxic effects have been reported in monotherapy with this antibody due nonspecific modulation of the immune system [45–47]. Furthermore, CTLA-4 blockade failed to induce rejection of less immunogenic tumors, such as B16 melanoma and SM1 mammary carcinoma [25, 48]. One strategy to minimize the secondary effects and increase the efficiency of anti-CTLA-4 therapy would be the stimulation of specific immune response in a combined therapy with immunological adjuvants or a tumor-specific vaccine [49]. Different studies have demonstrated that infection with T. cruzi provides increased expression of CTLA-4 and that although there is no consensus regarding the effect on CD8+ T cells, the use of anti-CTLA-4 has been reported to enhance host resistance to infection by even more virulent strains [50]. With this in mind, we combined the attenuated transgenic parasite stably expressing NY-ESO-1 as a vaccine with the blockade of CTLA-4, aiming to enhance the parasite action by blocking immunoregulation mechanisms as well as decreasing the several side effects of anti-CTLA-4 administration.
In conclusion, we report that the therapy combining CL14-NY-ESO-1 with anti-CTLA-4 is highly effective in melanoma-bearing mice. The efficiency of this protocol was dependent on the ability of anti-CTLA-4 antibodies to maintain the NY-ESO-1-specific CD8+ T cells induced by the transgenic parasite. We also demonstrated that the therapeutic vaccine contributed to antigen spreading, favoring the expansion and development of T cell response to related tumor antigens, as shown for gp100. Finally, we demonstrated that a consequence of the expansion of CD8+ TE in mice that were treated with CL14-NY-ESO-1 associated with CTLA-4 blockade is the enhanced frequency of CD8+ T cells as well as the CD8+ T/Treg lymphocyte ratio in the tumor microenvironment. As various studies have indicated the clinical relevance of CD8+ T cells and the improved prognosis of patients exhibiting CD8+ T cells in tumor infiltrates, this migration is a critical event in the effectiveness of CL14-NY-ESO-1/CTLA-4 immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank the Ludwig Institute of Cancer Research (LICR) Tetramer Facility for tetramer synthesis; the LICR–Cornell University for the recombinant NY-ESO-1 protein; Dr. James Allison from the Memorial Sloan-Kettering Cancer Center for providing the anti-CTLA-4 monoclonal antibody (9D9 hybridoma); Dr. Jonathan Cebon from LICR–Melbourne for providing the B16-NY-ESO-1 cell lines; Dr. Luiz Travassos from São Paulo University and Dr. Kevin Maloy from University of Oxford for incentive, scientific discussions, and suggestions during the development of this work. Grant support: Atlantic Philanthropies/Program of Clinical Discoveries from the LICR, Fundação de Amparo a Pesquisa de Minas Gerais, Fundação Oswaldo Cruz, and the National Institute of Science and Technology for Vaccines/Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- ACK
Ammonium Chloride Potassium
- CD8+TCM
Central Memory CD8+ T cells
- CD8+TE
Effector CD8+ T cells
- CD8+TEM
Effector Memory CD8+ T cells
- CTA
Cancer Testis Antigens
- CTLA-4
Cytotoxic T Lymphocyte Antigen 4
- FBS
Fetal Bovine Serum
- gzmB
Granzyme B
- HBSS
Hank’s Balanced Salt Solution
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- i.p.
Intraperitoneal injection
- IFN
Interferon
- IL
Interleukin
- MHC
Major Histocompatibility Complex
- PBS
Phosphate-buffered saline
- TI
Tumor Infiltrate
- Tregs
Regulatory T cells
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Zhang L, Wen W, Hao J, Zeng P, Qian X, Zhang Y, Yin Y. Induction of HCA587-specific antitumor immunity with HCA587 protein formulated with CpG and ISCOM in mice. PLoS One. 2012;7(10):e47219. doi: 10.1371/journal.pone.0047219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junqueira C, Guerrero AT, Galvao-Filho B, Andrade WA, Salgado AP, Cunha TM, Ropert C, Campos MA, Penido ML, Mendonca-Previato L, Previato JO, Ritter G, Cunha FQ, Gazzinelli RT. Trypanosoma cruzi adjuvants potentiate T cell-mediated immunity induced by a NY-ESO-1 based antitumor vaccine. PLoS One. 2012;7(5):e36245. doi: 10.1371/journal.pone.0036245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94(5):1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YT, Ross DS, Chiu R, Zhou XK, Chen YY, Lee P, Hoda SA, Simpson AJ, Old LJ, Caballero O, Neville AM. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS One. 2011;6(3):e17876. doi: 10.1371/journal.pone.0017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92(6):856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 9.Nicholaou T, Chen W, Davis ID, Jackson HM, Dimopoulos N, Barrow C, Browning J, Macgregor D, Williams D, Hopkins W, Maraskovsky E, Venhaus R, Pan L, Hoffman EW, Old LJ, Cebon J. Immunoediting and persistence of antigen-specific immunity in patients who have previously been vaccinated with NY-ESO-1 protein formulated in ISCOMATRIX. Cancer Immunol Immunother. 2011;60(11):1625–1637. doi: 10.1007/s00262-011-1041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, Lele S, duPont N, Edwards R, Shrikant P, Old LJ, Gnjatic S, Jager E. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA. 2012;109(15):5797–5802. doi: 10.1073/pnas.1117208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junqueira C, Santos LI, Galvao-Filho B, Teixeira SM, Rodrigues FG, DaRocha WD, Chiari E, Jungbluth AA, Ritter G, Gnjatic S, Old LJ, Gazzinelli RT. Trypanosoma cruzi as an effective cancer antigen delivery vector. Proc Natl Acad Sci USA. 2011;108(49):19695–19700. doi: 10.1073/pnas.1110030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin DL, Weatherly DB, Laucella SA, Cabinian MA, Crim MT, Sullivan S, Heiges M, Craven SH, Rosenberg CS, Collins MH, Sette A, Postan M, Tarleton RL. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2(8):e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padilla AM, Bustamante JM, Tarleton RL. CD8+ T cells in Trypanosoma cruzi infection. Curr Opin Immunol. 2009;21(4):385–390. doi: 10.1016/j.coi.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwek SS, Dao V, Roy R, Hou Y, Alajajian D, Simko JP, Small EJ, Fong L. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189(7):3759–3766. doi: 10.4049/jimmunol.1201529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maraskovsky E, Sjolander S, Drane DP, Schnurr M, Le TT, Mateo L, Luft T, Masterman KA, Tai TY, Chen Q, Green S, Sjolander A, Pearse MJ, Lemonnier FA, Chen W, Cebon J, Suhrbier A. NY-ESO-1 protein formulated in ISCOMATRIX adjuvant is a potent anticancer vaccine inducing both humoral and CD8+ T-cell-mediated immunity and protection against NY-ESO-1 + tumors. Clin Cancer Res. 2004;10(8):2879–2890. doi: 10.1158/1078-0432.CCR-03-0245. [DOI] [PubMed] [Google Scholar]
- 16.DaRocha WD, Silva RA, Bartholomeu DC, Pires SF, Freitas JM, Macedo AM, Vazquez MP, Levin MJ, Teixeira SM. Expression of exogenous genes in Trypanosoma cruzi: improving vectors and electroporation protocols. Parasitol Res. 2004;92(2):113–120. doi: 10.1007/s00436-003-1004-5. [DOI] [PubMed] [Google Scholar]
- 17.Streeck H, Frahm N, Walker BD. The role of IFN-gamma Elispot assay in HIV vaccine research. Nat Protoc. 2009;4(4):461–469. doi: 10.1038/nprot.2009.7. [DOI] [PubMed] [Google Scholar]
- 18.Mitsui J, Nishikawa H, Muraoka D, Wang L, Noguchi T, Sato E, Kondo S, Allison JP, Sakaguchi S, Old LJ, Kato T, Shiku H. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16(10):2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 19.Atayde VD, Neira I, Cortez M, Ferreira D, Freymuller E, Yoshida N. Molecular basis of non-virulence of Trypanosoma cruzi clone CL-14. Int J Parasitol. 2004;34(7):851–860. doi: 10.1016/j.ijpara.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J Immunol. 2008;181(4):2644–2650. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101(15):5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323(5913):505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6(4):e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T, Ramqvist T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One. 2012;7(6):e38711. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 29.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 30.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1(5):471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 31.Moreno M, Kramer MG, Yim L, Chabalgoity JA. Salmonella as live trojan horse for vaccine development and cancer gene therapy. Curr Gene Ther. 2010;10(1):56–76. doi: 10.2174/156652310790945566. [DOI] [PubMed] [Google Scholar]
- 32.Roskin G. Toxin therapy of experimental cancer; the influence of protozoan infections upon transplanted cancer. Cancer Res. 1946;6:363–365. [PubMed] [Google Scholar]
- 33.Klyueva NG, Roskin G. Cancerolytic substance of Schizotrypanum cruzi. Am Rev Sov Med. 1946;4(2):127–129. [PubMed] [Google Scholar]
- 34.Hauschka TS, Goodwin MB. Trypanosoma cruzi endotoxin (KR) in the treatment of malignant mouse tumors. Science. 1948;107(2788):600–602. doi: 10.1126/science.107.2788.600. [DOI] [PubMed] [Google Scholar]
- 35.Lima MT, Lenzi HL, Gattass CR. Negative tissue parasitism in mice injected with a noninfective clone of Trypanosoma cruzi. Parasitol Res. 1995;81(1):6–12. doi: 10.1007/BF00932410. [DOI] [PubMed] [Google Scholar]
- 36.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2(4):293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 37.Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195(10):1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartholomeu DC, Ropert C, Melo MB, Parroche P, Junqueira CF, Teixeira SM, Sirois C, Kasperkovitz P, Knetter CF, Lien E, Latz E, Golenbock DT, Gazzinelli RT. Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. J Immunol. 2008;181(2):1333–1344. doi: 10.4049/jimmunol.181.2.1333. [DOI] [PubMed] [Google Scholar]
- 39.Caetano BC, Carmo BB, Melo MB, Cerny A, dos Santos SL, Bartholomeu DC, Golenbock DT, Gazzinelli RT. Requirement of UNC93B1 reveals a critical role for TLR7 in host resistance to primary infection with Trypanosoma cruzi. J Immunol. 2011;187(4):1903–1911. doi: 10.4049/jimmunol.1003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2(5):415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 42.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27(6):985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Corse E, Allison JP. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J Immunol. 2012;189(3):1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 44.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13(1):29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 46.Heger M. Cancer immunotherapy shows promise in multiple tumor types. Nat Med. 2012;18(7):993. doi: 10.1038/nm0712-993. [DOI] [PubMed] [Google Scholar]
- 47.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M, Garbe C, Gutzmer R, Grabbe S, Hauschild A, Hein R, Hundorfean G, Justich A, Keller U, Klein C, Mateus C, Mohr P, Paetzold S, Satzger I, Schadendorf D, Schlaeppi M, Schuler G, Schuler-Thurner B, Trefzer U, Ulrich J, Vaubel J, von Moos R, Weder P, Wilhelm T, Goppner D, Dummer R, Heinzerling LM. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95(17):10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martins GA, Tadokoro CE, Silva RB, Silva JS, Rizzo LV. CTLA-4 blockage increases resistance to infection with the intracellular protozoan Trypanosoma cruzi. J Immunol. 2004;172(8):4893–4901. doi: 10.4049/jimmunol.172.8.4893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.